Abstract
The relationship between standardized neuropsychological test performance and functional magnetic resonance imaging (fMRI) response during cognitive tasks is largely unknown. This exploratory investigation examined the relationship between neuropsychological test performance and fMRI response to a spatial working memory (SWM) task among 49 typically developing adolescents. Participants were administered a variety of neuropsychological tests in the domains of working memory, visuospatial skills, executive functioning, attention, learning and memory, visuomotor skills and processing speed, and language functioning. Neuropsychological domain scores were used to predict fMRI response during a SWM task. Results suggest that in many brain regions, neuropsychological performance negatively predicts fMRI response, suggesting that those teens with better neuropsychological abilities required fewer neural resources to adequately perform the task. This study provides further understanding of how neuropsychological abilities relate to neural activity during fMRI tasks, and provides an important link between neuropsychological and fMRI research.
Introduction
The term working memory describes the cognitive processes that allow one to actively maintain and manipulate information over a short duration of time (Baddeley, 1986). Working memory abilities have been shown to improve from childhood through adolescence (Luciana & Nelson, 1998), which may be a function of increasing capacities in overall knowledge, processing speed, problem solving strategies, attention, and memory (Cowan, 1997). These abilities to store and manipulate information online are crucial to intact performance in a number of cognitive domains, including arithmetic skills (Adams & Hitch, 1997; Logie, Gilhooly, & Wynn, 1994), reading and language comprehension (Daneman & Merikle, 1996; de Jong, 1998; Leather & Henry, 1994), and overall academic achievement (Gathercole, Brown, & Pickering, 2003; Gathercole, Pickering, Knight, & Stegmann, 2004).
Working memory impairments have been documented in a number of developing populations including (but not limited to) youths with a history of substance use disorders (Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002), head injury (Levin et al., 2002), schizophrenia and related genetic predisposition (Niendam et al., 2003), attention deficit/hyperactivity disorder (McInnes, Humphries, Hogg-Johnson, & Tannock, 2003; Shallice et al., 2002), and learning disabilities (Swanson, 2003). Given the large number of pediatric populations with working memory impairments and its importance to many cognitive domains, understanding working memory and its neuroanatomical substrates in developing youth is critical.
Since the advent of functional magnetic resonance imaging (fMRI), emerging research is shedding light on working memory processes in the developing adolescent brain, and specific task-related patterns of activation have begun to be explored. Attempts have been made to identify and characterize adolescent brain response patterns during spatial working memory (SWM) tasks. Although brain response patterns in adolescents differ somewhat from those of adults (e.g., amount and dispersion of activation), the existing developmental fMRI literature suggests a pattern of prefrontal and parietal brain activation in response to accurate SWM performance (Kwon, Reiss, & Menon, 2002; Nelson et al., 2000; Thomas et al., 1999). These findings suggest that, as in adults, these brain regions are, at least in part, responsible for subserving SWM abilities in adolescent populations.
While understudied in developing populations, it has been consistently demonstrated in the normal adult literature that regional brain activation during working memory fMRI tasks varies as a function of performance and working memory capacity (Braver et al., 1997; Callicott et al., 1999; Rypma, Berger, & D'Esposito, 2002). Specifically, activation patterns typically evidence an inverted U-shaped pattern, whereby the amount of response is positively associated with working memory abilities until capacity has been reached, and then both performance and activation decline. One study in a typically developing population revealed that activation in regions associated with working memory performance (left superior frontal sulcus and intraparietal cortex) showed a positive correlation with working memory capacity measured outside the scanner (Klingberg, Forssberg, & Westerberg, 2002). Another study that examined the relationship between in-scanner working memory performance (accuracy and reaction time) and fMRI response in children and adolescents demonstrated that performance did not significantly account for variance in fMRI activation (Kwon et al., 2002). No studies, to date, have examined the relationship between other aspects of cognitive performance and fMRI response patterns. Therefore, it remains unclear how neuropsychological test performance, and specifically working memory performance (both in and outside of the scanner), relate to brain activation patterns during working memory fMRI tasks among adolescents.
In order to address this question, the current exploratory study used widely administered neuropsychological tests to predict fMRI blood oxygen level dependent (BOLD) response during a SWM task. It was hypothesized that performance on tests of working memory and visuospatial functioning would be correlated with BOLD response during a SWM task condition, while tests not thought to be associated with SWM (e.g., language tests) would not be related to SWM response. Specifically, based on the limited previous literature (Klingberg et al., 2002), it was hypothesized that better neuropsychological performance on tests of working memory, attention, and executive functioning would predict increased BOLD response in frontal brain regions, while better performance on tests of visuospatial functioning would predict increased BOLD response in parietal regions, during a SWM task.
Methods
Participants
The current study examined 49 typically developing adolescents (ages 12−17 years). Participants were recruited from local middle and high schools for involvement in a larger study on brain development in substance abusing and non-abusing adolescent populations. For the present study, only adolescents without a history of substance use problems were examined. Additional exclusion criteria included history of a DSM-IV psychiatric disorder, head trauma (with loss of consciousness greater than 2 minutes), learning disorder, mental retardation, or neurological or serious medical illness; family history of bipolar I or psychotic disorders; significant alcohol exposure in utero; sensory problems; inadequate English skills; use of psychoactive medications; irremovable metal on the body; and left-handedness. All participants and guardians underwent an extensive screening process to determine participant eligibility using a structured clinical interview (Brown, Vik, & Creamer, 1989), the Customary Drinking and Drug Use Record (Brown et al., 1998), the Family History Assessment Module Screener (Rice et al., 1995), and the Computerized Diagnostic Interview Schedule for Children 4.0 (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). In accordance with the University of California San Diego Institutional Review Board, written informed assent and consent (by the participant and legal guardian) were obtained for all teens prior to participation. Participant characteristics are provided in Table 1.
