Abstract
Myelination organizes axons into distinct domains that allow nerve impulses to propagate in a saltatory manner. The edges of the myelin sheath are sealed at the paranodes by axon–glial junctions that have a crucial role in organizing the axonal cytoskeleton. Here we propose a model in which the myelinated axons depend on the axon–glial junctions to stabilize the cytoskeletal transition at the paranodes. Thus paranodal regions are likely to be particularly susceptible to damage induced by demyelinating diseases such as multiple sclerosis.
Keywords: Myelin, axon-glial junctions, paranode, node of Ranvier, axonal cytoskeleton, Caspr (contactin-associated protein), NCP1, paranodin, cytoskeletal transition
INTRODUCTION
In vertebrates, neurons project axons over long distances and, to ensure fast nerve-impulse conduction in an energetically economic fashion, some of these axons are targeted for myelination and become insulated by specialized glia (oligodendrocytes in the CNS and Schwann cells in the PNS). Myelination, ultimately, allows the impulse to propagate in a saltatory manner by preventing ionic leakage along insulated segments of the axons. There are differences between myelination in the CNS and PNS such as protein composition, number of axonal segments myelinated by each myelinating cell, the type of glia contacting the nodes of Ranvier, and the presence of a basal lamina. Most of the features that we discuss here are thought to be shared by both the CNS and the PNS. We focus our discussion on the changes in axonal cytoskeleton promoted by myelination and speculate on the contribution of these changes to the dependence of myelinated axons on proper contacts with the myelinating glial cells.
The landmarks for the myelination process include: (1) axonal outgrowth and glial migration to the region of future white matter; (2) targeting of axons for myelination; (3) bidirectional signaling between axons and glia that triggers glial differentiation, polarization of glia and axons into nodes of Ranvier and adjacent molecular domains, and local changes in both glial and axonal architectures; and (4) dependence on cell–cell interactions for survival of both myelinating glia and myelinated axons (Barres and Barde, 2000; Witt and Brady, 2000; Pedraza et al., 2001; Corfas et al., 2004; Edgar and Garbern, 2004).
Myelination: a selective process
Axons must have a minimum axonal diameter to become a target for myelination, and proper contact between axons and glia is required for glial differentiation and survival mediated by the axonal signals (Sherman and Brophy, 2005). In the PNS, the protein Neuregulin 1 (Nrg1) type III, which is expressed on the axolemma (axonal membrane), is proposed to serve as a measurement tool for this selection. Because a threshold level of Nrg1 type III is required for further development of the Schwann cells, only axons that display more than this threshold level (i.e. those with more than the minimum membrane surface) provide sufficient Nrg1 type III signaling to be myelinated by Schwann cells (Michailov et al., 2004).
Myelination drives polarization of both the glia and the axons that is coordinated in time and space, resulting in the formation of distinct domains: nodes of Ranvier, paranodes, juxtaparanodes and internodes (Fig. 1A). Each segment of axon wrapped by a compact portion of the myelin sheath corresponds to an internode. Nodes of Ranvier are gaps in the myelin sheath where voltage-gated Na+ channels are clustered in the axonal membrane. The paranodes flank the nodes of Ranvier and form the axon–glial junctions (AGJs). Juxtaparanodes are a specialized region of the internodes that flank the paranodes. Here we provide a brief overview of these domains. Several recent reviews have focused on how the node of Ranvier and adjacent domains are established and maintained in myelinated axons (Arroyo and Scherer, 2000; Peles and Salzer, 2000; Pedraza et al., 2001; Salzer, 2003; Bhat, 2003; Rasband, 2004).
Fig. 1. Landmarks for changes in axons and glia after myelination.
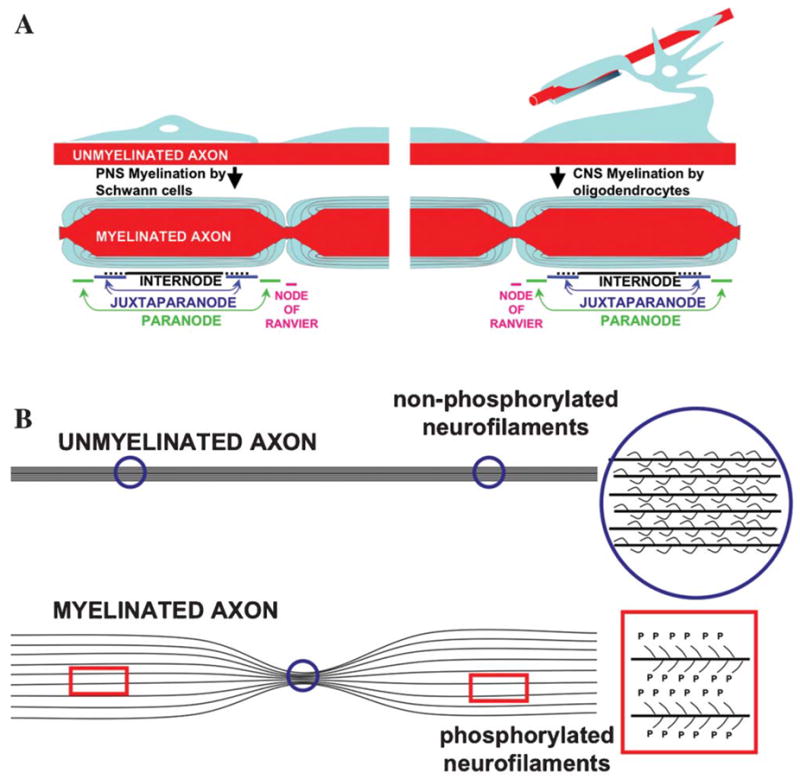
(A) Polarization of both axons and glia in the node of Ranvier and adjacent domains. The schematic is not to scale but the difference in axonal diameter between nodes of Ranvier and internodes is reported to be up to five fold. Similarities between myelination by oligodendrocytes (CNS) and Schwann cells (PNS) are presented. Nodes of Ranvier contact processes of perinodal astrocytes in the CNS and microvilli of Schwann cells in the PNS. We have omitted this information as well as the basal lamina in the PNS from the schematic, aiming to emphasize the similarities between the two myelination processes. (B) Phosphorylation of neurofilaments has a major role in increasing axonal diameter through myelination-dependent signaling.
