Abstract
Activated thrombin-activable fibrinolysis inhibitor (TAFIa) plays a significant role in the prolongation of fibrinolysis. During fibrinolysis, plasminogen is activated to plasmin, which lyses a clot by cleaving fibrin after selected arginine and lysine residues. TAFIa attenuates fibrinolysis by removing the exposed C-terminal lysine residues. It was recently reported that TAFI zymogen possesses sufficient carboxypeptidase activity to attenuate fibrinolysis through a mechanism similar to TAFIa. Here, we show with a recently developed TAFIa assay that when thrombin is used to clot TAFI-deficient plasma supplemented with TAFI, there is some TAFI activation. The extent of activation was dependent upon the concentration of zymogen present in the plasma, and lysis times were prolonged by TAFIa in a concentration-dependent manner. Potato tuber carboxypeptidase inhibitor, an inhibitor of TAFIa but not TAFI, abolished the prolongation of lysis in TAFI-deficient plasma supplemented with TAFI zymogen. In addition, TAFIa but not TAFI catalyzed release of plasminogen bound to soluble fibrin degradation products. The data presented confirm that TAFI zymogen is effective in cleaving a small substrate but does not play a role in the attenuation of fibrinolysis because of its inability to cleave plasmin-modified fibrin degradation products.
Thrombin-activable fibrinolysis inhibitor (TAFI)3 is a 60-kDa carboxypeptidase-like protein that circulates in plasma at a concentration of ∼75 nm (1). TAFI (also known as procarboxypeptidase U, plasma carboxypeptidase B, and carboxypeptidase R) was discovered independently by several groups (2–6), and its role in fibrinolysis was subsequently characterized (6). Thrombin in complex with thrombomodulin activates TAFI with a catalytic efficiency of 1.2 μm–1 s–1, which is ∼1250-fold greater than that with thrombin alone (7). Plasmin has also been shown to activate TAFI (0.008 μm–1 s–1), and the second-order rate constant of this reaction increases by 16-fold in the presence of unfractionated heparin (8).
Activated TAFI (TAFIa) plays a significant role in attenuating fibrinolysis. During fibrinolysis, plasminogen is activated to plasmin, which can then solubilize the fibrin clot by cleaving fibrin after specific arginine and lysine residues (9). TAFIa attenuates fibrinolysis by removing the exposed C-terminal lysine residues on fibrin (6, 10), thereby decreasing the tissue-type plasminogen activator (tPA) cofactor activity of plasmin-modified fibrin (11). Removal of these C-terminal lysine residues suppresses plasminogen activation (12, 13) and down-regulates the conversion of Glu-plasminogen to Lys-plasminogen, which is a 20-fold better substrate for tPA than Glu-plasminogen (14). TAFIa is intrinsically unstable, with its inactivation being highly temperature-dependent (15). The inactivation of TAFIa is thought to be accompanied by a large structural change (16). The existence of a TAFIa concentration threshold has been demonstrated, such that TAFIa at a concentration above the threshold inhibits fibrinolysis. Once TAFIa is thermally inactivated and its level falls below the threshold, however, lysis enters the propagation phase (17), and fibrin is quickly solubilized (17–19).
TAFI zymogen also has some carboxypeptidase activity. Previously, the carboxypeptidase activity of TAFI zymogen has been assigned to small synthetic substrates such as hippuryl-linked amino acids (20, 21) and N-(4-methoxyphenylazoformyl)-Lys-OH (22). Recently, TAFI zymogen has been shown to have carboxypeptidase activity toward the tetrapeptide PFGK and larger fibrin peptides (20). Like TAFI, procarboxypeptidase A exhibits carboxypeptidase activity toward short peptides (23), but neither has been shown to cleave macromolecules.