Table 1.
Participant characteristics and performance (N = 49)
| Mean (SD) or % | |
|---|---|
| Female | 51% |
| Years of age (range: 12 to 18) | 14.76 (1.73) |
| Caucasian | 78% |
| Annual household income | 89.45K (44.82) |
| Grade point average | 3.56 (0.48) |
| Number of grades completed | 8.08 (1.60) |
| Attention domain: | |
| Digits forward (raw score)* | |
| Age (12−14) | 9.54 (1.82) |
| Age (15−17) | 10.05 (2.74) |
| CVLT-C List A trial one | 53.78 (9.98) |
| Executive function domain: | |
| Trails number-letter switching time* | |
| Halstead TMT B | 53.30 (14.38) |
| DKEFS Trails Condition 3 | 68.23 (16.86) |
| Color-word interference time (in seconds)* | |
| Golden Stoop Test | 41.65 (8.35) |
| DKEFS Color-Word Interference Test Condition 3 | |
| Age (12−13.5) | 67.50 (14.91) |
| Age (13.5−14.9) | 52.75 (7.19) |
| Language domain: | |
| Vocabulary (T-score) | 57.76 (8.20) |
| Reading (Standard score) | 108.94 (9.30) |
| Learning/Memory domain: | |
| CVLT-C List A total recall (trials 1−5) (T-score) | 51.43 (9.28) |
| CVLT-C short-delay free recall (T-score) | 50.41 (7.96) |
| CVLT-C long-delay free recall (T-score) | 50.04 (9.39) |
| CVLT-C recognition discriminability (T-score) | 50.91 (7.12) |
| Rey-O delayed recall accuracy (raw score)* | 14.83 (4.75) |
| Visuomotor/Processing speed domain: | |
| Coding/Digit Symbol (T-score) | 50.65 (9.58) |
| Trails number sequencing time (in seconds)* | |
| Halstead TMT A | 16.25 (6.13) |
| DKEFS Trails Condition 1 | 32.08 (7.89) |
| Visuospatial domain: | |
| Block Design | 56.08 (8.87) |
| Rey-O copy accuracy (raw score)* | |
| Age (12−13.5) | 27.05 (2.92) |
| Age (13.5−14.9) | 27.14 (3.93) |
| Working memory domain: | |
| Arithmetic (T-score) | 50.39 (10.95) |
| Digits Backward (raw score)* | |
| Age (12−14) | 5.46 (1.88) |
| Age (15−17) | 6.71 (2.26) |
| Vigilance condition reaction time (in milliseconds) | 636.63 (73.12) |
| Vigilance condition accuracy | 95.62% |
| SWM condition reaction time (in milliseconds) | 605.00 (70.01) |
| SWM condition accuracy | 88.87% |
Scores were calculated based on each participant's raw score and the sample mean
Neuropsychological Testing
All teens underwent a two-hour neuropsychological evaluation by a trained bachelor-level psychometrist, in order to assess abilities in working memory, visuospatial, executive, learning/memory, processing speed, simple attention, and language domains. The assessment consisted of the following tests (dependent upon the age of the participant and their original study involvement): Vocabulary, Arithmetic, Digit Span, Block Design, and Coding/Digit Symbol subtests (from the Wechsler Intelligence Scale for Children-Third Edition (Wechsler, 1993) and the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) for those under age 17, or from the Wechsler Adult Intelligence Scale-Third Edition (Wechsler, 1981, 1997) for 17-year-olds; the Rey-Osterrieth Complex Figure Test (Rey-O) (Osterrieth, 1944); the California Verbal Learning Test-Children's version (CVLT-C) (Delis, Kramer, Kaplan, & Ober, 1994); the Reading subtest from the Wide Range Achievement Test-Third Edition (WRAT-3) (Wilkinson, 1993); the Stroop Color-Word Interference (Golden, 1978) or, for 12−14 year olds, the Color-Word Interference subtest of the Delis-Kaplan Executive Function System (D-KEFS) (Delis, Kaplan, & Kramer, 2001), and the Trail Making Test — Parts A and B (Reitan, 1958) or, for 12−14 year olds, the Trail Making subtest from the D-KEFS (Delis et al., 2001).
All test scores were converted to T-scores for purposes of analyses. For the Rey-O, Digit Span subtests (forward and backward performance), Color-Word Interference tests, and Trail Making tests, T-scores were calculated based upon each teen's raw score performance and the sample mean in order to provide an accurate standardized score for this sample and in an effort to control for differences in normative procedures across the tasks. For the Wechsler indices, the different age-based test versions were combined for analyses. Age-grouped sample means were calculated for those test scores that demonstrated significant age effects (Digits forward and backward: 12 years to 14 years-11 months and 15 years to 17 years-11 months; Rey-O Copy: 12 years to 14 years-11 months and 15 years to 17 years-11 months; Color-Word Interference: 12 years to 13 years-5 months, 13 years-6 months to 14 years-11 months, and 15 years to 17 years-11 months). Overall, the mean level of performance on neuropsychological tests for participants in this study was within the average to high average range, with a fairly wide distribution of scores falling from within the borderline to superior range of ability (see Table 1).
