Abstract
Over 200 disease-causing mutations have been identified in the NPC1 gene. The most prevalent mutation, NPC1I1061T, is predicted to lie within the cysteine-rich luminal domain and is associated with the classic juvenile-onset phenotype of Niemann-Pick type C disease. To gain insight into the molecular mechanism by which the NPC1I1061T mutation causes disease, we examined expression of the mutant protein in human fibroblasts homozygous for the NPC1I1061T mutation. Despite similar NPC1 mRNA levels between wild type and NPC1I1061T fibroblasts, NPC1 protein levels are decreased by 85% in NPC1I1061T cells. Metabolic labeling studies demonstrate that unlike wild type protein, which undergoes a glycosylation pattern shift from Endo H-sensitive to Endo H-resistant species, NPC1I1061T protein remains almost exclusively Endo H-sensitive and exhibits a reduced half-life (t½ 6.5 h) versus wild type Endo H-resistant species (t½ 42 h). Treatment with chemical chaperones, growth at permissive temperature, or inhibition of proteasomal degradation increases NPC1I1061T protein levels, indicating that the mutant protein is likely targeted for endoplasmic reticulum-associated degradation (ERAD) due to protein misfolding. Overexpression of NPC1I1061T in NPC1-deficient cells results in late endosomal localization of the mutant protein and complementation of the NPC mutant phenotype, likely due to a small proportion of the nascent NPC1I1061T protein that is able to fold correctly and escape the endoplasmic reticulum quality control checkpoints. Our findings provide the first description of an endoplasmic reticulum trafficking defect as a mechanism for human NPC disease, shedding light on the mechanism by which the NPC1I1061T mutation causes disease and suggesting novel approaches to treat NPC disease caused by the NPC1I1061T mutation.
Niemann-Pick type C (NPC)3 disease is a fatal neurodegenerative disease characterized by neuronal lipid storage and progressive Purkinje cell loss in the cerebellum. Mutations in the NPC1 gene are responsible for ∼95% of human NPC disease (1). The human NPC1 gene encodes a 1278-amino acid polytopic protein containing 13 transmembrane domains, including a pentahelical domain that is evolutionarily and functionally related to sterol-sensing domains found in five other polytopic proteins involved in sterol interactions or sterol metabolism (2, 3). The NPC1 sterol-sensing domain shares ∼30% identity with the sterol-sensing domains of 3-hydroxymethylglutaryl-CoA reductase, sterol regulatory element-binding protein cleavage-activating protein (SCAP), NPC1-L1 and Patched (4). A carboxyl-terminal dileucine motif targets NPC1 to the endocytic pathway, where it localizes to a late endosomal compartment that is LAMP-2-positive, Rab7-positive, and cation-independent mannose-6-P receptor-negative (5–7).
The NPC1 protein is a key participant in intracellular sterol trafficking. Cells harboring inactivating mutations in NPC1 exhibit marked impairment of low-density lipoprotein (LDL) cholesterol esterification and mobilization of newly hydrolyzed LDL cholesterol to the plasma membrane (8–10). As a result of these trafficking defects, NPC1 mutant cells demonstrate lysosomal sequestration of LDL cholesterol, delayed down-regulation of the LDL receptor and de novo cholesterol biosynthesis, and impaired ABCA1-mediated cholesterol efflux (11–14).
Over 200 disease-causing mutations have been identified in the NPC1 gene (15–17). The most prevalent mutation, NPC1I1061T, is predicted to lie within a cysteine-rich luminal domain of the NPC1 protein and represents 15–20% of all disease alleles (4, 15, 18). Human fibroblasts homozygous for the NPC1I1061T mutation exhibit markedly impaired LDL-stimulated cholesterol esterification and accumulation of unesterified cholesterol in aberrant lysosomes (15). Previous studies have shown that NPC1I1061T is expressed at lower levels and exhibits altered banding patterns on Western blotting as compared with wild type (WT) protein (19). However, the molecular mechanism through which the NPC1I1061T missense mutation results in NPC disease is poorly understood.
In the present study we examine the effect of the NPC1I1061T substitution on processing and stability of the NPC1 protein. We provide evidence that the NPC1I1061T protein is synthesized but fails to advance in the secretory pathway due to recognition as a misfolded protein by the endoplasmic reticulum (ER) quality control machinery and consequent targeting for proteasomal degradation. Overexpression of NPC1I1061T led to late endosomal localization of the mutant protein and functional complementation of the NPC mutant phenotype, likely as a result of a small proportion of NPC1I1061T mutant protein that folded correctly and was thus able to escape ER quality control. Our findings provide support for use of chemical chaperones as approaches to treat NPC disease caused by the NPC1I1061T mutation.
MATERIALS AND METHODS
Cell Culture and Chemicals—Normal human skin fibroblasts were obtained from ATCC (20). The human homozygous NPC1I1061T mutant human fibroblast cell lines (NIH 83.16, NIH 89.79, NIH 90.39, and NIH 95.47) were generously provided by Daniel Kraft and David Marks (Mayo) (15). M12 cells are mutant CHO-K1 cells that contain a deletion of the npc1 locus (8). To generate npc1-null cells expressing WT NPC1, NPC1I1061T, and NPC1P692S, M12 cells were infected with retrovirus prepared by transient transfection of 293GPG packaging cells with the ΔU3mnpc1-WT-GFP, ΔU3mnpc1-I1061T-GFP, and ΔU3mnpc1-P692S-GFP constructs, respectively, as previously described (3). Mouse embryonic fibroblasts were isolated from WT C57BL/6 mice. Cells were maintained in monolayer culture at 37 °C with 5% CO2. All fibroblasts cell lines were passaged in media containing Dulbecco's modified Eagle's medium with 10% (v/v) inactivated fetal bovine serum, 2 mm glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. CHO-derived cell lines were maintained in 1:1 Dulbecco's modified Eagle's medium:Ham's F-12 with 5% (v/v) fetal bovine serum, 2 mm glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin. Transfection of M12 cells was performed using Lipofectamine Plus reagent as previously described (3). Transient transfections of NPC1I1061T fibroblasts were performed using a primary cell line nucleofector kit and apparatus from Amaxa (20). Dulbecco's modified Eagle's medium, fetal bovine serum, glutamine, Ham's F-12 medium, Lipofectamine Plus, and penicillin/streptomycin were obtained from Invitrogen. Paraformaldehyde was obtained from EM Sciences. All restriction enzymes and endoglycosidases were obtained from New England Biolabs. [35S]Cys/Met (11.0 mCi/ml-EasyTag Express Labeling Kit), Western Lightning Chemiluminescent Reagent, and En3Hance were obtained from PerkinElmer Life Sciences. Complete Protease Inhibitor Mixture Tablets, phenylmethylsulfonyl fluoride, and Protein A-agarose were obtained from Roche Applied Science. The β-actin antibody, dialyzed fetal bovine serum, filipin, and glycerol were from Sigma. 4-Phenylbutyrate (sodium salt) was from EMD Biosciences. The NPC1 antibody used for immunoprecipitation and Western blotting was a rabbit anti-human NPC1 (raised against residues 1261–1278) (8). The p63 antibody was kindly provided by Jack Rohrer (21).
