Abstract
Mutations in SHANK3, which encodes a synaptic scaffolding protein, have been described in subjects with an autism spectrum disorder (ASD). To assess the quantitative contribution of SHANK3 to the pathogenesis of autism, we determined the frequency of DNA sequence and copy-number variants in this gene in 400 ASD-affected subjects ascertained in Canada. One de novo mutation and two gene deletions were discovered, indicating a contribution of 0.75% in this cohort. One additional SHANK3 deletion was characterized in two ASD-affected siblings from another collection, which brings the total number of published mutations in unrelated ASD-affected families to seven. The combined data provide support that haploinsufficiency of SHANK3 can cause a monogenic form of autism in sufficient frequency to warrant consideration in clinical diagnostic testing.
Autism (MIM 209850) is a neurodevelopmental disorder that manifests within the first 3 years of life. Narrowly defined “autism” has a population prevalence of 1.5–2 cases per 1,000 children and represents the classic pervasive developmental disorder (PDD).1 The group of PDDs that is also termed “autism spectrum disorders” (ASDs) includes autism, as well as PDD–not otherwise specified (PDD-NOS) and Asperger syndrome. ASDs have a prevalence of ∼6 cases per 1,000 children.1 The three core characteristics of autism are impairments of reciprocal social interactions, impairments in communication, and a restricted range of behaviors and interests. In contrast to those with autism, patients with Asperger syndrome differ in that they show an absence of clinically significant cognitive and language delay before age 3 years.2
ASDs are etiologically heterogeneous. They are associated with a recognized cause in ∼10% of cases, most commonly with fragile X syndrome (MIM 300624), tuberous sclerosis (MIM 191100), and cytogenetically detectable chromosome abnormalities.3,4 The most frequent cytogenetic anomaly is maternally derived duplication of chromosome 15q11-q13 (1%–3%)4; however, numerous other chromosomal regions are described,3,4 with a higher frequency of events observed in syndromic forms of ASD.5,6 Heritability estimates for ASDs, as determined from twin and family studies, are ∼90%,7 and a linkage scan from an international collaborative study has mapped putative risk loci in families with at least two affected individuals.8 The same study also reveals that submicroscopic copy-number variants (CNVs) can have a causal or susceptibility-related role. There is also the suggestion that de novo CNVs may be a more significant risk factor in sporadic compared with familial forms of ASD.9
ASD-associated mutations have been reported in two neuroligin genes (NLGN3 and NLGN4) on the X chromosome,10 RPL10 at Xq28,11 and SHANK3 (also termed “ProSAP2”) on chromosome 22q13.12 There have been other associations (reviewed in the work of Persico and Bourgeron13), but they represent extremely rare events, usually of unconfirmed pathogenicity (or associated with a comorbid phenotype), and, in most instances, they have not yet been validated by other studies.
The SHANK3 gene encodes a protein of the postsynaptic density (PSD) of excitatory synapses, where it may function as a master scaffolder forming large sheets that may represent the platform for the construction of the PSD complex.14 At the PSD complex, SHANK3 has been shown to bind to neuroligins,15 which, together with the neurexins, form a complex at glutamatergic synapses. SHANK3 was first identified in rats16 and then in humans17 as a gene expressed predominantly in cerebral cortex and cerebellum. Moreover, SHANK3 was found disrupted by a de novo balanced translocation in a child with severe expressive-language delay, mild mental retardation, and other features of the 22q13.3 deletion syndrome (MIM 606232).17 Durand and coworkers12 then identified two alterations in SHANK3 in subjects with an ASD but not observed in control individuals. One is a de novo insertion of a G nucleotide in exon 21 of SHANK3, which leads to a frameshift and presumed loss of function. Since this variant was present in two affected siblings, it was thought to result from germline mosaicism in the mother. A second alteration found in an unrelated family was a de novo deletion of terminal 22q13, with the breakpoint in intron 8 of SHANK3. The same study also reported a larger 800-kb deletion in a girl with an autism spectrum phenotype that includes severe language delay. Her brother with Asperger syndrome had a partial trisomy of terminal 22q; these unbalanced anomalies were the consequence of a paternal translocation.
Because significant genetic and functional data implicate SHANK3 as a potential monogenic cause of ASDs, we sought to further assess the types and frequency of mutations involved and the associated phenotypic outcomes. We therefore screened a sample of 400 probands with an ASD (and, in some cases, their families) for mutations in SHANK3, using Sanger dideoxy DNA sequencing and microarray-based comparative intensity analysis. As discussed below, we discovered variants in nearly 1% of these cases, further implicating SHANK3 and the neurexin-neuroligin complex in ASDs.
