Abdominal wall hernias occur when tissue structure and function is lost at the load-bearing muscle, tendon and fascial layer. The fundamental biological mechanisms are primary fascial pathology or surgical wound failure. In both cases, cellular and extra-cellular molecular matrix defects occur.
Abnormal collagen metabolism was an early biological mechanism proposed for the development of primary and incisional hernias.1, 2 Immature collagen isoforms were measured in patients with inguinal and incisional hernias.3, 4 Importantly, the collagen abnormality was detected in skin biopsies remote from the hernia site, supporting a genetic basis for hernia formation, although a large, population-based study of collagen expression in surgical patients needs to be done. Acquired collagen defects were ascribed to cigarette smoking or nutritional deficiencies.
Secondary fascial pathology occurs following acute laparotomy wound failure. This is in large part due to the replacement of fascial planes with scar tissue. It is well known that the incidence of recurrent incisional hernia increases with each attempt at repair.5, 6 Fibroblast and wound collagen disorders were observed in scar from incisional hernia patients. There is also evidence that mechanical strain, like coughing and weight lifting, can induce secondary changes in tissue fibroblast function within load-bearing tissues.7, 8 It is possible that chronic loading induces pathological changes in structural tissue cellular and molecular function, without an a priori biological defect.
Laparotomy wound failure and the loss of normal wound healing architecture may induce the selection of an abnormal population of wound repair fibroblasts, as occurs in chronic wounds.9, 10 This could result in the expression of abnormal structural collagen and also explain the high incidence of recurrent incisional hernias. One mechanism for phenotypic selection of abnormal laparotomy wound repair fibroblasts is the loss of abdominal wall load force signaling as the incision mechanically fails. It is recognized that mechanical load forces stimulate the repair of tendons.11 Wound ischemia also ensues during early acute wound failure, propagating deficient soft-tissue repair. The best studies of incisional hernia formation confirm that early laparotomy wound failure is an important mechanism of incisional hernia formation (Table 1).12 It is likely that early mechanical failure of the laparotomy wound induces pathological function of wound repair fibroblasts. By this mechanism, otherwise normal wound repair fibroblasts fail, without the primary expression of an extracellular matrix or wound repair disease. This hypothesis is now tested in at least one animal model where intentional mechanical laparotomy wound failure lead to pathological wound fibroblast function in vivo and in vitro.10 It is possible that a subset of incisional hernia patients expresses a defect in extra-cellular matrix and or wound repair function. It is hard to resolve that mechanism with the fact that the majority of surgical patients have no history of a wound healing defect (making them surgical candidates) and also do not express a defect at the primary surgical site (GI tract, vascular system, solid organs etc.). It is possible that mechanical failure is the major mechanism for incisional hernia formation and that the loss of mechanical load signaling or some other acute wound healing pathway induces defects in repair fibroblast biology. Since the tissue fibroblast is the major source for collagen synthesis and turnover, defects in fibroblast function are an important mechanism for subsequent tissue collagen disease.
Table 1.
Occult Laparotomy Wound Dehiscence by Post-Operative Day 30
| Outcome at 43 months | Less than 12 mm gap | More than 12 mm gap |
|---|---|---|
| % Healed | 95% (140/147) | 6% (1/18) |
| % Incisional Hernia | 5% (7/140) | 94% (17/18) |
With the limited information available, it is likely that primary hernias are the result of a connective tissue disorder, while secondary hernias (like incisional hernias) are most frequently due to technical failure, inducing a chronic wound. Recurrent hernias likely are a combination of both mechanisms.
Extra-cellular Matrix and Collagen Disease
Medicine provides many clues for the role of the extra-cellular matrix during hernia formation. Lathyrism is an acquired disorder of the connective tissue that predisposes to hernia formation. A diet high in chick peas inhibits collagen cross-linking leading to a laxity in fascial planes.13 Ehlers-Danlos syndrome is a collection of collagen isoform disorders, also predisposing to hernia formation. There is growing evidence that patients with large vessel aneurysmal disease express pathological extra-cellular matrix metabolism, predisposing to dilated aortas and hernias. Early studies found that the rectus sheath of direct inguinal hernia patients was thinned, displayed disordered collagen fibers and impaired hydroxylation of the collagen.1
Increased proteolytic activity may cause weakness in structural tissue. Matrix metalloprotease (MMP) −2 over-expression was measured in fibroblasts of patients with direct inguinal hernias and MMP-13 overexpression was detected in patients with recurrent inguinal hernias.14, 15 Studies like these are observational and it is not clear whether increased MMP expression leads to direct inguinal hernia formation. Alternatively, failing groin tissue may secondarily express increased MMP levels.
Many studies associate incisional hernias with impaired collagen and protease metabolism. Tissue from incisional hernias expressed more soluble (immature) collagen, increased ratios of early wound matrix collagen isoforms (collagen III) and increased tissue matrix metalloprotease levels.4, 16 A decreased ratio of type I: type III collagen mRNA and protein was measured in the hernia ring and skin specimens obtained from patients with incisional hernias. Morphologic changes were present not only in the fascial tissue, but also in the hernia sac, skin specimens, and scar tissue surrounding explanted meshes of hernia patients. These studies were the most compelling for the presence of a genetic collagen defect in patients that develop incisional hernias.