At the internodes, the glial cytoplasm is extruded during myelination, which results in the formation of layers of a compact lipoprotein coat (Fig. 2A). Accumulation of glial cytoplasm at the lateral edges of each layer form the paranodal loops. Beneath the glial internodes, in some axons the axonal internodes also experience a myelination-induced regulation in their volume, and axonal diameter increases by up to five-fold in the internodal region compared with the nodes of Ranvier (Figs. 1B and 2A) (Windebank et al., 1985). This expansion is a consequence of the trophic reorganization of the axonal cytoskeleton and correlates with the local phosphorylation of neurofilaments and a subsequent increase in neurofilament spacing (Fig. 1B) (de Waegh et al., 1992). Phosphorylation has been proposed to increase the spacing between the filaments in vitro by generating electrostatic repulsion of negatively-charged side-arms at the C-terminal of neurofilament subunits M and H (Kumar and Hoh, 2004). However studies of neurofilament gene replacement in mice indicate that electrostatic repulsion is not the sole mechanism by which phosphorylation increases neurofilament spacing in vivo (Garcia et al., 2003).
Fig. 2. Schematic of the main changes in axons and glia that are promoted by myelination.
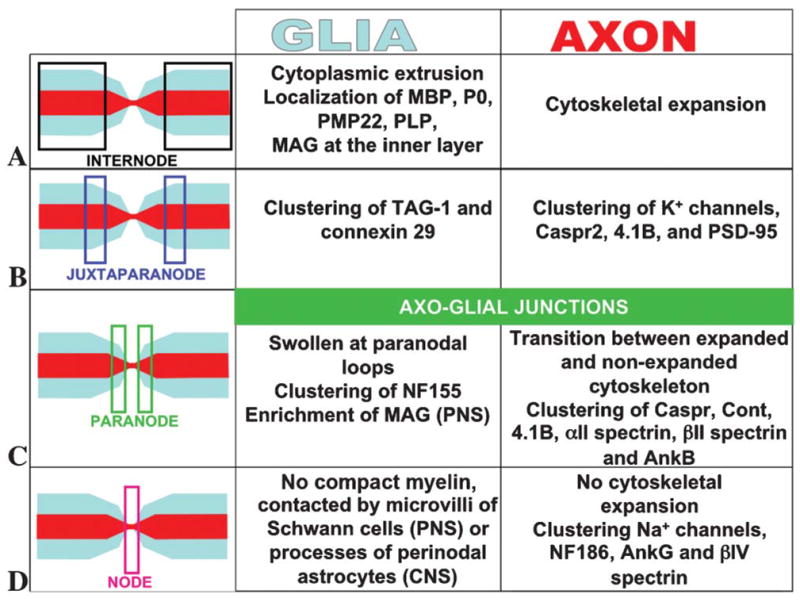
(A) In the internodal region the glial cytoplasm is extruded, which leads to compaction of the myelin sheath and remarkable expansion of the cytoskeleton in some axons. (B) In the juxtaparanodal region, the specific glial and axonal components cluster during myelination and establish an axon–glial scaffold with no specialized ultrastructure. (C) In the paranodal region, local clustering of glial NF155 and axonal Caspr, Cont, 4.1B, αII and βII spectrin, and ankyrin B results in the establishment of axon–glial junctions. (D) In the nodal region, either Schwann cell microvilli (PNS) or perinodal astrocytes (CNS) interact with nodal axolemmal components. Nodal cytoskeletal proteins NF186, ankyrinG and bIV spectrin coordinate the clustering of the voltage-gated Na+ channels.
The juxtaparanodes form at the region where compaction of the myelin sheath relaxes the inner layer to allow the formation of the paranodal loops. The function of the juxtaparanode is still unclear, but this domain is recognized by clustering of specific proteins such as TAG-1 and connexin-29 on the glial side and K+ channels, Caspr-2, protein 4.1B and PSD-95 on the axonal side (Fig. 2B) (Rasband, 2004).
The paranodal loops provide the region of closest contact between the myelinating glia and the axons. Paranodes are the site of assembly of AGJs, which resemble the invertebrate septate junctions (Einheber et al., 1997; Banerjee et al., 2006). In mouse mutants with disrupted AGJs, the paranodal domain does not form transverse bands and juxtaparanodal proteins on the axonal side localize at the region coordinated with the glial paranodal loops (Dupree et al., 1999; Bhat et al., 2001; Boyle et al., 2001). The consequence is a direct contact between proteins of juxtaparanodes and nodes of Ranvier, which indicates that AGJs normally serve as a ‘fence’ to segregate the axonal domains that flank the nodes of Ranvier (Rios et al., 2003). The molecular components of paranodes that are known are the 155 kDa isoform of neurofascin, NF155, on the glial side and Caspr (also known as Paranodin and NCP1), contactin (Cont), protein 4.1B, αII-spectrin, βII-spectrin and ankyrin-B on the axonal side (Fig. 2C) (Einheber et al., 1997; Menegoz et al., 1997; Bellen et al., 1998; Bhat et al., 2001; Charles et al., 2002; Denisenko-Nehrbass et al., 2003; Garcia-Fresco et al., 2006; Ogawa et al., 2006). Contactin is also synthesized by oligodendrocytes during maturation, but has not been detected on the glial side of the paranodes (Gennarini et al., 1989; Faivre-Sarrailh et al., 1992; Koch et al., 1997).
The node of Ranvier provides the site for clustering of the voltage-gated Na+ and K+ channels for ion exchange (Devaux et al., 2003; Poliak and Peles, 2003; Devaux et al., 2004; Schwarz et al., 2006). In addition to these channels, the main molecular components of the nodes are Nr-CAM and NF186 at the axolemma, and βIV-spectrin and ankyrin-G in the axonal cytoplasm (Fig. 2D) (Poliak and Peles, 2003).