Valnickova et al. (20) reported recently that the zymogen TAFI, acting as a carboxypeptidase, has the ability to suppress fibrinolysis (20). Willemse et al. (24) measured the generation of TAFIa activity during the clotting of human platelet-poor plasma and found that when clotting was initiated with thrombin plus calcium or calcium alone, TAFIa activity increased. They also confirmed that when coagulation was initiated with calcium, lysis was prolonged by the addition of TAFI in a concentration-dependent manner. However, when clotting was initiated with batroxobin, an enzyme that clots fibrinogen but does not activate TAFI, the prolongation of lysis was abolished. Consequently, the authors suggested that any prolongation of lysis is due to TAFIa generated upon the addition of thrombin plus calcium or calcium alone. Valnickova et al. (25) have rebutted these conclusions by pointing out that the carboxypeptidase activity assay used by Willemse et al. (24) is not specific for TAFIa and that clotting induced with batroxobin is not an optimal model because the mechanical properties, clot morphology, and protein composition differ from those of clotting induced with thrombin.
This study was undertaken to determine whether TAFI zymogen is antifibrinolytic or whether thrombin at a low level (5 nm) activates TAFI to a degree sufficient to attenuate lysis. A new assay specific for TAFIa (26) was used to measure TAFIa levels in human platelet-poor normal plasma supplemented with TAFI (66–1000 nm final concentrations) and clotted with thrombin plus calcium. The results indicate that TAFI zymogen does not attenuate fibrinolysis.
EXPERIMENTAL PROCEDURES
Materials—Newborn calf serum, Dulbecco's modified Eagle's medium/nutrient mixture F-12 (1:1), Opti-MEM, and penicillin/streptomycin/Fungizone mixture were obtained from Invitrogen. N,N-Dimethylformamide, 2-mercaptoethanol, and ε-aminocaproic acid were obtained from Fisher, Bio-Rad, and Sigma, respectively. Potato tuber carboxypeptidase inhibitor (PTCI), Phe-Pro-Arg-chloromethyl ketone, and Val-Phe-Lys-chloromethyl ketone were obtained from Calbiochem. Ancrod was obtained from Roche Applied Science GmbH (Mannheim, Germany). N-(4-Methoxyphenylazoformyl)-Arg-OH (AAFR) was purchased from Bachem Biosciences, Inc. (King of Prussia, PA). Baby hamster kidney cells and the mammalian expression vector pNUT were graciously provided by Dr. Ross MacGillivray (University of British Columbia). Recombinant human soluble thrombomodulin was a kind gift from Dr. Oliver Kops (Paion GmbH, Aachen, Germany). Methotrexate (Mayne Pharma Inc., Montreal, Quebec, Canada) and Activase (tPA) were obtained from Kingston General Hospital (Kingston, Ontario, Canada). The cysteine-specific fluorescent probe 5-iodoacetamidofluorescein and the cysteine-specific quencher QSY® 9 C5-maleimide were obtained from Invitrogen. 5-Iodoacetamidofluorescein-labeled plasminogen (5-IAF-Pg) (27), QSY 9 C5-maleimide-conjugated high molecular mass fibrin degradation products (QSY-FDPs) (26, 28), thrombin (28), recombinant human TAFI (29), TAFIa standards (30), TAFIa (29), and TAFI-deficient (30) and barium-absorbed (31) plasmas were prepared as described previously. The buffer used in all experiments (unless stated otherwise) was 0.2-μm filtered 20 mm HEPES, 150 mm NaCl, and 0.01% (v/v) Tween 80, pH 7.4 (HEPES-buffered saline). All plasmas used were dialyzed extensively against HEPES-buffered saline.
Clot Lysis Assays—TAFI-deficient plasma (TDP; 1:3 dilution) supplemented with 0–133 nm TAFI was clotted with 5 nm thrombin and 10 mm CaCl2 and lysed with 1 nm tPA in the presence or absence of 10 μm PTCI (final concentrations). Coagulation and lysis were monitored turbidometrically at 37 °C and 405 nm using a SpectraMax Plus plate reader (Molecular Devices, Sunnyvale, CA), and lysis times were determined. Lysis time is defined as the time required, after clot initiation, to decrease the absorbance to one-half of the plateau value achieved after clotting.