The 400 unrelated subjects with ASD were ascertained at The Hospital for Sick Children in Toronto (225) and in child diagnostic centers in Hamilton, Ontario (100), and in St. John’s, Newfoundland (75). All subjects met Autism Diagnostic Interview–Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) criteria conclusively or on a clinical best estimate.18 Affected and unaffected siblings were also assessed whenever possible, and 249 (62%) of these families had more than one child with an ASD. Blood samples were drawn from the index patients with ASD and their relatives, to facilitate ascertainment of the mode of inheritance of all detected variants. Most index patients (75%) were screened for fragile X mutations and were karyotyped. Wherever possible, experiments were performed on blood-derived genomic DNA (80%); otherwise, DNA from cell lines was used. Results from these families are described in table 1 and figure 1. Findings were confirmed on blood-derived genomic DNA or cells, apart from the exon 9 variant S341L, which was confirmed by its inheritance from the father.
Table 1. .
Summary of SHANK3 DNA Sequence and Identified CNVs
| DNA Variant or CNV |
||||||||||
| Subject | Exon | Amino Acid | Nucleotide | Sex of Proband | Transmission from | Family Type | Sibling Status | Population Ancestry | Frequency in ASD | No. of Controls Tested |
| SK0007 (ASD4) | 8 | Q321R | A962G | F | De novo | Simplex | Absent in unaffected sibling | Arabic Europeana | 1 in 400 | 186 |
| SK0042 | 9 | S341L | C1022T | F | Father | Simplex | Absent in unaffected sibling | Europeana | 1 in 400 | 57 |
| SK0041 | 21 | A970S | G2908T | M | Father | Simplex | Present in unaffected sibling | Europeana | 1 in 250 | 100 |
| SK0018 | 21 | A1173T | G3517A | M | Mother | Multiplex | Present in one of two affected siblings | Mixedb,c | 1 in 400 | 100 |
| SK0059 | 21 | P1263L | C3788T | M | Father | Simplex | CEUb | 1 in 400 | 100 | |
| MM0244 | 21 | L1406V | C4216G | M | Mother | Multiplex | Absent in affected sibling | CEUb | 1 in 400 | 100 |
| SK0266 | 21 | M1443T | T4328C | M | Mother | Simplex | Present in one of two unaffected siblings | CEUb | 1 in 400 | 100 |
| SK0203 | 21 | Del of 5 aa | Del 4358–4372 | M | Mother | Multiplex | Absent in affected sibling | Mixedb,c | 1 in 400 | 100 |
| SK0225 | 22 | G1557S | G4669A | M | Father | Simplex | CEUb | 1 in 400 | 200 | |
| SK0021 | 22 | P1654T | C4960A | M | Father | Multiplex | Present in unaffected sibling | Mixedb,c | 2 in 400 | 200 |
| SK0202 | 22 | P1654T | C4960A | M | Mother | Multiplex | Present in one of two unaffected siblings | CEUb | 2 in 400 | 200 |
| MM0109d (ASD1) | 277-kb Del at 22q13.33; 1.4-Mb gain at 20q13.33 | F | De novo | Simplex | Absent in two unaffected siblings | CEUb | 2 in 433 | 500e | ||
| NA0039d (ASD2f) | 3.2-Mb Del at 22q13.31-33 due to an unbalanced translocation der(22) t(14;22)(q32.33;q13.31) | F | Fatherg | Simplex | Sister with der(14) t(14;22)(q32.33;q13.31) has ADHD | CEUb | 2 in 433 | 500e | ||
| 3524d (ASD3h) | 4.4-Mb Del at 22q13.31-33 | M | De novo | Multiplex | Present in affected sister | CEUb | 500e | |||
Not assayed with 500K array; self reported ancestry.
Ancestry estimated using STRUCTURE, where CEU indicates European.
Individuals have mixed ancestry of CEU and Asian.
