Abstract
ATP/ADP-sensing (sulfonylurea receptor (SUR)/KIR6)4 KATP channels regulate the excitability of our insulin secreting and other vital cells via the differential MgATP/ADP-dependent stimulatory actions of their tissue-specific ATP-binding cassette regulatory subunits (sulfonylurea receptors), which counterbalance the nearly constant inhibitory action of ATP on the K+ inwardly rectifying pore. Mutations in SUR1 that abolish its stimulation have been found in infants persistently releasing insulin. Activating mutations in SUR1 have been shown to cause neonatal diabetes. Here, analyses of KIR6.2-based channels with diabetogenic receptors reveal that MgATP-dependent hyper-stimulation of mutant SUR can compromise the ability of KATP channels to function as metabolic sensors. I demonstrate that the channel hyperactivity rises exponentially with the number of hyperstimulating subunits, so small subpopulations of channels with more than two mutant SUR can dominate hyperpolarizing currents in heterozygous patients. I uncovered an attenuated tolbutamide inhibition of the hyperstimulated mutant, which is normally sensitive to the drug under non-stimulatory conditions. These findings show the key role of SUR in sensing the metabolic index in humans and urge others to (re)test mutant SUR/KIR6 channels from probands in physiologic MgATP.
Inborn errors of glucose homeostasis and metabolism (1) have illuminated a vital link between a metabolic index, the ATP/ADP ratio, and cellular excitability that utilizes ABCC8(9)/KCNJ11(8)-encoded KATP channels as metabolic sensors (2, 3). These tetradimeric channels are proposed to link the cell membrane potential, Vm, with the ATP/ADP ratio via the differential stimulatory actions of Mg2+-ATP/ADP on their cell type-specific regulatory sulfonylurea receptor (SUR)2 subunits (s). Like other members of the largest family of eukaryotic membrane transport proteins (4), SUR possess two non-equivalent nucleotide-binding domains, NBD1 and NBD2. Magnesium ·nucleotide-bound NBD1/NBD2 dimers counterbalance a magnesium-independent nucleotide inhibition of the KATP pore, an effect essentially saturated in intact cells by ATP present at >100 times the IC50(ATP) (5). Consistent with this mechanism, loss-of-stimulation mutations in NBD of SUR1 (ABCC8), the neuroendocrine-type receptor, have been discovered in infants with persistent hyperinsulinemic hypoglycemia (6), whereas mutations in ABCC8 that overactivate KIR6.2 (KCNJ11) in millimolar MgATP have been shown to cause permanent or transient neonatal diabetes, including ND with neurological symptoms (7). Several ND mutations map to the transmembrane (TM) domains of the ABCC8 core, TMD1 and TMD2, whose role in controlling the nucleotide-dependent open channel probability (Po) needs to be understood.
This study is the first analysis of permanent ND currents caused by a heterozygous mutation in a key TMD2-coding region of ABCC8, ABCC8Q1178R, found in a proband with normal KCNJ11 (8). To understand how ABCC8Q1178R (NDSUR1) hyperactivate the heterozygous KATP ensemble, I used direct approaches, including recordings of single channels with the differentially restricted receptor composition, as well as structural modeling of the receptor core. The results reveal a novel mechanism of channelopathies, explain why the smallest subpopulation of hyperstimulated KATP channels can make a large contribution to the pathogenic conductance in heterozygous patients, and reveal that normal tolbutamide sensitivity of non-stimulated mutant SUR does not guarantee their normal response to the drug in physiologic MgATP.
EXPERIMENTAL PROCEDURES
Mutagenesis, sequencing, cell culture and transfections were done as described previously (7). Gln-1178 is conserved in all SURs. All the described mutations were introduced into hamster SUR1 cDNA (9). Concatemers were engineered as described previously (5); different and similar subunits were fused via –TSGGG–and –SGGGASGGG–linkers, respectively. The cDNA construct(s) were co-expressed in COSm6 cells with enhanced green fluorescent protein as described previously (7); heterozygous cells are cells expressing SUR1 and NDSUR1 (1:1 construct ratio) plus KIR6.2.