AAFR Cleavage by TAFI and TAFIa—A solution (80 μl) containing AAFR in the presence or absence of PTCI was added to wells of a 96-well plate, and the absorbance (349 nm) was monitored continually (1-min intervals) at room temperature using the SpectraMax Plus plate reader. Between readings, TAFI or TAFIa (40 μl) was added to each well containing AAFR (±PTCI). In each experiment, the final concentrations were 120 μm AAFR, 0–20 μm PTCI, and 133 nm TAFI or 300 pm TAFIa.
Preparation of Samples for Assay of TAFIa—A 25-μl mixture containing thrombin, CaCl2, and tPA was added to a number of 1.5-ml Eppendorf tubes. A 50-μl aliquot comprising 25 μl of TDP and 25 μl of recombinant TAFI (prewarmed to 37 °C) was added to each tube with mixing, and the solutions were immediately incubated at 37 °C. The final concentrations were 5 nm thrombin, 10 mm CaCl2, 1 nm tPA, 66–1000 nm TAFI, and a 1:3 dilution of TDP. Ancrod (0.52 units/ml final concentration) instead of thrombin was used to initiate clotting in some experiments. Parallel clots were made in 96-well plates, and the absorbance (405 nm) was monitored at 37 °C using the SpectraMax Plus plate reader to determine the time courses of clotting and subsequent lysis. All 96-well plates were blocked with 1% Tween 80 for >1 h, washed with deionized distilled water, and dried before use. Clotting and fibrinolysis were arrested at various time points using 100 μm Phe-Pro-Arg-chloromethyl ketone (1 μl). Clots were vortexed for 10 s and centrifuged at 13,000 × g for 1 min at room temperature, and the supernatant was removed and immediately placed on ice. Samples were diluted 1:5 and 1:25 using TDP and assayed for TAFIa.
Effect of TAFI Zymogen on the Assay for TAFIa—TDP was supplemented with 500 nm TAFI zymogen, and the plasma was assayed for TAFIa activity as described (26).
Assay of TAFIa—QSY-FDPs were prepared and characterized essentially as described (26). 80 μl of a solution of 1.25 μm QSY-FDPs and 62.5 nm 5-IAF-Pg in HEPES-buffered saline was added to each well of an opaque 96-well plate and allowed to equilibrate at room temperature while continually (1-min intervals) monitoring the intensity with excitation and emission wavelengths of 480 and 520 nm, respectively, with a 495-nm emission cutoff filter using a SpectraMax Gemini XS fluorescence plate reader (Molecular Devices). TAFIa standards or samples (20 μl) then were added to the 96-well plate using a multichannel pipette between readings. Initial rates were measured for each standard, and a standard curve was generated. The initial rate of each sample was measured, and the standard curve was used to determine the TAFIa concentration.
RESULTS AND DISCUSSION
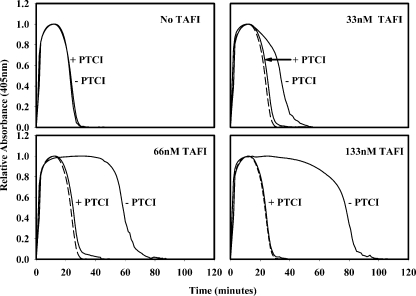
Effect of PTCI on tPA-induced Lysis—Clotting and lysis were initiated in TDP supplemented with 0–133 nm TAFI in the presence or absence of 10 μm PTCI. Clotting was initiated with 5 nm thrombin plus 10 mm CaCl2, and lysis was initiated with 1 nm tPA. Clot lysis profiles are presented in Fig. 1. In the absence of PTCI, increasing TAFI zymogen levels resulted in an increase in the lysis time, as reported previously by Valnickova et al. (20). This suggests that the addition of TAFI to TDP prolongs lysis. However, PTCI (10 μm) completely abolished this prolongation of lysis, giving a lysis time equal to that found in TDP without added TAFI (24 min).
FIGURE 1.