Axon–glial communication
Proper communication between axons and glial cells is a feature of the myelination process. Axons communicate their diameter to glia, which use this information to adjust accordingly the number of myelin wraps, resulting in a highly regulated ratio between axonal diameter and myelin thickness, known as the g ratio. The diameter of a myelinated axon also correlates with the length of the paranode (which correlates with the number of paranodal loops that contact the axons) and the length of the internodes. The mechanisms that dictate the internodal length in myelinated axons are unclear (Friede and Samorajski, 1967; Friede and Bischhausen, 1980; Friede and Bischhausen, 1982).
Analysis of myelin thickness in mice that are deficient in phosphorylation of neurofilaments because of deletion of the phosphorylable isoforms of neurofilament (NF-M, NF-H and NF-M/H) indicated that Schwann cells and oligodendrocytes read different axonal signals for establishment of the g ratio (Elder et al., 2001). In these mutants, the expected g ratio was maintained in CNS axons, whereas it was decreased in PNS axons. This suggests that the CNS axons relay to oligodendrocytes about their diameter after myelination-induced axonal expansion, whereas PNS axons relay to Schwann cells about their future diameter during initial contact or shortly thereafter. Because PNS axons of the above mutants do not undergo the axonal expansion that is regulated normally via phosphorylation of neurofilaments, the myelination thickness is increased in relation to the final axonal diameter, and the g ratio is decreased (Elder et al., 2001). Similar observations have been made in the sciatic nerve of periaxin-null mice that do not undergo significant myelination-induced axonal expansion and have a decreased g ratio (Williams and Brophy, 2002). Recent studies by Nave and colleagues have demonstrated that it is possible to alter the g ratio in the PNS through manipulating expression of Nrg1 type III in transgenic mice. This indicates that these axons communicate their diameter to glia through Nrg1 type III on the axolemma. At the glial membrane, ErbB2 binding to Nrg1 type III triggers signaling for glial extension into wrapping layers (Michailov et al., 2004). These studies indicate that CNS and PNS glia might receive axonal signaling for regulation of the g ratio at different stages during myelination. However, it is generally thought that myelinating glia perceive axonal diameter at the initial stages of myelination, because a minimum initial diameter is required for axons to be myelinated.
By contrast, axons rearrange their cytoskeleton in response to glial signaling. Myelination, thus, triggers a second expansion of axonal diameter (Windebank et al., 1985). Whereas the total number of neurofilaments grossly dictates the diameter of unmyelinated axons, the myelination-induced axonal expansion maximizes the initial diameter of axons by modulating the spacing between neurofilaments through phosphorylation (Mata et al., 1992; Hsieh et al., 1994b). Griffin and colleagues have developed a methodology based on electron micrographic data to quantify the levels of neurofilament packing in PNS axons through a parameter that the authors named nearest neighbour neurofilament distance (NNND). The NNND value indicates the spacing between orderly distributed neurofilaments and correlates nicely with the phosphorylation levels of neurofilaments and the diameter of normal myelinated axons (Hsieh et al., 1994a; Hsieh et al., 1994b; Lunn et al., 2002).
A key glial component in the signaling pathway of myelination-induced axonal expansion is myelin associated glycoprotein (MAG) (Yin et al., 1998). MAG localizes to the periaxonal membranes of glial internodes in both CNS and PNS. In the PNS, MAG is enriched at the paranodal loops of mature Schwann cells (Trapp et al., 1989). Cleveland and colleagues have analyzed mouse mutants that lack phosphorylation sites at the C-terminal of neurofilaments and proposed a model in which axonal expansion triggered by myelination starts with MAG-dependent signaling and, ultimately, targets phosphorylation of neurofilament-M via p75NTR-dependent activation of cyclin-dependent kinase 5 (cdk-5) and/or ERK1/2 (Garcia et al., 2003). Moreover, cdk5 mediates phosphorylation of important cytoskeletal proteins in axons such as tau and MAP1B, and, consequently, regulates the attachment of these proteins to microtubules, which modulates rates of axonal transport (Matsushita et al., 1996; Wada et al., 1998; Bu et al., 2002; Dashiell et al., 2002; Kawauchi et al., 2005).
Alternating axonal diameter: an Achilles’ heel of myelinated axons
Axonal expansion caused by phosphorylation of neurofilaments is reversible, thus, axons decrease their internodal diameter upon demyelination and expand again after remyelination. Consistent with this observation, axons do not undergo myelination-induced expansion in unmyelinated segments like at the nodes of Ranvier, where neurofilaments remain unphosphorylated and, hence, tightly packed (Hsieh et al., 1994b; Sanchez et al., 1996). Therefore, because of the axon–glial communication, some myelinated axons present alternating diameter along their length. Here we argue that this alternating pattern is a key contributor to the axonal dependence on the integrity of the axon–glial interactions after myelination (Fig. 1B).
While still unmyelinated, the diameter of the axons tends to be uniform along their length, which provides a linear track for axonal transport. After myelination, some axons display remarkably uneven diameter, with long segments of spaced phosphoneurofilaments interrupted by short segments of packed, non-phosphorylated neurofilaments (Fig. 1B). Alternation of diameter along these axons generates points of retention for axonal transport (i.e. nodal and paranodal regions present reduced rates of axonal transport and increased accumulation of organelles when compared with internodes) (reviewed in Salzer, 2003). Finally, one might picture the paranode as an Achilles’ heel in these myelinated axons, where the creation of an alternating pattern for axonal diameter might generate regions of potential complications in axonal transport. Does myelination provide an adaptation mechanism to ensure smooth transport at each change in axonal diameter?