Patch clamp recording and current analysis was done as described previously (7). The pipette solution contained (in mm): 145 KCl; 1 MgCl2; 1 CaCl2; 10 HEPES; pH 7.4 (KOH). The bath Mg2+-free ([Mg2+]i < 0.1 nm) internal solution contained (in mm): 140 KCl; 5 EDTA; 5 HEPES; 10 KOH; pH 7.2 (KOH). The bath intracellular solution contained (in mm): 140 KCl; 1 MgCl2; 5 EGTA; 5 HEPES; 10 KOH; pH 7.2 (KOH). The [Mg2+]i in nucleotide-containing solutions was kept at ∼0.7 mm by adding MgCl2. The holding potential was –40 mV. COSm6 cells have negligible background currents, permitting measurements of virtually any low mean KATP currents, I. I analyzed the inwardly directed currents through <102 KATP channel-containing patches, allowing me to verify the unitary current amplitude, i, in cell-attached configuration from all-points current amplitude histograms and thus accurately determine the on-cell activity of n identical channels with the mean open probability Po, n × Po = I × i–1. The Colquhoun-Hawkes test was used to evaluate the channel singularity. The ligand-independent Pomax determined from single-channel currents and from macro-current noise were similar. The ligand responses of KATP currents were obtained using a programmed rapid solution changer. To correct the ATP dose responses for run-down and/or refreshment, the I value in the presence of each ATP concentration was normalized to the arithmetic mean of the I values before application of each [ATP] and after washout. Similar corrections were applied when estimating the relative steady-state activity in the presence of other ligands. The averaged values were expressed as mean ± S.E. for n ≥ 5; differences were evaluated using unpaired t test (p values are given in text or figure legends).
Molecular modeling was done as described previously (10) using Sav1866 coordinates (11) and the sequence alignment shown in supplemental Fig. S1. supplemental Fig. S2 shows a stereo view of the NDSUR1 core model.
RESULTS AND DISCUSSION
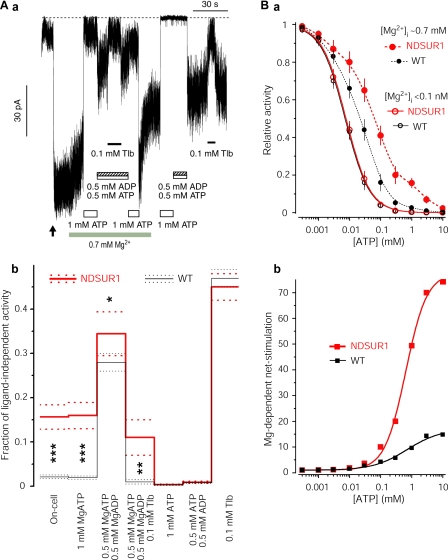
Fig. 1 shows that NDSUR1 hyperactivate normal KATP pores in the presence of millimolar MgATP in intact cells without changing their ligand-independent activity or apparent KD for inhibitory ATP. The effect of the mutation on the Po on-cell can be accounted for by its effect on the MgATP-dependent stimulatory action predicted to subsaturate at millimolar MgATP (5). The ADP in human insulin-secreting β-cells is unlikely to rise above 0.5 mm, and the intracellular ATP in these and other mammalian cells is unlikely to drop below 0.5 mm (12, 13). Fig. 1A, panel b, shows that in 0.5 mm MgATP + 0.5 mm MgADP, approximating severe catabolic conditions, the activity of the NDSUR1 channel is slightly higher than that of the WT channel. This implies that ADP makes a relatively small contribution to the hyperstimulation of NDSUR1 in vivo, where [ADP] should be lower and [ATP] + [ADP] > 1 mm, even in low glucose (12, 13). In the hyperglycemic NDSUR1 subject, the [ATP]/[ADP] ≫ 1. Therefore I determined the fraction of the maximal activity of the NDSUR1 versus WT channel in 1 mm MgATP + 0.1 mm MgADP and in 2 mm MgATP + 0.05 mm MgADP (0.159 ± 0.042 versus 0.035 ± 0.016 and 0.115 ± 0.033 versus 0.021 ± 0.007, respectively; n = 4 for each). The more significant (p < 0.005) effect of the mutation at these higher MgATP/ADP ratios indicates that the Q1178R markedly amplifies the stimulatory action of [MgATP] > 0.5 mm (see later). In 0.5 mm MgADP without ATP, or in 5 mm MgUDP, which subsaturates the stimulatory, but not adenine-selective, inhibitory sites (5, 14), both channels