To reduce the number of independent variables, individual test scores were grouped to form functional composite scores, determined by neuropsychological assessment theory and principal axis factoring with adolescents (Tapert & Brown, 2000). Individual T-test scores formed composite domains for attention, executive functioning, language, learning and memory, visuomotor skills/processing speed, visuospatial processing, and working memory. Domain scores were calculated as the mean T-score of tests comprising that domain. The domains were created as follows: working memory comprised Arithmetic and Digits backward; visuospatial skills included Block Design and Rey-O copy accuracy; executive functioning comprised Trails number-letter switching time and color-word interference time; attention consisted of Digits forward and CVLT-C List A trial one; learning and memory consisted of CVLT-C List A total recall (trials 1−5), short-delay free recall, long-delay free recall, and recognition discriminability, and Rey-O delayed recall accuracy; visuomotor/processing speed comprised Coding/Digit Symbol and Trails number sequencing time; and language included Vocabulary and Reading.
SWM Task
As previously described (Kindermann, Brown, Zorrilla, Olsen, & Jeste, 2004; Tapert et al., 2001), the SWM task was adapted from McCarthy and colleagues (McCarthy et al., 1994), and consisted of 18 20-second blocks that alternated between resting, baseline, and experimental conditions (see Figure 1). In the experimental condition (SWM), subjects were asked to remember the locations of abstract line drawings that were presented one at a time in one of eight spatial locations on a screen (see Figure 1). Subjects were instructed to respond to the stimuli by pressing a button with their right index finger every time a figure appeared in the same location as a previous design within that block. On average, three of the 10 stimuli presented in a block appeared in a repeat location (2-back) and required a response. The baseline condition (vigilance) consisted of the same abstract stimuli presented in the same spatial locations, and subjects were to respond by pressing a button with the right index finger every time a figure appeared with a dot above it. On average, 30% of the figures were shown with a dot. The active baseline condition was designed to control for activation related to the simple visual attention components of the working memory condition. In both active conditions, stimuli were presented for 1000 ms, with an interstimulus interval of 1000 ms (20 s/block; total task time=7 minutes 48 seconds). In the rest condition, a fixation cross appeared at the center of the screen. Subjects were trained on the task the day of the scan. Performance data were collected for both accuracy and reaction time in the experimental and baseline conditions during the fMRI experiment.
Figure 1.
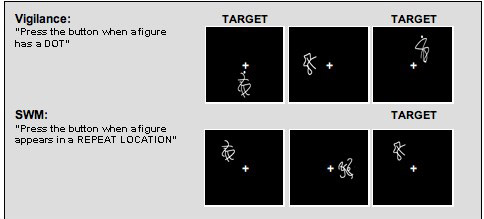
Examples of baseline and SWM task conditions. Note: The same abstract stimuli were used in both conditions.
Scanning Procedure
All imaging data were acquired using a 1.5 Tesla General Electric scanner. The scanning protocol consisted of a high-resolution structural image collected in the sagittal plane using an inversion recovery prepared T1-weighted 3D spiral fast spin echo (Wong, 2000) sequence (TR=2000ms, TE=16ms, FOV=240 mm, resolution=0.9375 × 0.9375mm × 1.328mm, 128 continuous slices, acquisition time=8:36). BOLD-weighted functional imaging was performed in the axial plane using T2*-weighted spiral gradient recall echo imaging (TR=3000ms, TE=40ms, flip angle=90°, FOV=240mm, 19−21 slices covering the whole brain, slice thickness=7mm, in-plane resolution=1.875 × 1.875mm, 156 repetitions). Spiral imaging was employed, as it can help reduce the effects of motion artifact on time series acquisitions (Meyer, Hu, Nishimura, & Macovski, 1992; Meyer & Macovski, 1987; Noll, Cohen, Meyer, & Schneider, 1995; Noll, Pauly, Meyer, Nishimura, & Macovski, 1991).
Data Analysis
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). Motion in the time series data was corrected by registering each acquisition to a selected repetition with an iterated least squares algorithm (Cox & Jesmanowicz, 1999) to estimate three rotational and three displacement parameters for each participant. An output file specifying adjustments made was subsequently used to control for spin history effects (Friston, Williams, Howard, Frackowiak, & Turner, 1996), and applied adjustments were correlated with age to see if motion indices would need to be corrected in subsequent analyses. The time series data were deconvolved (or correlated) with a reference vector that coded the hypothesized BOLD signal for alternating task conditions across the time series of the task while covarying for linear trends and the degree of motion correction previously applied. The reference vector was shifted forward six seconds to account for delays in the hemodynamic response (Bandettini, Jesmanowicz, Wong, & Hyde, 1993). All data were transformed into standardized space (Talairach & Tournoux, 1988). The functional data were resampled into 3.5mm cubic voxels, and a spatial smoothing Gaussian filter (full-width half maximum = 3.5 mm) was applied. The steps above resulted in a fit coefficient for each voxel representing BOLD response to SWM relative to the vigilance task condition.