Plasmids—The ΔU3mnpc1-I1061T-GFP construct was generated using the QuikChange XL Site-directed Mutagenesis Kit (Stratagene), using the ΔU3mnpc1-WT-GFP construct as a template (3). ΔU3mnpc1-I1061T was generated by deletion of the COOH-terminal GFP tag. The presence of the npc1I1061T mutation and the entire mnpc1 coding sequence in the expression construct was confirmed by ABI Prism automated sequencing.
Quantification of Human NPC1 Gene Expression—Total RNA was isolated from cells using TRIzol reagent (Invitrogen), and reverse transcribed to cDNA using SuperScriptII RNase H– reverse transcriptase and random hexamer primers (Invitrogen). cDNA was then amplified for 40 PCR cycles using SYBR Green PCR master mixture (Applied Biosystems) and template-specific primers (50 nm) in an ABI Prism 7500 sequence detector. Primer sequences were as follows: human NPC1 (forward, 5′-cagctggacaactatacccgaat-3′; reverse, 5′-tggcttcacccagtcgaaat-3′) and human glyceraldehyde-3-phosphate dehydrogenase (forward, 5′-cgagatccctccaaaatcaa-3′; reverse, 5′-catgcgtccttccacgataccaa-3′). Relative quantification of gene expression was performed using the comparative threshold (CT) method as described by the manufacturer. Changes in mRNA expression level were calculated following normalization to glyceraldehyde-3-phosphate dehydrogenase expression.
Protein Preparation and Western Blot Analysis—Detergent lysates were prepared by washing cell monolayers three times with phosphate-buffered saline. Cells were then scraped in 500 μl of TNEN+ (50 mm Tris, pH 8.0, 0.15 m NaCl, 2 mm EDTA, 0.5% Nonidet P-40, 1× Complete Protease Inhibitor Mixture, 1 mm phenylmethylsulfonyl fluoride) and incubated on ice for 10 min. Cell lysates were collected into microcentrifuge tubes and nuclei were pelleted by centrifugation at 1000 × g for 10 minutes at 4 °C. Supernatants were transferred to new tubes. Proteins in TNEN+ lysates were quantified using the bicinchoninic acid protein assay kit (Pierce). Isolation of microsomes from CHO cell lines was performed as described previously (22). Non-boiled samples were resolved by SDS-PAGE under reducing conditions. Proteins were transferred onto polyvinylidene difluoride (0.45 mm; Millipore) using a semi-dry electroblotter (Owl Scientific). Western blot analysis of NPC1 expression was performed using an affinity purified rabbit anti-human NPC1 at a dilution of 1:2000 and a peroxidase-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch) at 1:5000. Analysis of β-actin expression was performed using a rabbit anti-human β-actin at 1:500 and a peroxidase-conjugated donkey anti-rabbit IgG at 1:5000. Analysis of p63 expression was performed using a rabbit anti-human p63 at 1:5000 and a peroxidase-conjugated donkey anti-rabbit IgG at 1:5000. Detection was performed by chemiluminescence using Western Lightning reagents. Densitometry was performed using Quantity One software (Bio-Rad). For each of the non-peptide competition lanes, both the blot background and the region on the gel between 160 and 220 kDa in the peptide competition lanes (i.e. background bands), including the band denoted by the filled arrow, were subtracted from total NPC1 immunoreactivity.
Metabolic Labeling and Immunoprecipitation of NPC1 Protein—Pulse-chase labeling of NPC1 protein was achieved following a 1-h treatment in starvation media (Cys/Met-depleted) followed by a 1-h treatment in starvation media supplemented with [35S]Cys/Met (EasyTag Express Labeling Kit). After labeling, total cell lysate was collected in 500 μl of TNEN+ buffer (0.5% Nonidet P-40). The lysate was spun at 1000 × g for 10 min at 4 °C, the supernatant was collected, pre-cleared with Protein A-agarose, and incubated with NPC1 affinity purified antibody overnight at 4 °C. Protein-antibody complex was pulled down using Protein A-agarose at 4 °C for 45 min. Protein-antibody complex was removed from the Protein A-agarose using 1× Laemmli buffer (Bio-Rad) for 10 min at 37 °C followed by vortexing. For endoglycosidase treatments, immunoprecipitations (IPs) were treated overnight at 37 °C with either Endoglycosidase H (Endo H) or peptide:N-glycosidase F in the commercially supplied buffers. Protein was separated by 6% SDS-PAGE and analyzed by autoradiography.
Immunofluorescence Microscopy—Following transfection, cells were plated on 22-mm glass coverslips in 35-mm wells. Cells were cultured 24–72 h, and fixed with 4% paraformaldehyde in phosphate-buffered saline for 30 min. Immunocytochemistry for NPC1 and unesterified cholesterol (filipin staining) was performed as described (5). The coverslips were washed three times with phosphate-buffered saline, mounted (SlowFade, Molecular Probes), and examined by fluorescence microscopy on a Zeiss Axiovert epifluorescence microscope. The following filter sets (Chroma) were used: for filipin, excitation filter 360/40 nm, emission filter 460/50; for GFP, excitation filter 500/20, emission filter 535/30.
