Abstract
Activation of NFκB is a fundamental cellular event central to all inflammatory diseases. Hepatocyte growth factor (HGF) ameliorates both acute and chronic inflammation in a multitude of organ systems through modulating NFκB activity; nevertheless, the exact molecular mechanism remains uncertain. Here we report that HGF through inactivation of GSK3β suppresses NFκB p65 phosphorylation specifically at position Ser-468. The Ser-468 of RelA/p65 situates in a GSK3β consensus motif and could be directly phosphorylated by GSK3β both in vivo and in vitro, signifying Ser-468 of RelA/p65 as a putative substrate for GSK3β. In addition, the C terminus of RelA/p65 harbors a highly conserved domain homologue of the consensus docking sequence for GSK3β. Moreover, this domain was required for efficient phosphorylation of Ser-468 and was indispensable for the physical interaction between RelA/p65 and GSK3β. HGF substantially intercepted this interaction by inactivating GSK3β. Functionally, phosphorylation of Ser-468 of RelA/p65 was required for the induced expression of a particular subset of proinflammatory NFκB-dependent genes. Diminished phosphorylation at Ser-468 by HGF resulted in a gene-specific inhibition of these genes' expression. The action of HGF on proinflammatory NFκB activation was consistently mimicked by a selective GSK3β inhibitor or GSK3β knockdown by RNA interference but largely abrogated in cells expressing the mutant uninhibitable GSK3β. Collectively, our findings suggest that HGF has a potent suppressive effect on NFκB activation, which is mediated by GSK3β, an important signaling transducer controlling RelA/p65 phosphorylation specificity and directing the transcription of selective proinflammatory cytokines implicated in inflammatory kidney disease.
Hepatocyte growth factor (HGF)2 is a mesenchymally derived, pleiotropic, and multifunctional cytokine (1–3). Upon binding to its cognate tyrosine kinase receptor, c-Met, HGF activates multiple signaling pathways (4, 5), including phosphatidylinositol 3-kinase-Akt, Ras-Mek-Erk, and the Stat3 pathways, and thereby modulates a variety of cell processes, including mitogenesis, motogenesis, morphogenesis, and antiapoptosis/prosurvival in a number of cell types (1–7). In vivo, HGF has been shown to protect against acute and chronic injury in multiple organ systems, including liver (8), lung (9), intestine (10), and kidney (11). Among the mechanisms potentially responsible for this beneficial action, a growing body of evidence from this (12, 13) and other groups (9, 14–17) suggests that HGF has potent anti-inflammatory effects. One fundamental event that is central to the pathophysiology of inflammation is the activation of NFκB, a nuclear transcription factor that controls the expression of numerous proinflammatory molecules (18, 19). Studies show that HGF suppresses NFκB activation in cultured cells in vitro following TNF-α stimulation (13, 20, 21) and in vivo in inflamed organs, such as the kidney (20, 22). However, the molecular mechanism responsible for this inhibitory effect remains uncertain.
Activation and regulation of NFκB is a complex and highly orchestrated process, controlled by many signal transducers (23, 24), including GSK3β (25, 26). GSK3β is a well conserved ubiquitously expressed proline-directed serine/threonine protein kinase constitutively active in quiescent cells (27–29). Following HGF binding to c-Met, phosphatidylinositol 3-kinase-Akt is activated and phosphorylates GSK3β at Ser-9 (30). Once phosphorylated, GSK3β is inactivated (27). GSK3β resides at the nexus of multiple signaling pathways implicated in the regulation of NFκB activation and inflammatory response (27, 28). In fact, recent data have indicated that GSK3β is an essential element for NFκB activation (25, 27). Genetic disruption of GSK3β abrogates NFκB activation and NFκB-mediated inflammatory responses to TNF-α or IL-1β (interleukin-1β) (31). Moreover, GSK3β regulates NFκB target gene expression in a gene-specific manner (32). Consistent with this hypothesis, we demonstrated that GSK3β inactivation is required for HGF suppression of multiple NFκB-dependent proinflammatory chemokines and adhesion molecules (12, 30), strongly suggesting that GSK3β is involved in the anti-inflammatory action of HGF. In this study, we further examined the role of GSK3β in mediating HGF suppression of NFκB activation and the inflammatory response.
EXPERIMENTAL PROCEDURES
Cell Culture—Human proximal tubular epithelial cells (HKC-8) (courtesy of Dr. Racusen of John Hopkins University, Baltimore, MD) were maintained in Dulbecco's modified Eagle's medium/F-12 supplemented with 5% fetal bovine serum. Cells were plated at ∼70% confluence in the medium containing 5% fetal bovine serum for 24 h and then underwent serum starvation for another 24 h. Human recombinant HGF and human recombinant TNF-α (R&D Systems, Minneapolis, MN) were added to the culture with fresh serum-free medium at a final concentration of 20 and 2 ng/ml, respectively, or otherwise as indicated. Cell viability was assessed by trypan blue exclusion. At different time points, cells and conditioned media were harvested for further investigation.
Western Immunoblot Analysis—After different treatments, HKC cells were washed with PBS and lysed with radioimmune precipitation buffer supplemented with protease inhibitors (1% Nonidet P-40, 0.1% SDS, 100 μg/ml phenylmethysulfonyl fluoride, 0.5% sodium deoxycholate, 1 mm sodium orthovanadate, 2 μg/ml aprotin, 2 μg/ml leupeptin, 5 mm EDTA in PBS). Protein concentration was determined by using a bicinchoninic acid protein assay kit (Sigma). Samples with equal amounts of total protein (50 μg/ml) were fractionated by 7.5–15% SDS-polyacrylamide gels under reducing conditions and analyzed by Western immunoblot as described previously (12). The antibodies against p-GSK3β, p-NFκB RelA/p65, and p-IκBa were purchased from Cell Signaling Technology (Beverly, MA), and those for GSK3β and hemagglutinin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Plasmids and Transient Transfections—The expression plasmid for the p65, pGEX-p65-(354–551) was a kind gift of Dr. Michael Kracht (Frankfurt, Germany). GST fusion proteins were expressed in bacteria and purified on GSH-Sepharose using standard procedures. The eukaryotic expression vector encoding the influenza hemagglutinin (HA)-tagged wild type RelA/p65 (pcDNA3.1-p65-HA) was generously provided by Dr. Jonathan D. Licht (Chicago, IL). Standard PCR was applied to generate the vectors encoding each of the two truncation mutants of p65, Δ31 and Δ61, in which 31 or 61 amino acid residues of the C terminus of p65 were deleted. The vector encoding the mutant RelA/p65 in which the Ser-468 was replaced by alanine (S468A) was derived from pcDNA3.1-p65-HA using the standard technique of QuikChange (Stratagene, La Jolla, CA) site-directed mutagenesis according to the manufacturer's instructions and confirmed by sequencing. The eukaryotic expression vectors encoding the HA-tagged wild type (WT-GSK3β-HA/pcDNA3) (33) and uninhibitable mutant (S9A-GSK3β-HA/pcDNA3) (34) GSK3β were respectively provided by Dr. Jim Woodgett (Toronto, Canada) and Dr. Gail V. W. Johnson (Birmingham, AL). Transient transfection of HKC cells was carried out by using the Lipofectamine 2000 according to the instructions specified by the manufacturer (Invitrogen). After transfection with equal amounts of expression plasmid or empty vector pcDNA3 (Invitrogen), HKC cells were subjected to different treatments as indicated.