CNVs (from National Center for Biotechnology Information build 35 coordinates) present in families are as follows: ASD1 has a 286-kb (189,538,747–189,825,000) paternally inherited gain at 4q35.2, a 224-kb (190,742,803–190,967,000) paternally inherited gain at 4q35.2, a 1.66-Mb (18,427,100–20,089,400) maternally inherited gain at 15q11.2, a 1.25-Mb (21,441,805–22,688,093) maternally inherited gain at 16p12.2-12.1, a 534-kb (40,555,289–41,089,766) paternally inherited loss at 17q21.31, a 1.4-Mb (60,949,339–62,377,000) de novo gain at 20q13.33, and a 277-kb (49,243,247–49,519,949) de novo loss at 22q13.33. ASD2 has a 3.2-Mb (46,277,400–49,509,100) loss at 22q13.31-q13.33 due to unbalanced translocation. ASD3 has a 224-kb (82,972,800–83,197,200) maternally inherited gain at 12q21.31, a 1.4-Mb (103,544,000–104,968,000) paternally inherited loss at 13q33.1-q33.2, a 34.2-kb (27,536,400–27,570,600) maternally inherited loss at 14q12, a 253-kb (34,307,200–34,560,000) maternally inherited gain at 16p11.1-p11.2, and a 4.4-Mb (45,109,800–49,465,800) de novo loss at 22q13.31-q13.33. All CNV data have been submitted to the DECIPHER database. The CNVs should be compared with the Database of Genomic Variants to determine their status in the latest version of the control data.
No deletions of SHANK3 were present in European controls or in the HapMap sample controls, as assessed by 500K array CNV analysis.
The family was described in the work of Prasad et al.19
Has balanced translocation t(14;22)(q32.33;q13.31).
The family was described in the work of Szatmari et al.8
Figure 1. .
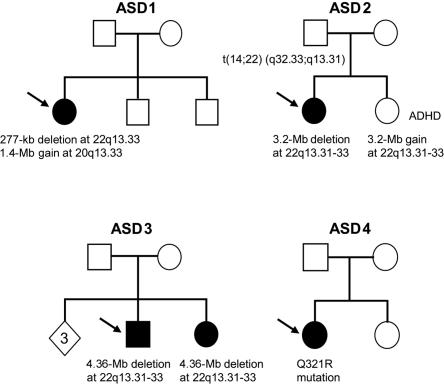
Pedigrees of the ASD-affected families with de novo variants or unbalanced translocation derivatives. Arrows denote the index subject. In ASD1, the proband has a de novo 277-kb deletion at 22q13.33 and a de novo 1.4-Mb duplication at 20q13.33. In ASD2, the father carries a balanced translocation t(14;22)(q32.33;q13.31); the elder daughter with ASD has inherited the der(22) t(14;22)(q32.33;q13.31), whereas the younger daughter with ADHD inherited the der(14) t(14;22)(q32.33;q13.31). In ASD3, both children have a de novo 4.4-Mb deletion at 22q13.31-33. In ASD4, the proband has a de novo Q321R mutation. The diamond indicates three siblings without ASD who were unavailable for sampling.
The genomic sequence of SHANK3 was initially derived by comparison of the rat Shank3 gene with human ESTs listed in the UCSC Genome Browser, as well as that described by Wilson et al.21 To ensure comparability of data, we maintained the nomenclature of the coding sequence used by Durand et al.12 Exons 1 and 11 could not be reproducibly amplified, even on repeated attempts with use of various conditions (the same exons posed problems elsewhere21). Primer sequences and PCR conditions used for amplification are available on request.
Samples were initially assessed for CNV content with use of signal intensities obtained from the Affymetrix 500K SNP array set. The complete data on all 400 subjects with ASD will be presented elsewhere (authors' unpublished data), and only those data relevant to families with SHANK3 CNVs are described here. The techniques used for validation of the initial Affymetrix comparative intensity analysis are described in figure 2, and all data were compared with the Database of Genomic Variants,22 to assess relevance with respect to population controls. The methodologies for calling the CNVs are (or will be) described elsewhere (authors' unpublished data).20,23 Quantitative PCR analysis included SYBR green methodology (Stratagene)24 and FISH experiments.25 A 12-marker forensic panel (Identifiler [Applied Biosystems]) was employed to ascertain that the parents in ASD-affected family 4 are indeed biological parents. Estimation of population ancestry of the subjects described in table 1 was obtained by STRUCTURE software,26,27 with use of 780 autosomal SNPs spaced at 3.5–4 Mb across the genome. For comparison, analysis also included genotypes from unrelated individuals representing populations from the HapMap collection (African, European, and Asian).28
Figure 2. .