display >90% of the ligand-independent activity, Pomax (three pairs of records not shown). Altogether, these observations argue that the hyperactivity of the NDSUR1 channel in physiological [ATP]/[ADP] is predominantly determined by MgATP, regardless of whether the magnesium ·nucleotide diphosphate-dependent stimulation of NDSUR1 is abnormal. ADP, normally a key regulator of the excitability of insulin-secreting cells (6), may not effectively control the basal KATP conductance in cells expressing NDSUR1/KIR6.2 channels as their hyperstimulation by submembrane MgATP ∼1–2 mm is sufficient to maintain large permanent hyperpolarizing currents. A much lower basal activity of KATP channels, ∼1% of their maximal activity (15), is essential for metabolic regulation of the β-cell excitability. Glucose metabolism-dependent increases in [ATP] and decreases in [ADP], which abolish this low basal activity of the WT KATP channels, may not be sufficient to nullify the NDSUR1 channel currents. Indeed these hyperpolarizing currents prevented excitation of insulin-secreting cells in vivo because the NDSUR1 patient responded to glibenclamide treatment (see also Ref. 7). Thus the experimental and clinical observations argue that the MgATP-dependent hyperstimulation of mutant SUR can compromise the ability of KATP channels to couple the ATP/ADP ratio with cellular excitability in humans.
FIGURE 1.
MgATP-dependent hyperstimulation of NDSUR1/KIR6.2 channels. A, panel a, inward currents through NDSUR1/KIR6.2 channels on-cell and in inside-out configuration indicating their similar hyperactivity in intact COSm6 cell and in millimolar ATP with, but not without, physiologic [Mg2+]i ∼ 0.7 mm. The arrow marks the time of the membrane patch excision. The dotted line shows the zero current level. Experimental conditions are described under “Experimental Procedures.” My similar recordings of SUR1/KIR6.2 channels expressed in COSm6 cells (7) showed much lower fractions of their maximal ligand-independent activity in cell-attached and inside-out patches in millimolar MgATP, an independently verified submembrane nucleotide concentration in COSm6 cells under similar experimental conditions (26). Tlb, tolbutamide. Panel b, the fractions of the Pomax of NDSUR1 versus WT channels from 10 versus 10 cells under different conditions tested as in panel a (see “Experimental Procedures” for details); 1.0 corresponds to normalized ligand-independent Po, which is unaffected by the ND mutation (supplemental Fig. S3). Solid and dotted lines show the mean and ±S.E. levels, respectively. The conditions resulting in higher activity of NDSUR1 versus WT channels are marked by *(p = 0.059), ** (p < 0.01) and *** (p < 0.0001). B, panel a, the ATP dose responses of the mutant versus WT channel currents from 10 versus 10 inside-out patches with and without physiologic free Mg2+ ∼ 0.7 mm. The dose responses in [Mg2+]i < 0.1 nm are fit with the inhibitory Hill (solid lines) with the R2 > 0.999, showing the similar IC50(ATP) and slope factor (h) values of 7.67 ± 0.22 versus 7.13 ± 0.24 μm and 1.12 ± 0.03 versus 1.11 ± 0.04 for the mutant versus WT channels, respectively. Hill-based functions do not fit with a comparable accuracy to the dose responses in [Mg2+]i ∼ 0.7 mm; the latter are interpolated by splines (dashed curves) for illustrative purpose only. Panel b, the ratios of the mean activities in MgATP versus corresponding ATP from A, panel a, fit with the logistic function with R2 > 0.996 (Origin 7 Pro, MicroCal, Northhampton, MA) indicating the apparent C50(MgATP) for NDSUR1 and SUR1 at ∼0.65 and ∼0.72 mm, respectively.