Surgical Wound Healing
The mechanism of primary hernia formation is important to understand to improve diagnosis, to provide prognosis and when modifying risks for hernia formation. Tissue matrix disorders that lead to primary hernia formation probably also impair surgical wound healing. Surgeons fundamentally seek a better understanding of the mechanism of successful and failed hernia repairs.
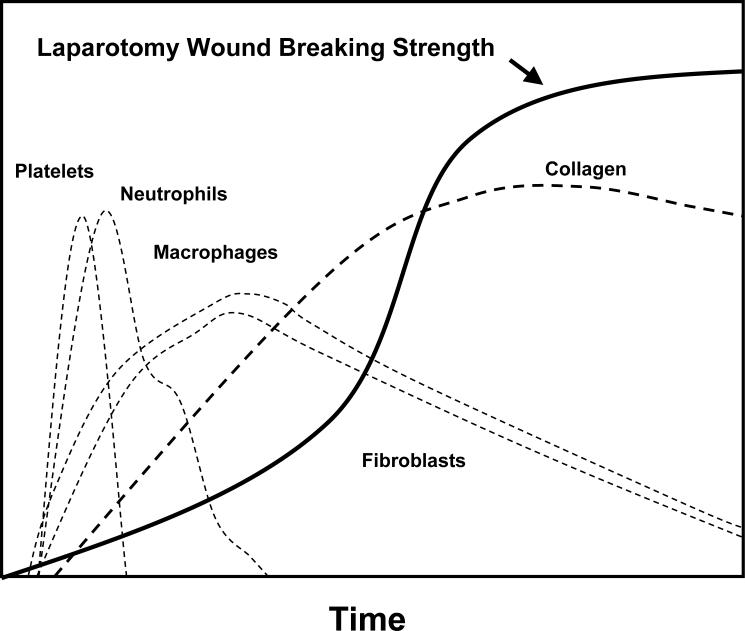
Acute wounds are defined by the loss of normal tissue structure and function in otherwise normal tissue where normal wound healing is expected to occur. An inter-regulated series of cellular and molecular events must be activated and modulated during the organization of a surgical wound matrix. (Fig. 1)17 It is the integrated summation of each pathway along the continuum of this host response to injury that results in acute wound healing. The phases of acute wound healing are described as hemostasis, inflammation, fibroproliferation (scar formation) and wound remodeling. A defect or delay in the activation of any of the repair pathways expressed during normal laparotomy and hernia repair may lead to hernia formation. Wound infection, wound ischemia and steroids all delay parts of the surgical wound healing pathway.18
Fig. 1.
A normal wound healing cascade. In otherwise normal tissue, without impediments to wound healing, sequential cellular and molecular elements of tissue repair are activated.
Abnormal wound matrix structure also contributes to the mechanism of recurrent hernias. Ideally, normal appearing aponeurotic and fascial structures would regenerate following hernia repairs. Smoking and malnutrition can impair collagen structure.1 Biological approaches for “normal” laparotomy wounds might be guided by information gained from identified genetic or epigenetic pathways associated with hernia formation like abnormal collagen matrix structure in Ehlers-Danlos syndrome or MMP/TIMP expression in abdominal aortic aneurysm (AAA) disease or in other chronic wounds.
Early mechanical wound failure (fascial dehiscence)
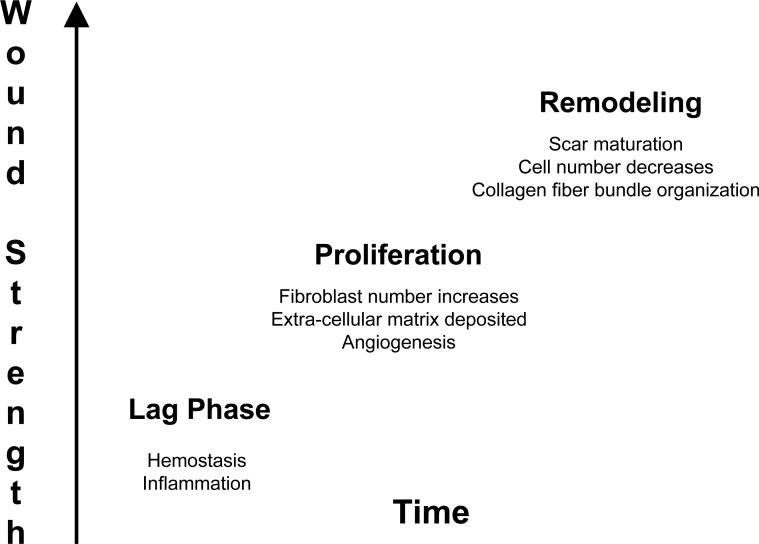
Growing evidence supports that incisional hernias and recurrent hernias are most often the result of early surgical wound healing failure. The majority of incisional hernias appear to develop following the mechanical disruption of laparotomy wounds occurring during the initial “lag phase” of the wound healing trajectory. (Fig. 2) Clinically evident laparotomy wound failure is a rare event, with reported dehiscence rates of 0.1%.19 Prior wound healing literature concluded that incisional hernias were the result of late laparotomy wound failure and scar breakdown.2 This concept was challenged by clinical studies of incisional hernias that recorded high primary and secondary recurrence rates after short-term follow-up, typically only 2−4 years.6 Prospective studies find that the true rate of laparotomy wound failure is closer to 11%, and that the majority of these (94%) go on to form incisional hernias during the first three years after abdominal operations.12 The real laparotomy wound failure rate is therefore 100 times what most surgeons think it is. By this mechanism, most incisional hernias and recurrent inguinal hernias originate from clinically occult dehiscences. The overlying skin wound heals, concealing the underlying myofascial defect. This mechanism of early mechanical laparotomy wound failure is more consistent with modern acute wound healing science. There are no other models of acute wound healing suggesting that a successfully healed acute wound goes on to breakdown and mechanically fail at a later date.