Increasing the TAFI zymogen concentration in TDP resulted in an increase in the lysis time when 1 nm tPA was used to trigger lysis. This effect was nullified by PTCI. The lysis time in TDP was the same in the presence or absence of 10 μm PTCI. As TAFI zymogen was added to TDP, the lysis time increased in the absence of PTCI. The lysis time in the presence of PTCI did not change regardless of the amount of TAFI zymogen added. As a reference, the clot lysis profile of TDP in the presence of PTCI is shown in each panel (– – –).
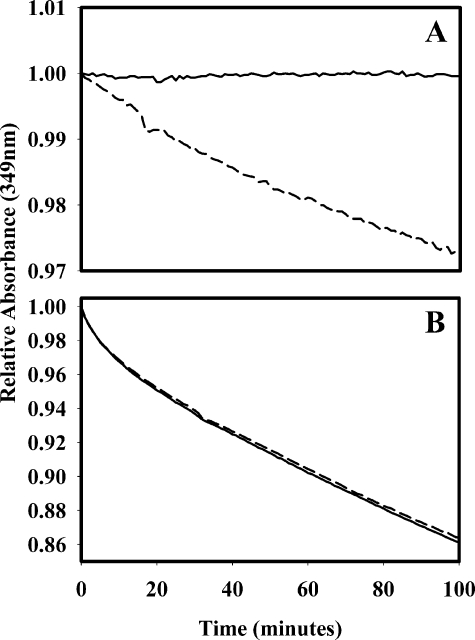
PTCI Inhibits TAFIa but Not TAFI—To determine whether the prolonged lysis seen in Fig. 1 was due to TAFI zymogen or TAFIa, the carboxypeptidase substrate AAFR, was used to determine the effect of PTCI on the activities of TAFI and TAFIa. PTCI (0–20 μm) was titrated into AAFR (120 μm) (data not shown), and the absorbance was monitored continually (1-min intervals). Once a base line had been established, TAFI zymogen (133 nm) or TAFIa (300 pm) was added. PTCI at a concentration of 10 μm was sufficient to completely inhibit the cleavage of AAFR by TAFIa (Fig. 2A), but it had no inhibitory effect on TAFI zymogen (Fig. 2B). The curvature in the progress curves for hydrolysis of AAFR is due to the depletion of substrate over time. Because ∼450-fold more TAFI was used compared with TAFIa, the higher rate of AAFR cleavage by TAFI was expected. Because TAFIa is inhibited by PTCI but TAFI zymogen is not, any prolongation of lysis in the presence of PTCI would be due strictly to TAFI zymogen in the plasma. In the presence of PTCI, no prolongation of lysis by TAFI was detected (Fig. 1). These observations indicate the enzyme TAFIa (not the zymogen TAFI) attenuates clot lysis.
FIGURE 2.
TAFIa (300 pm; A) or TAFI (133 nm;
B) was added to AAFR (120 μm) in the presence (–
– –) or absence
( ) of 10 μm
PTCI to determine the effect of PTCI on the carboxypeptidase activity of the
enzyme and zymogen. PTCI completely inhibited the activity of TAFIa but had no
effect on the carboxypeptidase activity of TAFI.
) of 10 μm
PTCI to determine the effect of PTCI on the carboxypeptidase activity of the
enzyme and zymogen. PTCI completely inhibited the activity of TAFIa but had no
effect on the carboxypeptidase activity of TAFI.
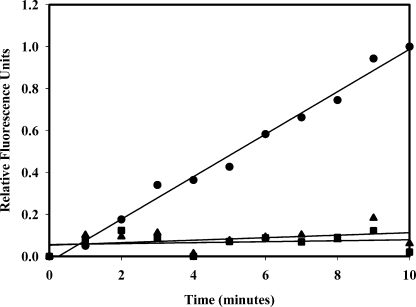
TAFI Zymogen Does Not Cleave Soluble Fibrin Degradation Products—The standard curve for our assay of TAFIa is generated by measuring the initial rate (over ∼10 min) of 5-IAF-Pg dissociation from soluble fibrin degradation products (QSY-FDPs) in the presence of known concentrations of TAFIa (26). TDP supplemented with 200 pm TAFIa showed an increase in fluorescence, suggesting cleavage of lysines on QSY-FDPs and subsequent dissociation of 5-IAF-Pg. When TDP was supplemented with 500 nm TAFI zymogen and assayed for TAFIa, the rate of 5-IAF-Pg dissociation was not significantly different from that in TDP (Fig. 3), indicating that TAFI zymogen is not able to cleave the macromolecular substrate QSY-FDPs, thus demonstrating that our assay is specific for TAFIa.