Cytoskeletal transition at the paranodes
Although perhaps unconventional, we refer to paranodes as the region of transition between alternations in diameter of myelinated axons. In axons that undergo alternating pattern, the paranodal cytoskeleton tapers as it approaches the nodes of Ranvier; and we suggest that this transition between expanded and nonexpanded cytoskeleton is maintained actively by paranodal modulation of local cytoskeleton (Figs. 1B and 2). The finding that AGJs are linked to axonal cytoskeleton through the Caspr-dependent recruitment of a paranodal complex with protein 4.1B, αII-spectrin, βII-spectrin, ankyrin B and actin supports this idea (Gollan et al., 2002; Denisenko-Nehrbass et al., 2003; Garcia-Fresco et al., 2006; Ogawa et al., 2006). CGT and Caspr mutants, which are deficient in proteins that are synthesized in glia and axons, respectively, exhibit disrupted AGJs stemming from very different mechanisms (Coetzee et al., 1996; Bhat et al., 2001). The study of these mutants reveals a previously unappreciated function for the Caspr-mediated link between AGJs and the axonal cytoskeleton in the maintenance of the cytoskeletal organization as well as axonal transport at the paranodal region. Disruption of this link leads to the development of axonal swellings at the paranodal region of the Purkinje axons (Fig. 3B). CGT- and Caspr-mutants exhibit normal myelination-induced expansion of the axonal cytoskeleton, but do not form the paranodal transverse bands. These mice, thus, provide a model to selectively study the role of AGJs in the intrinsic transition between expanded and nonexpanded cytoskeleton that normally takes place in the paranodal region (Fig. 3A). Ultrastructural analysis revealed that the paranodal regions in CGT and Caspr mutants are disorganized severely in their local cytoskeleton, as depicted in Fig. 2B. It is, thus, appealing to propose that the AGJ link with the cytoskeleton stabilizes the transition between expanded and nonexpanded cytoskeletons along the myelinated axons.
Fig. 3. Model for cytoskeletal transition at the paranodes.
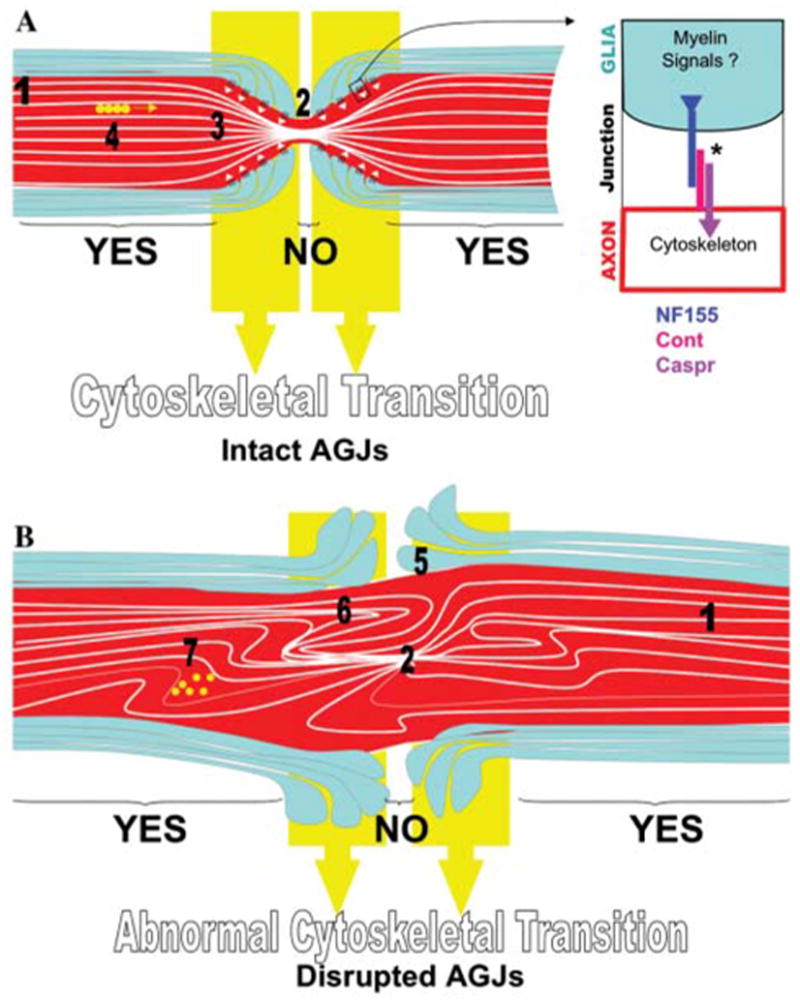
(A) Myelination induces phosphorylation of neurofilaments and increases the spacing between neurofilaments at the internodes (1), but not at the nodes of Ranvier where neurofilaments are more tightly packed (2). This periodic signaling along myelinated axons creates what we call the alternating pattern for axonal diameter. The transition between expanded and nonexpanded cytoskeleton happens at the paranodal region (3), where AGJs (white arrowheads) have a role in maintaining this smooth transition, ensuring organization of tracks for axonal transport (4). The inset shows the known molecular components of AGJs that link to the axonal cytoskeleton. Although Caspr is delivered to the paranodal axolemma only when associated with Cont, this association has been shown to inhibit Cont binding to NF155 (Charles et al., 2002; Gollan et al., 2003). * indicates that the association of Caspr to NF155/Cont might be mediated by other proteins. Yellow circles represent organelles that are transported along microtubules. (B) If AGJs are disrupted and the signaling for cytoskeletal expansion at the internodes is still intact, the axonal diameter undergoes alternating pattern (1 and 2) but the transition of diameters is not modulated by AGJs (5). In the absence of proper transition, expanded cytoskeleton invades regions of nonexpanded cytoskeleton, and vice-versa, causing cytoskeletal disorganization at the paranodal region (6) and generation of a site for impairment in transport (7) and potential formation of swellings.
The mechanisms and signaling cascades that direct the cytoskeleton transition at the paranodes are largely unknown. Does the AGJ/microfilament complex link the microtubules and/or neurofilaments to the axolemma? Are the paranodes sites for modifications of neurofilaments that promote a decrease in neurofilament spacing, such as dephosphorylation of C-terminal neurofilament sidearms or phosphorylation of the neurofilament N-terminal? Are paranodes sites for regulation of proteins that crosslink microtubules and neurofilaments? Although we do not have answers for these questions, the model proposed here suggests that they are relevant and need to be addressed. We propose that the AGJs actively modulate the cytoskeletal organization beneath the paranodes. This would ensure the spatial segregation and a smooth transition between expanded internodal and non-expanded nodal cytoskeletons.