GSK3β Gene Silencing—To selectively silence GSK3β, the RNA interference technique was applied. The complete coding sequence of human GSK3β (GenBank™ accession number NM_002093) was used for determining the small interference RNA (siRNA). The siRNA sequence was provided, synthesized, and validated by Ambion (Austin, TX) and did not show nearly exact matches to any other known sequence on a BLAST search, confirming sequence specificity for the human GSK3β. In addition, a scrambled sequence (5′-AATGTACTCACTACGAGTGCG-3′) with no nearly exact match to any known sequence was designed as control for GSK3β siRNA. HKC cells were seeded in 12-well plates at a cell density of 1 × 105 cells/well 24 h before transfection. Lipofectamine-mediated transfection of the siRNA was performed according to the manufacturer's instructions. Cells were maintained for 48 h and used for further treatments. RNA interference efficiency was assessed by Western immunoblot analysis of cellular content of GSK3β protein. Cells that were transfected with scrambled siRNA were used as control.
Luciferase Reporter Gene Assay—The reporter construct pGL-3κB-Luc, a firefly luciferase reporter gene driven by three copies of an NFκB consensus sequence, was applied as described previously. HKC cells were cultured to 70% confluence and then subjected to transfection of the reporter construct pGL-3κB-Luc by using the Lipofectamine 2000. A fixed amount (50 ng) of internal control reporter Renilla reniformis luciferase driven under thymidine kinase promoter (pRL-TK; Promega, Madison, WI) was co-transfected for normalizing the transfection efficiency. After different treatments, total cell lysates were collected, and luciferase activity was determined using the Dual-Glo luciferase assay kit (Promega). The relative NFκB transactivation activity was assessed as the -fold change of firefly luciferase activity after normalization for R. reniformis luciferase activity.
In Vitro Kinase Assay—Recombinant GSK3β (500 units; Cell Signaling Technology) was incubated with 50 ng of GST-p65 fusions proteins in 1× GSK3β reaction buffer supplemented with 135 μm ATP in a total volume of 30 μl for 30 min at 30 °C. Reactions were stopped by the addition of SDS-polyacrylamide gel electrophoresis sample buffer, and phosphorylation of proteins was detected and visualized by Western immunoblot analysis using specific antibodies.
Immunoprecipitation—Immunoprecipitation was carried out using an established method as described previously (35). Briefly, cells were washed with ice-cold PBS and then lysed with radioimmune precipitation buffer. After preclearing with normal IgG, cell lysates with equal amounts of total protein (0.5 mg of protein) were incubated overnight at 4 °C with 4 μg of specific agarose-conjugated antibodies. The precipitated complexes were collected, washed, and separated on SDS-polyacrylamide gels and blotted with various antibodies as indicated.
Fluorescent Immunocytochemistry—Indirect immunofluorescence staining was performed using an established procedure (30). Briefly, cells cultured on coverslips were washed twice with cold PBS and fixed with cold methanol/acetone (1:1) for 10 min at –20 °C. Following three extensive washings with PBS containing 0.5% bovine serum albumin, the cells were blocked with 20% normal donkey serum in PBS buffer for 30 min at room temperature and then incubated with the specific primary antibodies. Finally, cells were double-stained with 4′,6-diamidino-2-phenylindole to visualize the nuclei. Stained cells were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Results were interpreted using a fluorescence microscope.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)—Total RNA was extracted from ∼1 × 106 cultured HKC cells using TRIzol solution (Invitrogen) according to the instructions specified by the manufacturer. RNA was then diluted to 3 μg/μl in RNase-free distilled water. The first strand cDNA was prepared using 3 μg of RNA, Superscript RT reverse transcriptase (Invitrogen), and oligo(dT) primer according to the manufacturer's instructions. Semiquantitative RT-PCR of MCP-1 (monocyte chemoattractant protein-1), RANTES (regulated on activation normal T cell expressed and secreted), and glyceraldehyde-3-phosphate dehydrogenase was carried out using primers described before. PCR products resolved in ∼1.5–2% agarose gels were photographed under UV light. Alternatively, quantitative real time PCR was carried out on a Stratagene Mx4000 multiplex quantitative PCR system (Stratagene) using primers specific for human MCP-1, RANTES, IκBα, BCL-2, and glyceraldehyde-3-phosphate dehydrogenase (Table 1). All reactions were performed in triplicate with Brilliant® SYBR® Green QPCR Master Mix (Stratagene). Fluorescence values of SYBR Green I dye, representing the amount of product amplified at that point in the reaction, were recorded in real time at both the annealing step and the extension step of each cycle. The Ct value, defined as the point at which the fluorescence signal was statistically significant above background, was calculated for each amplicon in each experimental sample using Stratagene Mx4000 software. This value was then used to determine the relative amount of amplification in each sample by interpolating from the standard curve. The transcript level of each specific gene was normalized to glyceraldehyde-3-phosphate dehydrogenase amplification.
TABLE 1.
Primers used for real time RT-PCR
| Gene | Primers | GenBank™ accession number |
|---|---|---|
| RANTES | ||
| Forward | 5′-CAAAAAGAAGGTCTTCATTACACC-3′ | NM_000579 |
| Reverse | 5′-CCTGTGCCTCTTCTTCTCATTTCG-3′ | |
| MCP-1 | ||
| Forward | 5′-AGCAGCAAGTGTCCCAAAGA-3′ | NM_002982 |
| Reverse | 5′-TTGGGTTTGCTTGTCCAGGT-3′ | |
| IκBα | ||
| Forward | 5′-CCCTGTAATGGCCGGACTG-3′ | NM_020529 |
| Reverse | 5′-AGGAGTGACACCAGGTCAGGA-3′ | |
| BCL-2 | ||
| Forward | 5′-CATGTGTGTGGAGAGCGTCAA-3′ | NM_000657 |
| Reverse | 5′-GCCGGTTCAGGTACTCAGTCA-3′ | |
| Glyceraldehyde-3-phosphate dehydrogenase | ||
| Forward | 5′-CGAGATCCCTCCAAAATCAA-3′ | NM_002046 |
| Reverse | 5′-TTCACACCCATGACGAACAT-3′ |
Statistics—For immunoblot analysis, bands were scanned, and the integrated pixel density was determined using a densitometer and the NIH Image image analysis program. All data are expressed as mean ± S.D. Unless otherwise indicated, all experimental observations were made in triplicate. Statistical analysis of the data from multiple groups was performed by analysis of variance followed by Student-Newman-Kuels tests. Data from two groups were compared by Student's t test. Linear regression analysis was applied to examine possible relationships between two parameters. p < 0.05 was considered significant.