FIGURE 3.
Our assay for TAFIa is not sensitive to TAFI zymogen. TDP supplemented with 200 pm TAFIa (20 μl) was added to an 80-μl solution containing 1.25 μm QSY-FDPs and 62.5 nm 5-IAF-Pg. Upon the addition of 200 pm TAFIa, an increase in fluorescence was observed (•). When TDP was supplemented with 500 nm TAFI zymogen (▴), the extent of fluorescence increase was not significantly different from that in TDP (▪).
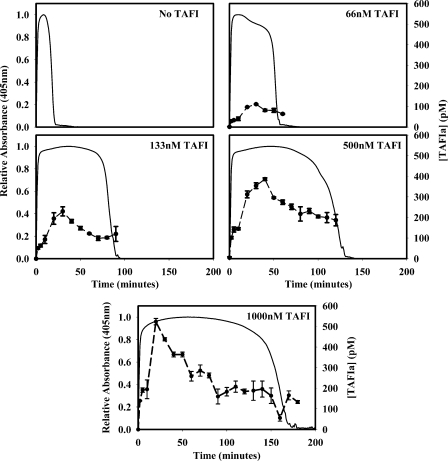
TAFIa Concentration Time Courses during tPA-induced Clot Lysis—TAFI zymogen at various concentrations was added to TDP to determine its effect on lysis when clotting was induced with 5 nm thrombin plus 10 mm CaCl2 and lysis was induced with 1 nm tPA. A recently described assay specific for TAFIa (26) was utilized to confirm that TAFIa was indeed present. In experiments designed to duplicate those of Valnickova et al. (20), it was demonstrated that the amount of TAFI activated was dependent upon the concentration of TAFI zymogen in the plasma (Fig. 4). In the absence of TAFI, the lysis time was ∼24 min. As the concentration of TAFI was increased, the lysis time also increased. When 66 nm TAFI was added, the lysis time doubled to 50 min, and 110 pm TAFIa was measured at its peak. At the highest TAFI concentration (1000 nm), the lysis time was 6-fold longer (155 min), and 525 pm TAFIa was measured at its peak (Fig. 4). Similar trends were observed when the plasma was supplemented with 133 and 500 nm TAFI. In all cases, TAFIa levels peaked between 20 and 40 min after coagulation was initiated, and the peak levels decreased as the concentration of TAFI zymogen decreased.
FIGURE 4.
The time course of TAFI activation was measured in plasma clotted with 5
nm thrombin and 10 mm CaCl2 and lysed with 1
nm tPA. TAFIa concentrations were reported over time in TDP
supplemented with 66–1000 nm TAFI zymogen (•). Turbidity
was monitored continuously at 405 nm
( ) to determine the timing
of lysis. TDP (1:3 dilution) had a lysis time of 22.6 min. As TDP was
supplemented with TAFI, lysis times increased, as did the peak concentration
of TAFIa. When TDP was supplemented with 1000 nm TAFI, the lysis
time increased to 155 min, and TAFIa levels peaked at 525 pm.
) to determine the timing
of lysis. TDP (1:3 dilution) had a lysis time of 22.6 min. As TDP was
supplemented with TAFI, lysis times increased, as did the peak concentration
of TAFIa. When TDP was supplemented with 1000 nm TAFI, the lysis
time increased to 155 min, and TAFIa levels peaked at 525 pm.