LDL-stimulated Cholesterol Esterification Assay—Cholesterol esterification assays were performed as described previously (3).
Statistics—All results are expressed as mean ± S.E. The statistical significance of differences in mean values was determined by single factor analysis of variance. Data shown are representative of at least three similar experiments and are presented as mean ± S.E.
RESULTS
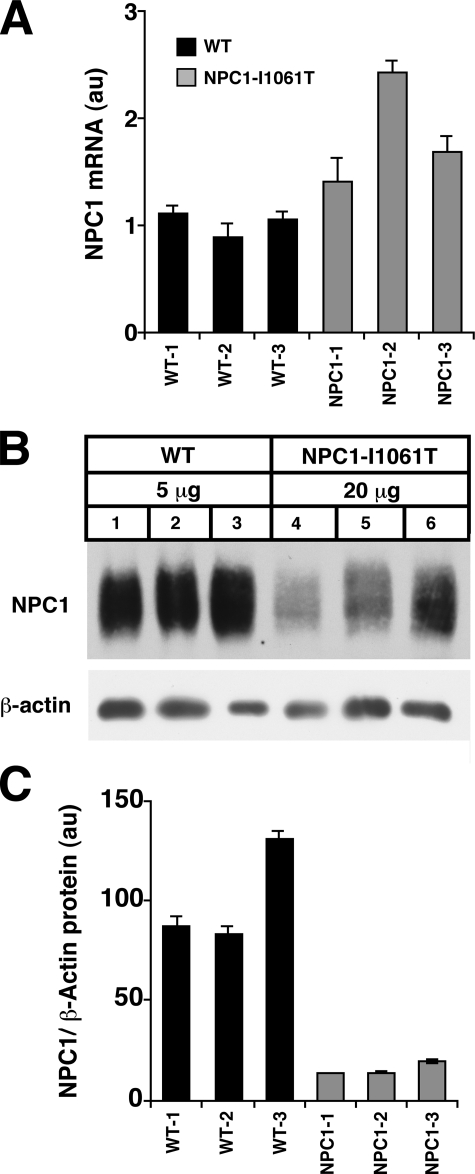
NPC1I1061T Mutant Protein Is Mislocalized and Expressed at Reduced Levels in Human Fibroblasts—In WT human fibroblasts NPC1 protein predominantly distributes to a multivesicular late endosomal compartment (5, 6) (Fig. 1). This contrasts with human fibroblasts homozygous for the NPC1I1061T missense mutation, in which NPC1 immunostaining is reduced and the mutant protein fails to localize to late endosomes. The resulting lysosomal free cholesterol accumulation in the NPC1I1061T fibroblasts is the hallmark of the NPC mutant phenotype (12). Whereas NPC1 mRNA transcript levels are elevated 1.4–2.4-fold in NPC1I1061T versus WT fibroblasts (Fig. 2A), NPC1 protein levels are reduced by 85% in the NPC1I1061T fibroblasts (Fig. 2, B and C). Thus, the NPC1I1061T substitution affects steady-state levels of endogenously expressed NPC1 protein, possibly by impairing translation of the NPC1 protein or by rendering the protein unstable.
FIGURE 1.
Immunofluorescence staining for NPC1 (A and C) and filipin staining for cholesterol (B and D) in WT (A and B) and NPC1I1061T mutant fibroblasts (C and D). Bar, 50 μm.
FIGURE 2.
Characterization of NPC1 expression in human fibroblasts homozygous for NPC1I1061T mutations. Data shown are for three independent WT and three independent NPC1I1061T human fibroblast cell lines. A, real-time quantitative PCR for NPC1 mRNA levels in WT (black bars) and NPC1I1061T mutant fibroblasts (gray bars). NPC1 mRNA expression is normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA. *, p = 0.056 for mean WT versus mean NPC1I1061T. B, Western blot analysis for NPC1 protein expression in WT and NPC1I1061T mutant fibroblasts. Note 4-fold greater loading of protein in NPC1I1061T lanes compared with WT lanes. NPC1 expression is normalized to β-actin expression (5 μg/lane). C, quantification of NPC1 protein expression in WT (black bars) and NPC1I1061T mutant fibroblasts (gray bars). *, p < 0.01 for mean WT versus mean NPC1I1061T.
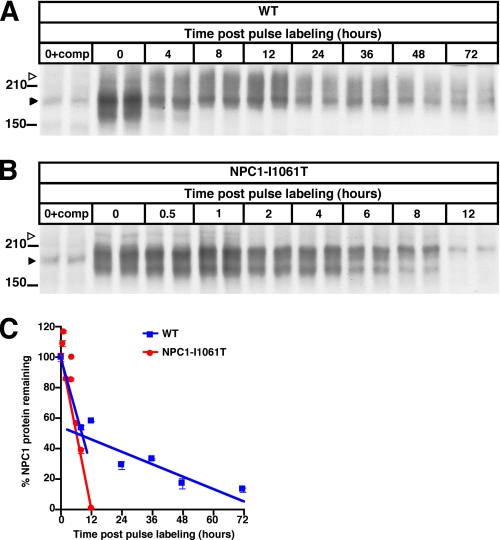
NPC1I1061T Missense Mutation Promotes Rapid Proteasomal Degradation of NPC1 Protein—To assess the effect of the NPC1I1061T mutation on stability of the NPC1 protein, we monitored the degradation of the protein in metabolic pulse-chase experiments over a 72-h period. WT and NPC1I1061T homozygous fibroblasts were labeled, and NPC1 protein immunoprecipitated and analyzed by autoradiography (Fig. 3, A and B). Two distinct rates for WT NPC1 protein degradation were observed. From 0–8 h post pulse labeling, WT NPC1 protein exhibited a t½ of 9 h, whereas from 8 to 72 h, the t½ was extended to 42 h (Fig. 3C). By contrast, NPC1I1061T was degraded with a t½ of 6.5 h, similar to that of the initial rate of degradation of WT protein (Fig. 3C). The maximal level of labeled NPC1 protein was comparable between WT and NPC1I1061T proteins, indicating similar rates in initiation of translation. An important difference, however, between WT and NPC1I1061T protein was the failure of NPC1I1061T to mature to higher molecular weight species (see open arrow, Fig. 3B).