Fig. 2.
During the initial “lag-phase” of healing, the laparotomy wound is mechanically weakest. As surgical patients recover, increasing abdominal wall loads can cause acute wound failure.
In summary, incisional hernias occur when the laparotomy wound fails to heal. The fundamental mechanism may be an underlying wound healing defect, or, inadequate surgical technique. When a laparotomy wound fails due to inadequate surgical technique, selective changes occur involving the wound fibroblasts and extra-cellular matrix molecules leading to a pathological chronic wound.
The Mechanism of Incisional Hernia Formation
Biological Components
Laparotomy wounds are totally dependent on suture until breaking strengths are achieved that are capable of off-setting the increased loads placed across an acute wound by a recovering patient. Tensile strength normalizes breaking strength to the surface area of the wound edge, thereby measuring a physical property of the particular wound and scar (tensile strength = breaking strength / wound-edge surface area). Wound breaking strength is more relevant for tissues placed under high loads. Burst abdomens, or acute fascial dehiscence with evisceration are an important extreme of acute wound failure. They are associated with mortalities of 50% or greater.20
Acute wound healing fails when there is a deficient quantity or quality of tissue repair. Ultimately, it is the time required for the recovery of wound breaking strength that determines the risk of acute wound failure. Inadequate hemostasis due to platelet dysfunction or poor technique can result in hematoma formation with ensuing mechanical disruption of a provisional wound matrix. A delayed or deficient inflammatory response can result in wound contamination or infection with abnormal signalling for progression into the fibro-proliferative phase of acute tissue repair.21 A prolonged inflammatory response due to the presence of a foreign material, like a mesh implant, or wound infection will delay the progression of acute wound healing into the fibroproliferative phase where rapid gains in breaking strength should occur.22 Delayed fibroblast responses in turn impede the synthesis of a provisional wound matrix, prolonging the period of time a surgical wound is subjected to increasing mechanical loads and dependent entirely on suture material and technique for strength.
Inflammation
Inflammatory cells marginate into injured tissue and an efflux of leukocytes and plasma proteins enter the wound site. Neutrophils arrive initially and function to phagocytose and debride the wound, but are not required for wound healing in clean wounds. Monocytes and tissue macrophages populate the inflammatory infiltrate within two to three days. Macrophages phagocytose injured tissue and debris as well as secrete multiple growth factors. The macrophage orchestrates tissue repair and appears to be the only inflammatory cell type absolutely required.23
Overall tissue strength of a wound is essentially zero during this inflammatory phase thus an excessive or prolonged inflammatory response as is seen with incisional foreign bodies, like suture or mesh material, or infections predispose to wound failure. Steroids can reduce wound inflammation but also inhibit collagen synthesis and wound contraction synergistically impeding tissue repair.24 Interestingly, there is minimal inflammatory cell infiltration seen in fetal wound repair during which the epidermis and dermis are restored to normal architecture without scar formation.25
Fibroblasts
Fibroblasts are responsible for collagen synthesis and the recovery of wound breaking strength. Fibroblasts migrate into acute wounds within two days and are the major cell type in granulation tissue by post-injury day four. At first, fibroblasts populate the wound site through migration and increase in number by proliferation. Wound fibroblast migration and proliferation are both influenced by soluble growth factors and inflammatory mediators.26 The chemical and structural composition of the provisional matrix on which fibroblasts move is equally important. Receptor mediated interactions are increasingly described between the wound extracellular matrix and activated repair fibroblasts. Very little, however, is known about defective fibroblast function during acute wound failure. Even less is known about the function of repair fibroblasts in tissue other than skin. Whether abdominal wall wound failure reflects a defect in tendon fibroblast recruitment and function during incisional hernia formation or whether abnormal mechanical signals following laparotomy wound failure subsequently results in impaired fibroblast function is not known.
In chronic ulcer studies, it was suggested that low wound growth factor levels might result in dermal fibroblast quiescence and even senescence.27 This may also be true in failing acute laparotomy and hernia wounds as an initially rapid rising growth factor signalling cascade became depleted. Relative fascial or tendon wound ischemia might also induce fibroblast cell-cycle arrest. This would occur, for example, when a suture line is closed too tight, or in a patient who is in shock and soft-tissue perfusion is reduced. An ischemic laparotomy repair might also be deficient in the components and co-factors required for DNA and protein synthesis, again resulting in repair fibroblast cell-cycle arrest. Finally, too little or too much tension across the laparotomy tendon repair may disturb the optimal set point of a normal mechanotransduction mechanism, again resulting in premature laparotomy wound fibroblast cell-cycle arrest.