Although the molecular link between AGJs and the cytoskeleton and its implications on axonal transport have been reported only recently, earlier studies by Griffin and others using the neurotoxin β,β′-iminodipropionitrile (IDPN) pioneered the correlation between paranodal integrity and disruption in axonal transport (Griffin et al., 1984; Griffin et al., 1987; Parhad et al., 1987; Griffin and Sheikh, 1999). It was demonstrated that impairments of axonal transport of neurofilaments induced by IDPN leads to the formation of neurofilament-enriched swellings and to a passive paranodal demyelination, with displacement of the paranodal loops towards the internodes (Griffin et al., 1987). These results are consistent with our model and indicate that the link between AGJs and the paranodal cytoskeleton is sufficiently strong to create interdependence. In other words, disorganization of the paranodal cytoskeleton leads to mechanical displacement of myelin loops through their link with the AGJs.
IDPN-induced paranodal swellings indicate that paranodes provide a cytoskeletal track that is highly susceptible to complications in axonal transport, which is consistent with the idea of a cytoskeletal transition at the paranodes. However, these experiments did not rule out the possibility that mechanical displacement of AGJs insults the axolemma, which might be the major cause of the formation of paranodal swellings. Trauma induced in myelinated axons is reported to lead to rapid formation of swellings at the nodes of Ranvier (nodal blebs), the formation of which has been attributed to Ca2+ influx into the axon through the axolemma (Maxwell et al., 1991; Maxwell and Graham, 1997). Other studies support the hypothesis that mechanical disruptions of the axolemma lead to formation of axonal swellings through influx of free Ca2+ and local activation of Ca2+-dependent enzymes, such as proteases (Fitzpatrick et al., 1998). The development of paranodal swellings in CGT- and Caspr-mutants, where AGJs do not form and no detectable paranodal demyelination is observed, indicate a causal link between the formation of AGJs and the organization of paranodal cytoskeleton (Garcia-Fresco et al., 2006).
Paranodal swellings in CGT and Caspr mice are observed only in a limited range of myelinated axon (i.e. axons of Purkinje neurons). This indicates that other compensatory mechanisms act to stabilize the cytoskeletal transition at the paranodes in the absence of AGJs in other neurons. AGJ disruption also leads to accumulation of mitochondria at the nodal region in older Caspr mutants (Einheber et al., in press). The presence of a basal lamina in the PNS nerves might physically protect the paranodes from developing swellings and, thus, might cause the accumulation of axonal transport cargo instead at the nodes. Because mitochondria are peculiar in the way they are transported through the axons with high rates of releases from the molecular motors, they might be more likely to be trapped on the disorganized paranodal cytoskeleton than other organelles (Einheber et al., in press).
Other possible mediators of cytoskeletal transition at the paranodes
AGJs are unlikely to be the sole mediators of cytoskeletal transition at the paranodes. PLP/DM20-null and mosaic mice develop paranodal swellings and myelination-induced axonal expansion but AGJs form normally in these mice (Griffiths et al., 1998). This observation indicates that isoforms of PLP might also affect paranodal cytoskeleton through unknown mechanisms.
Paranodal demyelination with the formation of swellings is also a feature of giant axonal neuropathy (GAN), an autosomal recessive disorder caused by mutations in the protein gigaxonin that interferes with axonal transport. In vitro, gigaxonin regulates microtubule stability by binding and inducing degradation of the microtubule-associated protein 1B (MAP1B) (Allen et al., 2005). Mutations in gigaxonin disrupt interaction between gigaxonin and MAP1B (Ding et al., 2002). Interestingly, MAP1B mediates cross talk between microtubules and actin filaments in axons. Cdk5 phosphorylates MAP1B and the phosphorylated form of MAP1B was reported recently to be enriched at the paranodes (Soares et al., 2002).
It is tempting to speculate that MAP1B has an additional role in the paranodal cytoskeletal transition. Most importantly, is the phosphorylation of MAP1B at the paranodes, a downstream event of MAG signaling triggered during myelination? Ablation of MAP1B leads to delayed myelination and myelination-induced axonal expansion (Takei et al., 1997). MAP1B localizes at neuronal membranes and immuno-precipitates with MAG; moreover, MAP1B phosphorylation increases when neurons are cocultured with cells that express MAG (Tanner et al., 2000; Franzen et al., 2001; Dashiell et al., 2002). Is there a correlation between disruption of gigaxonin binding to MAP1B in GAN and the formation of paranodal swellings? Identification of additional players in the cytoskeletal transition at the paranodes should help to elucidate the mechanisms by which myelination modifies the paranodal cytoskeleton.
Limitations of the cytoskeletal transition model
One of the premises of this model is that the feature of myelination-induced axonal expansion is, in some measure, shared by both the CNS and the PNS axons. At least for the CNS optic nerves, cytoskeletal expansion of the myelinated part of the axons is dependent on oligodendrocyte signaling, and the unmyelinated portion of the same axons does not expand (Sanchez et al., 1996). However, it must be pointed out that the key studies characterizing the differences between internodal and nodal diameter have been performed in the PNS. It is likely that the larger diameter of PNS axons together with the existence of a structurally protective basal lamina facilitates the preservation of local diameter in axonal segments during sample preparation and ultrastructural detection of the alternating pattern in the PNS rather than CNS.