RESULTS
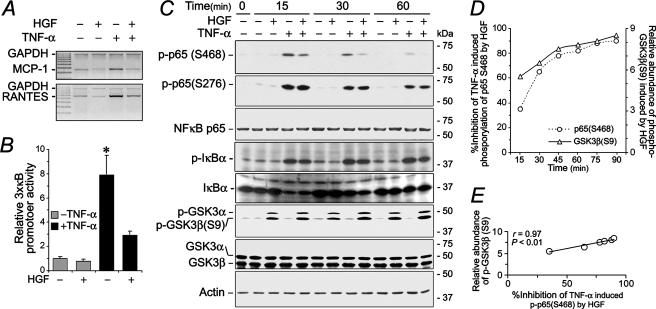
Suppression of NFκB Activity by HGF Is Associated with Inhibitory Phosphorylation of GSK3β—Our previous studies (20, 30) demonstrated that HGF ameliorates renal inflammation in animal models with chronic kidney disease and suppresses renal expression of multiple proinflammatory molecules, including MCP-1 and RANTES, two potent chemoattractant cytokines involved in the recruitment of mononuclear leukocytes to an inflammatory infiltrate. It is likely that HGF has a direct inhibitory effect on expression of these chemokines in tubular cells. As shown in Fig. 1A, HGF largely abrogated the augmented mRNA expression of MCP-1 and RANTES in HKC cells inflamed by TNF-α. Since both MCP-1 and RANTES are prototypical cytokines under tight control of NFκB, we next examined whether the suppressive effect of HGF is mediated by NFκB inhibition and occurs on the transcription level. To this end, an NFκB reporter gene construct was introduced into the HKC cells. (Fig. 1B). TNF-α treatment alone prominently elicited the transcriptional activity of the NFκB consensus promoter in the transfected luciferase reporter gene. This effect was substantially attenuated by HGF concomitant treatment, demonstrating that HGF suppresses NFκB transactivation activity. The NFκB signaling pathway was then dissected to further delineate the molecular mechanism responsible for this phenomenon. NFκB activation, characterized by phosphorylation of specific amino acid residues in the RelA/p65 subunit, is one important prerequisite for transactivation of the target genes. TNF-α treatment induced immediate serine phosphorylation at multiple sites in RelA/p65, including 276, 468, and 536 (data not shown). HGF markedly suppressed TNF-α induced phosphorylation of Ser-468 in a time-dependent fashion but had minimal effect on phosphorylation of Ser-276 (Fig. 1B) or Ser-536 (not shown), indicating that HGF inhibition of NFκB p65 serine phosphorylation is site-specific. In addition, the effect of HGF seems limited only to RelA/p65 activation, without affecting the upstream events of the canonical NFκB signaling pathway, since TNF-α-induced IκBα activation and degradation were minimally affected upon HGF concomitant treatment. Of note, HGF but not TNF-α simultaneously induced GSK3β phosphorylation at Ser-9 (Fig. 1C), which denotes inactivation of GSK3β kinase activity. Shown in Fig. 1C, HGF-induced inhibitory phosphorylation of GSK3β phosphorylation was sustained for at least 90 min and occurred in parallel with suppression of NFκB p65 phosphorylation at Ser-468, exhibiting a significantly strong linear correlationship (correlation coefficient, r = 0.97) in the densitometry values (Fig. 1, D and E). Thus, HGF regulates RelA/p65 phosphorylation at Ser-468, which, in turn, inhibits transactivation of its target genes, and this is associated with concomitant inhibitory phosphorylation of GSK3β.
FIGURE 1.
HGF inhibits NFκB transactivative activity and NFκB phosphorylation at Ser-468 in human kidney tubular epithelial cells, which is closely associated with the inhibitory phosphorylation of GSK3β induced by HGF. A, semiquantitative RT-PCR demonstrates that HGF has a direct inhibitory effect on TNF-α-elicited expression of proinflammatory genes like MCP-1 and RANTES. HKC cells were treated with HGF (20 ng/ml), with TNF-α (2 ng/ml), or in combination for 24 h. Then mRNA was extracted and processed for RT-PCR. B, luciferase reporter gene assay shows that HGF primarily inhibits NFκB transactive activity. HKC cells were transfected with a luciferase reporter construct driven by three copies of consensus NFκB element before different treatments as indicated for 8 h. Cells were then harvested for the luciferase assay. C, HGF suppresses NFκB phosphorylation specifically at Ser-468 but not at other sites like Ser-276; meanwhile, HGF induces inhibitory phosphorylation of GSK3β at Ser-9. D, densitometry analysis of Western immunoblots shows relative inhibition of TNF-α-induced phosphorylation of p65 Ser-468 by HGF as well as relative abundance of phosphorylated GSK3β (Ser-9) induced by HGF (n = 3). E, densitometry analysis of Western immunoblots demonstrates that HGF induced inhibitory phosphorylation of GSK3β at Ser-9 markedly correlates with HGF inhibition of TNF-α-elicited phosphorylation of p65 at Ser-468 with a statistically significant correlation coefficient (r = 0.97; p < 0.01). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
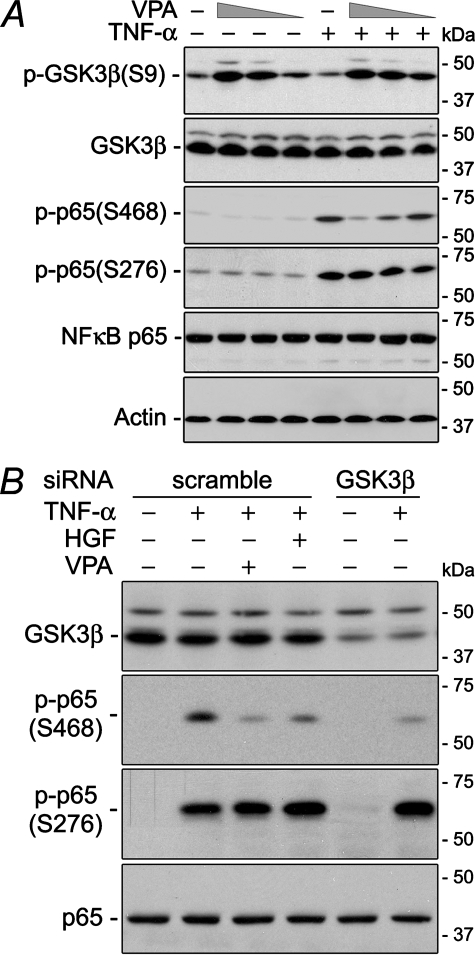
GSK3β Is Required for TNF-α-induced NFκB p65 Phosphorylation at Ser-468—Evidence suggests that GSK3β is involved in NFκB activation (25, 26, 31). In our study, sodium valproate (VPA), a selective pharmaceutical inhibitor of GSK3β (36, 37), induced inhibitory phosphorylation of GSK3β at Ser-9 and, in a parallel fashion, suppressed TNF-α-induced RelA/p65 phosphorylation at Ser-468 in a dose-dependent manner. Phosphorylation of p65 at other sites was minimally affected, reminiscent of the action of HGF (Fig. 2A). To rule out the possible nonspecific effects of the chemical inhibitor, this finding was verified by the gene silencing technique (Fig. 2B). In cells transfected with scrambled siRNA, GSK3β expression was barely altered, and TNF-α treatment consistently induced prominent RelA/p65 phosphorylation at both Ser-468 and Ser-276. Also, HGF or VPA markedly diminished p65 phosphorylation specifically at Ser-468 in these cells, congruent with the above observation. In contrast, in HKC cells where GSK3β was successfully silenced by GSK3β-specific siRNA, TNF-α-elicited RelA/p65 phosphorylation was consistently evident at Ser-276 but substantially obliterated at Ser-468, mimicking the action of HGF or VPA. These data suggest that an active GSK3β is required for TNF-α-induced RelA/p65 phosphorylation at Ser-468, and GSK3β inactivation via phosphorylation at Ser-9 is sufficient for HGF suppression of p65 phosphorylation at Ser-468 induced by TNF-α.