FIGURE 3.
Regulation of NPC1I1061T protein expression by ER quality control. A and B, metabolic labeling of newly synthesized NPC1 protein. WT (A) and NPC1I1061T mutant (B) fibroblasts were pulsed for 1 h with [35S]Cys/Met containing media followed by 0–72 h (WT) or 0–12 h (NPC1I1061T) chase and IP with NPC1 antibody. For each time point two independent samples are shown. Left lanes (0+comp) show IP in the presence of excess immunizing peptide (competition). Closed arrows identify nonspecific band. Note the shift to higher molecular weight species (open arrows) in WT cells from 0 to 4 h, which is absent in the NPC1I1061T mutant. C, the graph plots densitometric values for radiolabeled NPC1 versus time. From 0 to 8 h both WT (blue line) and NPC1I1061T fibroblasts (red line) show similar initial rapid rates of NPC1 protein degradation (WT t½ = 9 h, NPC1I1061T t½ = 6.5 h), whereas from 8 to 72 h WT (blue line) NPC1 protein degradation is decreased to t½ = 42 h.
NPC1I1061T Protein Is Sensitive to Endoglycosidase H—The maturation of the NPC1 protein in the secretory pathway was examined in metabolic pulse-chase experiments in which the sensitivity of the labeled protein to digestion by Endo H was determined. Endo H removes immature high-mannose N-linked glycans from proteins. Sensitivity to Endo H digestion indicates that the protein has not advanced beyond the ER in the secretory pathway. Conversely, resistance to Endo H digestion indicates that the glycan residues have been trimmed and/or further modified in the Golgi. Peptide:N-glycosidase F removes all N-linked glycan residues regardless of the glycan modification. In WT fibroblasts, the steady reduction in the level of Endo H-sensitive NPC1 protein (120-kDa band) is accompanied by a concomitant accumulation of Endo H-resistant NPC1 protein (180-kDa band) (Fig. 4A). By contrast, the NPC1I1061T mutant protein is present almost exclusively in the Endo H-sensitive form (Fig. 4B). As shown by the peptide competition controls, immunoreactivity detected at 180 kDa in the NPC1I1061T mutant blot is due to nonspecific banding, rather than accumulation of Endo H-resistant NPC1 protein. The more rapid loss of the Endo H-sensitive WT NPC1 species likely reflects the dynamic between degradation of ER-associated NPC1 protein and procession of the immature NPC1 within the secretory pathway (i.e. trafficking from ER to Golgi) to generate mature glycoprotein. The apparent molecular weight shift in NPC1 protein in the glycosidase experiments (Fig. 4 versus Fig. 3) is an artifact of the glycosidase treatment and buffer system used in the studies. Taken together, the metabolic labeling experiments demonstrate that ∼50% of WT NPC1 protein, and nearly all of the NPC1I1061T mutant protein, is ER-retained and targeted for degradation.
FIGURE 4.
Metabolic labeling of newly synthesized NPC1 protein followed by Endo H treatment. A, WT and NPC1I1061T mutant, and B, fibroblasts were pulse-labeled as above, followed by 0–8 h chase, IP with NPC1 antibody, and Endo H treatment. U, untreated, P, peptide:N-glycosidase F treated, and C, IP in the presence of excess immunizing peptide. Closed arrows and open arrows indicate Endo H-sensitive and Endo H-resistant species, respectively. Graphs show densitometric values for Endo H-sensitive (gray bars) and Endo H-resistant species (black bars) in WT (upper right panel) and NPC1I1061T mutant fibroblasts (lower right panel).
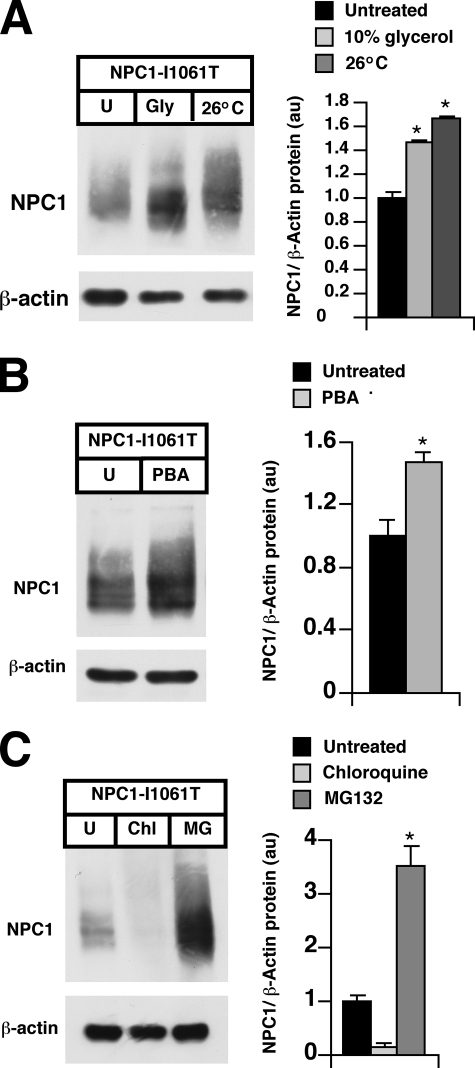
NPC1I1061T Protein Is Selected for ERAD—The ER quality control pathway selects for degradation misfolded and unassembled proteins in the ER (23). To examine whether the NPC1I1061T mutant protein is a substrate for ERAD, we subjected NPC1I1061T mutant fibroblasts to treatment with either glycerol or 4-phenylbutyric acid (PBA), chemical chaperones known to stabilize misfolded proteins (24–26). Glycerol and PBA treatments resulted in 1.4- and 1.5-fold increases, respectively, in the level of the mutant protein (Fig. 5, A and B). Similarly, growth of NPC1I1061T mutant fibroblasts at the permissive temperature of 26 °C, which partially rescues the ER processing block of cystic fibrosis transmembrane conductance regulator (CFTR) Δ508 mutant (24, 27), increased NPC1I1061T protein levels 1.7-fold. The stabilizing effects of the chemical chaperones and growth at permissive temperatures on NPC1I1061T provide compelling evidence that the mutant protein is selected for ERAD due to protein misfolding. To determine the metabolic fate of the mutant protein selected for ERAD, NPC1I1061T mutant fibroblasts were treated with MG132, a proteasome inhibitor, or chloroquine, a weak base that inhibits lysosomal proteolysis. In the presence of MG132, mutant protein levels were increased 3.5-fold, implicating the proteasomal pathway in degradation of the mutant protein (Fig. 5C). By contrast, NPC1I1061T protein levels were reduced 85% after treatment with chloroquine, clearly demonstrating that the mutant protein is not lysosomally degraded.
