Abstract
The ability of the human immunodeficiency virus, type 1 (HIV-1) protein Nef to induce cytoskeleton changes in infected host cells is a key event in viral replication. In renal podocytes, we found that Nef induced loss of stress fibers and increased lamellipodia, pathological changes leading to proteinuria in HIV-associated nephropathy. These morphological changes were mediated by Nef-induced Rac1 activation and RhoA inhibition. We identified a new interaction between Nef and diaphanous interacting protein (DIP), a recently described regulator of Rho and Rac signaling. We found that the Src homology 3 binding domain of DIP and the Nef PXXP motif were required for this interaction. Nef also interacts with Vav2 in podocytes. DIP and Vav2 both interact directly with Nef in a competitive manner. DIP interacts with p190RhoGAP, and intact DIP was required for Nef-induced phosphorylation of p190RhoGAP. DIP also interacts with Vav2, and although DIP enhanced baseline phosphorylation of Vav2, it was not required for Nef-induced Vav2 activation. In Nef-infected podocytes, Src kinase induces phosphorylation of DIP, p190RhoGAP, and Vav2, leading to RhoA inhibition and Rac1 activation. Inhibition of the Nef-induced signaling pathway by using a dominant negative of either Src or DIP or siRNA for DIP or p190RhoAGAP restored RhoA activity and stress fiber formation in Nef-infected podocytes, whereas siRNA for Vav2 reduced Rac1 activity and formation of lamellipodia. We conclude that in HIV-infected podocytes, Nef, through the recruitment of DIP and p190RhoAGAP to Nef-Src complex, activates p190RhoAGAP and down-regulates RhoA activity.
Nef is a 27–35-kDa HIV-14 accessory protein that plays a critical role in viral replication, immune evasion, and viral infectivity (1–5). The ability of Nef to alter the morphology of T lymphoctes and dendritic cell plays an important role in its pathogenecity (6–9). Several key functional domains enable Nef to perform these functions. The left-handed polyproline helix type II of Nef forms a (PXXP)3 sequence cluster, which mediates an interaction with the SH3 domain of members of the Src family kinase family and Vav (10, 11). The RR105 domain facilitates an interaction with Pak (12), and the N-terminal myristoylated region facilitates localization of Nef to the actin cytoskeleton and plasma membrane (13).
In the kidney, HIV-1 infection of renal epithelial cells induces the development of HIV-1-associated nephropathy (HIVAN), which is clinically associated with severe proteinuria and azotemia (14). Pathologically, HIVAN is characterized by the collapsing variant of focal segmental glomerulosclerosis, microcystic tubular dilatations, and tubulointerstitial nephritis (15, 16). Podocytes, the specialized epithelial cells that form the final filtration barrier of the glomerulus, are one of the primary cell types infected by HIV-1 (17, 18). The importance of podocyte involvement in HIVAN pathogenesis is supported by the recent finding that mice expressing HIV-1 genes in podocytes alone develop all the pathologic findings of HIVAN, including focal segmental glomerulosclerosis as well as tubular disease (19).
Nef has been shown to be a major determinant of the HIVAN podocyte phenotype in vitro (20, 21). Nef induces podocyte dedifferentiation and proliferation by activating the Ras-Raf-MAPK1/2 and Src-Stat3 pathways (22). A critical role for Nef was also identified in vivo, where podocyte-specific expression of Nef and Vpr induced glomerulosclerosis in a mouse model (23). The role of Nef or HIV in inducing podocyte morphologic change or effacement has yet to be explored.
Podocytes are quiescent and highly differentiated cells with interdigitating foot processes that wrap around the glomerular capillary tuft to form a filtration barrier (24). The actin cytoskeleton plays a critical role in the supporting the structure and function of the podocytes (25). In HIVAN, podocytes have been shown both in vivo and in vitro to lose differentiation markers and proliferate (17, 26, 27). They also exhibit structural changes with loss of the characteristic foot processes. Unlike in other glomerular diseases associated with podocyte effacement, the actin cytoskeleton gradually disappears on electron microscopy in human HIVAN biopsies rather than being rearranged into a continuous cytoskeletal mat (17).
The mechanism by which the actin cytoskeleton in the podocyte is affected in HIVAN has not been delineated. The Rho family of small GTPases regulates the actin cytoskeleton of eukaryotic cells in response to extracellular signals (28). Rho activation primarily causes formation of actin stress fibers, whereas Rac activation results in lamellipodia and membrane ruffling (29). We hypothesize that Nef may play a direct role in mediating the morphologic changes in podocytes, given its known ability to interact with regulators of the actin cytoskeleton. Here, we show that in podocytes, Nef inhibits RhoA activity and stress fiber formation while activating Rac1 and lamellipodia formation. It has been shown that Nef can interact with Vav2 via its SH3 domain, leading to Rac1 activation (9, 30). However, Nef regulation of RhoA activity has never been reported in any cell type. Further, it was not clear how Nef coordinately regulates the activity of RhoA and Rac1. p190RhoGAP is a GTPase-activating protein specific for RhoA that is regulated by Src phosphorylation in response to various extracellular signals (31, 32) but does not have a domain known to interact with those of Nef (31). Thus, the mechanism by which Nef regulates RhoA activity is not known.
To delineate the overall mechanisms by which Nef regulates actin cytoskeleton in podocytes, we studied the role of diaphanous interacting protein (DIP) in signaling from Nef to RhoA and Rac1. DIP has an SH3 binding domain and has been shown to mediate Src-induced phosphorylation of p190RhoGAP and Vav2 probably by localizing components of the signaling complex to the plasma membrane (33). We show here that DIP plays a critical role in Nef-induced changes on the actin cytoskeleton in podocytes through inhibition of RhoA activity.
MATERIALS AND METHODS
Podocyte Culture and Infection with HIV or Nef Constructs—Conditionally immortalized murine podocytes were isolated from control mice that express the SV40 T antigen (immortal mouse) as previously described (20, 27, 34). These cells proliferate under permissive conditions (IFN-γ at 33 °C) but differentiate under nonpermissive conditions (37 °C). Under nonpermissive conditions, the cells lose the expression of T antigen.
HIV-1 and Nef constructs were made as described (22). Briefly, a fragment containing the EGFP reporter gene (from pEGFP-C1; Clontech, Palo Alto, CA) was inserted in place of the gag/pol deletion in HIV-1 proviral construct, pNL4-3: d1443. The resulting construct (pNL4-3:ΔG/P-EGFP) was used for HIV-1 infection and to monitor the efficiency of infection in podocytes. To delineate the contribution of the nef gene, the nef gene was deleted from pNL4-3 by in vitro site-directed mutagenesis using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). A retroviral expression vector (pBabepuro) was used to clone nef into the BamHI-SalI site. The constructs with mutations in the PXXP (SH3) motif in Nef were generated using QuikChange site-directed mutagenesis kits. The PXXP-binding domain was mutated by changing the proline residues to alanines. Moloney murine leukemia virus gag/pol genes and VSV.G envelope were provided in trans to generate pseudotyped virus particles.