The precise histological origin of abdominal wall fibroblast repair cells in healed versus herniated wounds is also unknown. Differences may exist in the chemotactic response of ventral (anterior) myofascial vs. mesothelial surface fibroblasts following midline incisions. It is known, for example, that peritoneal surface defects heal by simultaneously re-epithelializing the entire wound surface as opposed to establishing an advancing epithelial edge as occurs in the skin.28, 29 Because epidermal to dermal communication is known to occur during the healing of skin, it is possible that a similar mechanism may be active on the peritoneal surfaces of abdominal wall (fascial) wounds. Peritoneal fluid itself may modulate acute repair in the abdominal wall. During fetal wound healing, amniotic fluid can act to accelerate the recovery of wound breaking strength in addition to minimizing the amount of scar formation.
Defects have been identified in the kinetic properties of fibroblasts cultured from laparotomy wound and hernia biopsies obtained from a rat model of incisional hernias (Franz, submitted). It was observed that fibroblasts cultured from incisional hernias expressed a defect in causing the contraction of fibroblast populated collagen lattices. Normally healing laparotomy wound fibroblasts caused 80% lattice contraction over five days while hernia fibroblasts caused only 50% lattice contraction. The same studies found no difference in the level of collagen gene expression between herniated and healed laparotomy wounds after 28 days. The results suggested that any difference in collagen gene expression occurs earlier than post-operative day 28 in this laparotomy repair model, or that the defect in herniated wounds is not one of collagen gene expression. Other possibilities included down-stream abnormalities in collagen protein synthesis and assembly, early scar crystallization and or fibroblast remodeling activity.
Collagen
Collagen is the predominant structural protein, especially of abdominal wall fascial layers, comprising 80% or more of structural tissue dry weight. Defects result in either delayed or abnormal collagen synthesis or increased wound protease activity leading to collagen degradation. The result is an imbalance in repair collagen homeostasis leading to a reduction in wound collagen levels, wound tensile strength and an increased risk of mechanical wound failure.30 Lathyrism, a disorder of collagen cross-linking, and lathyrogens were shown to be associated with herniation. Reduced hydroxyproline and collagen levels were measured in structural tissues of patients with direct inguinal hernias.31 Isolated fibroblasts from these patients expressed a proliferative defect and a reduced ability to translocate hydroxyproline. Subsequent to that work, apparent abnormalities in the ratios of collagen isoform expression, decreased collagen cross-linking and increased collagen solubility were observed. A two-fold increase in the amount of immature type III collagen was reported in the skin fibroblasts of patients with inguinal hernias when compared to non-hernia patients.15 A genetic predisposition to the formation of abdominal wall hernias was also suggested in large, controlled series of abdominal aortic aneurysm patients supporting the long held impression of a common extra-cellular matrix defect in both vascular wall and abdominal wall collagen metabolism.32-35
The mechanism by which the collagen-rich early laparotomy wound matrix attaches to uninjured tissue at the wound border is also poorly understood. This mechanism is important since acute laparotomy wounds most often fail at the scar to normal tissue interface.36 Animal modelling shows that a provisional wound matrix mechanically fails within the scar itself only during the first three to five days after injury. After that, mechanical failure is more likely to occur at the early scar to wound-edge interface. (Fig. 3) Different tissue also heals at different rates. Native tissues with collagen bundles organized in a parallel orientation, like fascia, ligament or tendon, re-gain breaking strength faster than tissue with a more complex, three dimensional fiber network, such as in the dermis.37 Another way to describe this is by measuring the recovery in relative breaking strength where wound progress is normalized to the uninjured tissue collagen content. The time required to achieve 50% wound breaking strength is greater in tissue with high collagen content, again, as in the case of dermis. Conversely, more “simply” arranged soft tissues like abdominal wall fascia, with lower tissue collagen content, but organized in a purely parallel manner along lines of tension, should achieve uninjured breaking strength faster.
Fig. 3.
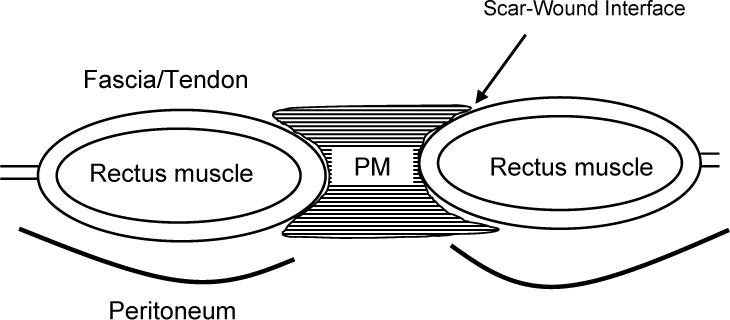
Incisional hernias occur when suture fails, suture lines are too loose, or suture pulls through the tissue adjacent to the wound. This develops before the laparotomy wound scar is mechanically capable of withstanding the distractive forces. The provisional matrix (PM) is comprised of immature and weak matrix glycoproteins and collagen isoforms. In addition, the scar to wound interface is not developed.