FIGURE 5.
NPC1I1061T protein is proteasomally degraded. A, NPC1I1061T cells were treated for 24 h in 10% glycerol or incubated at 26 °C, and cell lysates were subjected to Western blot analysis for NPC1 expression. A representative blot is shown and the graph displays NPC1 expression normalized toβ-actin. *, p < 0.05 for 26 °C versus untreated, p = 0.057 for glycerol versus untreated. B, NPC1I1061T cells were treated for 24 h with 20μm PBA, and lysates were subjected to Western blot analysis for NPC1 expression. A representative blot is shown and the graph displays NPC1 expression normalized to β-actin. *, p < 0.05 for PBA versus untreated. C, NPC1I1061T cells were treated for 24 h with the proteasome inhibitor MG132 (50 μm) or with the lysosome inhibitor chloroquine (10 μm), and lysates were subjected to Western blot analysis for NPC1 expression. A representative blot is shown and the graph displays NPC1 expression normalized to β-actin. *, p < 0.05 for MG132 versus untreated.
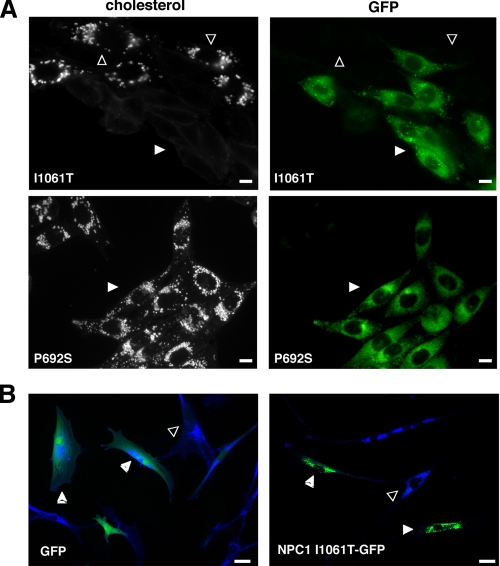
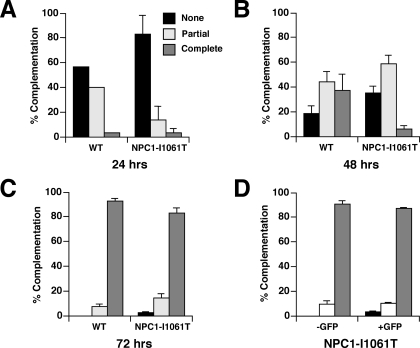
Overexpression of the NPC1I1061T Protein Rescues the NPC Mutant Phenotype—Previous studies have shown that overexpression of mutant proteins that are misfolded may allow for transit of a small proportion of the mutant proteins through the Golgi (28). Heterologous overexpression of CFTR Δ508 led to escape of mutant protein from ERAD and appropriate localization at the plasma membrane (29, 30). Therefore, we investigated whether overexpression of NPC1I1061T could lead to escape of NPC1I1061T molecules from ERAD and proper targeting to the late endosomal compartment. In transient transfection experiments, we found that overexpression of GFP-tagged NPC1I1061T in npc1-deficient CHO cells led to appropriate late endosomal localization of the mutant protein, and to clearance of lysosomal free cholesterol (Fig. 6A, top panels). By contrast, overexpression of the non-functional GFP-tagged NPC1P692S in npc1-deficient CHO cells failed to mobilize lysosomal free cholesterol (Fig. 6A, bottom panels) (3). This finding indicated that forced overexpression of an NPC1 mutant was not sufficient to complement the cholesterol accumulation phenotype. We likewise found that heterologous expression of NPC1I1061T could rescue the mutant phenotype in human NPC1I1061T mutant fibroblasts (Fig. 6B). To quantify the function of the NPC1I1061T mutant, we transiently expressed either WT NPC1 or NPC1I1061T GFP fusion proteins in the npc1-deficient CHO cells and monitored for clearance of unesterified lysosomal cholesterol by filipin staining (31). We found that the mutant protein was nearly as effective as WT protein in mobilizing lysosomal cholesterol, although at each time point examined the complementation efficiency of the npc1I1061T mutant was slightly less than that of WT (Fig. 7, A–C). To exclude the possibility that the NPC1I1061T mutant protein might have been artificially stabilized by the COOH-terminal GFP fusion, we compared the ability of the NPC1I1061T mutant expression constructs, with and without the GFP tag, to complement npc1-deficient CHO cells. At 72 h post-transfection, we found that expression of the npc1I1061T construct achieved 90.5% complementation, whereas expression of the npc1I1061T-GFP construct achieved 87.2% complementation (Fig. 7D). Thus, the functionality and/or stability of the GFP-tagged NPC1I1061T protein cannot be attributed to the presence of the GFP tag.
FIGURE 6.