Podocytes at passage 10 were infected with either control vector or Nef-containing vector as described previously (20). The expression of Nef in podocytes was confirmed by Western blot analysis (20). Podocytes were maintained in RPMI medium (Sigma) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Prior to all experiments, cells were kept at 37 °C on type I collagen-coated dishes for 10 days to inactivate the temperature-sensitive T antigen and to allow for differentiation. Western blot was used to confirm the absence of T antigen.
Nucleofection and siRNA—Transfections of differentiated podocytes were performed using the Amaxa nucleofection kit (Amaxa Biosystems, Gaithersburg, MD). Several protocols were tested based on the manufacturer's instructions. Podocytes were plated at a density of ∼50% on the day prior to transfection. After optimization, a transfection efficiency of 80–90% was achieved based on green fluorescent protein expression.
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% glutamine at 37 °C. Transfections were performed using Fugene (Roche Applied Science) per the manufacturer's protocol. A transfection efficiency of >90% was achieved. All experiments were performed between 1 and 3 days after transfection.
For siRNA transfection, we use a technique combining the Ambion siRNA synthesis kit for DIP, Vav2, and p190RhoAGAP combined with the Amaxa RNAi nucleofection kit to successfully introduce siRNA into podocytes. Two siRNA duplexes were made by the Ambion siRNA synthesis kit for each molecule with the following target sequences: DIP, 5′-ACCUUGUCUCGAAGAGGCAdU-3′ and 5′-UGCCUCUUCGAGACAAGGU-3′; Vav2, 5′-AAGAAACAGUGAGCUGUUUGA-3′ and 5′-AAGGAGAUGACAUUUAUGAGG-3′; p190RhoAGAP, 5′-AAGACAAAGGAAACUGUGGAG-3′ and 5′-AAUGGUGAAGATGGUGUAGAA-3. Dharmacon On TargetPlus SMARTpool siRNA reagents for Vav2 were also used as another control. We were able to knock down target genes by 60–70% based on Western blot analysis (35). miRIDIAN micro-RNA mimic negative control sequences are based on Caenorhabditis elegans miRNA for use as negative experimental controls in mammalian cells. siRNA for glyceraldehyde-3-phosphate dehydrogenase was also used as the control sequence. Efficiency of siRNA is confirmed by real time PCR for mRNA levels and Western blot for protein levels.
RhoA and Rac1 Activation Assays—Rho and Rac activation assay kits (Upstate Biotechnology, Inc.) were used to measure GTP-bound Rho and Rac, respectively. The manufacturer's instructions were followed; briefly, cells were lysed using a GST-Fish buffer containing 5 mm MgCl2, 10% glycerol, 50 mm Tris, pH 7.4, 100 mm NaCl, 1% Nonidet P-40, 2 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, leupeptin, pepstatin. Cell lysates were then incubated with rhotekin glutathione S-transferase fusion protein bound to glutathione beads to precipitate Rho-GTP- or PAK1-PBD-agarose beads to precipitate Rac-GTP overnight at 4 °C. Precipitated Rho-GTP and total Rho (from cell lysates) were detected by Western blotting with monoclonal anti-Rho (Upstate Biotechnology). Precipitated Rac-GTP and total Rac (from cell lysates) were detected by Western blotting with monoclonal anti-Rac (Upstate Biotechnology).
Immunoblot Analysis of Signaling Proteins—Control and Nef-infected podocytes were serum-starved for 24 h prior to lysis with a buffer containing 1% Nonidet P-40, a protease inhibitor mixture, and tyrosine and serine-threonine phosphorylation inhibitors. After determination of protein concentration, cell lysates were subjected to Western blot analysis using the following specific antibodies: polyclonal anti-rabbit p-Vav2, polyclonal anti-goat Vav2, anti-DIP (Santa Cruz Biotechnology), anti-p190RhoGAP (BD Transduction Laboratories), anti-Nef (FIT Biotech), anti-Myc (Sigma), and anti-β-actin (Sigma) antibodies.
Determination of Protein-Protein Interactions—293T cells were transfected with the following cDNAs: DIP/pcDNA3, DIPΔSH3/pcDNA3, and DIPΔPro/pcDNA3 (all provided by Dr. Tominaga). Transfected 293T cells as well as differentiated podocytes were lysed using GST-Fish buffer. Cell lysates were precleared by incubating with purified GST and glutathione beads for 1 h at 4°C. Unbound proteins were then incubated with either Nef-GST fusion protein bound to glutathione beads or GST bound to glutathione beads for 1 h at 4 °C. After extensive washing, precipitated proteins were dissociated from the beads using 2× SDS-PAGE buffer, and Western blotting was performed with polyclonal anti-DIP or anti-Vav2.
For co-immunoprecipitation, wild-type and Nef-infected podocytes were lysed using a lysis buffer containing 25 mm Tris, pH 7.4, 5 mm EDTA, 30 mm NaFl, 40 mm β-glycerophosphate, 10 mm sodium pyrophosphate, 1% Triton X-100, 10 mm NaVO4, 1 mm phenylmethylsulfonyl fluoride, 3 mm benzamidine, 5 μm pepstatin A, and 10 μm leupeptin. Cell lysates were incubated with either monoclonal anti-Nef (amino acids 191–205) (Advanced Biotechnologies) or control mouse myeloma IgG (Sigma) overnight at 4 °C. Protein G-Sepharose was added, and samples were allowed to rotate for an additional 90 min at 4 °C. After extensive washing, precipitated proteins were dissociated using 2× SDS-PAGE buffer, and Western blot analysis was performed using polyclonal anti-DIP. Where indicated, 10 μm pp2 was added to cell media for 1 h prior to lysis.
For co-immunoprecipitation with purified proteins, Nef was subcloned into pGEX vector for generating recombinant GST-Nef. HA-tagged Vav2 and Myc-tagged DIP were produced in HEK293 cells. Vav2-HA and DIP-Myc (constructs were gifts from Dr. Tomoko Fukumi-Tominaga) were precipitated using anti-HA or anti-Myc beads and eluted using HA or Myc peptide (Sigma). GST-Nef (1 μg) was immobilized on glutathione beads. After washing, 1 μg of purified Vav2-HA or DIP-Myc in 500 μl of PBS was added to the beads. Reactions were incubated under rotation for 2 h at 4°C. After washing, proteins were eluted in sample buffer and analyzed by Western blot using anti-HA or anti-Myc antibody.
Determination of Signaling Protein Activation—Control and Nef cells were serum-starved for 24 h prior to lysis with a modified radioimmune precipitation buffer containing 50 mm Tris-HCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm NaF. Lysates were incubated with recombinant anti-phosphotyrosine antibody conjugated to agarose beads (Upstate Biotechnology) at 4 °C overnight. After extensive washing, precipitated proteins dissociated from the beads using 2× SDS-PAGE sample buffer, and Western blot analysis was performed with monoclonal anti-p190RhoGAP (BD Bioscience); polyclonal anti-DIP was made by Dr. Tominaga as previously described (36).