Growth Factors
Growth factors are tissue repair signalling peptides up-regulated initially during the inflammatory phase of laparotomy wound healing. Five to seven days are required, however, before peak levels of fibroproliferative growth factors like TGF-β are reached within acute wounds.38-40 It is not known whether delays in the appearance of fibroproliferative growth factors contribute to the development of incisional hernias. Surgical wound therapy with proliferative growth factors is known to stimulate the appearance of fibroblasts and collagen into the wound thereby accelerating the gain in wound breaking strength.41
Nutrition
Tissue repair is an anabolic process that requires both energy and adequate nutritional building blocks. Patients who are malnourished or actively catabolic such as in the systemic inflammatory response syndrome demonstrate impaired healing. The National Surgery Quality Improvement Program sponsored by the Veterans Administration consistently measures low serum albumen as a risk factor for perioperative complications, including incisional hernia formation.42 Inadequate nutrition also impairs the immune response limiting opsonization of bacterial and sterilization of wounds. Several vitamin and mineral deficiencies also have been described that predispose to altered wound repair. Vitamins C, A, and B6 each are required for collagen synthesis and cross-linking. Deficiencies in Vitamins B1 and B2 as well as zinc and copper cause syndromes associated with poor wound repair. Finally, essential fatty acids are required for cell synthesis, particularly in areas of high cell turnover such as healing wounds.43
Tissue Perfusion
Perioperative shock is a well recognized risk factor for incisional hernia formation.44 Tissue oxygen levels of 30 mm Hg are required for healing to occur. Besides systemic hypotension, a too tight continuous suture line closure may exacerbate laparotomy wound ischemia. Emergency operations may also be associated with wound contamination and altered surgical technique. Laparotomies following gunshot wounds to the abdomen or for perforated viscous may leave devitalized tissue and high levels of bacteria in the surgical wound. High wound bacterial counts are known to lead to wound failure, and in this case, incisional hernia formation.45 During emergency operations, midline incisions are most common and they are usually longer than during elective procedures. There is evidence that midline incisions herniate more frequently than transverse incisions.20 The collagen bundles of the abdominal wall are predominantly oriented transversely.46 A transverse suture line is therefore mechanically more stable, as it encircles tissue collagen bundles, rather than splitting them.
The Effect of Surgical Technique on the Biology of Laparotomy and Hernia Repair
Most studies designed to improve laparotomy and hernia wound outcomes have focused on surgical technique and the mechanical properties of suture material and mesh.2, 47, 48 During the evolution of inguinal hernia repairs, it was assumed that a strong, stout tissue such as the conjoined tendon rigidly sutured to a similar structure like Cooper's ligament would produce a reliable hernia repair. Purely surgical approaches like this proved unreliable for many surgeons and recurrence rates remained unacceptably high.
Surgical wound failure is most often due to suture pulling through adjacent tissue and not suture fracture or knot slippage.20 Tissue failure occurs in a metabolically active zone adjacent to the acute wound edge where proteases activated during normal tissue repair result in a loss of native tissue integrity at the point where sutures are placed. The breakdown of the tissue matrix adjacent to the wound appears to be part of the mechanism for mobilizing the cellular elements of tissue repair.
Abdominal wall tendons and fascia are connective tissues placed under intrinsic and extrinsic loads that are likely dependant upon mechanical signals to regulate fibroblast homeostasis. Mechanotransduction pathways are being described in greater detail in ligament, tendon and bone repair.7, 36, 49 Mechanical signals are transmitted to the structural cell via integrin receptors, for example, and subsequently effect repair cell metabolism through the modulation of cytoskeleton anchoring proteins. In brief, a load imparted on a soft-tissue or bone is transmitted to structural cells through the extra-cellular matrix via transmembrane integrin receptors located on the cell surface. In one proliferative pathway, subsequent activation of the focal adhesion kinase (FAK) complex, leads to cytoskeletal changes, and the further activation of downstream signalling tyrosine kinases like c-src and the MAP kinase proliferation pathway.49
The varying mechanical forces exerted across anatomically different celiotomy incisions such as midline vs. transverse therefore may affect repair fibroblast activation, provisional matrix assembly and collagen deposition and ultimately the temporal recovery of laparotomy wound tensile strength. Surgical experience has long held that transverse abdominal wall incisions oriented parallel to the predominant myofascial fibers regain unwounded tissue strength faster, but a clear benefit on wound outcomes has never been proven.20
Biology or Surgery?
Optimized laparotomy wound healing therefore depends on the normal assimilation of both biological and mechanical signals. Factors that interfere with either or both of these pathways will result in delays or defects in the early phases of acute wound healing. From the “biological” perspective this most commonly includes infection, ischemia, malnutrition and pharmacological inhibitors. From the “mechanical” perspective this involves the reinforcing cycle of wound failure with a loss in optimal strain loads and a down-regulation of the mechanotransduction pathways normally activated to signal tissue repair. In one extreme this is due to acute wound overload and overt mechanical failure and in the other extreme may be due to acute wound under load due to a poor suturing technique or even the placement of a bridging mesh implant.