FIGURE 2.
VPA, a selective GSK3β inhibitor, as well as GSK3β knockdown suppresses TNF-α-elicited p65 phosphorylation at Ser-468 and induces inhibitory phosphorylation of GSK3β at Ser-9, reminiscent of the effect of HGF. A, HKC cells were treated with or without TNF-α (2 ng/ml) in the presence or absence of decreasing amounts (1 mm, 100 μm, 10 μm) of VPA for 60 min. Immunoblot assays were carried out on total cell lysates. B, HKC cells were transfected with scrambled or GSK3β-specific siRNA and then treated as indicated for 60 min. Immunoblot analysis was performed on total cell lysates.
Ectopic Expression of an Uninhibitable Mutant GSK3β Abolishes HGF Suppression of NFκB p65 Phosphorylation at Ser-468—To further examine the role of inhibitory phosphorylation of GSK3β in HGF regulation of NFκB p65 phosphorylation, we studied the effect of forced expression of GSK3β. Vectors encoding the HA-conjugated wild type GSK3β (GSK3β-WT) or uninhibitable mutant GSK3β, in which the regulatory Ser-9 residue was changed to alanine (GSK3β-S9A), were transfected to HKC. As a control, the empty vector (EV) was used in parallel. To evaluate the levels of expression, whole cell lysates were analyzed by immunoblotting for HA or HA-GSK3β (Fig. 3, A and B). The constructs were abundantly expressed 24 h after transfection. Immunofluorescent detection using an antibody against the HA epitope revealed that ∼70% of the cells expressed the HA-tagged constructs (Fig. 3A). Shown in Fig. 3B, HGF inhibition of TNF-α-induced phosphorylation of NFκB p65 at Ser-468 was evident in HKC transfected with the empty vector or GSK3β-WT. In contrast, the suppressive effect by HGF was completely abolished in cells expressing S9A, although the transfected vectors were equally expressed into HA-conjugated fusion protein. This finding was confirmed by densitometric analysis (Fig. 3C) of the immunoblots. Collectively, these data suggest that inhibitory phosphorylation of GSK3β at Ser-9 is required for HGF suppression of RelA/p65 phosphorylation at Ser-468.
FIGURE 3.
Forced expression of a mutant uninhibitable GSK3β (S9A) blocks the suppressive effect of HGF on TNF-α induced phosphorylation of p65 at Ser-468. A, HKC cells were transiently transfected with the pcDNA3 EV or the vector encoding the HA-conjugated wild type GSK3β or the mutant GSK3β in which the Ser-9 was replaced by alanine. Whole cell lysates were harvested and analyzed for different molecules by Western immunoblot. Fluorescent immunocytochemistry staining of HA demonstrated that ∼70% cells expressed the vector. B, after they were transfected with different vectors, HKC cells were subjected to different treatments as indicated. Whole cell lysates underwent immunoblot assay. C, densitometric analysis of the immunoblot in B shows that the inhibitory effect of HGF on TNF-α-induced phosphorylation of p65 at Ser-468 is obliterated in HKC cells expressing GSK3β S9A. *, p < 0.05 versus TNF-α-alone-treated cells with the same transfection.
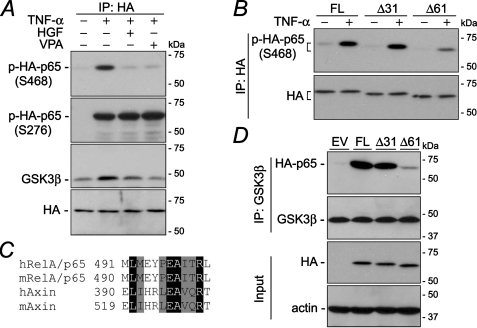
Ser-468 of RelA/p65 Is a Substrate for GSK3β—To further understand how GSK3β regulates selective p65 phosphorylation at Ser-468, the p65 sequence was analyzed and demonstrated that Ser-468 is located at a typical GSK3β phosphorylation motif (SXXXS) within the C-terminal transactivation domain (TAD) of RelA/p65 (Fig. 4A). In contrast, both Ser-276 and Ser-563 (not shown) fail to match the consensus sites for GSK3β, suggesting that position Ser-468 of RelA/p65 might be directly phosphorylated by GSK3β. This hypothesis was greatly supported by the in vitro kinase assay using recombinant RelA/p65 and GSK3β. As shown in Fig. 4B, mixture of GST-p65 with GST-GSK3β led to evident and efficient phosphorylation at Ser-468 but not at Ser-276, suggesting that Ser-468 of p65 is a suitable direct substrate for GSK3β.
FIGURE 4.
RelA/p65 Ser-468 is a suitable direct substrate for GSK3β. A, sequence analysis indicates that Ser-468 but not Ser-276 is located in a GSK3β consensus motif. B, GSK3β directly and efficiently phosphorylates RelA/p65 at Ser-468. Glutathione S-transferase (GST)-conjugated recombinant GSK3β and p65 were prepared for in vitro kinase assay as indicated. Total cell lysates extracted from TNF-α-stimulated HKC cells served as a positive control for different molecules probed.
The C terminus of RelA/p65 is Required for Efficient Phosphorylation at Ser-468—Whether and how Ser-468 is phosphorylated and regulated by GSK3β in vivo was explored next. HKC cells were transfected to ectopically express the HA-p65 fusion protein. TNF-α treatment induced prominent p65 phosphorylation at Ser-468 and Ser-276 (Fig. 5A). In agreement with the finding that GSK3β directly activates p65 Ser-468 in vitro, GSK3β co-immunoprecipitated with ectopic p65. TNF-α treatment enhanced this interaction, and this effect was remarkably attenuated by HGF or VPA, two known GSK3β inhibitors, accompanied with obliterated phsphorylation at Ser-468, strongly suggesting that GSK3β interacts with and phosphorylates p65 in vivo, and Ser-468 of p65 might be a direct substrate for GSK3β in vivo under physiological conditions. To further explore how GSK3β interacts with p65, HKC cells were transfected with vectors encoding full-length p65 (FL) or each of the two truncation mutants of p65, namely Δ31 and Δ61, in which 31 or 61 amino acid residues were deleted from the C terminus of p65 (Fig. 5B). After TNF-α treatment, HA was immunoprecipitated from total cell lysates, and samples were probed for phosphorylated Ser-468. TNF-α elicited abundant phosphorylation at Ser-468 in forcedly expressed full-length p65. This effect was minimally altered in cells expressing p65Δ31 but substantially blunted in cells with p65Δ61, suggesting that the segment harbored by Δ31 but not by Δ61 is vitally required for p65 Ser-468 phosphorylation. It is therefore of enormous interest to find that the sequence of this segment exhibits high similarity to the domain of axin that docks GSK3β and promotes GSK3β-mediated phosphorylation of β-catenin (Fig. 5C). To further test this idea, we examined whether the physical interaction between GSK3β and p65 in TNF-α-stimulated cells could be affected by truncation of the C terminus of p65. GSK3β was immunoprecipitated from whole cell lysates of TNF-α-treated cells that express full-length p65, p65Δ31, p65Δ61, or the empty vector (Fig. 5D). Subsequent immunoblot analysis demonstrated that abundant ectopic p65 co-immunopreciptated with GSK3β in cells transfected with full-length p65. This co-immunoprecipitation was not noticeably affected in cells introduced with p65Δ31 but was robustly reduced in cells expressing p65Δ61, although the ectopic HA-p65 fusion protein was equally expressed in all cells. Overall, these findings indicate that GSK3β phosphorylates RelA/p65 and physically interacts with p65 in vivo. This action is regulated by HGF and requires a C-terminal domain of p65 that is a homologue of the consensus docking sequence for GSK3β.
