Abstract
Objective
Women with preeclampsia and those who deliver small for gestational age (SGA) neonates are characterized by intravascular inflammation (T helper 1 (Th1)-biased immune response). There is controversy about the T helper 2 (Th2) response in preeclampsia and SGA. CD30, a member of the tumor necrosis factor receptor superfamily, is preferentially expressed in vitro and in vivo by activated T cells producing Th2-type cytokines. Its soluble form (sCD30) has been proposed to be an index of Th2 immune response. The objective of this study was to determine whether maternal serum concentration of sCD30 changes with normal pregnancy, as well as in mothers with preeclampsia and those who deliver SGA neonates.
Methods
This cross-sectional study included patients in the following groups: (1) non-pregnant women (N=49); (2) patients with a normal pregnancy (N=89); (3) patients with preeclampsia (N=100); and (4) patients who delivered an SGA neonates (N=78). Maternal serum concentration of sCD30 was measured by a specific and sensitive enzyme-linked immunoassay. Non-parametric tests with post-hoc analysis were used for comparisons. A p value <0.05 was considered statistically significant.
Results
(1) The median sCD30 serum concentration of pregnant women was significantly higher than that of non-pregnant women (median: 29.7 U/mL, range: 12.2-313.2 vs. median: 23.2 U/mL, range: 14.6-195.1, respectively; p=0.01); (2) Patients with preeclampsia had a significantly lower median serum concentration of sCD30 than normal pregnant women (median: 24.7 U/mL, range: 7.6-71.2 vs. median: 29.7 U/mL, range: 12.2-313.2, respectively; p<0.05); (3) Mothers with SGA neonates had a lower median concentration of sCD30 than normal pregnant women (median: 23.4 U/mL, range: 7.1-105.3 vs. median: 29.7 U/mL, range: 12.2-313.2, respectively; p<0.05); and (4) There was no significant correlation (r=-0.059, p=0.5) between maternal serum sCD30 concentration and gestational age (19-38 weeks) in normal pregnant women.
Conclusions
(1) Patients with preeclampsia and those who deliver a SGA neonate had a significantly lower serum concentration of sCD30 than normal pregnant women; (2) This finding is consistent with the view that preeclampsia and SGA are associated with a polarized Th1 immune response and, perhaps, a reduced Th2 response.
Keywords: Preeclampsia, sCD30, Th2 immune response, small for gestational age neonate, SGA
Introduction
Preeclampsia and small for gestational age (SGA) neonates are two of “the great obstetrical syndromes”[1]. That are characterized by: (1) multiple etiologies; (2) chronicity; (3) fetal involvment; (4) clinical manifestations that can be adaptive in nature; and (5) susceptibility to gene-environmetn interaction. Both entities share risk factors, such as advanced maternal age [2-4], chronic hypertension [5-8], renal disease [9-11], thrombophilia [12-14], and systemic lupus erythematous (SLE) [15,16]. In addition, mechanisms of disease shared by the two conditions include: (1) abnormal physiologic transformation of the spiral arteries [17-24]; (2) chronic uteroplacental ischemia [25-40]; (3) endothelial cell dysfunction [41-49]; (4) increased trophoblast apoptosis/necrosis [50] and (5) an anti-angiogenic state [51-83].
Preeclampsia and SGA are also characterized by an exaggerated maternal inflammatory response[84-89] and a predominantly T helper 1 (Th1)-biased immune response[90,91]. This is based on observations that a higher expression or concentration of Th1-type cytokines [tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin (IL)-1, IL-2, IL-12, IL-15 and IL-18] and a decreased expression or concentration of T helper 2 Th2-type cytokines (IL-4, IL-5, IL-6, IL-10 and IL-13) is seen in patients with preeclampsia [92-103] and mothers who deliver SGA neonates [104]. However, there is conflicting evidence whether there is an anti-inflammatory response in preeclampsia and SGA[105-107].
CD30, a member of the tumor necrosis factor receptor superfamily [108-110], is preferentially expressed by activated T cells producing Th2-type cytokines in vitro and in vivo [111,112]. Ligation of CD30 with its natural ligand (CD30L) has been associated with cell activation, proliferation, differentiation, and death [113-115]. The soluble form of CD30 (sCD30) results from cleavage of the CD30 extracellular domain by a metalloproteinase [116,117], and it has been proposed to be an index of a Th2 immune response [112]. The objective of this study was to determine the maternal serum concentration of sCD30 in patients with preeclampsia and those who delivered an SGA neonate.
Methods
Study design in population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, including patients in the following groups: (1) non-pregnant women (N=49); (2) patients with a normal pregnancy (N=89); (3) patients with preeclampsia (N=100); and (4) patients who delivered a SGA neonate (N=78). Women with multiple pregnancies and fetal anomalies were excluded.
Definitions
Non-pregnant women included healthy volunteers not taking oral contraceptives who donated blood in the secretory phase of their cycle. Patients were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complication of pregnancy, and delivered a term neonate with a birth weight above the 10th percentile for gestational age [118] without complications. Preeclampsia was diagnosed in the presence of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions 4 hours to 1 week apart, after the 20th week of gestation, and proteinuria ≥300 mg in a 24-hour urine collection, or two random urine specimens obtained 4 hours to 1 week apart containing ≥1+ protein by dipstick [119,120] or one dipstick measurement ≥2+ protein [121]. Severe preeclampsia was defined according to the criteria proposed by the American College of Obstetrics and Gynegology [120]. Patients with preeclampsia were sub-classified as either early-onset (<34 weeks) or late-onset (≥34 weeks) disease according to the gestational age at which preeclampsia was diagnosed. SGA neonate was defined as a birth weight below the 10th percentile for the gestational age at birth [118].
All women provided written informed consent prior to the collection of maternal blood samples. The utilization of samples for research purposes was approved by the iInstitutional review boards of both Wayne State University and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in non-pregnant women, normal pregnant women and those with complications.