The existence of nodes of Ranvier in CNS myelinated axons was received initially with much skepticism, because morphological features such as the ‘nodal constriction’ were not detected in these CNS myelinated axons. This was later attributed to difficulties in obtaining proper cytological fixation in the CNS because under optimal conditions the ‘nodal constrictions’ are detected in the CNS axons (Bodian, 1951; Nakai, 1954; Pease, 1955). However, CNS axons seem to be morphologically more diverse than PNS axons. Even in optimal tissue preparations, CNS nodes of Ranvier are more permeable than the internodes, which probably led to variability in the morphology and stain of these domains (Bodian, 1951). Another possible explanation for the variability of morphology in the CNS nodes of Ranvier is the fact that they are exposed to different environmental cues in a less patterned way compared to PNS fibers from, for example, the sciatic nerve. External cues modulate the dynamics of actin-based cytoskeleton and lead to different forms of structural plasticity such as synapse formation and axonal branching that influence the shape of the nodes of Ranvier (Rosenstein and Leure-DuPree, 1976; Yiu and He, 2006). This local, actin-based activity might lead to nodal protrusions and contractions and, thus, mask the morphological detection of neurofilament-based alternating pattern in the CNS.
Palay and Chan-Palay have described an anatomical point just before the onset of the myelin sheath as the narrowest portion of the initial segment of the Purkinje axons, where the diameter drops from about 1 μm to 0.5 μm, and expands again beneath the myelinated sheath (Palay and Chan-Palay, 1974), however, we are unaware of studies that show precisely that Purkinje axons indeed undergo alternating pattern. Together these circumstantial observations indicate that some CNS axons, including Purkinje axons, undergo alternating pattern after myelination, but further studies are needed to confirm this assumption.
Predictions of the cytoskeletal transition model
Understanding the mechanisms of degeneration of myelinated axons in demyelinating diseases is relevant to the development of treatments for MS and CMT because axonal degeneration is the major cause of neurological disabilities associated with these diseases (Bjartmar et al., 1999; Bjartmar et al., 2003). Here we present a view of the paranodes as a region of axonal susceptibility to injuries caused by demyelination. Consistent with this view, an early sign of myelin loss in MS is the mislocalization of Caspr and NF155 (Wolswijk and Balesar, 2003; Coman et al., 2006; Howell et al., 2006).
The model of cytoskeletal transition at the paranodes makes several predictions that can be tested and correlated with the available data. Probably the most important prediction is that partial myelination would insult some axons more than complete demyelination. This would be the case for myelinated axons that display alternating pattern in their diameter but have lost the adaptation mechanisms to modulate transition at each alternation. This prediction is consistent with the phenotype of mutants that have myelination-induced axonal expansion but lack proper AGJs, such as Caspr and CGT mutants, which develop paranodal swellings. By contrast, the model predicts that complete abolition of this alternating pattern on axonal diameter would not lead to the development of axonal swellings. This prediction is also consistent with the phenotype of mutants that are deficient in the signaling for phosphorylation of neurofilaments, such as MAG and mice deficient in phosphorylation of neurofilament by truncation or ablation of neurofilament subunits M or H/ M (Yin et al., 1998; Garcia et al., 2003).
A simplified summary of relevant observations is presented in Fig. 4, to conceptualize the predictions of our model. Interpretation of axonal degeneration in most demyelinating diseases and animal models should not be narrowed down to a single general mechanism. We have selected mutants for discussion that display changes in axonal diameter because of changes in neurofilament spacing, and we have analyzed a very specific parameter of axonal damage, the development of paranodal swellings. Studies using NNND as a parameter should be instrumental in better characterizing the intrinsic deficiencies in the alternating pattern generated by partial myelination and to test the model proposed here.
Fig. 4. Correlation between predictions of the cytoskeletal transition model and data from mouse mutants.
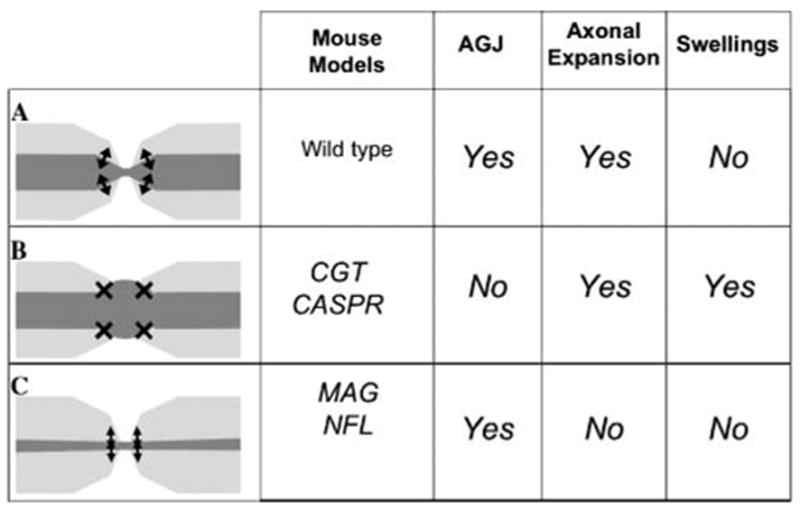
(A) Normal myelinated axons present myelination-induced axonal expansion at the internodes, intact AGJs and proper transition of cytoskeleton at the paranodes. (B) Disruption of AGJs in axons under proper myelination-induced cytoskeletal expansion leads to disorganized transition of cytoskeleton, impairments in axonal transport and the formation of paranodal swellings, which is consistent with the phenotypes of CGT and Caspr mutants (Garcia-Fresco et al., 2006). (C) Disruption of signaling in myelination-induced axonal expansion per se would not lead to formation of paranodal swellings as in the case of MAG and neurofilament mutants (Yin et al., 1998; Elder et al., 2001; Garcia-Fresco et al., 2006). Note that, as discussed for the PLP mutants under the heading ‘Other possible mediators of cytoskeletal transition at the paranodes’, not all the reported paranodal swellings correlate with disruptions of the axon–glial junctions.