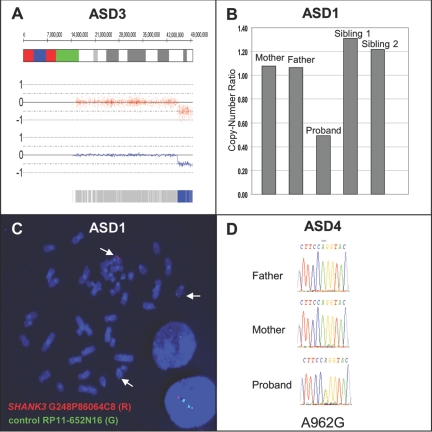
A, Copy-number analysis of ASD3 showing 4.4-Mb deletion at 22q13.31-q13.33. The genomic coordinates are shown at the top, with red dots and blue lines showing raw and smoothed intensities, respectively. The blue bar at the bottom is indicative of homozygous SNP calls. B, Quantitative PCR results depicting 277-kb loss in ASD1, where both parents and siblings are copy neutral. C, FISH analysis of the de novo deletion of the SHANK3 region in ASD1. The red signal (SHANK3 probe G248P86064C8) is deleted, and the green signal is a control probe mapping to chromosome 14 (RP11-652N16). D, Sequencing showing de novo A962G change in the ASD4 proband.
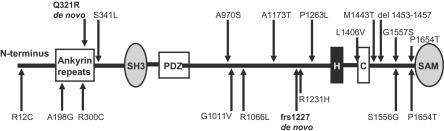
Through DNA sequencing of the majority of the coding sequences and splice junctions of SHANK3, we identified 10 novel nonsynonymous variants (table 1). One of these, found in the index patient of family ASD4, represents an apparent de novo mutation, with an A962G exchange in exon 8 leading to a heterozygous Q321R substitution. This change, found only in this single subject and in neither parent nor an unaffected sibling, was also absent from 372 control chromosomes and has not been described in any of the cases or controls reported elsewhere.12 The gestation and delivery, at 38 wk, of this female subject were uneventful, apart from an in vitro–fertilization procedure. She establishes no eye contact and displays narrow interests and verbal repetitive behaviors but understands complex instructions. The ADI exceeds the autism cutoff in all domains (see table 2 for clinical information). Her younger sister, who does not carry the mutation, is developing normally.
Table 2. .
Description of Clinical Features of Probands with ASD and SHANK3 Mutations or CNVs[Note]
| Results for Family and Index Patient |
||||
| Characteristic | MM0109 (ASD1) | NA0039 (ASD2) | 3524 (ASD3) | SK0007 (ASD4) |
| Sex | F | F | M | F |
| Exon | 277-kb deletion; 1.4-Mb gain | 3.2-Mb deletion | 4.36-Mb deletion | Q321R mutation |
| Delay: | ||||
| Speech | Nonverbal; no use of gestures or pointing | Vocabulary of 50 words at age 16 years, but uses mainly nonverbal communication | Nonverbal | Stopped using language at age 2–2.5 years; regained some words at age 3.5 years |
| Development | Global developmental delay; poor self-help skills | Global developmental delay; sat at age 13 mo; walked at age 17 mo | Yes | |
| Social interactions/hyperactivity/aggressive behavior | Score of 29 on ADI social reciprocity; no joint attention; not interested in peers; poor use of eyes and facial expression to interact socially | No eye contact; aversion to physical contact | Profound impairment in social interaction; hyperactivity and attention deficits, treated with methylphenidate and risperidone; aggression toward affected sister; mild self-injurious behavior | No eye contact; very limited use of gestures; social withdrawal; currently one friend with developmental delay; severe inattention and irritability, treated successfully with methylphenidate and atomoxetine; mild self-injurious behaviors (biting and hair pulling) |
| Restricted/repetitive behaviors and interests | Carries an object around all the time; score of 4 on ADI repetitive behaviors, Pica, sensitive to sensory stimuli | Self-stimulatory behaviors, such as rubbing clothes and sucking toys | Yes | Fascination with window blinds; understands complex instructions; limited reciprocal conversation about narrow interests; verbal repetitive behaviors; no motor stereotypes; hair pulling |
| Dysmorphic signs | Small head size; hypertelorism | Obesity; soft facial dysmorphism with low frontal hairline; submucous cleft palate with bifid uvula | No | |
| EEG results and seizures | Generalized slowing | Normal CT scan and EEG; history of febrile seizures | Abnormal EEG, with bilateral epileptiform discharges but no seizures; severe sleep disorder; normal CT scan | |
| Instrument scores | At age 7–8 years on Vineland, Communication SS=30, AE=0–11; Daily Living SS=19, AE=1–9; Social SS=41, AE=0–7 | ADI-R = 23 (cutoff 10); ADOS-1=15 (autism cutoff 12); WISC-IV nonverbal subtests + TONI−2: IQ <50 | ADI Social = 24; Communication = 13; Repetitive = 6; Early Abnormal Development = 5; ADOS scores: Communication = 6; Social = 14; Play = 4; Stereotyped Behaviors and Restricted Interests = 5 | Above cutoff in all domains on ADI; borderline ADOS; <1st percentile on old Leiter |
| Comments | Born at 34 wk; birth weight 2,282 g | Single umbilical artery; hand tremor; joint laxity | Sensory defensiveness toward loud noises; no self-injurious behaviors | Conceived by in vitro fertilization; parents are first cousins |
| Family history | Siblings unaffected; no family history of ASD | Sister has ADHD | One affected sister, family history otherwise negative. The affected sister has only a few words. She is noncompliant, with daily temper tantrums when shifted from a preferred to a nonpreferred activity | No family history of ASD |
Note.— AE = age equivalent; EEG = electroencephalogram; IQ = intelligence quotient; SS = standard score; TONI = test of nonverbal intelligence.