Preliminary observations found for the first time that an interactive biomechanical mechanism may be activated during acute laparotomy wound failure. In other words, “mechanical” failure alone might result in the abnormal function of repair cells. Fibroblasts isolated from otherwise normal rat hernias were observed to cause 50−75% less contraction of a fibroblast populated collagen lattice (FPCL) than those fibroblasts isolated from a normally healing wound. One possible mechanism for this loss in repair fibroblast kinetic and proliferative activity may be the reduction in mechanical signals that occurs as a structural soft tissue fails. As already discussed, it is known in tendon and ligament repair, that mechanotransduction is an important pathway for setting fibroblast repair function.49 From this perspective, an abdominal wall laparotomy wound behaves more like a ligament or tendon than skin during repair.
Abdominal Wall Physiology
As described above, well-controlled, prospective studies conclude that most laparotomy wound disruptions progressing to incisional hernias begin to form within 30 days of laparotomy wound closure.12 In the animal models of hernia formation, laparotomy wounds are temporarily repaired with rapidly absorbed suture. Laparotomy wound-edge separation occurs early, resulting in incisional hernias due to incompletely supported mechanical loads. The incisional hernias that develop have well-defined hernia rings, hernia sacs and visceral adhesions, all characteristic of the incisional hernias that develop in humans. (Fig. 4)
Fig. 4.
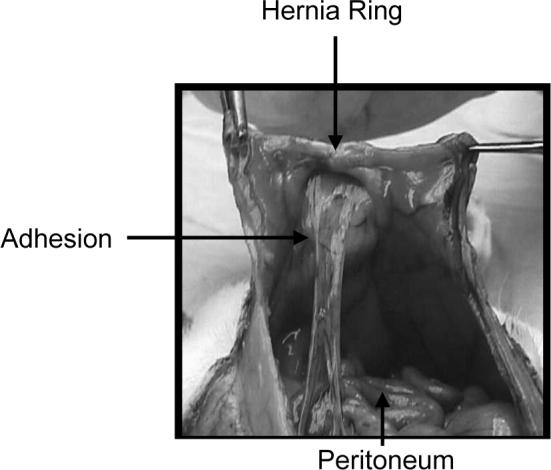
The peritoneal view of a rodent model of incisional hernias. The hernias develop well-defined hernia rings, hernia sacs and visceral adhesions, all characteristic of the incisional hernias that develop in humans. Modeling like this confirms biological limits of laparotomy wound repair and suggests that pathological changes occur in the wound and musculature of the abdominal wall following wound failure in otherwise normal tissue.
The function of intact abdominal wall structures during laparotomy repair and following hernia formation can be measured. This includes effects of the distractive load-forces generated by the lateral oblique and midline rectus muscle and fascial components. When the midline laparotomy mechanically fails, pathological disuse atrophy, fibrosis and muscle fiber type changes occur in the abdominal wall muscles.50 This pathological change in the lateral abdominal wall musculature supports the important role load signaling may play in abdominal wall wound repair. The observation is not surprising given the important function of the abdominal wall to support and animate the torso and to protect intra-abdominal organs. Surgically, it is equally likely that these pathological changes cause reduced abdominal wall compliance and contribute to the difficulty in achieving durable incisional hernia repairs.
Most evidence supports that primary hernia formation derives from a biological deficiency of the extra-cellular matrix of most patients. It is a soft-tissue disease. Risk factors like cigarette smoking and the metabolic syndrome likely contribute to the pathology. Once surgically repaired, the fundamental mechanism for recurrent hernia disease includes inadequate surgical technique and how that interacts with the biological limits of wound healing. Incisional hernias may be considered chronic wounds expressing abnormal tissue repair pathways.
Summary
The fundamental mechanism of abdominal wall hernia formation is the loss of structural integrity at the musculo-tendinous layer. This results in the inability to contain abdominal organs, support upright posture and maintain increased intra-peritoneal pressure during Valsalva. Primary abdominal wall hernias have been associated with extra-cellular matrix diseases. Incisional hernias and recurrent inguinal hernias more often involve a combination of technical and biological limitations. Defects in wound healing and extra-cellular matrix synthesis contribute to the high incidence of incisional hernia formation following laparotomy.