NDSUR1 has normal NBD. So I proposed that the Arg-1178 stabilizes the normally occurring Mg2+ ·nucleotide-bound state of the ABCC8 core (supplemental Fig. S2) with either two ATPs or one ATP/one ADP bound at its NBD1/NBD2 dimer interface. The proposal is consistent with the non-equivalent nucleotide binding properties of the two domains (16), similar ADP-bound versus AMP-PNP-bound conformers of Sav1866 with the closed NBD dimer and “outward-facing” (open) TMD (11, 17) and the maltose importer state with the similarly closed ATP-bound MalKE159Q dimer and open MalFG (18). The proposal passed two complementary tests. First, as Mn2+ substitutes for Mg2+ in ATP-dependent stimulation of SUR (19), I verified that hyperstimulation is reproduced with Mn2+ in place of Mg2+; the fraction of the maximal activity of the NDSUR1 channel in 1 mm Mn-ATP was 0.141 ± 0.031 (n = 3). Second, K719R plus K1384R in the Walker A motifs eliminated the differences between the two channel activities on-cell and in 1 mm MgATP; the fractions of the Pomax were 0.003 ± 0.0012 and 0.0034 ± 0.0018 versus 0.0025 ± 0.001 and 0.0028 ± 0.0011 for NDSUR1K719R+K1384R versus SUR1K719R+K1384R channel, respectively (n = 3 for each). These activities match the activities of the NDSUR1 and SUR1 channels in 1 mm ATP without Mg2+ (Fig. 1A, panel b).
Fig. 1A, panel b, also reveals that inhibition of the hyper-stimulated channels by tolbutamide is attenuated, whereas the same population of NDSUR1 channels under non-stimulatory conditions normally responds to the same drug. The IC50h = 1.8 ± 0.3 μm, hh = 1.01 ± 0.07, IC50l = 1305.5 ± 201.3 μm, hl = 1.09 ± 0.11 and L = 0.438 ± 0.017 describing the two-component tolbutamide dose response of the non-stimulated NDSUR1 channels were undistinguishable from the corresponding parameters for the WT channels under similar experimental conditions (14). Consistent with the latter report and my model, I hypothesize that the Q1178R mutation reduces the ability of the sulfonylurea-binding TM domain to destabilize the magnesium ·nucleotide-bound state of the receptor. This is in line with several observations. (i) The ND mutation is within the TM15–16 segment of the TMD2, which specifies the stronger inhibition of SUR1-versus SUR2A-containing channels by tolbutamide (14, 20). (ii) The coupling helix between TM15 and TM16 interacts with the canonical ATP-binding domain of the ABCC8 core (supplemental Fig. S2). (iii) The sulfonylurea binding releases ATP from ABCC8 and abolishes its stimulation (14, 21).
To further examine the proposed mechanism of metabolism-excitation uncoupling, I obtained full ATP dose responses of the ND versus WT metabolic sensors with versus without physiologic [Mg2+]i and derived their apparent net stimulation curves. Essential for this analysis, my assay resolves very low activities of KATP channels in supramillimolar ATP. The results (Fig. 1B) show the following. (i) The ATP dose response of both WT and mutant channels, in intracellular concentrations of free Mg2+ (∼0.7 mm), deviates from an inhibitory Hill function when the concentration of ATP is greater than either the IC50(ATP) or the KD for the magnesium-independent binding of ATP at the first Walker A-based site (<10 μm (16)). (ii) The deviation is greater for mutant channels in which the hyper-stimulating conformer is stabilized. (iii) The net stimulatory action of ATP, reflected by the ratio of the mean open channel probabilities in ATP with Mg2+ to ATP without Mg2+, is a sigmoidal (logistic) function, and C50 values for NDSUR1 and WT SUR1 are comparable with the apparent KD values for nucleotide binding, KD(ATP), (ADP), and KM for ATP hydrolysis at the lower affinity, second Walker A-based site of SUR1 and SUR1-KIR6.2 fusion (see Refs. 16 and 22, respectively, and note that all of the K values for the ABCC7 channel are also between 0.1 and 1 mm (23), whereas biochemical experiments on SUR1 indicate a very low, ADP-insensitive hydrolytic activity (16, 24)). (iv) The net stimulation saturates with a much higher maximum for the NDSUR1 channel. These findings reinforce the conclusion that the ND mutation in the TMD2 stabilizes the magnesium ·nucleotide-bound ABCC8 core whose lower affinity Walker A-based site specifies the stimulatory profile of the neuroendocrine-type metabolic sensors.