Overexpression of NPC1I1061T rescues the cholesterol accumulation phenotype in npc1-null cells. A, npc1-deficient CHO cells were transiently transfected with either npc1I1061T-GFP (top panels) or npc1P692S-GFP (bottom panels) constructs and stained with filipin for unesterified cholesterol. Cells were examined by immunofluorescence for cholesterol (left panels) and GFP expression (right panels). npc1-null cells expressing npc1I1061T-GFP (top panels, closed arrows) are filipin-negative, whereas non-transfected cells (top panels, open arrows) are filipin-positive indicative of NPC1 mutant phenotype. npc1-null cells expressing the non-functional mutant npc1P692Sbottom panels, closed arrows) remain filipin-positive. Bar, 10 μm. B, NPC1-deficient human fibroblasts were transiently transfected with either GFP (left panel) or npc1I1061T-GFP (right panel) constructs and tested for the ability to complement the NPC1 phenotype. Merged immunofluorescence images are shown for GFP (green) and cholesterol (blue) staining. In the right panel, npc1-null cells expressing npc1I1061T-GFP (closed arrows) are complemented (low cholesterol staining), whereas in the left panel GFP expressing cells (closed arrows) are not complemented (high cholesterol staining). Non-transfected cells (open arrows) do not exhibit complementation. Bar, 50 μm.
FIGURE 7.
Time course of complementation of npc1-null CHO cells by transient expression of WT npc1-GFP or npc1I1061T-GFP. Graphs show the extent of complementation of npc1-null CHO cells by the WT and npc1I1061T constructs at 24 (A), 48 (B), and 72 (C) h post-transfection. Transfected cells (GFP-positive) were either scored as none (<20% complementation), partial (20–80% complementation), or complete (>80% complementation). p = NS for WT npc1-GFP versus npc1I1061T-GFP at 72 h. D, comparison of the extent of complementation of npc1-null CHO cells by the npc1I1061T and npc1I1061T-GFP constructs at 72 h post-transfection. p = NS for npc1I1061T versus npc1I1061T-GFP.
As an independent approach to confirm the function of the NPC1I1061T mutant protein, we monitored the egress of LDL-derived cholesterol from the lysosomes using an LDL cholesterol esterification assay. For these experiments, we isolated npc1-deficient CHO cells stably re-expressing WT NPC1, NPC1I1061T, or NPC1P692S. In comparison to primary mouse embryonic fibroblasts, NPC1 protein was overexpressed ∼2-fold in the WT NPC1, NPC1I1061T, and NPC1P692S cell lines (Fig. 8, A and B). (Because the rabbit polyclonal antibody does not efficiently recognize hamster NPC1 protein, mouse embryonic fibroblasts rather than CHO cells were used to assess relative NPC1 expression.) We found that overexpression of NPC1I1061T corrected the biochemical defect to 53% of WT CHO cells (Fig. 8C). Overexpression of the NPC1P692S mutant protein, which appropriately localizes to late endosomes but is non-functional (3), failed to stimulate cholesterol esterification. Taken together, the complementation and esterification assay data demonstrate that if the NPC1I1061T mutant protein can escape ER quality control, it is properly localized to late endosomes and is functional with respect to mobilization of endosomal cholesterol.
FIGURE 8.
Overexpression of NPC1I1061T rescues the cholesterol esterification defect in npc1-null cells. A, Western blot analysis of NPC1 and p63 protein expression in microsomes (5 μg/lane) isolated from WT CHO cells; npc1-null CHO cells; npc1-null cell lines expressing WT NPC1, NPC1I1061T, and NPC1P692S; and WT mouse embryonic fibroblasts. The lower Mr for NPC1 protein in WT mouse embryonic fibroblasts likely represents differences in glycosylation patterns between the hamster and murine cell lines. B, quantification of NPC1 protein in npc1-null cell lines expressing WT NPC1, NPC1I1061T, and NPC1P692S. NPC1 expression is normalized to expression of p63, a resident ER membrane protein, and densitometry presented as mean of duplicate lanes. C, LDL-stimulated cholesterol esterification in WT CHO cells; npc1-null CHO cells; and npc1-null cell lines expressing WT NPC1, NPC1I1061T, and NPC1P692S. Assays were performed in triplicate and values represent mean ± S.E. *, p < 0.05 for mean cholesterol esterification in npc1-null cells versus npc1I1061T cells.
DISCUSSION
Protein misfolding has been implicated in the pathogenesis of over 30 human diseases, including cystic fibrosis, α1-antitrypsin deficiency, and lysosomal storage diseases (32, 33). In these disorders, mutant proteins that fail to achieve proper conformation are targeted for ERAD, resulting in loss of function and disease. In the present study, we demonstrate that the NPC1I1061T missense mutation, the most prevalent NPC disease allele, disrupts NPC1 protein trafficking by promoting ER-mediated degradation of the mutant protein. Overexpression of the NPC1I1061T mutant unexpectedly led to appropriate late endosomal localization of the mutant protein and correction of the mutant phenotype. We conclude that the NPC1I1061T mutant protein is functional with respect to cholesterol trafficking, but is unstable because it is misfolded and targeted by the ER quality control machinery for proteasomal degradation. Our study provides the first description of an ER trafficking defect as a mechanism for human NPC disease, and suggests novel approaches for treatment of this progressive neurodegenerative disorder.
The function of ER quality control machinery is to prevent delivery of proteins to sites of function until they are properly folded, thereby limiting cytotoxicity of accumulated misfolded proteins (25, 34). Proteins recognized as misfolded by quality control are degraded through the actions of the ubiquitin-proteasome pathway. ER quality control not only targets mutant proteins, but also wild-type proteins that have been exposed to damaging conditions or that are slow to achieve conformation because they require extensive post-translational modifications (35). Whereas mature WT NPC1 protein that has progressed to post-ER compartments (i.e. Endo H-resistant) exhibits a half-life of 42 h, the half-life of ER-associated (i.e. Endo H-sensitive) WT NPC1 protein is only 9 h. Our data indicate that nearly half of the newly synthesized WT NPC1 protein is degraded within the ER. Similar ER-mediated degradation of immature species has been reported for other large, polytopic proteins, such as CFTR (36), and is likely due to failure of these heavily glycosylated proteins to properly fold prior to arrival at the ER quality control checkpoints.