To test the hypotheses, regression analyses were performed in which each functional domain score was correlated with whole-brain BOLD response to the SWM task. Given the number of correlations being performed, the clusterwise α was Bonferroni corrected (p < .007) to control for Type I error. As determined by Monte Carlo simulations (Forman et al., 1995; Ward, 1997), this correction required that clusters exceed 1272 μL to be considered significant (voxelwise α < .05). From the resulting significant clusters, mean fit coefficients for each participant were computed, and stepwise regressions determined which neuropsychological test score, within significant functional domains, best predicted response within each cluster. Demographic variables that correlated with neuropsychological test performance (p < .01) were controlled in these follow-up analyses.
Results
SWM Activation
During the SWM condition (versus baseline task), participants demonstrated decreased BOLD response in left ventrolateral prefrontal cortex and Broca's area (Brodmann's Areas (BA) 44, 45, and 47); bilateral anterior medial frontal gyri (BA 10); bilateral anterior cingulate (BA 32); and in anterior regions of the left superior frontal gyrus (BA 9). Participants also demonstrated increased BOLD response during SWM in more posterior regions of bilateral anterior cingulate (BA 24 and 32) and bilateral medial and superior frontal gyri (BA 6 and 8). Increased BOLD response to the SWM condition was also observed in bilateral precuneus and superior and inferior parietal lobules (BA 40 and 7); left inferior, middle, and superior frontal gyri (BA 11); bilateral insula (anterior portions; BA 47) and subcortical structures (e.g., left claustrum and lentiform nucleus; right cau-date); and bilateral cerebellum (see Figure 2).
Figure 2.
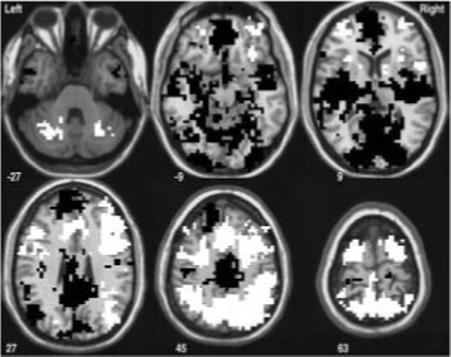
Significant clusters of activation for the SWM versus the baseline (Vigilance) task (p < .05). White areas indicate regions where SWM response was greater than baseline task activation — posterior regions of bilateral anterior cingulate (BA 24 and 32); bilateral medial and superior frontal gyri (BA 6 and 8); bilateral precuneus and superior and inferior parietal lobules (BA 40 and 7); left inferior, middle, and superior frontal gyri (BA 11); bilateral insula (anterior portions; BA 47) and subcortical structures (e.g., left claustrum and lentiform nucleus; right caudate); and bilateral cerebellum. Black areas indicate regions where the SWM task condition activated the brain less than the baseline (vigilance) condition — left ventrolateral prefrontal cortex and Broca's area (Brodmann's Areas (BA) 44, 45, and 47); bilateral anterior medial frontal gyri (BA 10); bilateral anterior cingulate (BA 32); and in anterior regions of the left superior frontal gyrus (BA 9).
Neuropsychological domain performances were not significantly correlated with task performance, with the exception of the visuomotor domain in which better performance was associated with faster SWM reaction time (r = −.30, p < .05). Task performance data are presented in Table 1. Despite limited associations between neuropsychological and task performance, neuropsychological domain performance scores significantly predicted SWM BOLD response as follows (see Table 2).
Table 2.
Regions where neuropsychological performance predicted SWM BOLD response
| Neuropsychological domain | Anatomic regions/BA | Hemisphere | Direction | Talairach coordinates | Volume (μL) | ||
|---|---|---|---|---|---|---|---|
| Working memory | Inferior Frontal Gyrus / Area 47 | Right | Negative | 30 | 10 | −11 | 5960 |
| Subcallosal Gyrus / Area 34 | Right | ||||||
| Insula, Putamen, Lentiform Nucleus, Globus | Right | ||||||
| Pallidus, Claustrum | Right | ||||||
| Insula and Superior Temporal Gyrus / Area 22 | Left | Negative | −40 | −19 | −4 | 3859 | |
| Inferior Frontal Gyrus / Area 47 | Left | Negative | −33 | 20 | −8 | 3602 | |
| Insula / Area 13 | Left | ||||||
| Claustrum | Left | ||||||
| Fusiform Gyrus / Area 18 | Right | Negative | 19 | −92 | −11 | 2187 | |
| Lingual Gyrus | Right | ||||||
| Inferior Occipital Gyrus | Right | ||||||
| Superior and Medial Frontal Gyrus / Areas 9 and 10 | Right | Negative | 2 | 55 | 28 | 2101 | |
| Superior and Medial Frontal Gyrus / Areas 9 and 10 | Left | Negative | −12 | 59 | 21 | 1929 | |
| Anterior Cingulate Gyrus / Area 32 | Left | Positive | −5 | 34 | −8 | 1886 | |
| Medial Inferior Frontal and Rectal Gyri / Area 11 | Left | ||||||
| Subcallosal Gyrus / Area 25 | Bilateral | ||||||
| Visuospatial functioning | Cerebellum | Left | Negative | −12 | −50 | −29 | 1415 |
| Caudate Nucleus | Right | Negative | 16 | 17 | 3 | 1329 | |
| Executive functioning | Inferior Parietal Lobule / Area 40 | Left | Positive | −30 | −47 | 38 | 1329 |
| Supramarginal and Angular Gyrus | Left | ||||||
| Attention | None | - | - | - | - | - | - |
| Learning/Memory | Middle Frontal Gyrus / Area 9 | Left | Positive | −40 | 24 | 35 | 1372 |
| Superior Frontal Gyrus / Area 24 | Right | Negative | 23 | 6 | 38 | 1286 | |
| Cingulate Gyrus | Right | ||||||
| Visuomotor/Processing | Cingulate Gyrus / Area 24 | Right | Negative | 19 | 3 | 45 | 1286 |
| Speed | Medial Frontal Gyrus | Right | |||||
| Language skills | None | - | - | - | - | - | - |
All clusters are p < .007. BA = Brodmann's Area. Direction = the direction of the association between domain score and BOLD response.