Immunostaining—Differentiated podocytes were used as the control. Nef-infected podocytes were treated with pp2 (10 μm) or U0126 (10 μm) or transfected with the following plasmids: control pcDNA3, DIPΔPro/pcDNA3 (provided by Dr. Tominaga), dominant negative Src and Stat3 (previously described (22)), RhoG14V, Rac1GV, dominant negative Rac1, siRNA for Vav2, p190RhoAGAP, DIP, or control oligonucleotide. After transfection, cells were directly cultured onto collagen-coated glass coverslips and incubated at 37 °C for 48–72 h to allow transgene expression. Podocytes were fixed and permeabilized with 4% paraformaldehyde in PBS with 0.3% Triton X-100 in PBS. After washing with PBS, cells were incubated with Alexa Fluor 488 phalloidin (Molecular Probes). After washing with PBS, cells were mounted onto glass slides with Slowfade Anti-fade kit (Molecular Probes) and visualized using an Olympus BX60 microscope (Olympus Optical Co.) with a digital camera.
RESULTS
Nef Expression Induces Actin Cytoskeleton Rearrangement in Podocytes in Vitro—HIVAN is associated with podocyte effacement and loss of cytoskeletal structure on electron microscopy (17). In order to visualize the effects of HIV-1 Nef on the actin cytoskeleton in vitro, differentiated podocytes expressing either a control vector, a replication-incompetent gag-pol-deleted HIV-1 proviral construct (pNL4-3Δgag-pol), a gag-pol-nef-deleted HIV-1 proviral construct (HIV-1ΔNef), nef-expressing vector, or a nef vector with mutation of the PXXP motif (NefPXXP) were stained with fluorescently labeled phalloidin to visualize the actin cytoskeleton. Control podocytes displayed prominent actin stress fibers throughout the cell cytoplasm, consistent with previous findings (37). In contrast, HIV-1-infected podocytes lacked stress fiber formation and had increased lamellipodia formation (Fig. 1A). Podocytes expressing the nef gene alone had a phenotype indistinguishable from HIV-1 podocytes, whereas those expressing HIV-1ΔNef or NefPXXP exhibited normal actin stress fiber-like control cells (Fig. 1A). These studies suggest that Nef is responsible for HIV-1-induced changes of actin cytoskeleton in podocytes and that an intact PXXP motif is required.
FIGURE 1.
A, HIV-1 Nef induces loss of stress fibers and formation of lamellipodia in podocytes. Differentiated control podocytes express prominent stress fibers throughout the cytoplasm. Podocytes expressing gag-pol-deleted HIV-1 lack stress fiber formation and form lamellipodia, which are absent in control podocytes. Podocytes expressing Nef alone develop a phenotype identical to that induced by the parental HIV-1 construct, whereas podocytes expressing Nef-deleted HIV-1 or NefPXXP mutant had similar actin cytoskeleton as control podocytes. Transfecting podocytes with constitutively active Rac1G14V and treating with C3 transferase (Cytoskeleton Inc.) induced actin morphology similar to HIV-1. B, Nef but not NefPxxP induces RhoA inactivation and Rac1 activation. Cell lysates from control and Nef- or NefPXXP-infected podocytes were incubated with GST-tagged rhotekin, which selectively binds RhoA-GTP or Pak1-PBD-agarose, a specific binder of Rac1-GTP. Western blot analysis with antibodies against RhoA and Rac1 was performed on precipitated fractions and whole cell lysates. Western blotting for Nef was performed in total cell lysates to verify Nef expression. Representative blots of three independent experiments are shown. C, densitometric analysis. The ratio of Rac1 and RhoA activities to total Rac1 and RhoA was obtained by analyzing the blots using densitometry (n = 3).
Nef Induces RhoA Inactivation and Rac1 Activation in Podocytes—The Rho family GTPases regulate eukaryotic cytoskeleton dynamics with RhoA primarily mediating stress fiber formation, Rac1 mediating lamellipodia formation and membrane ruffling, and Cdc42 causing filopodia formation (29). We therefore examined the activity level of RhoA, Rac1, and Cdc42 in podocytes infected with control vector, Nef, or NefPXXP. We found that RhoA activity was significantly suppressed in podocytes expressing Nef compared with podocytes infected with control vector or NefPXXP (Fig. 1, B and C). Rac1 activity was significantly higher in Nef cells than control or NefPXXP cells (Fig. 1, B and C). Cdc42 activity was unchanged in the setting of Nef expression (data not shown). These findings suggest that Nef-induced changes of actin structure are mediated by alterations in Rac1 and RhoA activities. To confirm that alteration of RhoA and Rac1 activity can induce a podocyte phenotype comparable with that of HIVAN, we examined actin cytoskeleton in podocytes transfected with constitutively active Rac1 (Rac1G14V) and treated with C3 transferase, an inhibitor of RhoA activity. These cells exhibited similar changes of actin cytoskeleton as Nef-infected podocytes (Fig. 1A).
Vav2 Interacts with Nef in Podocytes—An interaction between Vav2 and Nef has been previously shown in T cells (30). In order to verify this interaction in podocytes, cells were transfected with Nef or NefPXXP and lysed for immunoprecipitation with a Nef antibody specific to both Nef and NefPXXP. Vav2 co-precipitated with Nef but not NefPXXP (Fig. 2A). As a control, nonspecific IgG did not co-precipitate with Vav2. This was confirmed by reverse co-immunoprecipitation (IP with anti-Vav2 and IB with anti-Nef antibodies) in cells transfected with Nef or NefPXXP (Fig. 2B). Our results indicate that Vav2 interacts with the PXXP domain of Nef in podocytes. As controls, Vav2 and Nef expression were determined by Western blot in total cell lysates (Fig. 2, A and B).
FIGURE 2.
A, Vav2 interacts with Nef via its PXXP motif. 293T cells were transfected with Nef or NefPXXP. Cell lysates were immunoprecipitated (IP) with anti-Nef and immunoblotted (IB) with anti-Vav2. Nef but not NefPXXP co-precipitates with Vav2. Nef and Vav2 expression in input IP solution were verified by Western blotting. A representative blot of three experiments is shown. B, reverse co-immunoprecipitation. 293T cells were transfected with Nef or NefPXXP. Cell lysates were immunoprecipitated with anti-Vav2 and immunoblotted with anti-Nef.
DIP Interacts with Nef via Its SH3 Binding Domain—Although interaction between Nef and Vav2 has been reported previously in T cells and can mediate Rac1 activation, the mechanism by which Nef inhibits RhoA activation is not known. We therefore searched the data base of molecules containing the SH3 binding domain for adaptor proteins that could interact with Nef and mediate its effect on RhoA activity. One of the candidates in our search was DIP, which has a distinct SH3 binding domain and is able to regulate RhoA activity through interaction with p190RhoGAP (33). We first determined in vitro if DIP interacts with Nef. Cell lysates from 293T cells overexpressing DIP were incubated with Nef-GST fusion protein for a pull-down assay. We found a strong interaction between DIP and Nef (Fig. 3A). In addition to its SH3 domain, DIP also possesses a proline-rich domain (Pro) and a leucine-rich domain. We transfected 293T cells with DIP construct containing either a deletion in the proline-rich region (DIPΔPro) or the SH3 region (DIPΔSH3). Although full-length DIP and DIPΔPro were pulled down by Nef-GST, a significantly reduced interaction (>60% reduction based on densitometric analysis) was observed with DIPΔSH3, suggesting that the SH3 region of DIP mediates the interaction with Nef (Fig. 3A). As controls, expression of total input GST, GST-Nef, and DIP isoforms was determined by Western blot (Fig. 3A). Next, we tested whether the PXXP region of Nef was required for interaction. 293T cells were transfected with full-length DIP along with either Nef or Nef with a mutation in the PXXP region (NefPXXP) (proline was replaced by alanine). We found that the DIP and Nef interaction was abolished when Nef was mutated in the PXXP motif (Fig. 3B). Our results confirmed that the Nef proline-rich region is required to mediate an interaction between Nef and DIP.