Sample collection and soluble human CD30 (sCD30) immunoassays
Samples of peripheral blood from pregnant and non-pregnant women were obtained by venipuncture; blood was centrifuged at 1300 × g for 10 minutes at 4°C. The samples were stored at -70°C until assay. A specific and sensitive enzyme-linked immunoassay was used for the quantitation of human sCD30 in maternal serum. Immunoassay kits for human sCD30 were obtained from Bender MedSystems (Vienna, Austria). Briefly, maternal serum samples were incubated in duplicate wells of the microtiter plates, which had been pre-coated with a monoclonal antibody specific for human sCD30. During this incubation, any sCD30 present in the standards or maternal serum samples is bound by the immobilized antibodies. This step was followed by the addition of an enzyme-linked monoclonal anti-sCD30 antibody to the wells. Following a wash to remove excess and unbound materials, a substrate solution was added to the wells, and color developed in proportion to the amount of sCD30 bound in the initial step. The color development was terminated with the addition of an acid solution, after which the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of sCD30 in maternal plasma samples were determined by interpolation from individual standard curves composed of human sCD30. The calculated inter- and intra-assay coefficients of variation for sCD30 immunoassays in our laboratory were 6.72% and 5.20% respectively. The lower limit of detection (sensitivity) was calculated to be 0.655 U/mLl.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the data was normally distributed. Spearman's rho correlation was used to determine if the maternal serum concentration of sCD30 significantly changed with gestational age at blood draw in patients with normal pregnancies (19 to 38 weeks). Comparisons among groups were performed using the Kruskal-Wallis test with post-hoc test for continuous variables, and Mann-Whitney U test, as well as Chi-square or Fisher's exact test for categorical variables. A p value <0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
Three-hundred sixteen patients were included in this study. The demographic and clinical characteristics of the study groups are displayed in Table I. Among patients with preeclampsia, 63% (63/100) were classified as early-onset and 88% (88/100) as severe preeclampsia. In the SGA group, 76% (59/78) of the patients delivered a neonate with a birth weight below the 5th percentile.
Table I.
Demographic and clinical characteristics of the study groups
| Normal pregnancy (N=89) | Preeclampsia (N=100) | p | SGA (N=78) | p* | |
|---|---|---|---|---|---|
| Maternal age (years) † | 23
(17 - 34) |
25
(14 - 43) |
NS | 23.5
(15 - 43) |
NS |
| BMI (Kg/m2) † | 25.5
(16.3 – 51.6) |
26.3
(18.3 – 44.5) |
NS | 24.9
(14 - 36) |
NS |
| Nulliparity | 21.3
(19/89) |
29.3
(29/99) |
NS | 23.1
(18/78) |
NS |
| Smoking | 20.5
(17/83) |
13.2
(12/91) |
NS | 28.6
(20/70) |
NS |
| Race | |||||
| African-American | 83.1
(74/89) |
80.8
(80/99) |
NS | 84.6
(66/78) |
NS |
| Caucasian | 12.4
(11/89) |
13.1
(13/99) |
NS | 10.3
(8/78) |
NS |
| Other | 4.5
(4/89) |
6.1
(6/99) |
NS | 5.1
(4/78) |
NS |
| Gestational age at blood draw (weeks) † | 31.1
(19.4 – 38.3) |
32.6
(20.0 – 40.9) |
<0.05 | 36.6
(24.4 – 40.3) |
<0.05‡ |
| Gestational age at delivery (weeks) † | 39.6
(37 – 42) |
33.3
(20.1 – 40.9) |
<0.05 | 37.2
(24.9 – 41.7) |
<0.05‡ |
| Birthweight (g) † | 3342
(2550 – 4050) |
1700
(220 – 4460) |
<0.05 | 2130
(300 – 2895) |
<0.05§ |
Values are expressed as percentage (number) or median (range).
SGA: small for gestational age neonate; BMI: body mass index; NS: not significant.
p*: comparison between normal pregnancy and SGA.
: Kruskal-Wallis with post-hoc analysis.
: <0.05 between SGA and normal pregnancy, as well as SGA and preeclampsia.
: <0.05 between SGA and normal pregnancy.
Patients with a normal pregnancy had a significantly higher median serum concentration of sCD30 than non-pregnant women (median: 29.7 U/mL, range: 12.2-313.2 vs. median: 23.2 U/mL, range: 14.6-195.1, respectively; p=0.01) (Figure 1). No significant correlation was found between gestational age and maternal serum sCD30 concentrations in patients with a normal pregnancy (r= -0.059; p=0.5).
Figure 1.
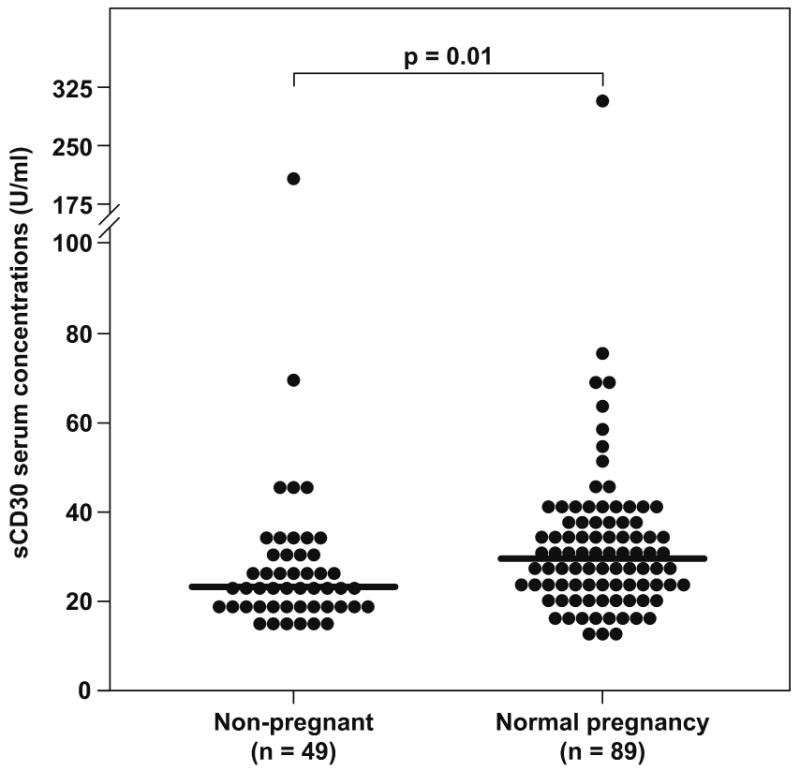
sCD30 serum concentrations in non-pregnant women and those with a normal pregnancy. Patients with a normal pregnancy had a significantly higher median serum concentration of sCD30 than non-pregnant women (median: 29.7 U/mL, range: 12.2-313.2 vs. median: 23.2 U/mL, range: 14.6-195.1, respectively; p=0.01).