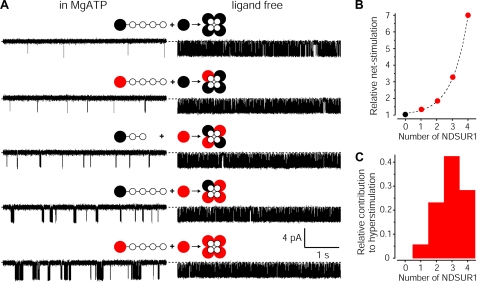
The ND mutation exerts its pathogenic action in the heterozygous state. To deduce which species of the heterogeneous ND-KATP ensemble dominate the pathogenic conductance, one must determine the effectiveness of NDSUR1 in mixed KATP channels. I solved the problem using heteroconcatemers (Fig. 2A). Fig. 2B shows an exponential dependence of channel hyperstimulation on NDSUR1 number. Macroscopic Imax from heterozygous, homozygous, and WT cells (10 for each) were similar, revealing no effect of the ND mutation on the density of KATP channels; the NHeterozygous/NWT and NHomozygous/NWT ratio was 0.97 ± 0.39 and 0.95 ± 0.36, respectively; thus channels with a different number of NDSUR1 should distribute binomially. In this case, the relative contribution of each channel species to the hyperconductance of cells expressing both ND and WT subunits is determined by the product of the exponential and binomial functions (Fig. 2C). The histogram shows that channels with three and four NDSUR1s, expected to comprise about one-third of a heterozygous population, generate ∼70% of the basal hyperconductance; (NDSUR1/KIR6.2)4 channels comprising only 6.25% of total n generate almost 30% of the pathogenic currents. Consistently, the heterozygous I × i–1 on-cell and in millimolar MgATP were between those for (NDSUR1/KIR6.2)4 and WT channels, e.g. 2.6 ± 0.5- and 2.7 ± 0.4-fold higher than the corresponding fractions of the n × Pomax for the WT channels (n = 5 for each). I conclude that tetradimeric channels with more than two NDSUR1 dominate the pathogenic conductance caused by the heterozygous mutation that stabilizes a Mg2+ ·nucleotide-bound conformer of the receptor.
FIGURE 2.
The NDABCC8 heterozygosity control of KATP currents. A, generating KATP channels with all possible predetermined numbers of NDSUR1 versus SUR1. The red, black, and white circles show NDSUR1, SUR1, and KIR6.2, respectively. Intersubunit linkers are indicated by —–. KIR6-based channels with less than four regulatory subunits do not reach the cell surface regardless of linkers (5); thus the subunit composition of each recorded concatemeric KATP channel is predetermined. Single-channel traces on the left indicate an exponential rise in the Po with the number of NDSUR1 subunits hyperstimulated in physiologic MgATP whereas traces on the right show similar ligand-independent activity of the same concatemeric channels, recorded as in Fig. 1 (see supplemental Table S1 and its legend for details). B, The dependence of the net stimulation at millimolar MgATP on the number of NDSUR1 subunits associated with the concatemeric KATP pore. The net stimulation was determined as in Fig. 1B and normalized to that of the concatemeric channel with four SUR1 subunits. The mean values of the relative net stimulation are from four cells for each channel type. The dashed line is an exponent, fit to the data with R2 > 0.995. C, the relative contribution of binomially distributed species of KATP channels with a different number of NDSUR1 to the IKATP hyperstimulation in heterozygous cells; the total area of the histogram corresponds to 100% of the effect. Concatemeric KATP with two same kind receptors adjacent to each other cannot be engineered, and one might speculate that two neighboring NDSUR1 might stimulate more like one or three of them. However neither of these scenarios can affect the histogram enough to change the conclusion that NDSUR1-containing channels comprising <50% of the normal n-large population dominate (control >50% of) the hyperconductance.