FIGURE 3.
A, the DIP SH3 region mediates an interaction with Nef. 293T cells were transfected with DIP, DIPΔPro, or DIPΔSH3. A Nef-GST fusion protein was used for pull-down, and fractions were immunoblotted (IB) with polyclonal anti-DIP. DIP and DIPΔPro were pulled down by Nef-GST. DIPΔSH3 showed a significantly weaker interaction with Nef. Densitometric analysis of pull-down experiments was shown (n = 3; *, p < 0.01). The input amount of GST and GST-Nef was verified by Western blotting using anti-GST antibody. The expression of DIP, DIPΔPro, or DIPΔSH3 was also verified by Western blotting using anti-DIP antibody. B, an intact Nef PXXP region was required for interaction with DIP. 293T cells were co-transfected with DIP plus either Nef or NefPXXP). Immunoprecipitation (IP) was performed with anti-Nef and immunoblotted with anti-DIP. Wild-type Nef but not NefPXXP co-precipitates with DIP. Western blotting was performed for Nef to verify its expression in cells transfected with either Nef or NefPXXP. The expression of DIP was also verified using anti-DIP antibody. C, Nef interacts with endogenous DIP in podocytes. Nef-GST fusion protein was used to pull-down endogenous Nef in podocytes. Immunoblotting was performed using polyclonal anti-DIP. The input amount of GST and GST-Nef was verified using anti-GST antibody. D, Nef associates with DIP in Nef-infected podocytes. Nef and NefPXXP podocytes were used for immunoprecipitation with monoclonal anti-Nef, and immunoblot was performed with polyclonal anti-DIP. Nef and DIP co-precipitate in podocytes. The expression of Nef, NefPXXP, and DIP were verified by Western blotting. All experiments were performed at least three times, and the representative blots are shown here.
To confirm this interaction in podocytes with endogenously expressed DIP, protein lysates were prepared from wild-type podocytes and incubated with Nef-GST fusion protein for the pull-down assay. Nef-GST interacted with the endogenous DIP in podocytes (see Fig. 3C). Finally, to confirm the interaction between Nef and DIP in podocytes transfected with either Nef or NefPXXP, cell lysates were immunoprecipitated with monoclonal anti-Nef and immunoblotted with anti-DIP antibody. We found that Nef expressed in podocytes also interacted with endogenous DIP, whereas NefPXXP did not (Fig. 3D). Together, these data indicate that Nef interacts with DIP in podocytes expressing HIV-1 Nef.
DIP Interacts with p190RhoGAP and Vav2 in Podocytes—Because p190RhoGAP is a known regulator of RhoA (33), we determined the interactions of p190RhoGAP with DIP. Cell lysates from control and Nef podocytes were immunoprecipitated with antibody against DIP. The precipitated fractions were immunoblotted with antibody against p190RhoGAP. We found that DIP co-precipitates with p190RhoGAP in both control and Nef-infected cells (Fig. 4). Protein fractions immunoprecipitated with DIP were also immunoblotted with antibody against Vav2. We found that DIP also co-precipitates with Vav2 in both control and Nef-infected podocytes (Fig. 4).
FIGURE 4.
DIP interacts with p190RhoGAP and Vav2 in podocytes. Lysates from control and Nef podocytes were immunoprecipitated (IP) with anti-DIP and immunoblotted (IB) with anti-p190 or anti-Vav2. DIP co-precipitates with both p190RhoGAP and Vav2 in both control and Nef podocytes. DIP and Nef expression was verified by Western blotting.
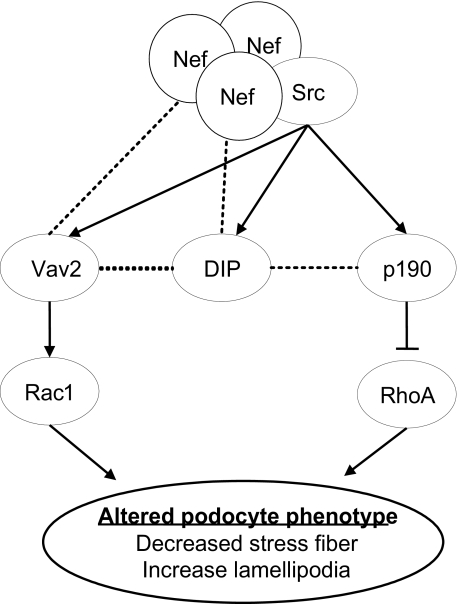
As described previously, p190RhoGAP does not have a domain known to interact directly with those of Nef (31). Our data suggest that p190RhoGAP interacts with Nef through DIP, whereas Vav2 interacts directly with Nef as well as DIP in podocytes (see Fig. 10).
FIGURE 10.
Nef interacts with Src, DIP, and Vav2. DIP interacts with both Nef and p190RhoAGAP, mediating Nef-Src-induced phosphorylation of p190RhoGAP. Nef interacts directly with Vav2 to induce Src-mediated Vav2 phosphorylation. The interaction between DIP and Vav2 is not required for Nef-induced Vav2 phosphorylation. Vav2 and p190 phosphorylation leads to Rac1 activation and RhoA inhibition and changes of actin cytoskeleton, as observed in HIV-infected podocytes.
Nef Interacts with DIP and Vav2 Directly and in a Competitive Manner—Since we found that Nef interacted with both DIP and Vav2 via the same motif, we explored whether or not the Nef-DIP and Nef-Vav2 interactions are direct. Purified DIP-Myc and Vav2-HA were immunoprecipitated with GST-Nef. A direct interaction with GST-Nef was seen with both DIP-Myc and Vav2-HA. GST was used as a control for nonspecific interaction. Total DIP-Myc and Vav2-HA are also shown (Fig. 5A). To confirm whether the same binding site on Nef mediates these interactions, we performed a competition assay. We found that by increasing the DIP-Myc input into a GST-Nef pull-down with Vav2, we were able to decrease the binding affinity of GST-Nef with Vav2 (Fig. 5B). This suggests that DIP and Vav2 bind to the same or a similar site (PXXP motif) of Nef (38).
FIGURE 5.