Figure 2 displays the maternal serum concentrations of sCD30 among the study groups. Patients with preeclampsia had a significantly lower median maternal serum concentration of sCD30 than those with a normal pregnancy (median: 24.7 U/mL, range: 7.6-71.2 vs. median: 29.7 U/mLl, range: 12.2-313.2, respectively; p<0.05). The same results were found between patients who delivered a SGA neonate and those with a normal pregnancy (median: 23.4 U/mLl, range: 7.1-105.3 vs. median: 29.7 U/mL, range: 12.2-313.2, respectively; p<0.05). No differences were found between patients who delivered a SGA neonate and those with preeclampsia (median: 23.4 U/mL, range: 7.1-105.3 vs. median: 24.7 U/mL, range: 7.6-71.2, respectively; p>0.05).
Figure 2.
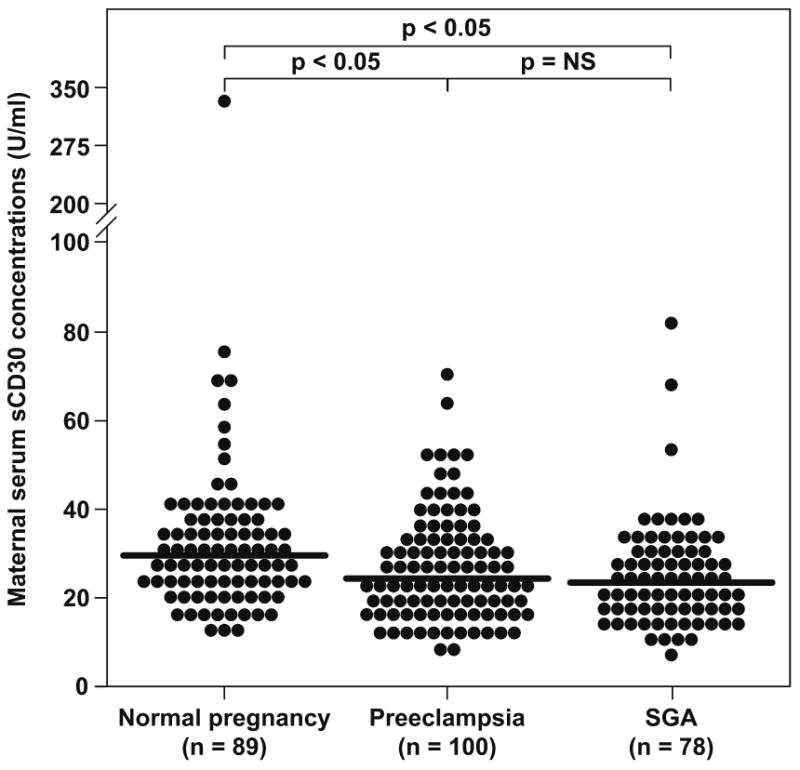
Maternal serum sCD30 concentrations among the study groups. Patients with preeclampsia had a significantly lower median maternal serum concentration of sCD30 than those with a normal pregnancy (median: 24.7 U/mL, range: 7.6-71.2 vs. median: 29.7 U/mL, range: 12.2-313.2, respectively; p<0.05). The same results were found between patients who delivered a SGA neonate and those with a normal pregnancy (median: 23.4 U/mL, range: 7.1-105.3 vs. median: 29.7 U/mLl, range: 12.2-313.2, respectively; p<0.05). No differences were found between patients with a SGA neonate and those with preeclampsia (median: 23.4 U/mL, range: 7.1-105.3 vs. median: 24.7 U/mL, range: 7.6-71.2, respectively; p>0.05). SGA: small for gestational age neonate; NS: not significant.
A sub-analysis among preeclamptic patients demonstrated no significant differences in the median maternal serum concentrations of sCD30 between early- and late-onset preeclampsia (median: 23 U/mL, range: 7.6-71.2 vs. median: 27.4 U/mL, range: 9.6-69.5, respectively; p=0.3), as well as between mild and severe preeclampsia (median: 20.8 U/mL, range: 12.3-39 vs. median: 25.2 U/mL, range: 7.6-71.2, respectively; p=0.2). Similarly, there were no significant differences in the maternal serum concentration of sCD30 between patients who delivered a SGA neonate with a birthweight below the 5th percentile and those with a birthweight between the 5th and 9th percentile (median: 23.5 U/mL, range: 7.1-80.8 vs. median: 22.4 U/mL, range: 13.4-105.3, respectively; p=0.8).
DISCUSSION
Principal findings of this study
(1) Patients with preeclampsia, and those who delivered a SGA neonate, had a significantly lower median serum concentration of sCD30 than normal pregnant women.; (2) Normal pregnancy was associated with a significantly higher sCD30 serum concentration when compared to the non-pregnant state. (3) There was no significant correlation between maternal serum sCD30 concentration and gestational age.
What is sCD30?
CD30, a 120-kD glycoprotein, [122,123] was originally identified as a surface molecule expressed by Hodgkin's and Reed-Sternberg cells in patients with Hodgkin's disease [124,125]. This cytokine receptor is a member of the TNF/nerve growth factor receptor superfamily [108,109,114], considered to be type I transmembrane proteins [126]. The extracellular domain of the CD30 molecule is cleaved by a metalloproteinase [116,117] into an 88-kD soluble CD30 antigen (sCD30) which is released from activated T cells in vitro and in vivo [127]. The CD30 gene is located on chromosome 1p36[128]. Serum concentrations of sCD30 have been found to correlate with CD30 expression, predict allograft rejection [129-132], and to be closely associated with activity, stage, and prognosis of several disorders including SLE, HIV-1, and Hodgkin's disease [133-143].
The conventional view is that CD30 expression is normally restricted to lymphocytes [125,144] and endometrial cells with decidual changes [145]. Indeed, it has been reported that between 3 and 31% of T cells in the peripheral blood of normal healthy donors express CD30 (most of them were found in the CD8+ cell population) [146], as well as in approximately 15% of CD45RO+ T cells after activation with different stimuli [147]. In addition, a subset of CD4+ CD8+ cells in Hassal's corpuscles and thymic medullary epithelial cells show a high expression of CD30L, the natural ligand of CD30 [148]. Macrophages and monocytes do not express CD30 [114].
Depending on cell type and stage of differentiation, the interaction between CD30 and its ligand is associated with cell activation, proliferation, differentiation, and death [113-115]. On activated B cells, CD30 can induce polyclonal immunoglobulin secretion and stimulate B cells proliferation, whereas on activated T cells, CD30 can act as a co-stimulatory receptor, induce cell surface molecules (CD54, CD80, CD86) and cytokine expression, and promote T helper type-2 lymphocytes [149].