FIGURE 5.
Physical interaction between RelA/p65 and GSK3β is regulated by HGF and requires the C terminus of p65 protein. A, HKC cells were transfected with vectors encoding wild type full-length p65. After the indicated treatments for 60 min, cell lysates were collected for immunoprecipitation (IP) of HA. Immunoprecipitation samples were subjected to immunoblot analysis for the indicated molecules. B, HKC cells were transfected with vectors encoding the HA-conjugated wild type full-length p65 (FL) or each of the two truncation mutants of p65, namely Δ31 and Δ61. After the indicated treatments for 60 min, cell lysates were collected for immunoprecipitation of HA. Immunoprecipitation samples were subjected to immunoblot analysis for the indicated molecules. C, sequence alignment of the GSK3β-interacting region of axin and part of the C terminus region of RelA/p65 that exists in p65Δ31 but not in p65Δ61. Identical residues are highlighted with black, conserved residues with gray, and mismatched ones with white; h, Homo sapiens; m, Mus musculus. D, HKC cells were transfected with EV or vectors encoding the HA-conjugated wild type full-length p65 or each of the two truncation mutants of p65, namely Δ31 and Δ61. After TNF-α stimulation for 60 min, cell lysates were collected for immunoprecipitation of GSK3β. Immunoprecipitation samples as well as input samples were subjected to immunoblot analysis for the indicated molecules.
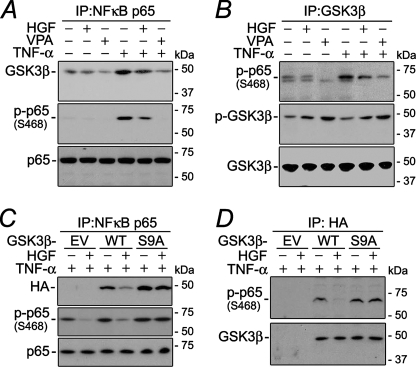
HGF Modulates the Physical Interaction between GSK3β and NFκB p65—The above described interactions between GSK3β and RelA/p65 were made in ectopically expressed fusion protein. Whether the interaction exists between endogenous GSK3β and p65 under physiological conditions was further explored by co-immunoprecipitation. GSK3β was minimally associated with RelA/p65 in untreated cells but markedly co-precipitated with RelA/p65 following TNF-α stimulation (Fig. 6A), suggesting that GSK3β physically interacts with RelA/p65 during the inflammatory response. HGF significantly blocked the TNF-α-induced interaction between GSK3β and RelA/p65, reminiscent of the effect of VPA, the specific GSK3β inhibitor. Moreover, the extent of the association between GSK3β and p65 highly correlated with the magnitude of p65 phosphorylation at Ser-468, consistent with the aforementioned observation that Ser-468 of p65 is a direct physiological substrate for GSK3β. This was further strengthened by the finding that the amount of phosphorylated p65 (Ser-468) co-precipitated with GSK3β was also greatly increased after exposure to TNF-α (Fig. 6B), diminished by HGF or VPA, and strongly correlated with the activity of GSK3β, as inversely denoted by the level of phosphorylation at Ser-9. To further examine whether HGF modulates the physical interaction between GSK3β and p65 through inhibitory phosphorylation of GSK3β, vectors encoding HA-conjugated wild type GSK3β (GSK3β-WT) or GSK3β-S9A or empty vector were introduced into HKC cells (Fig. 6, C and D). Upon TNF-α stimulation, ectopically expressed GSK3β conjugated to HA clearly co-precipitated with NFκB p65 in both WT- and S9A-transfected cells (Fig. 6C). HGF markedly reduced HA-GSK3β fusion protein to co-precipitate with p65 in GSK3β-WT-transfected cells; however, this inhibitory effect was considerably abrogated in HKC cells expressing GSK3β-S9A, in parallel with the attenuation of the suppressive action of HGF on Ser-468 phosphorylation. Similarly, phosphorylated RelA/p65 (Ser-468) co-precipitated with HA-tagged GSK3β following TNF-α stimulation (Fig. 6D). This physical association was significantly overridden by HGF in GSK3β-WT-transfected cells. In contrast, the inhibitory effect of HGF was largely abolished in cells expressing GSK3β-S9A. Taken together, these findings suggest that HGF modulates the physical interaction between endogenous GSK3β and RelA/p65 through inhibitory phosphorylation of GSK3β at Ser-9.
FIGURE 6.
The suppressive effect of HGF on physical interaction between GSK3β and RelA/p65 mimics the action of valproic acid but is overridden in cells expressing the mutant uninhibitable GSK3β. A and B, after different treatments as indicated, whole cell lysates were subjected to immunoprecipitation (IP) by anti-RelA/p65 (A) or anti-GSK3β antibody (B). Immunoprecipitates were then probed for different molecules. C and D, HKC cells were transfected with EV or vectors encoding the HA-conjugated wild type GSK3β (WT) or mutant GSK3β (S9A), in which the serine 9 was replaced by alanine. Then cells were treated with HGF, with TNF-α, or in combination before whole cell lysates were collected and subjected to immunoprecipitation of RelA/p65 (D) or HA (E). Immunoprecipitates were then probed for different molecules as indicated.
GSK3β Inactivation Mediates the Selective Suppression by HGF of NFκB-dependent Proinflammatory Gene Expression—Recent evidence suggests that distinct posttranslational modifications of p65, including serine phosphorylation at specific positions (e.g. Ser-468), control the transcription of different subsets of NFκB target genes (38–42). To understand what is the functional sequel after gain or loss of phosphorylation at Ser-468, vectors encoding the wild type p65 (p65-WT), mutant p65 in which Ser-468 is changed to alanine (p65-S468A), and EV were introduced into HKC cells. Quantitative real time RT-PCR revealed that in response to TNF-α stimulation (Fig. 7A), overexpression of wild type p65 robustly augmented the mRNA expression of NFκB target genes, including MCP-1, RANTES, IL-8 (not shown), IκBα, and BCL-2, which virtually belong to categories of a variety of NFκB biological functions, such as immune response and inflammatory reaction (MCP-1, RANTES, and IL-8), anti-inflammation and NFκB repression (IκBα), and antiapoptosis/prosurvival cally for expression of proinflammatory genes like MCP-1 and RANTES. Provided that HGF specifically suppresses the induced p65 phosphorylation at Ser-468 by GSK3β inactivation, we next asked whether HGF regulates the expression of these NFκB target genes. As shown in Fig. 7B, mRNA expression of all of the above mentioned NFκB target genes was profiled by real time RT-PCR. HGF markedly suppressed TNF-α-induced expression of MCP-1 and RANTES, the proinflammatory chemoattractant cytokines. Besides, HGF also suppressed induced mRNA expression of IL-8 (data not shown). In contrast, TNF-α-induced mRNA expression of IκBα, an anti-inflammatory transcription factor, as well as BCL-2, a prosurvival factor, was not significantly altered by HGF. Consistent with the hypothesis that the anti-inflammatory effects of HGF are mediated by GSK3β, the specific GSK3β inhibitor VPA mimicked the action of HGF on the pattern of expression of these genes. Subsequently, studies were repeated in cells forcedly expressing the wild type (GSK3β-WT) or mutant (GSK3β-S9A) GSK3β or those transfected with the EV. HGF suppression of TNF-α-induced MCP-1 and RANTES expression was preserved in cells transfected with EV or WT but was largely prevented in cells expressing GSK3β-S9A, demonstrating that inhibitory phosphorylation of GSK3β at Ser-9 is essential for HGF suppression of MCP-1 and RANTES expression. Collectively, these findings suggest that HGF selectively inhibits the induced expression of a subset of NFκB target proinflammatory genes through GSK3β inactivation.