My findings (also see Ref. 7) suggest that magnesium ·nucleotide-dependent hyperstimulation of mutant KATP channels is a common pathogenic mechanism and that the measured on-cell fraction of the n × Pomax, not the IC50(ATP), for a recombinant mutant channel is the most universal and direct predictor of the severity of KATP channelopathies. A >2-fold overstimulation of KATP channels seems to be sufficient to uncouple excitability from the metabolic index in at least two human cell types with high input impedance, e.g. β-cells and neurons, thus illuminating the key role of cell type-specific regulators of ubiquitous KIRs in fine-tuning the Vm response to the tissue-specific dynamic metabolic rate. The regulatory signal (differential stimulation) is specified by the lower affinity, second Walker A-based site of ABCC8. The disease due to the gain in the ABCC8 response to physiologic [MgATP] implies that evolution has optimized SUR/KIR6 channels to monitor the submillimolar ADP at this lower affinity site of the NBD1/NBD2 dimer, positioned several nm away from the membrane phospholipids, when all of the other nucleotide-binding sites in the channel are essentially saturated by intracellular [ATP]. ATP-sensitive K+ pores themselves cannot function as sensors of the metabolic index. Nature created high fidelity, low noise metabolic sensors by coupling intrinsically low active weak inward rectifiers with ABC-based integral proteins that decrease the IC50(ATP) despite increasing the Pomax (25) and are large enough to encircle and thus shield the KIR pore from promiscuous activators.
The attenuated response of NDSUR1 channels to tolbutamide in the presence, but not absence, of MgATP is consistent with the proposed mechanism of hyperstimulation and reveals a potential overlooked mechanism of sulfonylurea tolerance, a compromised inhibition of receptor stimulation (14). Predicting the effectiveness of the sulfonylurea treatment of ND requires testing recombinant ND channels at physiologic [MgATP]. The daily dose of tolbutamide (per kg of body weight) needed to transfer the NDSUR1 patient from insulin therapy to sulfonylurea treatment is predicted to exceed the currently recommended dose for treatment of type 2 diabetes.
The exponential dependence in Fig. 2B is consistent with the view of KATP channels as concerted tetradimers (5) and may close debates on the stoichiometry of KATP stimulation as four NDSUR1s with intact NBDs stimulate more than three NDSUR1 + one SUR1, in physiologic MgATP. Given a similar, exponential dependence of Pomax on the number of KIR6 subunits with a higher stability of the active state (5), I anticipate that 50% of the offspring of parents with mild diabetes or glucose intolerance secondary to heterozygous mutations in either ABCC8 and/or KCNJ11 may have severe neuroendocrine disorders.
Supplementary Material
Acknowledgments
Discussions of the manuscript with J. Bryan were invaluable. I thank H. B. T. Christesen, M. Polak, and L. Aguilar-Bryan for discussions of clinical features of the NDSUR1 patient (8); P. Froguel and M. Vaxillaire for discussions of similar NDABCC8 cases; and G. Zhao for preparation of the mutant plasmids and cell transfections.
This work was supported by grants from American Heart Association and NIDDK, National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains three supplemental figures and a supplemental table, as well as supplemental references.
Footnotes
The abbreviations used are: SUR, sulfonylurea receptor; ABC, ATP-binding cassette; WT, wild type; TM, transmembrane; TMD, transmembrane domain; ND, neonatal diabetes; AMP-PNP, adenosine 5′-(β,γ-imino)triphosphate.