Valnickova et al. (20) showed that when soluble thrombomodulin was included during the clotting and lysis of normal plasma, the lysis time was extended by >3-fold compared with that in the absence of soluble thrombomodulin. When PTCI was added with soluble thrombomodulin to inhibit TAFIa, the lysis time was the same as in the absence of soluble thrombomodulin, an observation from which Valnickova et al. inferred that TAFI is not activated in the absence of soluble thrombomodulin. However, TAFI was activated in plasma supplemented with TAFI, as shown in Fig. 4, and attenuation of lysis can be attributed specifically to TAFIa. The activation of TAFI by thrombin has a relatively high Km compared with its plasma concentration (7), which implies that the addition of TAFI zymogen would increase the rate of TAFI activation in a concentration-dependent manner. As a result, as the TAFI concentration is increased, the TAFIa concentration increases, and the extent to which lysis is prolonged increases.
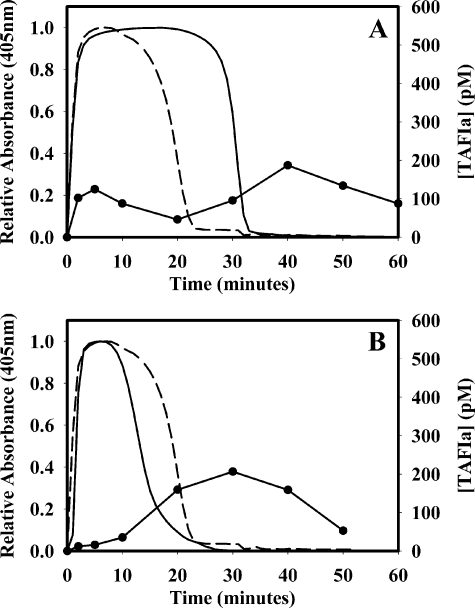
Because TAFI activation occurs during the propagation phase of coagulation, TAFI-deficient, barium citrate-adsorbed plasma was supplemented with 500 nm TAFI to determine the extent of TAFI activation in plasma lacking an intrinsic coagulation pathway. When clotting was initiated with 5 nm thrombin plus 10 mm CaCl2 and lysis was induced with 1 nm tPA, the lysis time was 30.5 min, and TAFIa appeared transiently at 5 min and again at 40 min (Fig. 5A). This is consistent with the timing of both thrombin addition and subsequent plasmin production. This demonstrates that even in plasma lacking an intrinsic coagulation pathway, 5 nm thrombin is sufficient to activate some TAFI and to prolong lysis in the absence of soluble thrombomodulin. Clots formed in TAFI-deficient, barium citrate-adsorbed plasma with no added TAFI lysed in 19.3 min under the same conditions. When clotting was induced in TAFI-deficient, barium citrate-adsorbed plasma with 500 nm TAFI and 0.52 units/ml ancrod in place of thrombin plus 10 mm CaCl2 in the presence of 1 nm tPA, the lysis time was 13.3 min, and the TAFIa level peaked at 207 pm 10 min after the clot had fully lysed (Fig. 5B). This peak was probably due to TAFI activation by plasmin. The extent of TAFI activation post-lysis was similar to that observed when coagulation was induced with thrombin. When ancrod (instead of thrombin) was used to initiate coagulation, the TAFIa peak that corresponded with thrombin addition was absent, whereas the secondary peak due to TAFI activation by plasmin remained (Fig. 5B). The lysis time was much shorter than that observed when clotting was initiated with thrombin (13.3 min compared with 30.5 min). Clotting induced with ancrod in the presence of 500 nm TAFI had shorter lysis times compared with clotting initiated with thrombin in the absence of TAFI, which may be due to differences in clot structure (25).
FIGURE 5.