Is sCD30 a specific marker for Th2 immune response?
In vitro experiments have demonstrated that sCD30 is preferentially released by human T cells producing Th2-type cytokines. Del Prete et al [111] examined the expression of CD30 and release of sCD30 in CD4+ T cell clones with an established Th0, Th1, and Th2 cytokine secretion profile, generated from peripheral blood mononuclear cells (PBMC) of healthy donors. The results showed that most Th2 clones have both CD30 mRNA and surface CD30 expression, and also released sCD30. In contrast, Th1 clones did not express or express poorly CD30 mRNA and surface CD30, and had low or undetectable concentrations of sCD30, while Th0 clones had an intermediate pattern. Similar findings have been observed in CD8+ T cell clones [150]. Moreover, circulating CD4+ T cells in serum from symptomatic grass-sensitive donors were divided into CD30- and CD30+, and stimulated with an antigen known to induce Th2-type cytokines, showing that only CD30+ T cells produced IL-4 and IL-5 [111].
In addition, increased concentrations of sCD30 have been found in serum of patients with Hodgkin's disease [133,141]. anaplastic large cell lymphomas [136], Wegener's granulomatosis [138], Grave's disease and Hashimoto's thyroiditis [139], HIV-1 [140], chronic hepatitis B [151] measles [152], and in other diseases characterized by a shift towards a Th2 immune response, such as SLE [137], systemic sclerosis [153,154] and Omen's syndrome [155].
Despite compelling evidence supporting the association of sCD30 is associated with a Th2 immune response, some observations have caused some to question this view: 1) knockout mice for CD30 can have normal Th2 differentiation and effector response [156]; and 2) in vitro CD30 expression can be found in Th0- and Th1-type T cell clones [157,158], although CD30 expression was sustained over time by Th2 cells compared to Th0 and Th1 cells [115,157]. In addition, Pellegrini et al [159] reported that CD30 regulates balanced production of different Th1- and Th2-type cytokines, and that the inhibition of CD30/CD30L interaction may be responsible for positive selection of Th1 cells and/or Th2 suppressor. Thus, rather than being a specific marker for Th2 immunity, it has been proposed that sCD30 should be considered as a modulator of the Th1 and Th2 cytokine network [159].
sCD30 and pregnancy
The observation that normal pregnancy is associated with a higher median maternal serum concentration of sCD30 than the non-pregnant state supports the view that normal pregnancy is associated with a shift towards a Th2 immune response [160]. However, this result is not consistent with previous reports [161,162], possibly because of differences in the sample size (those studies included only 10 non-pregnant women). In addition, the maternal serum concentration of sCD30 did not change with advancing gestational age in the present study. However, this is at variance with the results of previous studies in which the maternal serum concentration of sCD30 decreased with gestational age [161,162]. The latter studies included patients in the first trimester. In contrast, the gestational age range of the study presented herein is 19-38 weeks. Thus, it is possible that the maternal plasma concentration of sCD30 may decrease with gestational age as previously reported.
In addition, it has been reported that atopic pregnant patients have a significantly higher serum concentration of sCD30 compared to non-atopic pregnant women. However, no differences were found between the groups in the cord blood sCD30 serum concentration and the placental expression of CD30 and CD30L [163].
Th1/Th2 immunity in Preeclampsia and SGA
Normal pregnancy has been proposed as a Th2-biased state[160]. Evidence supporting this view includes: (1) Th2-type cytokines (IL-3, IL-4, IL-5 and IL-10) are synthesized in the placenta[164,165]; (2) activated splenocytes of pregnant mice produce significantly less IL-2 and more IL-3, IL-4 and IL-6 as pregnancy progresses[166]; (3) mitogen-activated PBMC from pregnant women produce significantly higher concentrations of IL-10 compared to non-pregnant women[167]; (4) studies in murine models demonstrated that administration of pro-inflammatory cytokines is associated with fetal resorption, whereas injection of IL-10 (a Th2-type cytokine) and either anti-IFN-γ or pentoxifillin (an anti-TNF-α agent) can reduce the rate of abortion[168]; (5) a Th1-biased immune response is associated with recurrent abortions[169-174]; and (6) pregnancy has been associated with clinical improvement of Th1-predominant diseases such as rheumatoid arthritis[175] and multiple sclerosis[176]
Although there is a shift towards anti-inflammatory cytokines production, normal pregnancy is considered a systemic pro-inflammatory state. Recent studies have shown that leukocyte activation in patients with normal pregnancy, as well as those with preeclampsia, is similar from patients with Sepsis [84], but these metabolic and phenotypic changes are more remarkable in patients with preeclampsia [84,88]. Thus, preeclampsia is characterized by an excessive maternal pro-inflammatory response to pregnancy. A solid body of evidence supports this view, including: (1) high maternal plasma or serum concentration of IL-2 [92], TNF- [48,95,177-180] and IFN-γ [181]; (2) low maternal plasma concentration of IL-4 [181], IL-10 [182], and IL-18 [183]; and (3) upregulation of mRNA and protein expression of IL-1β [184] and TNF- [184,185] in the placenta. Some evidence also indicates that SGA is associated with a predominant Th1 response [89,90,104,186,187]. In summary, preeclampsia and SGA are considered to be characterized by a Th1 immunity.
sCD30 in preeclampsia and SGA
The observation that preeclampsia and SGA are associated with a lower median serum concentration of sCD30 than normal pregnancy is Novel, and suggests that a reduced Th2 response in these pregnancy complications may also contribute to the imbalance in the Th1/Th2 immune response. This is consistent with previous studies that have reported lower maternal plasma or serum concentrations of Th2-type cytokines in patients with either preeclampsia or SGA, compared to those with normal pregnancies [104,181,182]. Moreover, no significant differences in the maternal serum concentration of sCD30 were found between patients with early- and late-onset preeclampsia, mild and severe preeclampsia, as well as between patients who delivered an SGA neonate with a birth weight below the 5th percentile and those with a birth weight between the 5th and 9th percentile. These findings suggest that a reduced maternal serum concentration of sCD30 is not associated with the severity of preeclampsia and SGA.
Limitations of the study
Limitations of this study include its retrospective nature, which does not allow the performance of flow cytometry in these patients, and that the maternal serum concentrations of pro- and anti-inflammatory cytokines were not measured. Although the measurement of Th2-type cytokines such as IL-4 and IL-10 could be a different approach to demonstrate a Th2 response in pregnant women with preeclampsia sCD30 has been widely proposed and used as an index of Th2 immune response.