Working Memory Domain
Several clusters of SWM BOLD response were predicted by working memory domain scores ( p < .007) as shown in Figure 3. The only cluster to be positively predicted by working memory performance was the left anterior cingulate and medial inferior frontal gyrus (BA 32 and 11) and bilateral subcallosal gyri (BA 25). In all other clusters, working memory performance negatively predicted BOLD response, including: bilateral inferior frontal gyri (BA 47), insula, and claustrum; bilateral superior and medial frontal gyri (BA 9 and 10); right basal ganglia (caudate and lentiform nucleus); right fusiform, lingual, and inferior occipital gyri (BA 18); and left superior temporal gyrus and insula (BA 22). Follow-up analyses provided support for both Digits backward and Arithmetic in predicting these clusters; however activation in the left anterior cingulate, medial frontal gyrus (BA 32 and 11), and bilateral subcallosal gyri (BA 25) was predicted solely by Arithmetic scores (F (1,47) = 12.15, p < .001; β = .453, p < .001; R2 = 21%, = .205, p < .002).
Figure 3.
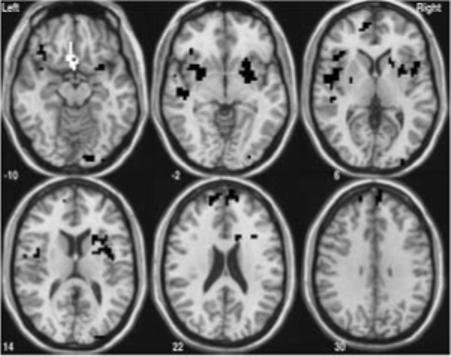
SWM activation clusters significantly predicted by neuropsychological assessment of working memory functioning (p < .007). White areas indicate regions where there was a positive association between working memory performance and SWM brain response — left anterior cingulate and medial inferior frontal gyrus (BA 32 and 11) and bilateral subcallosal gyri (BA 25). Black areas show where there was a negative relationship between working memory scores and SWM activation — bilateral inferior frontal gyri (BA 47), insula, and claustrum; bilateral superior and medial frontal gyri (BA 9 and 10): right basal ganglia (caudate and lentiform nucleus); right fusiform, lingual, and inferior occipital gyri (BA 18); and left superior temporal gyrus and insula (BA 22).
Visuospatial Domain
The visuospatial domain negatively predicted BOLD response in two regions: the left cerebellum and the right caudate nucleus ( p < .007). Follow-up analyses of the tests comprising this domain revealed that, while both visuospatial scores contributed to variance in activation in both clusters, the best predictor of cerebellar activation was the Rey-O Copy (F (1,45) = 10.94, p < .01; β = −.442, p < .01; R2 = 20%, = .196, p < .01), and variance in caudate activation was most accounted for by the Block Design subtest (F (1,45) = 14.50, p < .001; β = −.494, p < .001; R2 = 24%, = .244, p < .001).
Executive Functioning Domain
The executive functioning domain positively predicted BOLD response in the left inferior parietal lobule and the supramarginal and angular gyri (BA 40) (p < .007). Follow-up analyses revealed that the Trail Making number-letter switching task was the best executive functioning predictor of response within these regions (F (1,47) = 13.70, p < .001; β = .475, p < .001; R2 = 23%, = .226, p < .001), while Color-Word Interference also explained significant variance (ΔR2 = 1%, = .085, p < .05) above and beyond the number-letter switching task.
Learning/Memory Domain
SWM BOLD response was significantly predicted by learning/memory performance in two clusters. Learning/memory domain scores positively predicted BOLD response in the left middle frontal gyrus (BA 9), and negatively predicted BOLD response in the right superior frontal and cingulate gyri (BA 24) (p < .007). Because age was significantly correlated with several scores in the learning/memory domain (p < .01) and the groups significantly differed on several scores on the basis of gender (t(1,47) = 2.81, p < .008), these variables were controlled in follow-up analyses. The follow-up analyses revealed that, after controlling for the effects of gender and age, response in the left precentral and middle frontal gyri (BA 9 and 10) was solely predicted by CVLT-C List A total recall (trials 1−5) (F (3,43) = 7.18, p < .001; β = .551, p < .001; ΔR2 = 25%, = .245, p < .001), whereas response in the right superior frontal and cingulate gyri (BA 24) was predicted only by CVLT-C Short-Delay Free Recall (F (3,43) = 20.63, p < .001; β = −.764, p < .001; ΔR2 = 52%, = .522, p < .001).