The nine other novel nonsynonymous variants found in patients with ASD but not in controls were inherited from an unaffected parent. Four of these nonsynonymous changes also occur in unaffected siblings, indicating that these are likely not pathogenic. In the index subject of family SK0203, we detected a heterozygous deletion of 15 nt in exon 21 that is predicted to eliminate 5 aa from the protein. This deletion alteration was inherited from the mother and was absent in the affected sibling, which suggests that it is not pathogenic. The absence of an ASD in the transmitting parent may reflect incomplete penetrance of the allele in question, but it could also suggest an effect caused by other inherited alleles in the affected family members.
From the CNV screen, two chromosomal deletions were detected (table 1). In ASD-affected family 1 (ASD1), a heterozygous deletion of ∼277 kb encompassing the MAPK8IP2, ARSA, and SHANK3 genes was found in the female proband but in neither parent nor in two unaffected brothers (fig. 1). The proband has autism associated with severe intellectual disability (table 2). She has global developmental delay and could not be examined using standard cognitive tests. She had been evaluated clinically for Rett syndrome (MIM 312750) but had delayed development from birth and no evidence of regression.
Interestingly, in addition to the de novo loss at 22q13.33 in the ASD1 proband, we detected a second de novo CNV gain encompassing ∼1.4 Mb at 20q13.33. The gain was not present in either of the unaffected siblings or controls. There are 44 genes in the region, including some potentially interesting functional candidates, such as KCNQ2, CHRNA4, MYT1, and OPRL1. We also detected three other CNVs (see table 1) in this sample not described (at the time of writing) in the Database of Genomic Variants. Although they are all inherited, they could also contribute to the ASD etiology.
In ASD-affected family 2 (ASD2), the female index patient was found to carry a 3.2-Mb heterozygous deletion of chromosome 22q13.31-33. We later determined that this individual was described earlier as a case of monosomy 22q13,19 but further analysis here shows that the 22q13 deletion is due to an unbalanced 22q13.31-33 monosomy: der(22) t(14;22)(q32.33;q13.31), as confirmed by cytogenetic and FISH analysis. Her derivative chromosome is inherited from the father, who is phenotypically normal and has a de novo balanced translocation: t(14;22)(q32.33;q13.31). Her sister was found to have a partial 22q13.3 trisomy, which is due to inheritance of the other derivative chromosome: der(14) t(14;22)(q32.33;q13.31). The index patient has typical autism, with ADOS scores on module 1 above the autism cutoffs and ADI-R scores also above the autism cutoffs (table 2). The sister carrying the reciprocal trisomic event received the diagnosis of attention-deficit/hyperactivity disorder (ADHD) at age 5 years and was treated with stimulant medication at age 5–12 years. She has mild cognitive impairment.
ASD-affected family 3 was first examined in the Autism Genome Project multiplex ASD study,8 and here we define the deletion in the index patient to be 4.4 Mb in size and occurring on the maternally inherited chromosome. The affected male proband is nonverbal and has profound impairment in social interaction. The same maternally derived deletion was also present in the affected sister. The deletion was not observed in either parent, suggesting a mechanism of gonadal mosaicism in the mother (fig. 1).
The deletions encompassing SHANK3 were validated experimentally by quantitative PCR analysis and FISH (fig. 2). No deletions of SHANK3 were present in European controls or in the HapMap sample controls, as assessed by 500K array CNV analysis. Conversely, 10 calls of 22q13.33 deletions described in the HapMap sample by BAC arrays20 and displayed in the Database of Genomic Variants were determined to be false-positive results with use of quantitative PCR to represent true-positive results. Thus, the deletions encompassing SHANK3 described here are autism specific and are not present in the control populations.
Our findings of de novo, apparently disorder-causing mutations in 3 (0.75%) of 400 subjects with ASD (table 1 and fig. 3), along with those from the study by Durand and colleagues,12 in which mutations were found in 3 (1.4%) of 222 subjects, suggest a combined discovery rate of disorder-associated sequence changes or deletions in as much as 1% of ASD cases. Our prevalence rate was obtained from a well-characterized convenience sample and should be replicated in other representative samples. We note that the sequence characteristics of the SHANK3 gene make it difficult to assay, and other mutations in our families could remain unidentified.