Conclusions
(1) Normal pregnancy is associated with a higher maternal serum concentration of sCD30 than the non-pregnant state; and (2) Patients with preeclampsia and those who deliver a SGA neonate had a significantly lower serum concentration of sCD30 than normal pregnant women. These findings are consistent with the view that normal pregnancy is characterized by a Th2-biased immune response, and that preeclampsia and SGA are associated with a polarized Th1 response, maybe due to a reduced Th2 response.
Acknowledgments
The authors wish to acknowledge the contributions of the nursing staff of the Perinatology Research Branch and Detroit Medical Center: Nancy Hauff, Sandy Field, Lorraine Nikita, Vicky Ineson, Mahbubeh Mahmoudieh, Julie McKinley, Sue Rehel, Shannon Donegan, Carolyn Sudz, Sylvia Warren, Gail Barley, Denise Bayoneto, Judy Kerman, Barbara Steffy, and Lynn Laity.
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R. Prenatal medicine: The child is the father of the man. Prenatal and Neonatal Medicine. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH. Risk factors for preeclampsia in healthy nulliparous women: A prospective multicenter study. American Journal Of Obstetrics And Gynecology. 1995;172:642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727–733. doi: 10.1097/01.AOG.0000140682.63746.be. [DOI] [PubMed] [Google Scholar]
- 4.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23:325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 5.Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. American Journal Of Obstetrics And Gynecology. 1994;171:410–416. doi: 10.1016/0002-9378(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: Definitions and standards. Clinical Obstetrics And Gynecology. 1997;40:704–714. doi: 10.1097/00003081-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: A population based study. BMC Pregnancy Childbirth. 2004;4:17. doi: 10.1186/1471-2393-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zetterstrom K, Lindeberg SN, Haglund B, Hanson U. Chronic hypertension as a risk factor for offspring to be born small for gestational age. Acta Obstet Gynecol Scand. 2006;85:1046–1050. doi: 10.1080/00016340500442654. [DOI] [PubMed] [Google Scholar]
- 9.Chao AS, Huang JY, Lien R, Kung FT, Chen PJ, Hsieh PC. Pregnancy in women who undergo long-term hemodialysis. Am J Obstet Gynecol. 2002;187:152–156. doi: 10.1067/mob.2002.123200. [DOI] [PubMed] [Google Scholar]
- 10.Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC., III Chronic renal disease in pregnancy. Obstet Gynecol. 2006;108:1531–1539. doi: 10.1097/01.AOG.0000246790.84218.44. [DOI] [PubMed] [Google Scholar]
- 11.Germain S, Nelson-Piercy C. Lupus nephritis and renal disease in pregnancy. Lupus. 2006;15:148–155. doi: 10.1191/0961203306lu2281rr. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, August P. Genetic thrombophilias and preeclampsia: A meta-analysis. Obstet Gynecol. 2005;105:182–192. doi: 10.1097/01.AOG.0000146250.85561.e9. [DOI] [PubMed] [Google Scholar]
- 13.Brenner B. Thrombophilia and pregnancy complications. Pathophysiol Haemost Thromb. 2006;35:28–35. doi: 10.1159/000093540. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub AY, Sheiner E, Levy A, Yerushalmi R, Mazor M. Pregnancy complications in women with inherited thrombophilia. Arch Gynecol Obstet. 2006;274:125–129. doi: 10.1007/s00404-006-0133-3. [DOI] [PubMed] [Google Scholar]
- 15.Carmona F, Font J, Cervera R, Munoz F, Cararach V, Balasch J. Obstetrical outcome of pregnancy in patients with systemic Lupus erythematosus. A study of 60 cases. Eur J Obstet Gynecol Reprod Biol. 1999;83:137–142. doi: 10.1016/s0301-2115(98)00312-1. [DOI] [PubMed] [Google Scholar]
- 16.Moroni G, Quaglini S, Banfi G, Caloni M, Finazzi S, Ambroso G, Como G, Ponticelli C. Pregnancy in lupus nephritis. American Journal Of Kidney Diseases: The Official Journal Of The National Kidney Foundation. 2002;40:713–720. doi: 10.1053/ajkd.2002.35678. [DOI] [PubMed] [Google Scholar]
- 17.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J Pathol. 1970;101:vi. [PubMed] [Google Scholar]
- 18.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 19.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 20.De Wolf F, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol. 1980;87:678–685. doi: 10.1111/j.1471-0528.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 21.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 22.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 23.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 24.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 25.Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–127. [PubMed] [Google Scholar]
- 26.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4:573–593. [PubMed] [Google Scholar]
- 27.Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 28.Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, Teague MJ. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983;1:675–677. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 29.Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol. 1991;42 Suppl:S14–S20. [PubMed] [Google Scholar]
- 30.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol. 1993;100:989–994. doi: 10.1111/j.1471-0528.1993.tb15139.x. [DOI] [PubMed] [Google Scholar]
- 31.Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultrasound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol. 1996;7:182–188. doi: 10.1046/j.1469-0705.1996.07030182.x. [DOI] [PubMed] [Google Scholar]
- 32.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 33.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 34.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks' gestation. Obstet Gynecol. 2000;96:559–564. doi: 10.1016/s0029-7844(00)00946-7. [DOI] [PubMed] [Google Scholar]
- 35.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18:441–449. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 36.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 37.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–111. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 38.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- 39.Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2004;18:383–396. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53. doi: 10.1186/1477-7827-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 42.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol. 1992;166:962–968. doi: 10.1016/0002-9378(92)91372-h. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 44.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 45.Poston L, Chappell LC. Is oxidative stress involved in the aetiology of pre-eclampsia? Acta Paediatr Suppl. 2001;90:3–5. doi: 10.1111/j.1651-2227.2001.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 46.Bretelle F, Sabatier F, Blann A, D'Ercole C, Boutiere B, Mutin M, Boubli L, Sampol J, Dignat-George F. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG. 2001;108:1277–1282. doi: 10.1111/j.1471-0528.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 47.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 48.Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002;109:836–839. doi: 10.1111/j.1471-0528.