A, Nef interacts directly with DIP and Vav2. Myc-tagged DIP and HA-tagged Vav2 were purified from 293T cells using anti-Myc or anti-HA beads. Co-immunoprecipitation was performed between Nef-GST and Vav2-HA or DIP-Myc. Western blotting was performed using anti-Myc or anti-HA. Western blotting was also performed to verify the expression of input proteins using GST, Myc, or HA antibodies. B, competitive binding between Nef-Vav2 and Nef-DIP. Nef-GST (1μg) was incubated with 1μg of Vav2-HA and increasing amounts of DIP-Myc (0.5, 1, and 2 μg). Western blotting was performed using anti-Myc and anti-HA antibodies for Nef-IP and input as indicated. The representative blots of three independent experiments are shown. IP, immunoprecipitation; IB, immunoblotting.
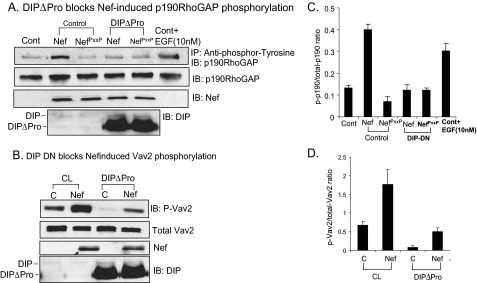
DIP Is Required for Nef-induced Phosphorylation of p190RhoGAP—We next determined whether Nef could induce tyrosine phosphorylation of p190RhoGAP activity. 293T cells transfected with either Nef or NefPXXP were lysed and incubated with an anti-phosphotyrosine-agarose conjugate to pull down tyrosine-phosphorylated proteins. The precipitates were subjected to Western blot analysis with anti-p190RhoGAP antibody. We found that Nef-infected cells had significant up-regulation of p190RhoGAP phosphorylation, suggesting that it mediates Nef-induced RhoA down-regulation (Fig. 6, A and C). NefPXXP had no effect, indicating that the Nef PXXP region is required for this activation to take place. We next sought to determine the functional significance of DIP in the protein complex with Nef and p190RhoGAP. We used the DIPΔPro mutant as a dominant negative, since it has been shown that the proline-rich domain is required for DIP phosphorylation and for DIP to mediate phosphorylation of Vav2 and p190RhoGAP. 293T cells transfected with Nef or NefPXXP were co-transfected with DIPΔPro. Compared with cells transfected with control, Nef did not induce tyrosine phosphorylation of p190RhoGAP in the presence of DIPΔPro (Fig. 6, A and C). This suggests that DIP is required for Nef-mediated activation of p190RhoGAP. Next, we examined the role of DIP in Nef-induced Vav2 phosphorylation.
FIGURE 6.
A, DIP dominant negative (DN) blocks Nef-induced p190RhoGAP phosphorylation. 293T cells were transfected with either control, Nef, or NefPXXP along with either control or DIPΔPro. Control with EGF was used as a positive control. Pull-down was performed with anti-phosphotyrosine conjugated to agarose beads, and samples were immunoblotted (IB) with anti-p190RhoGAP. DIPΔPro and Nef expression in these cells was verified by Western blotting. B, DIP dominant negative does not inhibit Nef-induced vav2 phosphorylation in podocytes. Control and Nef podocytes were transfected with either vector or DIPΔPro. Cell lysates were blotted with anti-phospho-Vav2 or Vav2 or Nef. DIPΔPro and Nef expression in these cells was verified by Western blotting. C and D, densitometric analysis. Blots in A and B were analyzed using densitometry. All experiments were repeated at least three times. IP, immunoprecipitation.
Podocytes were transfected with DIPΔPro or control and immunoblotted with antibody specific to phospho-Vav2. We found that DIPΔPro inhibited both base-line Vav2 phosphorylation in control cells and Nef-induced Vav2 activation (Fig. 6, B and D) in Nef-infected cells. These data suggest that DIP may help to recruit Vav2 to Nef and mediate Vav2 activation in response to endogenous growth factors in control cells as previously reported (33). However, DIPΔPro had no effect on Nef-induced Vav2 phosphorylation, indicating that the Nef-Vav2 interaction is sufficient to cause Vav2 activation.
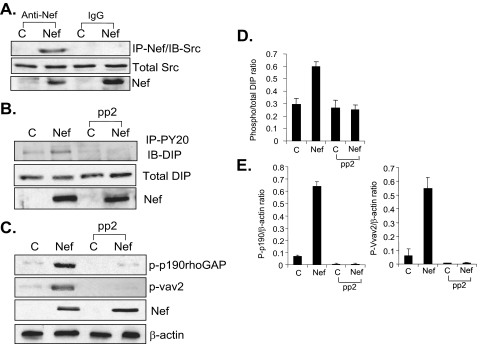
Src Is Responsible for Phosphorylation of DIP as Well as p190RhoGAP and Vav2 in Nef-infected Podocytes—It is well known that Nef interacts with Src family kinases, including c-Src, mediating its downstream signaling activation (33). DIP phosphorylation by Src kinase in its proline-rich region has been shown to be required for activation of p190RhoGAP and Vav2 (33). We have previously shown that Nef is able to activate Src in podocytes. Therefore, we examined here whether activation of Src plays a role in DIP function. First, we confirmed that Nef interacted with Src in podocytes (Fig. 7A). By co-immunoprecipitation using anti-tyrosine phosphorylation and anti-DIP antibodies, we found that a significantly higher proportion of DIP was phosphorylated in Nef-infected podocytes. Treatment of the cells with the Src inhibitor pp2 abrogated Nef-associated DIP phosphorylation (Fig. 7, B and D). Our results indicate that Src mediates DIP phosphorylation in the presence of Nef.
FIGURE 7.
A, Nef interacts with Src in podocytes. Nef-infected podocytes were lysed and immunoprecipitated (IP) with anti-Nef antibody. Western blotting was performed using monoclonal anti-Src antibody (Upstate Cell Signaling). B, DIP is tyrosine-phosphorylated in Nef podocytes in a Src-dependent manner. Control and Nef podocytes were treated with either control or pp2 (10 μm) for 1 h. Cell lysates were obtained and pulled down with anti-phosphotyrosine conjugated to agarose beads. Samples were immunoblotted (IB) with polyclonal anti-DIP. Nef expression was verified by Western blotting. C, Nef induces Src-mediated phosphorylation of p190RhoGAP and Vav2. Control and Nef-infected cells were treated with either control or pp2 (10 μm) for 1 h prior to lysis. Lysates were incubated with phosphotyrosine antibody conjugated to agarose beads. Precipitated fractions were subjected to Western blotting with monoclonal anti-p190 or polyclonal anti-vav2. Total cell lysates were subjected to Western blotting using anti-Nef or anti-α-actin antibodies. D and E, densitometric analysis. The blots in B and C were analyzed by densitometry. All experiments were repeated at least three times.
We also tested the effect of pp2 on Nef-induced phosphorylation of p190RhoGAP and Vav2. Cells were treated with either control vehicle or 10 μm pp2 and then lysed for pull-down with anti-phosphotyrosine-agarose conjugate. Western blotting on the precipitated protein fractions was performed with anti-p190RhoGAP and anti-Vav2. Pp2 significantly inhibited the activation of p190RhoGAP and Vav2 by Nef (Fig. 7, C and D). Together, these data indicate that Src acts upstream of DIP to activate p190RhoGAP and Vav2.