Figure 3. .
Mutations and variations of SHANK3. The top half gives the location of the mutations and rare nonsynonymous variations found in the present study, whereas the bottom half shows those described elsewhere.12 De novo mutations are shown in bold. SH3 = Src homology-3 domain; PDZ = PDZ domain; H = homer–binding site; C = cortactin-binding site; SAM = sterile α motif.
The observation in family ASD1 of a second de novo duplication (in addition to the SHANK3 deletion) of ∼1.4 Mb encompassing ∼44 genes on chromosome 20q13.33 is also of interest. Genes encompassed by this duplication, such as KCNQ2 (potassium channel important in neuron excitability; diverse mutations cause benign familial neonatal convulsions type 129 and can be associated with delayed psychomotor development or mental retardation30), CHRNA4 (encoding nicotinic acetylcholine receptor that mediates fast signal transmission at synapses; mutations appear to account for a proportion of the cases of nocturnal frontal-lobe epilepsy); MYT1 (myelin transcription factor that is highly expressed in the developing brain31), and OPRL1 (encoding opiate-like G-protein–coupled receptor that is thought to be involved in the regulation of instinctive and emotional behaviors), could also contribute to severity of phenotype in this subject.32 Finally, we report other inherited nonsynomymous sequence variants (table 1 and fig. 3) that will need further investigation to more definitively assess their possible role in ASD.33
From our data, however, there is no definitive genotype-phenotype correlation that can yet be drawn, suggesting that all ASDs are equal candidates for SHANK3 testing. The observation that four of five of the de novo cases reported here with SHANK3 alterations are female (see table 1, noting that family ASD1 has two female siblings with ASD) may have relevance, given the typical 4:1 male:female ratio (Durand et al.12 reported five of nine as female).
Gonadal mosaicism may be an important general mechanism for the generation of de novo pathogenic CNV. Two female siblings with autism were recently described as having an identical CNV loss eliminating coding exons from the neurexin 1 gene (NRXN1).8 This 300-kb deletion was not present in either parent. Microsatellite analysis of the siblings demonstrated an identical maternal chromosomal segment but no paternal DNA. Thus, the likely explanation for this deletion of NRXN1-coding exons is paternal gonadal mosaicism. Another example from the same study is an ∼1-Mb de novo duplication at 17p12 in a male-female sib pair who had received ASD diagnoses.8 Given these results and the maternal germline mosaicism described elsewhere for SHANK3,12 both paternal and maternal germlines may contribute to the formation of de novo CNVs and to the pathogenesis of autism. Thus, although the majority of ASD-associated CNVs observed may be in apparently sporadic cases of ASD, de novo rearrangements are not confined to simplex families. These observations of de novo germline mutations in multiplex families help to unify the phenomena of sporadic mutation and high heritability of ASDs.
As discussed elsewhere,12,34,35 fine tuning of the gene expression of critical genes such as SHANK3 can be crucial for the development of speech and language and/or social communication in humans. In family ASD2, described here with unbalanced cytogenetic abnormalities inherited from a paternal balanced translocation, the children developed different pathologies. Whereas the child who inherited the 22q13 deletion developed an ASD phenotype, her sister who inherited a 22q13 partial trisomy presented with ADHD. Similarly, in a family with a paternal balanced translocation described by Durand and colleagues,12 the daughter with a 22q13 deletion developed autism, whereas her brother with a 22q13 partial trisomy, albeit the diagnosis of Asperger syndrome, demonstrated precocious language development. The terminal 3.2 Mb and 800 kb were affected in the former and latter families, respectively, which may explain the different outcomes observed.