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 49.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 50.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 51.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, Gull I, Lessing JB. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod Immunol. 1997;38:302–306. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 52.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:223–228. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 53.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 54.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 58.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 59.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 60.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 61.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 62.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 64.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 66.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 67.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 68.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Levine RJ, Karumanchi SA. Circulating Angiogenic Factors in Preeclampsia. Clin Obstet Gynecol. 2005;48:372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 70.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 71.Malamitsi-Puchner A, Boutsikou T, Economou E, Sarandakou A, Makrakis E, Hassiakos D, Creatsas G. Vascular endothelial growth factor and placenta growth factor in intrauterine growth-restricted fetuses and neonates. Mediators Inflamm. 2005;2005:293–297. doi: 10.1155/MI.2005.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne's syndrome) J Matern Fetal Neonatal Med. 2006;19:607–613. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69:621–624. doi: 10.1038/sj.ki.5000075. [DOI] [PubMed] [Google Scholar]
- 74.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 75.Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med. 2006;354:1857–1858. doi: 10.1056/NEJMc052721. [DOI] [PubMed] [Google Scholar]
- 76.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 77.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Espinoza J, Nien JK, Kusanovic JP, Goncalves LF, Medina LH, Gomez R, Romero R. The combined use of uterine artery Doppler and maternal plasma placental growth factor concentrations identifies patients at risk for early onset and/or severe preeclampsia. Ultrasound Obstet Gynecol. 2006;28:387–388. [Google Scholar]
- 79.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–259. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 80.Boutsikou T, Malamitsi-Puchner A, Economou E, Boutsikou M, Puchner KP, Hassiakos D. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum Dev. 2006;82:235–239. doi: 10.1016/j.earlhumdev.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Padavala S, Pope N, Baker P, Crocker I. An imbalance between vascular endothelial growth factor and its soluble receptor in placental villous explants of intrauterine growth-restricted pregnancies. J Soc Gynecol Investig. 2006;13:40–47. doi: 10.1016/j.jsgi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–57. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 84.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 85.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 86.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 87.Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–522. doi: 10.1016/s1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- 88.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 89.Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82:1099–1102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 90.Hahn-Zoric M, Hagberg H, Kjellmer I, Ellis J, Wennergren M, Hanson LA. Aberrations in placental cytokine mRNA related to intrauterine growth retardation. Pediatr Res. 2002;51:201–206. doi: 10.1203/00006450-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 91.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 92.Sunder-Plassmann G, Derfler K, Wagner L, Stockenhuber F, Endler M, Nowotny C, Balcke P. Increased serum activity of interleukin-2 in patients with pre-eclampsia. J Autoimmun. 1989;2:203–205. doi: 10.1016/0896-8411(89)90156-x. [DOI] [PubMed] [Google Scholar]
- 93.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin Exp Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1757. [PubMed] [Google Scholar]
- 95.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 96.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86:165–170. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 97.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 98.Ida A, Tsuji Y, Muranaka J, Kanazawa R, Nakata Y, Adachi S, Okamura H, Koyama K. IL-18 in pregnancy; the elevation of IL-18 in maternal peripheral blood during labour and complicated pregnancies. J Reprod Immunol. 2000;47:65–74. doi: 10.1016/s0165-0378(00)00058-9. [DOI] [PubMed] [Google Scholar]
- 99.Rein DT, Schondorf T, Gohring UJ, Kurbacher CM, Pinto I, Breidenbach M, Mallmann P, Kolhagen H, Engel H. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J Reprod Immunol. 2002;54:133–142. doi: 10.1016/s0165-0378(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 100.Darmochwal-Kolarz D, Rolinski J, Leszczynska-Goarzelak B, Oleszczuk J. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am J Reprod Immunol. 2002;48:381–386. doi: 10.1034/j.1600-0897.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 101.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47:91–97. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 102.Sakai M, Shiozaki A, Sasaki Y, Yoneda S, Saito S. The ratio of interleukin (IL)-18 to IL-12 secreted by peripheral blood mononuclear cells is increased in normal pregnant subjects and decreased in pre-eclamptic patients. J Reprod Immunol. 2004;61:133–143. doi: 10.1016/j.jri.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Jonsson Y, Matthiesen L, Berg G, Ernerudh J, Nieminen K, Ekerfelt C. Indications of an altered immune balance in preeclampsia: a decrease in in vitro secretion of IL-5 and IL-10 from blood mononuclear cells and in blood basophil counts compared with normal pregnancy. J Reprod Immunol. 2005;66:69–84. doi: 10.1016/j.jri.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Omu AE, Makhseed M, al-Qattan F. The comparative value of interleukin-4 in sera of women with preeclampsia and cord sera. Nutrition. 1995;11:688–691. [PubMed] [Google Scholar]
- 106.Omu AE, al-Qattan F, Diejomaoh ME, Al-Yatama M. Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet Gynecol Scand. 1999;78:675–680. [PubMed] [Google Scholar]
- 107.Engel SA, Olshan AF, Savitz DA, Thorp J, Erichsen HC, Chanock SJ. Risk of small-for-gestational age is associated with common anti-inflammatory cytokine polymorphisms. Epidemiology. 2005;16:478–486. doi: 10.1097/01.ede.0000164535.36412.6b. [DOI] [PubMed] [Google Scholar]