Visuomotor/Processing Speed Domain
Better performance on tests within the visuomotor/processing speed domain was associated with less BOLD response in the right medial frontal and cingulate gyri (BA 24) (p < .007). Follow-up analyses examining the contribution of each score within the domain controlled for gender, due to a significant difference between the genders on Coding scores (t (1,46) = 4.06, p < .001). Follow-up analyses revealed that, after controlling for the effects of gender, number-sequencing trail making was the sole significant contributor to variance in BOLD response (F (2,45) = 13.99, p < .001; β = −.567, p < .001; ΔR2 = 32%, = .322, p < .001).
Attention Domain
Using stringent Bonferroni correction (p < .007), the attention domain did not significantly predict SWM BOLD response. However, there was a trend for better attention scores to predict greater BOLD response in the left precentral, middle, and inferior frontal gyri (BA 9) (p < .025).
Language Domain
With the correction level employed, language domain scores did not significantly predict SWM BOLD response. A trend was found for the language domain to negatively predict BOLD response in bilateral precuneus and the right cingulate gyrus (BA 31) (p < .025).
Task Performance Related Activation Patterns
SWM accuracy and reaction times (see Table 1) predicted BOLD response to SWM relative to vigilance. Specifically, more accurate SWM performance (fewer errors) was associated with increased BOLD response in the left superior and inferior parietal lobules (BA 7 and 40) and decreased response in the left medial regions of the inferior parietal lobule (BA 40) and left fusiform, middle occipital (BA 19), and lingual gyri (BA 18). Faster reaction times during the SWM task were related to decreased activation in bilateral cerebellum, right middle frontal and precentral gyri (BA 6), and left inferior frontal and precentral gyri (BA 6 and 44) and insula. Faster reaction times were associated with increased activation in bilateral superior and inferior parietal lobules (BA 7 and 40), right postcentral gyrus (BA 5), right superior and middle frontal gyri (BA 8 and 6), and right middle temporal (BA 20 and 21), fusiform (BA 37), and parahippocampal gyri.
Discussion
Consistent with previous fMRI studies of SWM, participants in the current study demonstrated a pattern of bilateral dorsolateral prefrontal and intraparietal brain activation (Kwon et al., 2002; Nelson et al., 2000; Thomas et al., 1999). In addition to frontal and parietal activation, participants demonstrated increased brain response in bilateral subcortical structures, right caudate, and the cerebellum during the SWM condition. Subcortical and cerebellar activation have been demonstrated in previous adult studies of SWM (for review see Cabeza & Nyberg, 2000).
As hypothesized, tests of working memory and visuospatial functioning were related to BOLD response during the SWM task condition. In addition, tests of executive functioning, learning/memory, and visuomotor/processing speed were also associated with patterns of SWM activation. The only two neuropsychological domain scores that did not significantly predict SWM response were the language and attention domain scores. While the absence of correlation between language domain scores and SWM BOLD response was not surprising and consistent with hypotheses, the lack of relationship between attention domain scores and SWM response was unexpected. However, the simple attention demands of the SWM task were controlled for by the baseline task condition (vigilance), which may help to explain this finding.
Overall, the direction of the associations between neuropsychological domain performance and task activation were contrary to the hypotheses. While better domain scores were associated with increased response in some brain regions, more often than not, better neuropsychological domain scores predicted less activation in working memory areas. The predominantly negative relationships between neuropsychological performance and BOLD response are in contrast to other fMRI studies that have suggested increases in task-related BOLD response as a function of better working memory performance (Klingberg et al., 2002) and better verbal memory performance (Johnson, Saykin, Flashman, McAllister, & Sparling, 2001). In contrast, better performance on the SWM task in the scanner (fewer errors and/or faster reaction time) predicted increased BOLD response in a number of SWM areas, including intraparietal cortex, which is consistent with the work of Klingberg and colleagues (2002).
Given that working memory abilities are inherently present in a number of cognitive processes (Gathercole, 1999), it is not surprising that many of the neuropsychological domain scores were associated with BOLD response during the working memory task condition. As was expected however, the most robust associations (both in terms of volume of each cluster and number of distinct clusters) were found between the working memory domain scores and activation during the SWM task. One plausible explanation for the negative association may be that individuals with a propensity for better working memory abilities require fewer neural resources in working memory-related brain areas to achieve adequate SWM performance. Given that activation appears to increase with working memory load until a capacity has been reached (Braver et al., 1997; Callicott et al., 1999), perhaps these findings suggest that individuals who perform better on working memory tasks are farther from reaching working memory capacity in the scanner, and thus demonstrate less brain response in dorsal and ventrolateral prefrontal brain regions. In this study, the only regions of activation that demonstrated a positive relationship with working memory abilities were the anterior cingulate and inferior frontal gyrus. This finding is consistent with previous adult literature suggesting that among those with greater working memory ability (e.g., high-span performers), there is greater anterior cingulate and inferior frontal response (Osaka et al., 2004). These authors suggested that, among those with better working memory skills, there is greater effective connectivity between these brain regions, thus supporting a stronger working memory system (Kondo et al., 2004).