FIGURE 7.
HGF selectively suppresses TNF-α-induced expression of specific NFκB target genes, which requires p65 phosphorylation at Ser-468. This effect is reminiscent of the action of the GSK3β inhibitor VPA and obliterated in cells expressing the mutant uninhibitable GSK3β. A, HKC cells were transfected with EV or vectors encoding the HA-conjugated wild type full-length p65 (FL) or the mutants of p65S468A, in which the Ser-468 was replaced by alanine. Cells were then stimulated with TNF-α for 12 h before mRNA extraction. RNA expression levels of MCP-1, RANTES, IκBα, or BCL-2 were then profiled by real time PCR and expressed as -fold induction versus EV-transfected cells treated by TNF-α. B, TNF-α-stimulated HKC cells were treated with HGF or VPA for 12 h before mRNA extraction. In parallel, HKC cells, transfected with empty vector or vectors encoding the wild type GSK3β or the mutant uninhibitable GSK3β (S9A), in which the Ser-9 position was replaced by alanine, were treated with or without HGF upon TNF-α stimulation for 12 h before mRNA extraction. RNA expression levels of MCP-1 (a), RANTES (b), IκBα (c), or BCL-2 (d) were profiled by real time PCR and expressed as -fold induction versus non-TNF-α-treated cells of the same group. *, p < 0.05 versus TNF-α-alone-treated cells in the same experiment.
DISCUSSION
NFκB is a pluripotent nuclear transcription factor implicated in the regulation of multiple cellular processes, including the inflammatory response, innate immunity, antiapoptosis/prosurvival, proliferation, and differentiation (23, 24, 43). NFκB is under the control of multiple signaling pathways and transducers, including GSK3β, which has recently been identified as one important regulator (27–29, 44). In this study, we provided compelling evidence that HGF suppresses NFκB activation and downstream proinflammatory events by inducing inhibitory phosphorylation and inactivation of GSK3β. Our findings suggest that GSK3β controls NFκB activation, directs NFκB-dependent gene expression, and might be used as a novel marker or therapeutic target of injury in human kidney disease.
NFκB is the collective name for a group of structurally related and evolutionally conserved homo- or heterodimeric DNA-binding proteins (45); the most frequent form is the heterodimer of p50 and p65 (RelA). In addition to a Rel homology domain in the N-terminal half that is shared by all members of the NFκB family, RelA/p65 contains two additional strong, acidic transactivation domains in its C-terminal portion named TAD1 and TAD2 (45, 46). Once activated, NFκB transcriptional activity is further regulated by inducible post-translational modifications, including phosphorylation, acetylation, ubiquitilation, or prolyl isomerization (45, 46). A number of different phosphorylation sites have been identified on the RelA/p65 subunit. Several sites located in the N-terminal Rel homology domain, including Ser-276, are essential for NFκB nuclear transportation, subunit dimerization, and DNA binding (46). Other sites located within the C terminus TAD region appear to play a pivotal role in the finer regulation of NFκB transcriptional activity (46, 47). Different phosphorylation patterns may recruit different transcriptional cofactors to the subunit and induce distinct profiles of gene expression (46–48). In support of this hypothesis, Okazaki et al. showed that Ser-276, but not Ser-529 or Ser-536, phosphorylation is required for TNF-α-induced p65-dependent IL-6 expression and protection from cellular death (39). In another study, broad specificity dephosphorylation mediated by active protein phosphatases diminished the TNF-α-provoked NFκB p65/RelA activation, reduced cellular inflammatory response, and substantially promoted cell apoptosis (49), suggesting that nonspecific inhibition of p65 phosphorylation markedly alters diverse NFκB-controlled cellular responses.
Highly selective phosphorylation of specific sites on RelA/p65 may also be an essential element of the regulation of specific components of the inflammatory response. This notion is supported by the work of Mattioli et al. (40), who found that T-cell costimulation, which selectively induces phosphorylation at Ser-468 in human peripheral T lymphocytes, elicited the expression of a specific set of Th1 cytokines, including IL-2 and INF-α. On the other hand, these cytokines were not induced by CD43 stimulation, which also failed to phosphorylate Ser-468 on p65. Similarly, in this study, we found that Ser-468 is required for the induced expression of proinflammatory genes like MCP-1, RANTES, and IL-8 but not dispensable for anti-inflammatory or antiapoptotic/prosurvival genes like IκBα or BCL-2.
HGF, a pleiotropic ligand of the tyrosine kinase receptor c-Met, suppresses both acute and chronic inflammation and injury in a variety of organ systems (12–17). This protective action was recently ascribed, at least in part, to potent anti-inflammatory actions. HGF suppresses NFκB activation induced by multiple proinflammatory stimuli in a variety of cell types (13, 20, 21); however, the specific molecular mechanism by which HGF regulates NFκB is still unclear. Recent evidence suggests that HGF only affects downstream events of NFκB activation, since upstream events like IκBα phosphorylation and degradation are barely altered by HGF. This is again confirmed in our present study in kidney tubular cells. Of note, GSK3β, a downstream substrate of the phosphatidylinositol 3-kinase-Akt signaling pathway triggered by HGF/c-Met, has also been shown to modulate the inflammatory response (50, 51). Furthermore, inhibition of GSK3β only affects NFκB target gene transcription and has no effect on upstream events of NFκB signaling, including nuclear accumulation of NFκB (52). A ubiquitously expressed proline directed serine threonine kinase, GSK3β, is a unique intracellular signal transducer in that it is constitutively active under basal conditions. GSK3β is inactivated in response to phosphatidylinositol 3-kinase-Akt-mediated inhibitory phosphorylation. Recent evidence suggests that GSK3β is critically involved in NFκB signaling transduction and is essential for NFκB activation. Genetic disruption of GSK3β abrogates NFκB activation and NFκB-mediated inflammatory responses to TNF-α or IL-1β (31). In another study, Schwabe and Brenner (53) reported that active GSK3β directly phosphorylated and activated NFκB p65, although the exact substrate position on p65 was not defined. Steinbrecher et al. (32) also reported that GSK3β gene knock-out profoundly altered expression of NFκB-dependent molecules in a gene-specific manner. RelA/p65 sequence analysis demonstrates that Ser-468 is located in the GSK3β consensus motif, suggesting that Ser-468 may be a suitable specific substrate site for GSK3β. Indeed, GSK3β knockdown by RNA interference as well as inhibition of GSK3β activity by HGF or VPA led to loss of p65 phosphorylation specifically at Ser-468, whereas recombinant GSK3β directly and efficiently phosphorylated p65 in vitro specifically at Ser-468 but not at other sites like Ser-276. Thus, our studies demonstrated that GSK3β is essential and sufficient for p65 Ser-468 phosphorylation. In fact, a recent report implicated GSK3β phosphorylation of p65 at Ser-468 in the regulation of basal and cytokine-induced p65 transactivation in HeLa cells (54). In addition, in our study, HGF inhibited phosphorylation of NFκB p65 at Ser-468 but not at Ser-276 or Ser-563. Furthermore, the inhibitory effect on Ser-468 phosphorylation was closely linked with HGF-induced GSK3β inactivation. In fact, GSK3β inactivation was required for HGF-induced inhibition of p65 phosphorylation at Ser-468, since ectopic expression of an uninhibitable GSK3β abolished the inhibitory effect of HGF on Ser-468 phosphorylation. Moreover, we also provide evidence that GSK3β phosphorylation of p65 Ser-468 is dependent on the physical interaction between GSK3β and p65. To efficiently execute the catalytic activity of GSK3β on its substrate requires physical docking and interaction with the substrate protein, as illustrated by the canonical Wnt pathway, in which GSK3β binds to the corresponding docking domain on axin, and GSK3β-mediated phosphorylation of β-catenin is subsequently allowed. Further evidence revealed that a C terminus sequence shows high similarity to the consensus GSK3β-docking domain present in axin and is indispensable for GSK3β interaction with p65 as well as efficient p65 phosphorylation at Ser-468. HGF treatment intercepts this interaction by inactivating GSK3β.