Conclusions and remarks
Here, we propose a model in which alternations in axonal diameter induced by myelination generate axonal dependence on the proper cytoskeletal transition at each paranode. An important prediction from this model is that disruptions in AGJs will promote less damage if axons do not present the alternating pattern in their diameter. One might wonder whether the development of paranodal swellings in mice with disrupted AGJs (CGT and Caspr) would be attenuated in a mouse background in which phosphorylation of neurofilaments induced by myelination is abolished (neurofilament-M mutants). In addition, this raises the hypothesis that drugs that disrupt phosphorylation of neurofilament-M specifically might have the potential to either attenuate or partially prevent paranodal swellings that are promoted by disruption in the AGJs. Finally, the model for cytoskeletal transition at the paranodes provides a novel perspective to speculate on the role of axon–glial interactions beyond the membrane specializations, down into the core of the specialized axonal cytoskeleton.
Acknowledgments
We thank E. Anton, H. Bellen, J. Dupree, A. Fanning, B. Philpot, R. Petralia and M. Rasband for helpful comments on this manuscript. We also thank anonymous reviewers for their insightful suggestions and comments which led to an improved manuscript. Research in our laboratory is supported by grants from the NIH (GM63074 and NS050356) and by funds from the State of North Carolina.
References
- Allen E, Ding J, Wang W, Pramanik S, Chou J, Yau V, et al. Gigaxonin-controlled degradation of MAP1B light chain is critical to neuronal survival. Nature. 2005;438:224–228. doi: 10.1038/nature04256. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochemistry and Cell Biology. 2000;113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochemistry and Biophysics. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Barres BA, Barde Y. Neuronal and glial cell biology. Current Opinion in Neurobiology. 2000;10:642–648. doi: 10.1016/s0959-4388(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin–novel members of the neurexin family: encounters of axons and glia. Trends in Neurosciences. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axoglial junctions. Current Opinion in Neurobiology. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, et al. Axon–glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. Journal of Neurological Science. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. Journal of Neurocytology. 1999;28:383–395. doi: 10.1023/a:1007010205037. [DOI] [PubMed] [Google Scholar]
- Bodian D. A note on nodes of Ranvier in the central nervous system. Journal of Comparative Neurology. 1951;94:475–483. doi: 10.1002/cne.900940309. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. Journal of Neuroscience. 2002;22:6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, et al. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Current Biology. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree JL, Shi R, Blight A, Suzuki K, et al. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Coman I, Aigrot MS, Seilhean D, Reynolds R, Girault JA, Zalk B, et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3186–3195. doi: 10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. Journal of Neuroscience. 2004;24:9250–9260. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiell SM, Tanner SL, Pant HC, Quarles RH. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. Journal of Neurochemistry. 2002;81:1263–1272. doi: 10.1046/j.1471-4159.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, et al. Kv3.1b is a novel component of CNS nodes. Journal of Neuroscience. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. Journal of Neuroscience. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, et al. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. European Journal of Neuroscience. 2003;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Ding J, Liu JJ, Kowal AS, Nardine T, Bhattacharya P, Lee A, et al. Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. Journal of Cell Biology. 2002;158:427–433. doi: 10.1083/jcb.200202055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. Journal of Cell Biology. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JM, Garbern J. The myelinated axon is dependent on the myelinating cell for support and maintenance: molecules involved. Journal of Neuroscience Research. 2004;76:593–598. doi: 10.1002/jnr.20063. [DOI] [PubMed] [Google Scholar]
- Einheber S, Bhat MA, Salzer JL. Disrupted axo–glial junctions result in accumulation of abnormal mitochondria at the nodes of Ranvier. Neuron Glia Biology. doi: 10.1017/S1740925X06000275. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate–like paranodal junctions that assemble during myelination. Journal of Cell Biology. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Friedrich VL, Jr, Lazzarini RA. Schwann cells and oligodendrocytes read distinct signals in establishing myelin sheath thickness. Journal of Neuroscience Research. 2001;65:493–499. doi: 10.1002/jnr.1179. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/ F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. Journal of Neuroscience. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick MO, Maxwell WL, Graham DI. The role of the axolemma in the initiation of traumatically induced axonal injury. Journal of Neurology, Neurosurgery and Psychiatry. 1998;64:285–287. doi: 10.1136/jnnp.64.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen R, Tanner SL, Dashiell SM, Rottkamp CA, Hammer JA, Quarles RH. Microtubule-associated protein 1B: a neuronal binding partner for myelin-associated glycoprotein. Journal of Cell Biology. 2001;155:893–898. doi: 10.1083/jcb.200108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Bischhausen R. The precise geometry of large internodes. Journal of Neurological Science. 1980;48:367–381. doi: 10.1016/0022-510x(80)90109-4. [DOI] [PubMed] [Google Scholar]
- Friede RL, Bischhausen R. How are sheath dimensions affected by axon caliber and internode length? Brain Research. 1982;235:335–350. doi: 10.1016/0006-8993(82)91012-5. [DOI] [PubMed] [Google Scholar]