Our frequency estimate for SHANK3 mutations is in line with previous findings for other genes of the neurexin-neuroligin-SHANK3 biochemical complex. Thus, putative rare mutations have been described in the neuroligins NLGN3 and NLGN4 in autism.10 The de novo deletion of NRXN1-coding exons in an affected sibling pair is a rare variant, since it was not detected in 1,167 other autism-affected families with two or more affected individuals.8 Moreover, rare missense variants in the NRXN1 β signal peptide found in ASD, although not de novo, were not found in control individuals.36 Variants in the ribosomal protein gene RPL10 have recently been described in two ASD-affected pedigrees.11 RPL10 is located on Xq28, and the variants (L206M and H213Q) were inherited from the carrier mother and are thought to cause ASD in the hemizygous state in the sons.11 In that study, 296 independent ASD-affected families were investigated, yielding an apparent frequency of pathogenic RPL10 variants of 0.68%. At this stage, confirmation of the relevance of the RPL10 gene for ASD is required before routine clinical screening of RPL10 in subjects with ASD can be recommended. Mutations of NLGN3 and NLGN4 in ASD are apparently very rare. An NLGN3 mutation has been found in only one autism-affected family to date,10 and apparently pathogenic variants of NLGN4 were detected in <1% of patients with ASD.13
Neuroligins have been shown to bind to SHANK315 and are required for the maturation of glutamatergic synapses.37 Moreover, the neurexins are ligands of the neuroligins, thus supporting a pathogenic construct of a network of interrelated molecules, from the transsynaptic interaction between the neurexins and neuroligins to the postsynaptic density complex that includes the SHANK3-scaffolding protein. Given that SHANK3 promotes the formation, maturation, and enlargement of dendritic spines,38 a functional network or complex emerges in which perturbations at a number of potential molecules, acting alone or in combination, may lead to a similar clinical end point.
Acknowledgments
We thank Dr. Fei Yu Han, Memorial University of Newfoundland and Labrador, for performing the FISH analysis on family ASD2; Dr. Susan McGrew and Genea Crockett, for assessment of the VAN3524 (ASD3) family; and Dr. Jonathan Haines, Michelle Lee, and Maryam Mashreghi-Mohammadi of The Centre for Applied Genomics, for technical assistance. This work is supported by Genome Canada/Ontario Genomics Institute, the Canadian Institutes of Health Research (CIHR), the McLaughlin Centre for Molecular Medicine, the Canadian Institute of Advanced Research, the Hospital for Sick Children (SickKids) Foundation (all to S.W.S.), and National Institutes of Health grant MH061009 (to J.S.S.). C.R.M. is supported by the SickKids Foundation and the National Alliance for Research on Schizophrenia and Depression. D.P. is supported by Netherlands Organization for Scientific Research–Rubicon fellowship 2007/02470/ALW and Royal Netherlands Academy of Arts and Sciences–Ter Meulen Funds fellowship TMF/DA/5801. S.W.S. is an Investigator of CIHR and the GlaxoSmithKline/CIHR Pathfinder Chair in Genetics and Genomics at SickKids and the University of Toronto.
Web Resources
The URLs for data presented herein are as follows:
- Database of Genomic Variants, http://projects.tcag.ca/variation/
- DECIPHER, http://www.sanger.ac.uk/PostGenomics/decipher/ (for the Database of Chromosomal Imbalance and Phenotype in Humans using Ensembl Resources) [DOI] [PMC free article] [PubMed]
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for autism, fragile X syndrome, tuberous sclerosis, 22q13.3 deletion syndrome, and Rett syndrome)
References
- 1.Chakrabarti S, Fombonne E (2005) Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 162:1133–1141 10.1176/appi.ajp.162.6.1133 [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Association, Washington, DC [Google Scholar]
- 3.Xu J, Zwaigenbaum L, Szatmari P, Scherer SW (2004) Molecular cytogenetics of autism. Curr Genomics 5:347–364 10.2174/1389202043349246 [DOI] [Google Scholar]
- 4.Veenstra-Vanderweele J, Christian SL, Cook EH Jr (2004) Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet 5:379–405 10.1146/annurev.genom.5.061903.180050 [DOI] [PubMed] [Google Scholar]
- 5.Jacquemont ML, Sanlaville D, Redon R, Raoul O, Cormier-Daire V, Lyonnet S, Amiel J, Le Merrer M, Heron D, de Blois MC, et al (2006) Array-based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. J Med Genet 43:843–849 10.1136/jmg.2006.043166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L (2006) Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry 11:18–28 10.1038/sj.mp.4001757 [DOI] [PubMed] [Google Scholar]
- 7.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25:63–77 [DOI] [PubMed] [Google Scholar]
- 8.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, et al (2007) Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39:319–328 10.1038/ng1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al (2007) Strong association of de novo copy number mutations with autism. Science 316:445–449 10.1126/science.1138659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, et al (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34:27–29 10.1038/ng1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klauck SM, Felder B, Kolb-Kokocinski A, Schuster C, Chiocchetti A, Schupp I, Wellenreuther R, Schmotzer G, Poustka F, Breitenbach-Koller L, et al (2006) Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol Psychiatry 11:1073–1084 10.1038/sj.mp.4001883 [DOI] [PubMed] [Google Scholar]