The presence of the NPC1I1061T mutation further accelerates degradation of immature NPC1 protein by the ER quality control machinery. We find that the half-life of ER-associated NPC1I1061T protein is reduced to 6.5 h, and that almost none of the endogenously expressed protein escapes the ER. The NPC1I1061T substitution could affect NPC1 protein stability by introducing structural features into the NPC1 protein that cause its recognition as a misfolded protein. The significant increase in NPC1I1061T protein levels in cells grown at permissive temperatures or in cells administered chemical chaperones (e.g. glycerol and PBA) provide evidence that the mutant form of the protein is misfolded. Alternatively, newly synthesized NPC1I1061T protein may be slower to achieve conformation, thus prolonging association with ER chaperones and increasing the likelihood of selection for ERAD.
A key finding in our study was the demonstration that overexpression of the NPC1I1061T protein rescues the mutant phenotype in npc1-deficient cells. We show that at least a portion of the overexpressed mutant protein was correctly targeted to late endosomes, and importantly, properly functioned in mobilization of free cholesterol from this compartment, as determined by clearance of lysosomal free cholesterol and re-esterification of LDL-derived cholesterol. Overexpression of misfolded proteins leading to ER escape has been shown previously for other proteins, such as the CFTR Δ508 mutant (29). A possible explanation for “leakiness” of the mutant NPC1 protein is that enforced overexpression of the protein saturated the ER quality control machinery (37). Perhaps a more plausible explanation, based on a kinetic model, is that a small proportion of the nascent NPC1I1061T protein is able to fold correctly and therefore escape the quality control checkpoints (38). Assuming a fixed rate for leakiness for the I1061T mutant (e.g. 2–5% of the I1061T molecules achieve proper conformation), a sufficient quantity of correctly folded I1061T molecules could escape the ER as a result of overexpression, leading to complementation of the mutant phenotype. In the present study we demonstrate that a 2-fold overexpression of NPC1I1061T protein is able to partially correct the biochemical phenotype in npc1-null cells. Assuming that the level of properly targeted NPC1 protein required for complete complementation of the cholesterol trafficking defect is ∼10%, akin to the level of hydrolases required for enzymatic correction of other lysosomal disorders (39), it is reasonable to conclude that in human fibroblasts as much as 2.5–5% of the endogenous NPC1I1061T protein could exit the ER in the proper conformation. Whereas steady-state NPC1 protein levels were increased when the mutant fibroblasts were cultured at permissive temperatures or exposed to chemical chaperones that stabilize protein, the overall effect on efficiency of NPC1I1061T protein folding does not appear to be sufficient to correct the cholesterol trafficking defect (not shown). On the other hand, identification of chemical chaperones that enhance the efficiency of NPC1I1061T protein folding through high throughput small molecule screens has the potential to develop new and effective approaches for the treatment of NPC1 disease. Such an approach might not only benefit subjects with the NPC1I1061T genotype, but subjects with other missense mutations, in particular the G992W and P1007A genotypes that likewise effect the cysteine-rich luminal domain. In future studies these and other NPC1 mutants could be screened to determine whether the mutant proteins are similarly misfolded and respond to treatment with chemical chaperones. If this is indeed the case, there is the potential that a significant percentage of NPC1 subjects could benefit from small molecule-based chaperone therapy.
Acknowledgments
We are grateful for Stuart Kornfeld for critical review of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: NPC1, Niemann-Pick type C1; CFTR, cystic fibrosis transmembrane regulator; CHO, Chinese hamster ovary; GFP, green fluorescent protein; Endo H, Endoglycosidase H; ER, endoplasmic reticulum; ERAD, ER-associated degradation; IP, immunoprecipitation; LDL, low density lipoprotein; WT, wild type; PBA, 4-phenylbutyric acid.
References
- 1.Ory, D. S. (2000) Biochim. Biophys. Acta 1529 331–339 [DOI] [PubMed] [Google Scholar]
- 2.Carstea, E. D., Morris, J. A., Coleman, K. G., Loftus, S. K., Zhang, D., Cummings, C., Gu, J., Rosenfeld, M. A., Pavan, W. J., Krizman, D. B., Naqle, J., Polymeropoulos, M. H., Sturley, S. L., Ioannou, Y. A., Higgins, M. E., et al. (1997) Science 277 228–231 [DOI] [PubMed] [Google Scholar]
- 3.Millard, E., Gale, S., Dudley, N., Zhang, J., Schaffer, J., and Ory, D. (2005) J. Biol. Chem. 280 28581–28590 [DOI] [PubMed] [Google Scholar]
- 4.Davies, J. P., and Ioannou, Y. A. (2000) J. Biol. Chem. 275 24367–24374 [DOI] [PubMed] [Google Scholar]
- 5.Frolov, A., Srivastava, K., Daphna-Iken, D., Traub, L. M., Schaffer, J. E., and Ory, D. S. (2001) J. Biol. Chem. 276 46414–46421 [DOI] [PubMed] [Google Scholar]