- 108.Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, Dower SK, Cosman D, Goodwin RG. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248:1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- 109.Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 110.Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, Brannan CI, Copeland NG, Jenkins NA, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 111.Del Prete G, De CM, Almerigogna F, Daniel CK, D'Elios MM, Zancuoghi G, Vinante F, Pizzolo G, Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 112.Romagnani S, Del PG, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leukoc Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 113.Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994;83:2045–2056. [PubMed] [Google Scholar]
- 114.Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, Martelli MF, Stein H. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 115.Lee SY, Park CG, Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J Exp Med. 1996;183:669–674. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen HP, Kisseleva T, Kobarg J, Horn-Lohrens O, Havsteen B, Lemke H. A zinc metalloproteinase is responsible for the release of CD30 on human tumor cell lines. Int J Cancer. 1995;63:750–756. doi: 10.1002/ijc.2910630524. [DOI] [PubMed] [Google Scholar]
- 117.Hansen HP, Dietrich S, Kisseleva T, Mokros T, Mentlein R, Lange HH, Murphy G, Lemke H. CD30 shedding from Karpas 299 lymphoma cells is mediated by TNF-alpha-converting enzyme. J Immunol. 2000;165:6703–6709. doi: 10.4049/jimmunol.165.12.6703. [DOI] [PubMed] [Google Scholar]
- 118.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 119.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 120.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 121.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 122.Froese P, Lemke H, Gerdes J, Havsteen B, Schwarting R, Hansen H, Stein H. Biochemical characterization and biosynthesis of the Ki-1 antigen in Hodgkin-derived and virus-transformed human B and T lymphoid cell lines. J Immunol. 1987;139:2081–2087. [PubMed] [Google Scholar]
- 123.Nawrocki JF, Kirsten ES, Fisher RI. Biochemical and structural properties of a Hodgkin's disease-related membrane protein. J Immunol. 1988;141:672–680. [PubMed] [Google Scholar]
- 124.Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982;299:65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 125.Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 126.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 127.Josimovic-Alasevic O, Durkop H, Schwarting R, Backe E, Stein H, Diamantstein T. Ki-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989;19:157–162. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- 128.Fonatsch C, Latza U, Durkop H, Rieder H, Stein H. Assignment of the human CD30 (Ki-1) gene to 1p36. Genomics. 1992;14:825–826. doi: 10.1016/s0888-7543(05)80203-4. [DOI] [PubMed] [Google Scholar]
- 129.Kim KH, Oh EJ, Jung ES, Park YJ, Choi JY, Kim DG, Lee KY, Kang CS. Evaluation of pre- and posttransplantation serum interferon-gamma and soluble CD30 for predicting liver allograft rejection. Transplant Proc. 2006;38:1429–1431. doi: 10.1016/j.transproceed.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 130.Ayed K, Abdallah TB, Bardi R, Abderrahim E, Kheder A. Plasma levels of soluble CD30 in kidney graft recipients as predictors of acute allograft rejection. Transplant Proc. 2006;38:2300–2302. doi: 10.1016/j.transproceed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Dong W, Shunliang Y, Weizhen W, Qinghua W, Zhangxin Z, Jianming T, He W. Prediction of acute renal allograft rejection in early post-transplantation period by soluble CD30. Transpl Immunol. 2006;16:41–45. doi: 10.1016/j.trim.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 132.Sengul S, Keven K, Gormez U, Kutlay S, Erturk S, Erbay B. Identification of patients at risk of acute rejection by pretransplantation and posttransplantation monitoring of soluble CD30 levels in kidney transplantation. Transplantation. 2006;81:1216–1219. doi: 10.1097/01.tp.0000203324.49969.30. [DOI] [PubMed] [Google Scholar]
- 133.Pizzolo G, Vinante F, Chilosi M, Dallenbach F, Josimovic-Alasevic O, Diamantstein T, Stein H. Serum levels of soluble CD30 molecule (Ki-1 antigen) in Hodgkin's disease: relationship with disease activity and clinical stage. Br J Haematol. 1990;75:282–284. doi: 10.1111/j.1365-2141.1990.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 134.Nadali G, Vinante F, Ambrosetti A, Todeschini G, Veneri D, Zanotti R, Meneghini V, Ricetti MM, Benedetti F, Vassanelli A, et al. Serum levels of soluble CD30 are elevated in the majority of untreated patients with Hodgkin's disease and correlate with clinical features and prognosis. J Clin Oncol. 1994;12:793–797. doi: 10.1200/JCO.1994.12.4.793. [DOI] [PubMed] [Google Scholar]
- 135.Pizzolo G, Vinante F, Morosato L, Nadali G, Chilosi M, Gandini G, Sinicco A, Raiteri R, Semenzato G, Stein H, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–745. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Nadali G, Vinante F, Stein H, Todeschini G, Tecchio C, Morosato L, Chilosi M, Menestrina F, Kinney MC, Greer JP, et al. Serum levels of the soluble form of CD30 molecule as a tumor marker in CD30+ anaplastic large-cell lymphoma. J Clin Oncol. 1995;13:1355–1360. doi: 10.1200/JCO.1995.13.6.1355. [DOI] [PubMed] [Google Scholar]
- 137.Caligaris-Cappio F, Bertero MT, Converso M, Stacchini A, Vinante F, Romagnani S, Pizzolo G. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol. 1995;13:339–343. [PubMed] [Google Scholar]
- 138.Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross WL. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener's granulomatosis. Am J Med. 1997;102:517–523. doi: 10.1016/s0002-9343(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 139.Okumura M, Hidaka Y, Kuroda S, Takeoka K, Tada H, Amino N. Increased serum concentration of soluble CD30 in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1997;82:1757–1760. doi: 10.1210/jcem.82.6.4000. [DOI] [PubMed] [Google Scholar]
- 140.Pizzolo G, Vinante F, Nadali G, Krampera M, Morosato L, Chilosi M, Raiteri R, Sinicco A. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997;108:251–253. doi: 10.1046/j.1365-2249.1997.d01-1005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nadali G, Tavecchia L, Zanolin E, Bonfante V, Viviani S, Camerini E, Musto P, Di RN, Carotenuto M, Chilosi M, et al. Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin's disease at high risk of unfavorable outcome. Blood. 1998;91:3011–3016. [PubMed] [Google Scholar]
- 142.Zinzani PL, Pileri S, Bendandi M, Buzzi M, Sabattini E, Ascani S, Gherlinzoni F, Magagnoli M, Albertini P, Tura S. Clinical implications of serum levels of soluble CD30 in 70 adult anaplastic large-cell lymphoma patients. J Clin Oncol. 1998;16:1532–1537. doi: 10.1200/JCO.1998.16.4.1532. [DOI] [PubMed] [Google Scholar]
- 143.Kadin ME. Regulation of CD30 antigen expression and its potential significance for human disease. Am J Pathol. 2000;156:1479–1484. doi: 10.1016/S0002-9440(10)65018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwarting R, Gerdes J, Durkop H, Falini B, Pileri S, Stein H. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood. 1989;74:1678–1689. [PubMed] [Google Scholar]
- 145.Ito K, Watanabe T, Horie R, Shiota M, Kawamura S, Mori S. High expression of the CD30 molecule in human decidual cells. Am J Pathol. 1994;145:276–280. [PMC free article] [PubMed] [Google Scholar]