Plasma lacking an intrinsic coagulation pathway can yield TAFIa when low
levels (5 nm) of thrombin are added. TAFI-deficient,
barium-absorbed plasma was supplemented with 500 nm TAFI and
clotted with thrombin (5 nm) and calcium (A) or ancrod
(0.52 units/ml) and calcium (B) to determine the extent of TAFI
activation in plasma lacking an intrinsic coagulation pathway. In all
experiments, lysis was initiated by the addition of 1 nm tPA. With
thrombin (A), TAFIa levels (•) peaked at 125 and 187
pm after 5 and 40 min, respectively. The lysis time was 30.5 min in
TAFI-deficient, barium-absorbed plasma supplemented with 500 nm
TAFI ( ) compared with 19.3
min in TAFI-deficient, barium-absorbed plasma with no added TAFI (–
– –). Ancrod was also used to clot TAFI-deficient, barium-absorbed
plasma (B). There was very little TAFI activated until after the clot
had lysed. TAFIa levels peaked at 207 pm at 30 min (•),
whereas the lysis time was 13.3 min
(
) compared with 19.3
min in TAFI-deficient, barium-absorbed plasma with no added TAFI (–
– –). Ancrod was also used to clot TAFI-deficient, barium-absorbed
plasma (B). There was very little TAFI activated until after the clot
had lysed. TAFIa levels peaked at 207 pm at 30 min (•),
whereas the lysis time was 13.3 min
( ). The thrombin-induced
clotting and subsequent lysis of TAFI-deficient, barium-absorbed plasma (no
added TAFI) are shown as a reference (– – –).
). The thrombin-induced
clotting and subsequent lysis of TAFI-deficient, barium-absorbed plasma (no
added TAFI) are shown as a reference (– – –).
In summary, our results show that whereas TAFI zymogen catalyzes cleavage of a small substrate, it does not catalyze cleavage of plasmin-modified fibrin (fibrin degradation products). Consequently, TAFI zymogen is not antifibrinolytic. The fact that increased TAFI zymogen added at time 0 results in increased lysis times is due to increased levels of TAFIa appearing as a consequence of activation by thrombin used to form the clot.
This work was supported in part by National Institutes of Health Grant PO1 HL046703 and Heart and Stroke Foundation of Ontario Grant T5575. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TAFI, thrombin-activable fibrinolysis inhibitor; TAFIa, activated TAFI; tPA, tissue-type plasminogen activator; PTCI, potato tuber carboxypeptidase inhibitor; AAFR, N-(4-methoxyphenylazoformyl)-Arg-OH; 5-IAF-Pg, 5-iodoacetamidofluorescein-labeled plasminogen; QSY-FDPs, QSY® 9C5-maleimide-conjugated high molecular mass fibrin degradation products; TDP, TAFI-deficient plasma.
References
- 1.Bajzar, L., Nesheim, M. E., and Tracy, P. B. (1996) Blood 88 2093–2100 [PubMed] [Google Scholar]
- 2.Hendriks, D., Wang, W., Scharpe, S., Lommaert, M. P., and van Sande, M. (1990) Biochim. Biophys. Acta 1034 86–92 [DOI] [PubMed] [Google Scholar]
- 3.Wang, W., Hendriks, D. F., and Scharpe, S. S. (1994) J. Biol. Chem. 269 15937–15944 [PubMed] [Google Scholar]
- 4.Eaton, D. L., Malloy, B. E., Tsai, S. P., Henzel, W., and Drayna, D. (1991) J. Biol. Chem. 266 21833–21838 [PubMed] [Google Scholar]
- 5.Tan, A. K., and Eaton, D. L. (1995) Biochemistry 34 5811–5816 [DOI] [PubMed] [Google Scholar]
- 6.Bajzar, L., Manuel, R., and Nesheim, M. E. (1995) J. Biol. Chem. 270 14477–14484 [DOI] [PubMed] [Google Scholar]