- 6.Neufeld, E. B., Wastney, M., and Patel, S., Suresh, S., Cooney, A. M., Dwyer, N. K., Roff, C. F., Ohno, K., Morris, J. A., Carstea, E. A., Incardona, J. P., Strauss, J. F., III, Vanier, M. T., Patterson, M. C., Brady, R. O., Pentchev, P. G., and Blanchette-Mackie, E. J. (1999) J. Biol. Chem. 274 9627–9635 [DOI] [PubMed] [Google Scholar]
- 7.Zhang, M., Dwyer, N., Neufeld, E. B., Love, D. C., Cooney, A., Comly, M., Patel, S., Watari, H., Strauss, J. F., III, Pentchev, P. G., Hanover, J. A., and Blanchette-Mackie, E. J. (2001) J. Biol. Chem. 276 3417–3425 [DOI] [PubMed] [Google Scholar]
- 8.Millard, E. E., Srivastava, K., Traub, L., Schaffer, J. E., and Ory, D. S. (2000) J. Biol. Chem. 275 38445–38451 [DOI] [PubMed] [Google Scholar]
- 9.Neufeld, E. B., Cooney, A. M., Pitha, J., Dawidowicz, E. A., Dwyer, N. K., Pentchev, P. G., and Blanchette-Mackie, E. J. (1996) J. Biol. Chem. 271 21604–21613 [DOI] [PubMed] [Google Scholar]
- 10.Wojtanik, K. M., and Liscum, L. (2003) J. Biol. Chem. 278 14850–14856 [DOI] [PubMed] [Google Scholar]
- 11.Liscum, L., and Faust, J. R. (1987) J. Biol. Chem. 262 17002–17008 [PubMed] [Google Scholar]
- 12.Pentchev, P. G., Comly, M. E., Kruth, H. S., Vanier, M. T., Wenger, D. A., Patel, S., and Brady, R. O. (1985) Proc. Natl. Acad. Sci. 82 8247–8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, W., Sun, Y., Welch, C., Gorelik, A., Leventhal, A. R., Tabas, I., and Tall, A. R. (2001) J. Biol. Chem. 276 43564–43569 [DOI] [PubMed] [Google Scholar]
- 14.Choi, H. Y., Karten, B., Chan, T., Vance, J. E., Greer, W. L., Heidenreich, R. A., Garver, W. S., and Francis, G. A. (2003) J. Biol. Chem. 278 32569–32577 [DOI] [PubMed] [Google Scholar]
- 15.Park, W. D., O'Brien, J. F., Lundquist, P. A., Kraft, D. L., Vockley, C. W., Karnes, P. S., Patterson, M. C., and Snow, K. (2003) Hum. Mutat. 22 313–325 [DOI] [PubMed] [Google Scholar]
- 16.Scott, C., and Ioannou, Y. A. (2004) Biochim. Biophys. Acta 1685 8–13 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Valero, E. M., Ballart, A., Iturriaga, C., Lluch, M., Macias, J., Vanier, M. T., Pineda, M., and Coll, M. J. (2005) Clin. Genet. 68 245–254 [DOI] [PubMed] [Google Scholar]
- 18.Millat, G., Marcais, C., Rafi, M. A., Yamamoto, T., Morris, J. A., Pentchev, P. G., Ohno, K., Wenger, D. A., and Vanier, M. T. (1999) Am. J. Hum. Genet. 65 1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watari, H., Blanchette-Mackie, E. J., Dwyer, N. K., Watari, M., Burd, C. G., Patel, S., Pentchev, P. G., and Strauss, J. F., 3rd (2000) Exp. Cell Res. 259 247–256 [DOI] [PubMed] [Google Scholar]
- 20.Frolov, A., Zielinski, S. E., Crowley, J. R., Dudley-Rucker, N., Schaffer, J. E., and Ory, D. S. (2003) J. Biol. Chem. 278 25517–25525 [DOI] [PubMed] [Google Scholar]
- 21.Schweizer, A., Rohrer, J., Slot, J. W., Geuze, H. J., and Kornfeld, S. (1995) J. Cell Sci. 108 2477–2485 [DOI] [PubMed] [Google Scholar]
- 22.Gale, S. E., Frolov, A., Han, X., Bickel, P. E., Cao, L., Bowcock, A., Schaffer, J. E., and Ory, D. S. (2006) J. Biol. Chem. 281 11082–11089 [DOI] [PubMed] [Google Scholar]
- 23.Werner, E. D., Brodsky, J. L., and McCracken, A. A. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 13797–13801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, S., Ward, C. L., Krouse, M. E., Wine, J. J., and Kopito, R. R. (1996) J. Biol. Chem. 271 635–638 [DOI] [PubMed] [Google Scholar]
- 25.Shearer, A. G., and Hampton, R. Y. (2004) J. Biol. Chem. 279 188–196 [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein, R. C., Egan, M. E., and Zeitlin, P. L. (1997) J. Clin. Investig. 100 2457–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denning, G. M., Anderson, M. P., Amara, J. F., Marshall, J., Smith, A. E., and Welsh, M. J. (1992) Nature 358 761–764 [DOI] [PubMed] [Google Scholar]
- 28.Spear, E. D., and Ng, D. T. (2003) Mol. Biol. Cell 14 2756–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalemans, W., Barbry, P., Champigny, G., Jallat, S., Dott, K., Dreyer, D., Crystal, R. G., Pavirani, A., Lecocq, J. P., and Lazdunski, M. (1991) Nature 354 526–528 [DOI] [PubMed] [Google Scholar]
- 30.Sharma, M., Benharouga, M., Hu, W., and Lukacs, G. L. (2001) J. Biol. Chem. 276 8942–8950 [DOI] [PubMed] [Google Scholar]
- 31.Watari, H., Blanchette-Mackie, E. J., Dwyer, N. K., Watari, M., Neufeld, E. B., Patel, S., Pentchev, P. G., and Strauss, J. F., 3rd (1999) J. Biol. Chem. 274 21861–21866 [DOI] [PubMed] [Google Scholar]
- 32.Aridor, M., and Hannan, L. A. (2000) Traffic 1 836–851 [DOI] [PubMed] [Google Scholar]
- 33.Aridor, M., and Hannan, L. A. (2002) Traffic 3 781–790 [DOI] [PubMed] [Google Scholar]
- 34.Sifers, R. N. (2003) Science 299 1330–1331 [DOI] [PubMed] [Google Scholar]
- 35.Sitia, R., and Braakman, I. (2003) Nature 426 891–894 [DOI] [PubMed] [Google Scholar]
- 36.Ward, C. L., and Kopito, R. R. (1994) J. Biol. Chem. 269 25710–25718 [PubMed] [Google Scholar]
- 37.Gelman, M. S., and Kopito, R. R. (2002) J. Clin. Investig. 110 1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kopito, R. R. (1999) Physiol. Rev. 79 S167–173 [DOI] [PubMed] [Google Scholar]
- 39.Schueler, U. H., Kolter, T., Kaneski, C. R., Zirzow, G. C., Sandhoff, K., and Brady, R. O. (2004) J. Inherit. Metab. Dis. 27 649–658 [DOI] [PubMed] [Google Scholar]