Another expected relationship was demonstrated between visuospatial domain performance and SWM activation. However, while it was hypothesized that this relationship would be evidenced in parietal brain regions, it was found that visuospatial abilities were related to SWM activation in the cerebellum and caudate nucleus. Interestingly, while the group as a whole showed increased activation in these regions during the SWM condition, individuals with better visuospatial skills demonstrated decreased activation in these areas. Faster reaction times on the SWM task were also related to decreased cerebellar activation. These findings may suggest that those individuals with superior visuospatial abilities require fewer neural resources in cerebellar regions associated with spatial attention (Akshoomoff, Courchesne, & Townsend, 1997) and planned or anticipated motor responding (Horwitz, Deiber, Ibanez, Sadato, & Hallett, 2000). These findings may also suggest that teens with better visuospatial abilities required less neural response in the caudate, an area shown to evidence greater response during manipulation of information held in working memory (Lewis, Dove, Robbins, Barker, & Owen, 2004).
The associations between the executive functioning, learning/memory, and visuomotor/processing speed domains and SWM activation were fewer, but nonetheless deserve mention. One of the few neuropsychological domains to positively predict SWM BOLD response was the executive functioning domain. This positive relationship was demonstrated in the left inferior parietal lobe and supramarginal/angular gyrus region. This greater inferior parietal activation may be explained by those individuals with better executive functioning making greater use of spatial strategies during the task (Glabus et al., 2003). An additional positive association was demonstrated between learning/memory domain scores and left dorsolateral prefrontal activation, which may have been related to the encoding and retrieval of spatial information during the task (Ranganath, Johnson, & D'Esposito, 2003), particularly as activation in this region was best predicted by CVLT-C list A total recall — a measure that is thought to assess ability to encode and retrieve information. A possible explanation for the decreased right superior and medial frontal and cingulate activation in association with better learning/memory and visuomotor/processing speed scores is that those with stronger abilities in these domains demonstrate decreased susceptibility to interference or competing responses, as the cingulate region has been associated with monitoring for and detecting the presence of response conflict (Botvinick, Braver, Barch, Carter, & Cohen, 2001). This explanation is especially parsimonious for the relationship between the learning/memory domain and this region, as the best predictor from this domain was CVLT-C short-delay free recall — a measure that should be particularly vulnerable to interference effects.
While this study had the benefit of a relatively large and well-distributed sample, there are several limitations that need to be addressed. First, due to the exploratory nature of the study, gender and age effects were not examined. Although these variables were statistically controlled for when they demonstrated significant associations with the independent variables, and age effects were managed in neuropsychological score computation, BOLD response during fMRI tasks varies as a function of age and development (Klingberg et al., 2002; Kwon et al., 2002), as well as by gender (Speck et al., 2000). Another limitation of the current study concerns the neuropsychological domain scores. The domain scores in this study, although similar to those in a statistically-driven study with adolescents (Tapert & Brown, 2000), were chiefly theoretically driven. A larger sample size would have provided enough power to perform factor analysis and determine more accurate assignments of neuropsychological tests to functional domains. In addition, the inclusion of an out-of-scanner test of visuospatial working memory in the working memory domain may have provided more information regarding the relationship between working memory abilities and SWM BOLD response. Also, on several occasions, somewhat different versions of tasks were combined in the analyses. While there has been support for the comparability of different versions of Wechsler IQ tests (Thompson & Sota, 1998), the combining of different test versions based on different normative populations (e.g., D-KEFS Trail Making and the Halstead Trail Making Test, D-KEFS Color-Word Interference and the Golden Stroop Test) presents another source of potential variability into the data (Kalechstein, van Gorp, & Rapport, 1998).
Despite methodological limitations, this is the first known study of its kind to attempt to bridge the gap between standardized neuropsychological assessment measures and neuroimaging research. The results of this study provide important information into how neuropsychological functioning influences brain response in normally developing adolescents with varying ability levels across different behavioral tasks, and suggest that many aspects of neuropsychological performance directly affect brain response during fMRI tasks. These findings suggest that individuals with different neuropsychological profiles allocate different brain resources in order to perform tasks. This may have important clinical relevance for the role of premorbid neuropsychological functioning when characterizing expected patterns of impairment in relation to neurological insults, and may help to explain findings in which neurologically compromised populations demonstrate greater brain activation to working memory tasks than controls (McAllister et al., 1999). Future studies should be directed toward examining the relationships between other of types of neuropsychological and fMRI tasks, while attending to potential contributions made by gender and developmental/age differences.
Acknowledgments
This study was supported by grants R21 AA12519 and R01 AA13419 from the National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD (Dr. Tapert) and the UCSD Fellowship in Biological Psychiatry and Neuroscience. Portions of this study were presented at the annual meeting for the International Neuropsychological Society, February 2003, Honolulu, Hawaii. We would like to thank Greg Brown, Sandra Kindermann, MJ Meloy, Lisa Caldwell, and Lauren Killeen for their valuable contributions to this research.