To examine the functional consequences of HGF suppression of NFκB phosphorylation at Ser-468, the expression of multiple NFκB target genes was profiled in renal tubular epithelial cells stimulated with TNF-α in the presence or absence of HGF. Consistent with the findings by Steinbrecher et al. (32) that GSK3β regulates only a subset of NFκB target genes and the contention that Ser-468 is specifically responsible for the induced expression of a selective number of genes, HGF only suppressed the expression of proinflammatory genes like MCP-1, RANTES, and IL-8 but had no effect on other NFκB-dependent genes, including IκBα and BCL-2. This gene-specific suppressive effect of HGF was mimicked by a GSK3β inhibitor and prevented by ectopic expression of the mutant uninhibitable GSK3β, supporting our hypothesis that GSK3β mediates HGF regulation of NFκB activity and expression of a specific subset of proinflammatory target genes. This result is partially consistent with recent work by Chen et al. (55). They reported that GSk3β inhibition suppressed the liver expression of a number of NFκB-responsive proinflammatory molecules, including inducible nitric-oxide synthase, IL-6, and COX-2, in rats with partial hepatectomy; however, the expression of CDK2 or CDK4, NFκB target genes involved in cell proliferation and cycle regulation, was not affected. Taken together, a growing body of evidence suggests that GSK3β through regulation of NFκB activity might direct the transcription of selective target genes. Nevertheless, the exact mechanism by which GSK3β selectively regulates the expression of specific NFκB-dependent proinflammatory genes is uncertain. Further studies are certainly merited to address this issue.
In summary, this study demonstrates that HGF suppresses NFκB p65 phosphorylation specifically at the Ser-468 position through GSK3β inactivation. This effect involves HGF regulation of GSK3β-mediated phosphorylation and interaction with RelA/p65, leading to loss of phosphorylation at Ser-468 of RelA/p65, which is located in a GSK3β consensus motif. Functionally, expression of NFκB target proinflammatory genes requires p65 Ser-468 phosphorylation and is selectively suppressed by HGF in a gene-specific manner through GSK3β inactivation. Our findings suggest that HGF has a potent suppressive effect on NFκB activation, which is mediated by GSK3β, an important signaling transducer controlling RelA/p65 phosphorylation specificity, directing the transcription of selective target genes, and presumably implicated in the pathogenesis of inflammatory kidney disease as well. Moreover, manipulation of GSK3β activity might represent a novel strategy to treat kidney diseases in which inflammation plays an important role.
Acknowledgments
We thank Drs. Kracht, Licht, Johnson, and Woodgett for gifts of expression vectors.
This work was supported by the Young Investigator Research Award from the Rhode Island Foundation for Health and Lifespan Developmental Grant (to R. G.) and National Institutes of Health Grant RO1-DK52314 (to L. D. D.) and AT001465-01A2 (to A. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HGF, hepatocyte growth factor; NFκB, nuclear factor-κB; TNF, tumor necrosis factor; VPA, sodium valproate; HA, influenza hemagglutinin; WT, wild type; PBS, phosphate-buffered saline; siRNA, small interfering RNA; RT, reverse transcription; EV, empty vector; TAD, transactivation domain.
References
- 1.Stoker, M., Gherardi, E., Perryman, M., and Gray, J. (1987) Nature 327 239–342 [DOI] [PubMed] [Google Scholar]
- 2.Montesano, R., Matsumoto, K., Nakamura, T., and Orci, L. (1991) Cell 67 901–908 [DOI] [PubMed] [Google Scholar]
- 3.Rosen, E. M., Nigam, S. K., and Goldberg, I. D. (1994) J. Cell Biol. 127 1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furge, K. A., Zhang, Y. W., and Vande Woude, G. F. (2000) Oncogene 19 5582–5589 [DOI] [PubMed] [Google Scholar]
- 5.Stuart, K. A., Riordan, S. M., Lidder, S., Crostella, L., Williams, R., and Skouteris, G. G. (2000) Int. J. Exp. Pathol. 81 17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boros, P., and Miller, C. M. (1995) Lancet 345 293–295 [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier, C., and Gherardi, E. (1998) Trends Cell Biol. 8 404–410 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto, K., and Nakamura, T. (1991) J. Gastroenterol. Hepatol. 6 509–519 [DOI] [PubMed] [Google Scholar]
- 9.Ware, L. B., and Matthay, M. A. (2002) Am. J. Physiol. 282 L924–L940 [DOI] [PubMed] [Google Scholar]
- 10.Dignass, A. U., and Sturm, A. (2001) Eur. J. Gastroenterol. Hepatol. 13 763–770 [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y. (2004) Am. J. Physiol. 287 F7–F16 [DOI] [PubMed] [Google Scholar]
- 12.Gong, R., Rifai, A., and Dworkin, L. D. (2006) Kidney Int. 69 1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong, R., Rifai, A., and Dworkin, L. D. (2006) J. Am. Soc. Nephrol. 17 2464–2473 [DOI] [PubMed] [Google Scholar]
- 14.Oh, K., Iimuro, Y., Takeuchi, M., Kaneda, Y., Iwasaki, T., Terada, N., Matsumoto, T., Nakanishi, K., and Fujimoto, J. (2005) Am. J. Physiol. 288 G729–G735 [DOI] [PubMed] [Google Scholar]