Acknowledgments
This work was supported by grant no. R01 GM078288 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Read RC. Introduction. Hernia. 2006 December;10(6):454–5. [Google Scholar]
- 2.Peacock J. Fascia and muscle. In: Peacock J, editor. Wound repair. 3rd ed. W.B. Saunders; Philadelphia: 1984. pp. 332–62. [Google Scholar]
- 3.Junge K, Klinge U, Rosch R, et al. Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbecks Archives of Surgery. 2004 February;389(1):17–22. doi: 10.1007/s00423-003-0429-8. [DOI] [PubMed] [Google Scholar]
- 4.Klinge U, Zheng H, Si ZY, Schumpelick V, Bhardwaj R, Klosterhalfen B. Synthesis of type I and III collagen, expression of fibronectin and matrix metalloproteinases −1 and −13 in hernial sac of patients with inguinal hernia. International Journal of Surgical Investigation. 1999;1(3):219–27. [PubMed] [Google Scholar]
- 5.Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population based analysis. Annals of Surgery. 2003 January;237(1):129–35. doi: 10.1097/00000658-200301000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luijendijk RW, Hop WCJ, van den Tol P, et al. A comparison of suture repair with mesh repair for incisional hernia. New England Journal of Medicine. 2000 August 10;343(6):392–8. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 7.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. European Journal of Applied Physiology. 2001 November;86(1):48–52. doi: 10.1007/s004210100502. [DOI] [PubMed] [Google Scholar]
- 8.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. Journal of Biological Chemistry. 2005 April 29;280(17):16546–9. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 9.Franz MG, Smith PD, Wachtel TL, et al. Fascial incisions heal faster than skin: a new model of abdominal wall repair. Surgery. 2001 February;129(2):203–8. doi: 10.1067/msy.2001.110220. [DOI] [PubMed] [Google Scholar]
- 10.Dubay DA, Wang X, Adamson BS, Kuzon WM, Jr, Dennis RG, Franz MG. Progressive fascial wound failure impairs subsequent abdominal wall repairs: a new animal model of incisional hernia formation. Surgery. 2005 April;137(4):463–71. doi: 10.1016/j.surg.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin M, Hillen B. Mechanical influences on cells, tissues and organs - ‘mechanical morphogenesis’. European Journal of Morphology. 2003 February;41(1):3–7. doi: 10.1076/ejom.41.1.3.28102. [DOI] [PubMed] [Google Scholar]
- 12.Pollock AV, Evans M. Early prediction of late incisional hernias. British Journal of Surgery. 1989;76:953–4. doi: 10.1002/bjs.1800760926. [DOI] [PubMed] [Google Scholar]
- 13.Conner WT, Peacock EE,, Jr. Some studies on the etiology of inguinal hernia. Am J Surg. 1973 December;126(6):732–5. doi: 10.1016/s0002-9610(73)80059-5. [DOI] [PubMed] [Google Scholar]
- 14.Bellon JM, Bajo A, Ga-Honduvilla N, et al. Fibroblasts from the transversalis fascia of young patients with direct inguinal hernias show constitutive MMP-2 overexpression. Annals of Surgery. 2001 February;233(2):287–91. doi: 10.1097/00000658-200102000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Si ZY, Kasperk R, et al. Recurrent inguinal hernia: disease of the collagen matrix? World Journal of Surgery. 2002 April;26(4):401–8. doi: 10.1007/s00268-001-0239-5. [DOI] [PubMed] [Google Scholar]
- 16.Rosch R, Junge K, Knops M, Lynen P, Klinge U, Schumpelick V. Analysis of collagen-interacting proteins in patients with incisional hernias. Langenbecks Archives of Surgery. 2003 February;387(11−12):427–32. doi: 10.1007/s00423-002-0345-3. [DOI] [PubMed] [Google Scholar]
- 17.Dubay DA, Franz MG. Acute wound healing: the biology of acute wound failure. Surgical Clinics of North America. 2003 June;83(3):463–81. doi: 10.1016/S0039-6109(02)00196-2. [DOI] [PubMed] [Google Scholar]
- 18.Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg. 2000;135:773–7. doi: 10.1001/archsurg.135.7.773. [DOI] [PubMed] [Google Scholar]
- 19.Santora TA, Roslyn JJ. Incisional hernia. Surgical Clinics of North America. 1993 June;73(3):557–70. doi: 10.1016/s0039-6109(16)46037-8. [DOI] [PubMed] [Google Scholar]
- 20.Carlson MA. Acute wound failure. Surgical Clinics of North America. 1997 June;77(3):607–36. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 21.Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001 February;38(2):72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 22.Robson MC, Shaw RC, Heggers JP. The reclosure of postoperative incisional abscesses based on bacterial quantification of the wound. Ann Surg. 1970;171:279–82. doi: 10.1097/00000658-197002000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riches DWH. Macrophage involvement in wound repair, remodeling and fibrosis. In: Clark RAF, editor. The cellular and molecular biology of wound healing. Second Edition ed. Plenum Press; New York: 1995. pp. 95–141. [Google Scholar]
- 24.Levenson SM, Demetriou AA. Metabolic Factors. In: Cohen IH, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical and clinical aspects. Saunders; Philadelphia: 2001. pp. 248–73. [Google Scholar]