References
- Adams JW, Hitch GJ. Working memory and children's mental addition. J Exp Child Psychol. 1997;67(1):21–38. doi: 10.1006/jecp.1997.2397. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E, Townsend J. Attention coordination and anticipatory control. Int Rev Neurobiol. 1997;41:575–598. doi: 10.1016/s0074-7742(08)60371-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addictive Behaviors. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cowan N. The development of working memory. In: Cowan N,, editor. The development of memory in childhood. Psychology Press; Hove, UK: 1997. pp. 163–199. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: A meta-analysis. Psychonomic Bull Rev. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- de Jong PF. Working memory deficits of reading disabled children. J Exp Child Psychol. 1998;70(2):75–96. doi: 10.1006/jecp.1998.2451. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive Function System (D-KEFS) Psychological Corp; San Antonio, TX: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test-Children's Version. Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Cognitive approaches to the development of short-term memory. Trends Cogn Sci. 1999;3(11):410–419. doi: 10.1016/s1364-6613(99)01388-1. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Brown L, Pickering SJ. Working memory assessments at school entry as longitudinal predictors of national curriculum attainment levels. Educational and Child Psychology. 2003;20(3):109–122. [Google Scholar]
- Gathercole SE, Pickering SJ, Knight C, Stegmann Z. Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology. 2004;18(1):1–16. [Google Scholar]
- Glabus MF, Horwitz B, Holt JL, Kohn PD, Gerton BK, Callicott JH, et al. Inter-individual differences in functional interactions among prefrontal, parietal and parahippocampal regions during working memory. Cereb. Cortex. 2003;13(12):1352–1361. doi: 10.1093/cercor/bhg082. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A manual for clinical and experimental uses. Stoelting Co; Wood Dale, IL: 1978. [Google Scholar]
- Horwitz B, Deiber MP, Ibanez V, Sadato N, Hallett M. Correlations between reaction time and cerebral blood flow during motor preparation. Neuroimage. 2000;12(4):434–441. doi: 10.1006/nimg.2000.0632. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Saykin AJ, Flashman LA, McAllister TW, Sparling MB. Brain activation on fMRI and verbal memory ability: Functional neuroanatomic correlates of CVLT performance. J Int Neuropsychol Soc. 2001;7(1):55–62. doi: 10.1017/s135561770171106x. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, van Gorp WG, Rapport LJ. Variability in clinical classification of raw test scores across normative data sets. Clinical Neuropsychologist. 1998;12(3):339–349. [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophr Res. 2004;68(2−3):203–116. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H. Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage. 2004;21(1):2–14. doi: 10.1016/j.neuroimage.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather CV, Henry LA. Working memory span and phonological awareness tasks as predictors of early reading ability. J Exp Child Psychol. 1994;58(1):88–111. doi: 10.1006/jecp.1994.1027. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Chang CC, Zhang L, Schachar R, Ewing-Cobbs L, et al. Working memory after traumatic brain injury in children. Ann Neurol. 2002;52(1):82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: A functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19(3):755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Logie RH, Gilhooly KJ, Wynn V. Counting on working memory in arithmetic problem solving. Mem Cognit. 1994;22(4):395–410. doi: 10.3758/bf03200866. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia. 1998;36(3):273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, et al. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology. 1999;53(6):1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, et al. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes A, Humphries T, Hogg-Johnson S, Tannock R. Listening comprehension and working memory are impaired in attention-deficit hyperactivity disorder irrespective of language impairment. J Abnorm Child Psychol. 2003;31(4):427–443. doi: 10.1023/a:1023895602957. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn. Reson. Med. 1992;28:202–213. doi: 10.1002/mrm.1910280204. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Macovski A. Proceedings, Sixth Annual Meeting. : Society of Magnetic Resonance in Medicine. 1987.
- Nelson CA, Monk CS, Lin J, Carver L.qJ., Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36(1):109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160(11):2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral K space MR imaging of cortical activation. J Magn Reson Imaging. 1995;5(1):49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- Noll DC, Pauly JM, Meyer CH, Nishimura DG, Macovski A. Magn. Reson. Med. 1991;25(2):319. doi: 10.1002/mrm.1910250210. [DOI] [PubMed] [Google Scholar]
- Osaka N, Osaka M, Kondo H, Morishita M, Fukuyama H, Shibasaki H. The neural basis of executive function in working memory: An fMRI study based on individual differences. Neuroimage. 2004;21(2):623–631. doi: 10.1016/j.neuroimage.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Archives of Psychology. 1944;30:206–356. [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol. 2002;21(1):43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11(11):2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Swanson HL. Age-related differences in learning disabled and skilled readers' working memory. J Exp Child Psychol. 2003;85(1):1–31. doi: 10.1016/s0022-0965(03)00043-2. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional proportional system: An approach to cerebral imaging. Thieme; New York: 1988. Coplanar stereotaxic atlas of the human brain. [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert S, Brown G, Meloy M, Dager A, Cheung E, Brown S. fMRI measurement of brain function in alcohol use disordered adolescents. Alcoholism: Clinical and Experimental Research. 2001;25:80A. [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy N, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thompson AP, Sota DD. Comparison of the WAIS-R and the WISC-III scores with a sample of 16-year-old youth. Psychological Reports. 1998;82(3):1339–1346. doi: 10.2466/pr0.1998.82.3c.1339. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale -Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-III. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III. Psychological Corp; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp; San Antonio, TX: 1999. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proc Intl Magn Reson Med, 2000;8:683. [Google Scholar]