References
- 1.Sperling, M. A. (2006) N. Engl. J. Med. 355 507–510 [DOI] [PubMed] [Google Scholar]
- 2.Babenko, A. P., Aguilar-Bryan, L., and Bryan, J. (1998) Annu. Rev. Physiol. 60 667–687 [DOI] [PubMed] [Google Scholar]
- 3.Seino, S. (1999) Annu. Rev. Physiol. 61 337–362 [DOI] [PubMed] [Google Scholar]
- 4.Dean, M., Rzhetsky, A., and Allikmets, R. (2001) Genome. Res. 11 1156–1166 [DOI] [PubMed] [Google Scholar]
- 5.Babenko, A. P., and Bryan, J. (2001) J. Biol. Chem. 276 49083–49092 [DOI] [PubMed] [Google Scholar]
- 6.Nichols, C. G., Shyng, S. L., Nestorowicz, A., Glaser, B., Clement, J. P. t., Gonzalez, G., Aguilar-Bryan, L., Permutt, M. A., and Bryan, J. (1996) Science 272 1785–1787 [DOI] [PubMed] [Google Scholar]
- 7.Babenko, A. P., Polak, M., Cave, H., Busiah, K., Czernichow, P., Scharfmann, R., Bryan, J., Aguilar-Bryan, L., Vaxillaire, M., and Froguel, P. (2006) N. Engl. J. Med. 355 456–466 [DOI] [PubMed] [Google Scholar]
- 8.Christesen, H. B. T., Sjoblad, S., Brusgaard, K., Papadopoulou, D., and Jacobsen, B. B. (2005) Horm. Res. 64 135. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar-Bryan, L., Nichols, C. G., Wechsler, S. W., Clement, J. P. t., Boyd, A. E., III, Gonzalez, G., Herrera-Sosa, H., Nguy, K., Bryan, J., and Nelson, D. A. (1995) Science 268 423–426 [DOI] [PubMed] [Google Scholar]
- 10.Babenko, A. P., and Bryan, J. (2003) J. Biol. Chem. 278 41577–41580 [DOI] [PubMed] [Google Scholar]
- 11.Dawson, R. J., and Locher, K. P. (2007) FEBS Lett. 581 935–938 [DOI] [PubMed] [Google Scholar]
- 12.Detimary, P., Dejonghe, S., Ling, Z., Pipeleers, D., Schuit, F., and Henquin, J. C. (1998) J. Biol. Chem. 273 33905–33908 [DOI] [PubMed] [Google Scholar]
- 13.Fridlyand, L. E., Ma, L., and Philipson, L. H. (2005) Am. J. Physiol. 289 E839–E848 [DOI] [PubMed] [Google Scholar]
- 14.Babenko, A. P., Gonzalez, G., and Bryan, J. (1999) FEBS Lett. 459 367–376 [DOI] [PubMed] [Google Scholar]
- 15.Cook, D. L., Satin, L. S., Ashford, M. L., and Hales, C. N. (1988) Diabetes 37 495–498 [DOI] [PubMed] [Google Scholar]
- 16.Matsuo, M., Tanabe, K., Kioka, N., Amachi, T., and Ueda, K. (2000) J. Biol. Chem. 275 28757–28763 [DOI] [PubMed] [Google Scholar]
- 17.Dawson, R. J., and Locher, K. P. (2006) Nature 443 180–185 [DOI] [PubMed] [Google Scholar]
- 18.Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L., and Chen, J. (2007) Nature 450 515–521 [DOI] [PubMed] [Google Scholar]
- 19.Schwanstecher, M., Sieverding, C., Dorschner, H., Gross, I., Aguilar-Bryan, L., Schwanstecher, C., and Bryan, J. (1998) EMBO J. 17 5529–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashfield, R., Gribble, F. M., Ashcroft, S. J., and Ashcroft, F. M. (1999) Diabetes 48 1341–1347 [DOI] [PubMed] [Google Scholar]
- 21.Ueda, K., Komine, J., Matsuo, M., Seino, S., and Amachi, T. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 1268–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikhailov, M. V., Campbell, J. D., de Wet, H., Shimomura, K., Zadek, B., Collins, R. F., Sansom, M. S., Ford, R. C., and Ashcroft, F. M. (2005) EMBO J. 24 4166–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg, M. F., Kamis, A. B., Aleksandrov, L. A., Ford, R. C., and Riordan, J. R. (2004) J. Biol. Chem. 279 39051–39057 [DOI] [PubMed] [Google Scholar]
- 24.de Wet, H., Mikhailov, M. V., Fotinou, C., Dreger, M., Craig, T. J., Venien-Bryan, C., and Ashcroft, F. M. (2007) FEBS J. 274 3532–3544 [DOI] [PubMed] [Google Scholar]
- 25.Babenko, A. P., Gonzalez, G., Aguilar-Bryan, L., and Bryan, J. (1999) FEBS Lett. 445 131–136 [DOI] [PubMed] [Google Scholar]
- 26.Gribble, F. M., Loussouarn, G., Tucker, S. J., Zhao, C., Nichols, C. G., and Ashcroft, F. M. (2000) J. Biol. Chem. 275 30046–30049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.