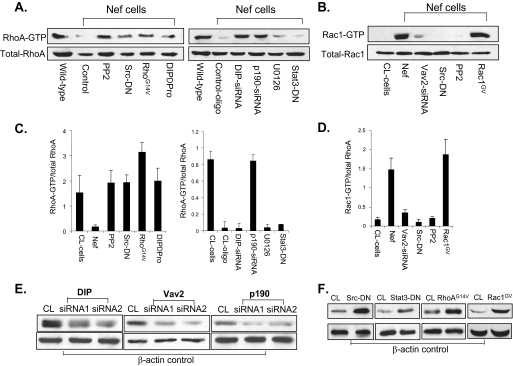
Role of Nef-mediated Signaling Pathway in Regulation of Rac1 and RhoA Activities—We wanted to confirm that Src, DIP, p190RhoGAP, and Vav2 together mediate RhoA inhibition and Rac1 activation in Nef podocytes. We performed RhoA and Rac1 activation assays on Nef-infected podocytes that were either treated with pp2 or transfected with Src-DN, DIPΔPro, siRNA for DIP, siRNA for p190RhoAGAP, or siRNA for Vav2. As positive controls, Nef podocytes were transfected with constitutively active mutants RhoAG14V and Rac1G14V. Since the MAPK1/2 and Stat3 pathways have previously been implicated in Nef pathogenicity on podocytes, we also treated Nef-infected podocytes with the MAPK1/2 inhibitor, U0126, or transfected with a dominant negative form of Stat3 (Stat3-DN). Nef-induced down-regulation of RhoA activity was restored in cells treated with pp2 or transfected with Src-DN, RhoAG14V, DIPΔpro, and siRNA for DIP and for p190RhoAGAP, whereas no effects were observed in cells treated with U0126 or transfected with Stat3-DN (Fig. 8, A and C). Nef-induced Rac1 activation was inhibited with pp2 Src-DN and siRNA for Vav2 (Fig. 8, B and D). These data indicate that Src-DIP-p190RhoAGAP and Src-Vav2 interactions mediate Nef regulation of RhoA and Rac1 activity, respectively, whereas the Stat3 and MAPK pathways are not involved. To confirm the siRNA data in these experiments, we have used two siRNA duplexes for each molecule as described under “Materials and Methods.” As shown in Fig. 8E, both siRNA duplexes for each molecule were able to reduce significantly the expression of DIP, Vav2, or p190RhoAGAP, respectively. Knockdown of the expression of DIP, Vav2, or p190RhoAGAP using the second siRNA duplex led to similar findings to those shown in Fig. 8. We have also confirmed the expression of Src-DN, Stat3-DN, Rac1GV, and RhoAG14V in podocytes (Fig. 8F).
FIGURE 8.
A and B, effect of Src/DIP-mediated signaling pathways in Nef-regulated RhoA and Rac1 activities. Nef-infected podocytes were treated with pp2 (10 μm), U01260 (10 μm) overnight or transfected with Src-DN, Stat3-DN, RhoG14V, Rac1GV, DIPΔPro, and siRNA for DIP, Vav2, and p190RhoAGTP for 3 days. Podocytes infected with empty vector were used as the control. Cells were lysed for RhoA and Rac1 activation assays. C and D, densitometric analysis. The blots from A and B were analyzed by densitometry. All experiments were repeated at least three times. E, inhibitory effects of two siRNA duplexes for DIP, Vav2, and p190RhoAGAP expression were confirmed by Western blotting using their specific antibodies, respectively. β-Actin was used as loading control. F, Western blotting was also performed to confirm the expression of Src-DN, Stat3-DN, RhoG14V, and Rac1GV with β-actin as loading control.
Role of Nef-mediated Signaling Pathway in Regulation of Stress Fiber Formation in Nef-expressing Podocytes—We wanted to determine the functional significance of these actin regulatory pathways in mediating morphologic changes induced by Nef in podocytes. We found that Src-DN restored the normal appearance of podocytes with increased stress fiber formation and reduced lamellipodia (Fig. 9). Suppression of DIP activity by using DIPΔPro as a dominant negative or siRNA for DIP also resulted in increased stress fiber formation (Fig. 9). Likewise, transfecting the Nef-infected podocytes with a constitutively active RhoA mutant, RhoG14V, or siRNA for p190RhoAGAP recovered stress fiber formation (Fig. 9). Transfecting Nef cells with siRNA for Vav2 diminished lamillopedia and increased stress fiber formation in podocytes, whereas U0126 and Stat3-DN did not change the actin cytoskeleton in Nef-podocytes (Fig. 9). Knockdown of DIP, p190RhoAGAP, or Vav2 expression using another siRNA duplex caused the similar morphological changes to those shown in Fig. 9. These morphological changes are consistent with Nef regulation of Rac1 and RhoA activities.
FIGURE 9.
Inhibition of the Nef-Src-DIP pathway restores normal podocyte actin cytoskeletal structure. Nef-infected podocytes were treated with U0126 (10 μm) or transfected with Src-DN, Stat3-DN, RhoG14V, DIPΔPro, or siRNA for DIP, Vav2, or p190RhoAGAP. Cells were stained for F-actin by phalloidin for stress fiber and lamellipodia formation.
DISCUSSION
We have identified a new interaction between Nef and DIP, a recently described regulator of actin cytoskeleton dynamics. This interaction allows for signal from Nef to inhibit RhoA activity through stimulation of p190RhoAGAP. Simultaneously, Nef activates Rac1. Src functions as the transducer of Nef signals to both Rac and Rho (Fig. 10). These findings provide insight into the mechanisms by which Nef influences podocyte morphology. Our findings are also important for understanding the role of Nef in HIV infection through regulation of actin cytoskeleton.
In this study, we characterize the in vitro phenotype of podocytes infected with HIV-1. Like undifferentiated podocytes at 33 °C, HIV-1-infected podocytes lost characteristic stress fibers (39). Furthermore, a large number of lamellipodia, which are not normally observed in cultured podocytes, were seen. Coordinated inhibition of RhoA and activation of Rac1 signaling is critical to determining cell morphology (40, 41). The Nef-DIP, Nef-Vav2, and Nef-Src interactions complete the upstream circuitry by which Nef regulates coordinated activity of RhoA and Rac1 and, therefore, the actin cytoskeleton (Fig 10). This function of Nef may be a critical determinant of the unique ability of HIV-1-infected podocytes to migrate into Bowman's space, where proliferation occurs (42). Further studies using an in vivo model will help to clarify the physiological consequences of these findings.