- Friede RL, Samorajski T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. Journal of Comparative Neurology. 1967;130:223–231. doi: 10.1002/cne.901300304. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, et al. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proceedings of the National Academy of Sciences of the USA. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ML, Lobsiger CS, Shah SB, Deerinck TJ, Crum J, Young D, et al. NF-M is an essential target for the myelin-directed ‘‘outside-in’’ signaling cascade that mediates radial axonal growth. Journal of Cell Biology. 2003;163:1011–1020. doi: 10.1083/jcb.200308159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G, Cibelli G, Rougon G, Mattei M-G, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidy-linositol-anchored member of the immunoglobulin superfamily related to chicken contactin. Journal of Cell Biology. 1989;109:775–778. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. Journal of Cell Biology. 2002;157:1247–1256. doi: 10.1083/jcb.200203050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. Journal of Cell Biology. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Anthony DC, Fahnestock KE, Hoffman PN, Graham DG. 3,4-Dimethyl-2,5-hexanedione impairs the axonal transport of neurofilament proteins. Journal of Neuroscience. 1984;4:1516–1526. doi: 10.1523/JNEUROSCI.04-06-01516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Drucker N, Gold BG, Rosenfeld J, Benzaquen M, Charnas LR, et al. Schwann cell proliferation and migration during paranodal demyelination. Journal of Neuroscience. 1987;7:682–699. doi: 10.1523/JNEUROSCI.07-03-00682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Sheikh K. Schwann cell-axon interactions in Charcot-Marie-Tooth disease. Annals of the New York Academy of Sciences. 1999;883:77–90. [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, et al. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3173–3185. doi: 10.1093/brain/awl290. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Crawford TO, Griffin JW. Neurofilament distribution and organization in the myelinated axons of the peripheral nervous system. Brain Research. 1994a;642:316–326. doi: 10.1016/0006-8993(94)90937-7. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Kidd GJ, Crawford TO, Xu Z, Lin WM, Trapp BD, et al. Regional modulation of neurofilament organization by myelination in normal axons. Journal of Neuroscience. 1994b;14:6392–6401. doi: 10.1523/JNEUROSCI.14-11-06392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochemical and Biophysical Research Communications. 2005;331:50–55. doi: 10.1016/j.bbrc.2005.03.132. [DOI] [PubMed] [Google Scholar]
- Koch T, Brugger T, Bach A, Gennarini G, Trotter J. Expression of the immunoglobulin superfamily cell adhesion molecule F3 by oligodendrocyte-lineage cells. Glia. 1997;19:199–212. doi: 10.1002/(sici)1098-1136(199703)19:3<199::aid-glia3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hoh JH. Modulation of repulsive forces between neurofilaments by sidearm phosphorylation. Biochemical and Biophysical Research Communications. 2004;324:489–496. doi: 10.1016/j.bbrc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- Lunn MP, Crawford TO, Hughes RA, Griffin JW, Sheikh KA. Anti-myelin-associated glycoprotein antibodies alter neurofilament spacing. Brain. 2002;125:904–911. doi: 10.1093/brain/awf072. [DOI] [PubMed] [Google Scholar]
- Mata M, Kupina N, Fink DJ. Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier. Journal of Neurocytology. 1992;21:199–210. doi: 10.1007/BF01194978. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Lu YF, Moriwaki A, Tokuda M, Itano T, et al. Distinct cellular compartment of cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activator protein (p35nck5a) in the developing rat cerebellum. Brain Research. 1996;734:319–322. [PubMed] [Google Scholar]
- Maxwell WL, Graham DI. Loss of axonal microtubules and neurofilaments after stretch-injury to guinea pig optic nerve fibers. Journal of Neurotrauma. 1997;14:603–614. doi: 10.1089/neu.1997.14.603. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Irvine A, Graham, Adams JH, Gennarelli TA, Tipperman R, et al. Focal axonal injury: the early axonal response to stretch. Journal of Neurocytology. 1991;20:157–164. doi: 10.1007/BF01186989. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, et al. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Nakai J. The osmic acid injection method for demonstrating nodes in the central nervous system. The Anatomical Record. 1954;119:267–273. doi: 10.1002/ar.1091190209. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. Journal of Neuroscience. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay VC, editors. Cytology and organization. Springer; 1974. Cerebellar cortex. [Google Scholar]
- Parhad IM, Clark AW, Griffin JW. Effect of changes in neurofilament content on caliber of small axons: the beta, beta’-iminodipropionitrile model. Journal of Neuroscience. 1987;7:2256–2263. doi: 10.1523/JNEUROSCI.07-07-02256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease DC. Nodes of Ranvier in the central nervous system. Journal of Comparative Neurology. 1955;103:11–15. doi: 10.1002/cne.901030103. [DOI] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Current Opinion in Neurobiology. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Rasband MN. It’s ‘‘juxta’’ potassium channel! Journal of Neuroscience Research. 2004;76:749–757. doi: 10.1002/jnr.20073. [DOI] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, et al. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. Journal of Neuroscience. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM, Leure-DuPree AE. Electron microscopic observations of nodes of Ranvier in the external cuneate nucleus. Journal of Comparative Neurology. 1976;170:461–483. doi: 10.1002/cne.901700406. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. Journal of Neuroscience. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao T-C, Nodera H, Tabuena D, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. Journal of Physiology. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Soares S, von Boxberg Y, Lombard MC, Ravaille-Veron M, Fischer I, Eyer J, et al. Phosphorylated MAP1B is induced in central sprouting of primary afferents in response to peripheral injury but not in response to rhizotomy. European Journal of Neuroscience. 2002;16:593–606. doi: 10.1046/j.1460-9568.2002.02126.x. [DOI] [PubMed] [Google Scholar]
- Takei Y, Kondo S, Harada A, Inomata S, Noda T, Hirokawa N. Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene. Journal of Cell Biology. 1997;137:1615–1626. doi: 10.1083/jcb.137.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner SL, Franzen R, Jaffe H, Quarles RH. Evidence for expression of some microtubule-associated protein 1B in neurons as a plasma membrane glycoprotein. Journal of Neurochemistry. 2000;75:553–562. doi: 10.1046/j.1471-4159.2000.0750553.x. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Cootauco C, Quarles R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. Journal of Cell Biology. 1989;109:2417–2426. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Ishiguro K, Itoh TJ, Uchida T, Hotani H, Saito T, et al. Microtubule-stimulated phosphorylation of tau at Ser202 and Thr205 by cdk5 decreases its microtubule nucleation activity. Journal of Biochemistry (Tokyo) 1998;124:738–746. doi: 10.1093/oxfordjournals.jbchem.a022174. [DOI] [PubMed] [Google Scholar]
- Williams AC, Brophy PJ. The function of the Periaxin gene during nerve repair in a model of CMT4F. Journal of Anatomy. 2002;200:323–330. doi: 10.1046/j.1469-7580.2002.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank AJ, Wood P, Bunge RP, Dyck PJ. Myelination determines the caliber of dorsal root ganglion neurons in culture. Journal of Neuroscience. 1985;5:1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt A, Brady ST. Unwrapping new layers of complexity in axon/glial relationships. Glia. 2000;29:112–117. doi: 10.1002/(sici)1098-1136(20000115)29:2<112::aid-glia3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, et al. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. Journal of Neuroscience. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nature Reviews Neuroscience. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