- 25.Ferguson MW, Whitby DJ, Shah M. Scar formation: The spectral nature of fetal and adult wound repair. Plast Reconstr Surg. 1996;97:854–60. doi: 10.1097/00006534-199604000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Morgan CJ, Pledger WJ. Fibroblast Proliferation. In: Cohen IH, Diegelmann RF, Lindblad WJ, editors. Wound Healing: biochemical and clinical aspects. First ed. Saunders; Philadelphia: 1992. pp. 63–76. [Google Scholar]
- 27.Vande BJ, Rudolph R, Hollan C, Haywood-Reid PL. Fibroblast senescence in pressure ulcers. Wound Repair and Regeneration. 1998;6(1):38–49. doi: 10.1046/j.1524-475x.1998.60107.x. [DOI] [PubMed] [Google Scholar]
- 28.Ellis H, Harrison W, Hugh TB. The healing of peritoneum under normal and pathological conditions. Brit J Surg. 1965;52:471. doi: 10.1002/bjs.1800520616. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard TB, Jr, Khan MZ, Carag VR. The pathology of peritoneal repair: its relation to the formation of adhesions. Ann Surg. 1967;165:908. doi: 10.1097/00000658-196706000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro HN, Cuthbertson DP. The response of protein metabolism to injury. Biochemical J. 1943;37:129. [Google Scholar]
- 31.Jorgensen LN, Kellehave F, Karlsmark T, Gottrup F. Reduced collagen accumulation after major surgery. Brit J Surg. 1996;83:1591–4. doi: 10.1002/bjs.1800831133. [DOI] [PubMed] [Google Scholar]
- 32.Hall KA, Peters B, Smyth SH. Abdominal wall hernias in patients with abdominal aortic aneurysm versus aortoiliac occlusive disease. Am J Surg. 1995;170:572. doi: 10.1016/s0002-9610(99)80018-x. [DOI] [PubMed] [Google Scholar]
- 33.Ayde B, Luna G. Incidence of abdominal wall hernia in aortic surgery. Am J Surg. 1998;175:400–2. [PubMed] [Google Scholar]
- 34.Lehnert B, Wadouh F. High coincidence of inguinal hernias and abdominal aortic aneurysms. Ann Vasc Surg. 1992;6:134–7. doi: 10.1007/BF02042733. [DOI] [PubMed] [Google Scholar]
- 35.Friedman DW, Boyd CD, Norton P. Increases in type III collagen gene expression and protein synthesis in patients with inguinal hernias. Annals of Surgery. 1993;218:754–60. doi: 10.1097/00000658-199312000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viidik A, Gottrup F. Mechanics of healing soft tissue wounds. In: Schmidt-Schonbein GW, Woo SLY, Zweifach BW, editors. Frontiers In Biomechanics. Springer; New York: 1986. pp. 263–79. [Google Scholar]
- 37.Viidik A. The dynamic connective tissue. In: Kaupa J, editor. Annals of Estonia Medical Association.; Malmo, Sweden, Foerlags AB Eesti Post.: 1975. pp. 155–169. Ref Type: Serial (Book,Monograph) [Google Scholar]
- 38.Cromack DT, Sporn MB, Roberts AB, Merino MJ, Dart LL, Norton JA. Transforming growth factor beta levels in rat wound chambers. J Surg Res. 1987;42:622–8. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- 39.Franz MG, Kuhn MA, Nguyen K, et al. Transforming growth factor β2 lowers the incidence of incisional hernias. Journal of Surgical Research. 2001 May 15;97(2):109–16. doi: 10.1006/jsre.2001.6083. [DOI] [PubMed] [Google Scholar]
- 40.Roberts AB. Transforming growth factor beta: activity and efficacy in animal models of wound healing. Wound Rep Reg. 1995;3(4):408–18. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 41.Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-α. Science. 1987;237:1333–6. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- 42.Best WR, Khuri SF, Phelan Identifying patient preoperative risk factors and postoperative adverse events in administrative databases: results from the department of veterans affairs national surgical quality improvement program. Journal of the American College of Surgeons. 2002;194(3):257–66. doi: 10.1016/s1072-7515(01)01183-8. [DOI] [PubMed] [Google Scholar]
- 43.Williams JG, Barbul A. Nutrition and wound healing. Surg Clin N Am. 2003;83:571–96. doi: 10.1016/S0039-6109(02)00193-7. [DOI] [PubMed] [Google Scholar]
- 44.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. British Journal of Surgery. 1985;72(1):70–1. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 45.Robson MC. Infection in the surgical patient: an imbalance in the normal equilibrium. Clin Plast Surg. 1979;6:493. [PubMed] [Google Scholar]
- 46.Korenkov M, Beckers A, Koebke J, Lefering R, Tiling T, Troidl H. Biomechanical and morphological types of the linea alba and its possible role in the pathogenesis of midline incisional hernia. European Journal of Surgery. 2001 December;167(12):909–14. doi: 10.1080/110241501753361596. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins TPN. Incisional hernia repair: a mechanical approach. British Journal of Surgery. 1980;67(5):335–6. doi: 10.1002/bjs.1800670511. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson E. The relative rate of wound healing in longitudinal and transverse Incisions. Acta Chirurg Scand. 1982;148:251–6. [PubMed] [Google Scholar]
- 49.Schmidt C, Pommerenke H, Durr F. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. Journal of Biological Chemistry. 1998;273:5081–5. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- 50.Dubay DA, Choi W, Urbanchek MG, et al. Incisional herniation induces decreased abdominal wall compliance via oblique muscle atrophy and fibrosis. Ann Surg. 2007 January;245(1):140–6. doi: 10.1097/01.sla.0000251267.11012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]