The ability of Nef to regulate actin cytoskeleton dynamics in other cell types plays a critical role in HIV-1 infectivity and survival (6, 7, 9, 43). The N-terminal myristoylation of Nef is known to mediate an interaction with the actin cytoskeleton in B and T lymphocytes (8). In previous studies, the Nef PXXP has been shown to interact directly with the C-terminal SH3 domain of Vav2, leading to activation of Rac1 and Pak2 (30). In dendritic cells, Nef has been shown to cause up-regulation of Rac1 via Src-mediated Vav2 phosphorylation (9). We show that Nef interacts with Vav2 in podocytes, leading to pronounced lamellipodia in HIV-infected podocytes. However, the change in Rac1 activity alone is not sufficient to explain morphological changes observed in HIV-infected podocytes. We show for the first time that DIP is a critical molecule mediating the effects of Nef on RhoA activity through its interaction with both Nef and p190RhoAGAP. We also show that in the presence of Nef, Src mediates tyrosine phosphorylation of DIP, thus permitting the assembly of the functional complex of Nef-Src-DIP and p190RhoAGAP. This allows Src to phosphorylate and thus activate RhoAGAP. Although DIP is not required for the Nef-Vav2 interaction, it does enhance Src-mediated phosphorylation of Vav2 in both control and Nef cells. Through this coordinate regulation of the signaling pathways to Rac1 and RhoA, Nef is able to decrease stress fibers and increased lamellipodia formation in podocytes (Fig. 10).
The Nef PXXP motif allows for its interaction with both Vav2 and DIP in a competing manner. How Nef is able to interact with multiple proteins in a dynamic way or recruit multiple proteins simultaneously is unclear. Previous work has shown that Nef forms oligomers that allow Nef to interact with multiple proteins simultaneously. Oligomerization of Nef is also critical to many of its functions (38, 44, 45). Although function of Nef oligomers were not the focus of this study, such oligomerization provides a plausible explanation for how Nef interacts with both Vav2 and DIP via the same motif. Furthermore, Nef is usually expressed in mammalian cells (podocytes) in excess amounts compared with endogenous signaling molecules, providing another explanation of why it could interact with multiple proteins in a single cell.
The podocyte phenotype observed in HIVAN is complex, with loss of differentiation markers, morphological changes, and proliferation. Nef has been shown to induce podocyte dedifferentiation and proliferation by activating the Src-Stat3 pathway and Ras-Raf-MAPK1, 2 pathways (22). However, our data suggest that Stat3 and MAPK pathways do not mediate Nef-induced changes of actin cytoskeleton in podocytes. Actin-based podocyte differentiation markers, such as synaptopodin or α-actinin-4, may contribute to the morphological changes seen, since both of these proteins have been demonstrated to play roles in maintenance of the actin cytoskeleton and adhesion in podocytes (39, 46, 47). We have previously shown that Nef reduced expression of synaptopodin, which is a stabilizer of RhoA protein (46). Consistent with this, our data suggest that Nef also partially diminished total RhoA protein levels, probably through down-regulation of synaptopodin expression (Fig. 1A). In our study, inhibition of Nef-Src-DIP-p190RhoAGAP and Nef-Src-Vav2 pathways appears to return the podocyte to a normal morphology establishing a direct relationship between alterations of these signaling pathways and the podocyte morphology phenotype. Further physiological studies are required to analyze the relative contribution of these pathways in mediating HIV-1-induced podocyte injury.
In summary, we find that DIP is a new interacting partner of Nef. DIP is the first adaptor protein known to mediate Nef-regulated RhoA activity. The findings in this study provide a rationale to further study the role of DIP in cytoskeleton regulation in vivo. The findings in this study may also provide insight into the mechanism of how Nef enhances HIV-1 replication through regulation of actin cytoskeleton in T cells.
This work was supported in part by National Institutes of Health (NIH) Grants P01 DK056492 (to P. K.) and R01 GM54508 (to R. I.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HIV-1, human immunodeficiency virus, type 1; SH3, Src homology 3; HIVAN, HIV-1-associated nephropathy; DIP, diaphanous interacting protein; siRNA, small interfering RNA; GST, glutathione S-transferase; HA, hemagglutinin; PBS, phosphate-buffered saline; MAPK, mitogen-activated protein kinase.
References
- 1.Deacon, N. J., Tsykin, A., Solomon, A., Smith, K., Ludford-Menting, M., Hooker, D. J., McPhee, D. A., Greenway, A. L., Ellett, A., Chatfield, C., Lawson, V. A., Crowe, S., Maerz, A., Sonza, S., Learmont, J., Sullivan, J. S., Cunningham, A., Dwyer, D., Dowton, D., and Mills, J. (1995) Science 270 988–991 [DOI] [PubMed] [Google Scholar]
- 2.Hanna, Z., Kay, D. G., Rebai, N., Guimond, A., Jothy, S., and Jolicoeur, P. (1998) Cell 95 163–175 [DOI] [PubMed] [Google Scholar]
- 3.Kestler, H. W., III, Ringler, D. J., Mori, K., Panicali, D. L., Sehgal, P. K., Daniel, M. D., and Desrosiers, R. C. (1991) Cell 65 651–662 [DOI] [PubMed] [Google Scholar]
- 4.Kirchhoff, F., Greenough, T. C., Brettler, D. B., Sullivan, J. L., and Desrosiers, R. C. (1995) N. Engl. J. Med. 332 228–232 [DOI] [PubMed] [Google Scholar]
- 5.Fackler, O. T., and Baur, A. S. (2002) Immunity 16 493–497 [DOI] [PubMed] [Google Scholar]
- 6.Campbell, E. M., Nunez, R., and Hope, T. J. (2004) J. Virol. 78 5745–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller, C., Rauch, S., Michel, N., Hannemann, S., Lehmann, M. J., Keppler, O. T., and Fackler, O. T. (2006) J. Biol. Chem. 281 19618–19630 [DOI] [PubMed] [Google Scholar]
- 8.Fackler, O. T., Kienzle, N., Kremmer, E., Boese, A., Schramm, B., Klimkait, T., Kucherer, C., and Mueller-Lantzsch, N. (1997) Eur. J. Biochem. 247 843–851 [DOI] [PubMed] [Google Scholar]