- 146.Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996;157:3229–3234. [PubMed] [Google Scholar]
- 147.Ellis TM, Simms PE, Slivnick DJ, Jack HM, Fisher RI. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993;151:2380–2389. [PubMed] [Google Scholar]
- 148.Romagnani P, Annunziato F, Manetti R, Mavilia C, Lasagni L, Manuelli C, Vannelli GB, Vanini V, Maggi E, Pupilli C, et al. High CD30 ligand expression by epithelial cells and Hassal's corpuscles in the medulla of human thymus. Blood. 1998;91:3323–3332. [PubMed] [Google Scholar]
- 149.Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10:457–470. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 150.Manetti R, Annunziato F, Biagiotti R, Giudizi MG, Piccinni MP, Giannarini L, Sampognaro S, Parronchi P, Vinante F, Pizzolo G, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180:2407–2411. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fattovich G, Vinante F, Giustina G, Morosato L, Alberti A, Ruol A, Pizzolo G. Serum levels of soluble CD30 in chronic hepatitis B virus infection. Clin Exp Immunol. 1996;103:105–110. doi: 10.1046/j.1365-2249.1996.915607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vinante F, Krampera M, Morosato L, Rigo A, Romagnani S, Pizzolo G. Peripheral T lymphocyte cytokine profile (IFNgamma, IL-2, IL-4) and CD30 expression/release during measles infection. Haematologica. 1999;84:683–689. [PubMed] [Google Scholar]
- 153.Mavalia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, Emmi L, Pupilli C, Pizzolo G, Maggi E, Romagnani S. Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–1758. [PMC free article] [PubMed] [Google Scholar]
- 154.D'Elios MM, Romagnani P, Scaletti C, Annunziato F, Manghetti M, Mavilia C, Parronchi P, Pupilli C, Pizzolo G, Maggi E, et al. In vivo CD30 expression in human diseases with predominant activation of Th2-like T cells. J Leukoc Biol. 1997;61:539–544. [PubMed] [Google Scholar]
- 155.Chilosi M, Facchetti F, Notarangelo LD, Romagnani S, Del PG, Almerigogna F, De CM, Pizzolo G. CD30 cell expression and abnormal soluble CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–334. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 156.Barner M, Kopf M, Lefrang K. CD30 is a specific marker for Th2 cells but is not required for their development. 1997;62:54. [Google Scholar]
- 157.Bengtsson A, Johansson C, Linder MT, Hallden G, van dP I, Scheynius A. Not only Th2 cells but also Th1 and Th0 cells express CD30 after activation. J Leukoc Biol. 1995;58:683–689. doi: 10.1002/jlb.58.6.683. [DOI] [PubMed] [Google Scholar]
- 158.Hamann D, Hilkens CM, Grogan JL, Lens SM, Kapsenberg ML, Yazdanbakhsh M, van Lier RA. CD30 expression does not discriminate between human Th1- and Th2-type T cells. J Immunol. 1996;156:1387–1391. [PubMed] [Google Scholar]
- 159.Pellegrini P, Berghella AM, Contasta I, Adorno D. CD30 antigen: not a physiological marker for TH2 cells but an important costimulator molecule in the regulation of the balance between TH1/TH2 response. Transpl Immunol. 2003;12:49–61. doi: 10.1016/S0966-3274(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 160.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 161.Hoshimoto K, Ohta N, Ohkura T, Inaba N. Changes in plasma soluble CD26 and CD30 during pregnancy: markers of Th1/Th2 balance? Gynecol Obstet Invest. 2000;50:260–263. doi: 10.1159/000010328. [DOI] [PubMed] [Google Scholar]
- 162.Ostensen M, Forger F, Nelson JL, Schuhmacher A, Hebisch G, Villiger PM. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann Rheum Dis. 2005;64:839–844. doi: 10.1136/ard.2004.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ekstrom ES, Bengtsson A, Svensson A, Nilsson C, Ostlund E, Sandstedt B, Bremme K, Lilja G, Scheynius A. Presence of CD30(+) and CD30L(+) cells in human placenta and soluble CD30 levels in cord blood are independent of maternal atopy. Placenta. 2001;22:372–379. doi: 10.1053/plac.2000.0619. [DOI] [PubMed] [Google Scholar]
- 164.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 165.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dudley DJ, Chen CL, Mitchell MD, Daynes RA, Araneo BA. Adaptive immune responses during murine pregnancy: pregnancy-induced regulation of lymphokine production by activated T lymphocytes. Am J Obstet Gynecol. 1993;168:1155–1163. doi: 10.1016/0002-9378(93)90361-l. [DOI] [PubMed] [Google Scholar]
- 167.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 168.Chaouat G, Assal MA, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- 169.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–1936. [PubMed] [Google Scholar]
- 170.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 171.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MM, Hassan NA, Bandar A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol. 1999;42:273–281. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 172.Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod. 2000;15:713–718. doi: 10.1093/humrep/15.3.713. [DOI] [PubMed] [Google Scholar]
- 173.Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16:2219–2226. doi: 10.1093/humrep/16.10.2219. [DOI] [PubMed] [Google Scholar]
- 174.Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am J Reprod Immunol. 2002;48:77–86. doi: 10.1034/j.1600-0897.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 175.Ostensen M, Aune B, Husby G. Effect of pregnancy and hormonal changes on the activity of rheumatoid arthritis. Scand J Rheumatol. 1983;12:69–72. doi: 10.3109/03009748309102886. [DOI] [PubMed] [Google Scholar]
- 176.Al-Shammri S, Rawoot P, Azizieh F, AbuQoora A, Hanna M, Saminathan TR, Raghupathy R. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J Neurol Sci. 2004;222:21–27. doi: 10.1016/j.jns.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 177.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin Exp Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 179.Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 180.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 181.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J Soc Gynecol Investig. 2005;12:335–342. doi: 10.1016/j.jsgi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 182.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 183.Adams KM, Mandel LS, Guthrie KA, Atkinson MW. Interleukin-18 in the plasma of women with preeclampsia. Am J Obstet Gynecol. 2003;188:1234–1237. doi: 10.1067/mob.2003.349. [DOI] [PubMed] [Google Scholar]
- 184.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 186.Heinig J, Wilhelm S, Muller H, Briese V, Bittorf T, Brock J. Determination of cytokine mRNA-expression in term human placenta of patients with gestational hypertension, intrauterine growth retardation and gestational diabetes mellitus using polymerase chain reaction. Zentralbl Gynakol. 2000;122:413–418. doi: 10.1055/s-2000-10606. [DOI] [PubMed] [Google Scholar]
- 187.Holcberg G, Huleihel M, Sapir O, Katz M, Tsadkin M, Furman B, Mazor M, Myatt L. Increased production of tumor necrosis factor-alpha TNF-alpha by IUGR human placentae. Eur J Obstet Gynecol Reprod Biol. 2001;94:69–72. doi: 10.1016/s0301-2115(00)00321-3. [DOI] [PubMed] [Google Scholar]