- 15.Arthur, L. G., Schwartz, M. Z., Kuenzler, K. A., and Birbe, R. (2004) J. Pediatr. Surg. 39 139–143 [DOI] [PubMed] [Google Scholar]
- 16.Arthur, L. G., Kuenzler, K. A., and Schwartz, M. Z. (2003) J. Gastrointest. Surg. 7 1062–1068 [DOI] [PubMed] [Google Scholar]
- 17.Ito, W., Kanehiro, A., Matsumoto, K., Hirano, A., Ono, K., Maruyama, H., Kataoka, M., Nakamura, T., Gelfand, E. W., and Tanimoto, M. (2005) Am. J. Respir. Cell Mol. Biol. 32 268–280 [DOI] [PubMed] [Google Scholar]
- 18.Xiao, C., and Ghosh, S. (2005) Adv. Exp. Med. Biol. 560 41–45 [DOI] [PubMed] [Google Scholar]
- 19.Baeuerle, P. A. (1998) Cell 95 729–731 [DOI] [PubMed] [Google Scholar]
- 20.Gong, R., Rifai, A., Tolbert, E. M., Biswas, P., Centracchio, J. N., and Dworkin, L. D. (2004) J. Am. Soc. Nephrol. 15 2868–2881 [DOI] [PubMed] [Google Scholar]
- 21.Min, J. K., Lee, Y. M., Kim, J. H., Kim, Y. M., Kim, S. W., Lee, S. Y., Gho, Y. S., Oh, G. T., and Kwon, Y. G. (2005) Circ. Res. 96 300–307 [DOI] [PubMed] [Google Scholar]
- 22.Herrero-Fresneda, I., Torras, J., Franquesa, M., Vidal, A., Cruzado, J. M., Lloberas, N., Fillat, C., and Grinyo, J. M. (2006) Kidney Int. 70 265–274 [DOI] [PubMed] [Google Scholar]
- 23.Chen, L. F., and Greene, W. C. (2004) Nat. Rev. Mol. Cell. Biol. 5 392–401 [DOI] [PubMed] [Google Scholar]
- 24.Tak, P.P., and Firestein, G. S. (2001) J. Clin. Invest. 107 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haefner, B. (2003) Drug Discov. Today 8 1062–1063 [DOI] [PubMed] [Google Scholar]
- 26.Deng, J., Xia, W., Miller, S. A., Wen, Y., Wang, H. Y., and Hung, M. C. (2004) Mol. Carcinog. 39 139–146 [DOI] [PubMed] [Google Scholar]
- 27.Doble, B. W., and Woodgett, J. R. (2003) J. Cell Sci. 116 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jope, R. S., and Johnson, G. V. (2004) Trends Biochem. Sci. 29 95–102 [DOI] [PubMed] [Google Scholar]
- 29.Cohen, P., and Frame, S. (2001) Nat. Rev. Mol. Cell. Biol. 2 769–776 [DOI] [PubMed] [Google Scholar]
- 30.Gong, R., Rifai, A., and Dworkin, L. D. (2005) Biochem. Biophys. Res. Commun. 330 27–33 [DOI] [PubMed] [Google Scholar]
- 31.Hoeflich, K. P., Luo, J., Rubie, E. A., Tsao, M. S., Jin, O., and Woodgett, J. R. (2000) Nature 406 86–90 [DOI] [PubMed] [Google Scholar]
- 32.Steinbrecher, K. A., Wilson, W., III, Cogswell, P. C., and Baldwin, A. S. (2005) Mol. Cell. Biol. 25 8444–8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xavier, I. J., Mercier, P. A., McLoughlin, C. M., Ali, A., Woodgett, J. R., and Ovsenek, N. (2000) J. Biol. Chem. 275 29147–29152 [DOI] [PubMed] [Google Scholar]
- 34.Cho, J. H., and Johnson, G. V. (2004) J. Neurochem. 88 349–358 [DOI] [PubMed] [Google Scholar]
- 35.Gong, R., Rifai, A., Tolbert, E. M., Centracchio, J. N., and Dworkin, L. D. (2003) J. Am. Soc. Nephrol. 14 3047–3060 [DOI] [PubMed] [Google Scholar]
- 36.Cohen, P., and Goedert, M. (2004) Nat. Rev. Drug Discov. 3 479–487 [DOI] [PubMed] [Google Scholar]
- 37.Kim, A. J., Shi, Y., Austin, R. C., and Werstuck, G. H. (2005) J. Cell Sci. 118 89–99 [DOI] [PubMed] [Google Scholar]
- 38.Perkins, N. D. (2006) Oncogene 25 6717–6730 [DOI] [PubMed] [Google Scholar]
- 39.Okazaki, T., Sakon, S., Sasazuki, T., Sakurai, H., Doi, T., Yagita, H., Okumura, K., and Nakano, H. (2003) Biochem. Biophys. Res. Commun. 300 807–812 [DOI] [PubMed] [Google Scholar]
- 40.Mattioli, I., Dittrich-Breiholz, O., Livingstone, M., Kracht, M., and Schmitz, M. L. (2004) Blood 104 3302–3304 [DOI] [PubMed] [Google Scholar]
- 41.Ghosh, S. (1999) Immunol. Res. 19 183–189 [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto, K., and Nakamura, T. (2001) Kidney Int. 59 2023–2038 [DOI] [PubMed] [Google Scholar]
- 43.Gilmore, T. D. (2006) Oncogene 25 6680–6684 [DOI] [PubMed] [Google Scholar]
- 44.Pomerantz, J. L., and Baltimore, D. (2000) Nature 406 26–27 [DOI] [PubMed] [Google Scholar]
- 45.Ghosh, S., May, M. J., Kopp, E. B. (1998) Annu. Rev. Immunol. 16 225–260 [DOI] [PubMed] [Google Scholar]
- 46.Hayden, M. S., and Ghosh, S. (2004) Genes Dev. 18 2195–2224 [DOI] [PubMed] [Google Scholar]
- 47.May, M. J., and Ghosh, S. (1998) Immunol. Today 19 80–88 [DOI] [PubMed] [Google Scholar]
- 48.Sheppard, K. A., Rose, D. W., Haque, Z. K., Kurokawa, R., McInerney, E., Westin, S., Thanos, D., Rosenfeld, M. G., Glass, C. K., and Collins, T. (1999) Mol. Cell. Biol. 19 6367–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sreenivasan, Y., Sarkar, A., and Manna, S. K. (2003) Oncogene 22 4356–4369 [DOI] [PubMed] [Google Scholar]
- 50.Jope, R. S., Yuskaitis, C. J., and Beurel, E. (2007) Neurochem. Res. 32 577–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dugo, L., Collin, M., and Thiemermann, C. (2007) Shock 27 113–123 [DOI] [PubMed] [Google Scholar]
- 52.Ougolkov, A. V., Bone, N. D., Fernandez-Zapico, M. E., Kay, N. E., and Billadeau, D. D. (2007) Blood 110 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwabe, R. F., and Brenner, D. A. (2002) Am. J. Physiol. 283 G204–G211 [DOI] [PubMed] [Google Scholar]
- 54.Buss, H., Dorrie, A., Schmitz, M. L., Frank, R., Livingstone, M., Resch, K., and Kracht, M. (2004) J. Biol. Chem. 279 49571–49574 [DOI] [PubMed] [Google Scholar]
- 55.Chen, H., Yang, S., Yang, Z., Ma, L., Jiang, D., Mao, J., Jiao, B., and Cai, Z. (2007) J. Cell. Biochem. 102 1281–1289 [DOI] [PubMed] [Google Scholar]