- 7.Bajzar, L., Morser, J., and Nesheim, M. (1996) J. Biol. Chem. 271 16603–16608 [DOI] [PubMed] [Google Scholar]
- 8.Mao, S. S., Cooper, C. M., Wood, T., Shafer, J. A., and Gardell, S. J. (1999) J. Biol. Chem. 274 35046–35052 [DOI] [PubMed] [Google Scholar]
- 9.Suenson, E., Lutzen, O., and Thorsen, S. (1984) Eur. J. Biochem. 140 513–522 [DOI] [PubMed] [Google Scholar]
- 10.Redlitz, A., Tan, A. K., Eaton, D. L., and Plow, E. F. (1995) J. Clin. Investig. 96 2534–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker, J. B., and Nesheim, M. E. (2001) J. Biol. Chem. 276 3138–3148 [DOI] [PubMed] [Google Scholar]
- 12.Wang, W., Boffa, M. B., Bajzar, L., Walker, J. B., and Nesheim, M. E. (1998) J. Biol. Chem. 273 27176–27181 [DOI] [PubMed] [Google Scholar]
- 13.Sakharov, D. V., Plow, E. F., and Rijken, D. C. (1997) J. Biol. Chem. 272 14477–14482 [DOI] [PubMed] [Google Scholar]
- 14.Horrevoets, A. J. G., Pannekoek, H., and Nesheim, M. E. (1997) J. Biol. Chem. 272 2183–2191 [DOI] [PubMed] [Google Scholar]
- 15.Schneider, M., Boffa, M., Stewart, R., Rahman, M., Koschinsky, M., and Nesheim, M. (2002) J. Biol. Chem. 277 1021–1030 [DOI] [PubMed] [Google Scholar]
- 16.Boffa, M. B., Bell, R., Stevens, W. K., and Nesheim, M. E. (2000) J. Biol. Chem. 275 12868–12878 [DOI] [PubMed] [Google Scholar]
- 17.Leurs, J., Nerme, V., Sim, Y., and Hendriks, D. (2004) J. Thromb. Haemostasis 2 416–423 [DOI] [PubMed] [Google Scholar]
- 18.Walker, J. B., and Bajzar, L. (2004) J. Biol. Chem. 279 27896–27904 [DOI] [PubMed] [Google Scholar]
- 19.Walker, J. B., and Bajzar, L. (2007) J. Thromb. Haemostasis 5 1257–1264 [DOI] [PubMed] [Google Scholar]
- 20.Valnickova, Z., Thogersen, I. B., Potempa, J., and Enghild, J. J. (2007) J. Biol. Chem. 282 3066–3076 [DOI] [PubMed] [Google Scholar]
- 21.Willemse, J. L., Polla, M., and Hendriks, D. F. (2006) Anal. Biochem. 356 157–159 [DOI] [PubMed] [Google Scholar]
- 22.Schneider, M., Nagashima, M., Knappe, S., Zhao, L., Morser, J., and Nesheim, M. (2002) J. Biol. Chem. 277 9944–9951 [DOI] [PubMed] [Google Scholar]
- 23.Reverter, D., Garcia-Saez, I., Catasus, L., Vendrell, J., Coll, M., and Aviles, F. X. (1997) FEBS Lett. 420 7–10 [DOI] [PubMed] [Google Scholar]
- 24.Willemse, J. L., Heylen, E., and Hendriks, D. F. (2007) J. Thromb. Haemostasis 5 1334–1336 [DOI] [PubMed] [Google Scholar]
- 25.Valnickova, Z., Thogersen, I. B., Potempa, J., and Enghild, J. J. (2007) J. Thromb. Haemostasis 5 1336–1337 [DOI] [PubMed] [Google Scholar]
- 26.Kim, P. Y., Foley, J., Hsu, G., Kim, P. Y., and Nesheim, M. E. (2008) Anal. Biochem. 372 32–40 [DOI] [PubMed] [Google Scholar]
- 27.Horrevoets, A. J. G., Pannekoek, H., and Nesheim, M. E. (1997) J. Biol. Chem. 272 2176–2182 [DOI] [PubMed] [Google Scholar]
- 28.Walker, J. B., and Nesheim, M. E. (1999) J. Biol. Chem. 274 5201–5212 [DOI] [PubMed] [Google Scholar]
- 29.Boffa, M. B., Wang, W., Bajzar, L., and Nesheim, M. E. (1998) J. Biol. Chem. 273 2127–2135 [DOI] [PubMed] [Google Scholar]
- 30.Neill, E. K., Stewart, R. J., Schneider, M. M., and Nesheim, M. E. (2004) Anal. Biochem. 330 332–341 [DOI] [PubMed] [Google Scholar]
- 31.Bajzar, L., Fredenburgh, J. C., and Nesheim, M. E. (1990) J. Biol. Chem. 265 16948–16954 [PubMed] [Google Scholar]