- 12.Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, et al (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39:25–27 10.1038/ng1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persico AM, Bourgeron T (2006) Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci 29:349–358 10.1016/j.tins.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 14.Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU (2006) An architectural framework that may lie at the core of the postsynaptic density. Science 311:531–535 10.1126/science.1118995 [DOI] [PubMed] [Google Scholar]
- 15.Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N (2004) The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology 47:724–733 10.1016/j.neuropharm.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 16.Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, Wex H, Langnaese K, Bockmann J, Garner CC, et al (1999) Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci 19:6506–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O (2001) Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet 69:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH Jr, Leventhal BL, Pickles A (2006) Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 45:1094–1103 10.1097/01.chi.0000227880.42780.0e [DOI] [PubMed] [Google Scholar]
- 19.Prasad C, Prasad AN, Chodirker BN, Lee C, Dawson AK, Jocelyn LJ, Chudley AE (2000) Genetic evaluation of pervasive developmental disorders: the terminal 22q13 deletion syndrome may represent a recognizable phenotype. Clin Genet 57:103–109 10.1034/j.1399-0004.2000.570203.x [DOI] [PubMed] [Google Scholar]
- 20.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, et al (2006) Global variation in copy number in the human genome. Nature 444:444–454 10.1038/nature05329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, Phelan MC, Hu S, Marshall J, McDermid HE (2003) Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet 40:575–584 10.1136/jmg.40.8.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C (2004) Detection of large-scale variation in the human genome. Nat Genet 36:949–951 10.1038/ng1416 [DOI] [PubMed] [Google Scholar]
- 23.Fiegler H, Redon R, Andrews D, Scott C, Andrews R, Carder C, Clark R, Dovey O, Ellis P, Feuk L, et al (2006) Accurate and reliable high-throughput detection of copy number variation in the human genome. Genome Res 16:1566–1574 10.1101/gr.5630906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plumet S, Gerlier D (2005) Optimized SYBR green real-time PCR assay to quantify the absolute copy number of measles virus RNAs using gene specific primers. J Virol Methods 128:79–87 10.1016/j.jviromet.2005.03.020 [DOI] [PubMed] [Google Scholar]
- 25.Scherer SW, Cheung J, MacDonald JR, Osborne LR, Nakabayashi K, Herbrick JA, Carson AR, Parker-Katiraee L, Skaug J, Khaja R, et al (2003) Human chromosome 7: DNA sequence and biology. Science 300:767–772 10.1126/science.1083423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437:1299–1320 10.1038/nature04226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Haan GJ, Pinto D, Carton D, Bader A, Witte J, Peters E, van Erp G, Vandereyken W, Boezeman E, Wapenaar MC, et al (2006) A novel splicing mutation in KCNQ2 in a multigenerational family with BFNC followed for 25 years. Epilepsia 47:851–859 10.1111/j.1528-1167.2006.00552.x [DOI] [PubMed] [Google Scholar]
- 30.Steinlein OK, Conrad C, Weidner B (2007) Benign familial neonatal convulsions: always benign? Epilepsy Res 73:245–249 10.1016/j.eplepsyres.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 31.Romm E, Nielsen JA, Kim JG, Hudson LD (2005) Myt1 family recruits histone deacetylase to regulate neural transcription. J Neurochem 93:1444–1453 10.1111/j.1471-4159.2005.03131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuk L, Marshall CR, Wintle RF, Scherer SW (2006) Structural variants: changing the landscape of chromosomes and design of disease studies. Hum Mol Genet Spec 15:R57–R66 10.1093/hmg/ddl057 [DOI] [PubMed] [Google Scholar]
- 33.Kryukov GV, Pennacchio LA, Sunyaev SR (2007) Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am J Hum Genet 80:727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP (2001) A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413:519–523 10.1038/35097076 [DOI] [PubMed] [Google Scholar]
- 35.Somerville MJ, Mervis CB, Young EJ, Seo EJ, del Campo M, Bamforth S, Peregrine E, Loo W, Lilley M, Perez-Jurado LA, et al (2005) Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med 353:1694–1701 10.1056/NEJMoa051962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH Jr, Skinner C, Schwartz CE, Sommer SS (2006) High frequency of neurexin 1β signal peptide structural variants in patients with autism. Neurosci Lett 409:10–13 10.1016/j.neulet.2006.08.017 [DOI] [PubMed] [Google Scholar]
- 37.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N (2006) Neuroligins determine synapse maturation and function. Neuron 51:741–754 10.1016/j.neuron.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 38.Roussignol G, Ango F, Romorini S, Tu JC, Sala C, Worley PF, Bockaert J, Fagni L (2005) Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci 25:3560–3570 10.1523/JNEUROSCI.4354-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]