- 9.Quaranta, M. G., Mattioli, B., Spadaro, F., Straface, E., Giordani, L., Ramoni, C., Malorni, W., and Viora, M. (2003) FASEB J. 17 2025–2036 [DOI] [PubMed] [Google Scholar]
- 10.Geyer, M., Fackler, O. T., and Peterlin, B. M. (2001) EMBO Rep. 2 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, C. H., Saksela, K., Mirza, U. A., Chait, B. T., and Kuriyan, J. (1996) Cell 85 931–942 [DOI] [PubMed] [Google Scholar]
- 12.Renkema, G. H., Manninen, A., Mann, D. A., Harris, M., and Saksela, K. (1999) Curr. Biol. 9 1407–1410 [DOI] [PubMed] [Google Scholar]
- 13.Kaminchik, J., Margalit, R., Yaish, S., Drummer, H., Amit, B., Sarver, N., Gorecki, M., and Panet, A. (1994) AIDS Res. Hum. Retroviruses 10 1003–1010 [DOI] [PubMed] [Google Scholar]
- 14.Rao, T. K., Friedman, E. A., and Nicastri, A. D. (1987) N. Engl. J. Med. 316 1062–1068 [DOI] [PubMed] [Google Scholar]
- 15.D'Agati, V., Suh, J. I., Carbone, L., Cheng, J. T., and Appel, G. (1989) Kidney Int. 35 1358–1370 [DOI] [PubMed] [Google Scholar]
- 16.Chander, P., Soni, A., Suri, A., Bhagwat, R., Yoo, J., and Treser, G. (1987) Am. J. Pathol. 126 513–526 [PMC free article] [PubMed] [Google Scholar]
- 17.Barisoni, L., Kriz, W., Mundel, P., and D'Agati, V. (1999) J. Am. Soc. Nephrol. 10 51–61 [DOI] [PubMed] [Google Scholar]
- 18.Winston, J. A., Bruggeman, L. A., Ross, M. D., Jacobson, J., Ross, L., D'Agati, V. D., Klotman, P. E., and Klotman, M. E. (2001) N. Engl. J. Med. 344 1979–1984 [DOI] [PubMed] [Google Scholar]
- 19.Zhong, J., Zuo, Y., Ma, J., Fogo, A. B., Jolicoeur, P., Ichikawa, I., and Matsusaka, T. (2005) Kidney Int. 68 1048–1060 [DOI] [PubMed] [Google Scholar]
- 20.Husain, M., Gusella, G. L., Klotman, M. E., Gelman, I. H., Ross, M. D., Schwartz, E. J., Cara, A., and Klotman, P. E. (2002) J. Am. Soc. Nephrol. 13 1806–1815 [DOI] [PubMed] [Google Scholar]
- 21.Sunamoto, M., Husain, M., He, J. C., Schwartz, E. J., and Klotman, P. E. (2003) Kidney Int. 64 1695–1701 [DOI] [PubMed] [Google Scholar]
- 22.He, J. C., Husain, M., Sunamoto, M., D'Agati, V. D., Klotman, M. E., Iyengar, R., and Klotman, P. E. (2004) J. Clin. Invest. 114 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo, Y., Matsusaka, T., Zhong, J., Ma, J., Ma, L. J., Hanna, Z., Jolicoeur, P., Fogo, A. B., and Ichikawa, I. (2006) J. Am. Soc. Nephrol. 17 2832–2843 [DOI] [PubMed] [Google Scholar]
- 24.Mundel, P., and Shankland, S. J. (2002) J. Am. Soc. Nephrol. 13 3005–3015 [DOI] [PubMed] [Google Scholar]
- 25.Ichimura, K., Kurihara, H., and Sakai, T. (2003) J. Histochem. Cytochem. 51 1589–1600 [DOI] [PubMed] [Google Scholar]
- 26.Barisoni, L., Bruggeman, L. A., Mundel, P., D'Agati, V. D., and Klotman, P. E. (2000) Kidney Int. 58 173–181 [DOI] [PubMed] [Google Scholar]
- 27.Schwartz, E. J., Cara, A., Snoeck, H., Ross, M. D., Sunamoto, M., Reiser, J., Mundel, P., and Klotman, P. E. (2001) J. Am. Soc. Nephrol. 12 1677–1684 [DOI] [PubMed] [Google Scholar]
- 28.Ridley, A. J. (1999) Nat. Cell Biol. 1 E64–E66 [DOI] [PubMed] [Google Scholar]
- 29.Ridley, A. J., and Hall, A. (1992) Cold Spring Harb. Symp. Quant. Biol. 57 661–671 [DOI] [PubMed] [Google Scholar]
- 30.Fackler, O. T., Luo, W., Geyer, M., Alberts, A. S., and Peterlin, B. M. (1999) Mol. Cell 3 729–739 [DOI] [PubMed] [Google Scholar]
- 31.Settleman, J., Narasimhan, V., Foster, L. C., and Weinberg, R. A. (1992) Cell 69 539–549 [DOI] [PubMed] [Google Scholar]
- 32.Chang, J. H., Gill, S., Settleman, J., and Parsons, S. J. (1995) J. Cell Biol. 130 355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng, W., Numazaki, M., Takeuchi, K., Uchibori, Y., Ando-Akatsuka, Y., Tominaga, M., and Tominaga, T. (2004) EMBO J. 23 760–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem, M. A., O'Hare, M. J., Reiser, J., Coward, R. J., Inward, C. D., Farren, T., Xing, C. Y., Ni, L., Mathieson, P. W., and Mundel, P. (2002) J. Am. Soc. Nephrol. 13 630–638 [DOI] [PubMed] [Google Scholar]
- 35.Chuang, P. Y., Yu, Q., Fang, W., Uribarri, J., and He, J. C. (2007) Kidney Int. 72 965–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satoh, S., and Tominaga, T. (2001) J. Biol. Chem. 276 39290–39294 [DOI] [PubMed] [Google Scholar]
- 37.Mundel, P., Reiser, J., Zuniga Mejia Borja, A., Pavenstadt, H., Davidson, G. R., Kriz, W., and Zeller, R. (1997) Exp. Cell Res. 236 248–258 [DOI] [PubMed] [Google Scholar]
- 38.Ye, H., Choi, H. J., Poe, J., and Smithgall, T. E. (2004) Biochemistry 43 15775–15784 [DOI] [PubMed] [Google Scholar]
- 39.Asanuma, K., Kim, K., Oh, J., Giardino, L., Chabanis, S., Faul, C., Reiser, J., and Mundel, P. (2005) J. Clin. Invest. 115 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zondag, G. C., Evers, E. E., ten Klooster, J. P., Janssen, L., van der Kammen, R. A., and Collard, J. G. (2000) J. Cell Biol. 149 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sander, E. E., ten Klooster, J. P., van Delft, S., van der Kammen, R. A., and Collard, J. G. (1999) J. Cell Biol. 147 1009–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Agati, V., and Appel, G. B. (1997) J. Am. Soc. Nephrol. 8 138–152 [DOI] [PubMed] [Google Scholar]
- 43.Thoulouze, M. I., Sol-Foulon, N., Blanchet, F., Dautry-Varsat, A., Schwartz, O., and Alcover, A. (2006) Immunity 24 547–561 [DOI] [PubMed] [Google Scholar]
- 44.Dennis, C. A., Baron, A., Grossmann, J. G., Mazaleyrat, S., Harris, M., and Jaeger, J. (2005) Proteins 60 658–669 [DOI] [PubMed] [Google Scholar]
- 45.Liu, L. X., Heveker, N., Fackler, O. T., Arold, S., Le Gall, S., Janvier, K., Peterlin, B. M., Dumas, C., Schwartz, O., Benichou, S., and Benarous, R. (2000) J. Virol. 74 5310–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asanuma, K., Yanagida-Asanuma, E., Faul, C., Tomino, Y., Kim, K., and Mundel, P. (2006) Nat. Cell Biol. 8 485–491 [DOI] [PubMed] [Google Scholar]
- 47.Kos, C. H., Le, T. C., Sinha, S., Henderson, J. M., Kim, S. H., Sugimoto, H., Kalluri, R., Gerszten, R. E., and Pollak, M. R. (2003) J. Clin. Invest. 111 1683–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]