Abstract
Liver repopulation by transplanted normal hepatocytes has been described in a number of experimental settings. Extensive repopulation can also occur from the selective proliferation of endogenous normal hepatocytes, both in experimental animals and in the human liver. This review highlights the intriguing association between clinical and experimental conditions related to liver repopulation and an increased risk for development of hepatocellular carcinoma. It is suggested that any microenvironment that is able to sustain the clonal growth of normal transplanted (or endogenous) hepatocytes is also geared to select for the emergence of rare resistant cells with an altered phenotype. Whereas the first pathway leads to liver repopulation with normal histology, the latter results in the growth of focal proliferative lesions and carries an increased risk of neoplastic disease. The implications of this association are discussed, both in terms of pathogenetic significance and possible therapeutic exploitation.
The liver is the only solid organ that can be efficiently repopulated through transplantation of normal isolated hepatocytes. A number of experimental systems have been developed over the past 10 to 15 years describing this remarkable phenomenon.1,2,3,4,5,6,7,8,9,10,11 The analysis of these systems has revealed that two key requirements must be fulfilled for the process to occur: (1) transplanted cells must be endowed with a growth and/or survival advantage compared with the hepatocytes in the recipient organ, and (2) there must be space for donor-derived cells to expand, in that transplanted cells only divide at the expense of preexisting resident hepatocytes.12 In fact, liver repopulation is a coordinated process of cell replacement, during which no increase in total liver mass is observed, whereas normal transplanted hepatocytes take the lead over the endogenous damaged counterpart. The above paradigm applies to virtually all available models of liver repopulation via transplanted cells, including the albumin-urokinase-plasminogen activator (uPA) transgenic mouse,1,2 the fumaryl-acetoacetate-hydroxylase (Fah)-null mouse,3 the multidrug resistance-2 (mdr-2) knockout mouse,9 the bcl-2-transduced mouse hepatocyte model,8 the retrorsine-treated4,5,6 or monocrotaline-treated11 rat model, and the radiation-treated7 rat models.
An intriguing aspect that has received little attention so far is that most, if not all of these model systems capable of sustaining liver repopulation are also associated with an increased risk of cancer development in the liver (Table 1).12,13 Such increased risk is inherent to these model systems, ie, it is independent of cell transplantation. Furthermore, the human diseases related to some of these animals models, including hypertyrosinemia (modeled by the Fah-null mouse) and familial intrahepatic cholestasis (modeled by the mdr-2 knockout mouse), are also burdened with an increased risk of hepatocellular carcinoma, developing at childhood age.14,15 Is this a pure chance phenomenon, or does it suggest the existence of a fundamental linkage between the two processes, two sides of the same coin per se? In this review, the evidence for this intriguing association will be examined, and its possible biological significance and clinical implications will be discussed.
Table 1.
Animal Models of Liver Repopulation Associated with Increased Risk of Liver Cancer Development
Models for Liver Repopulation and Increased Risk of Cancer
(i) The Albumin-Urokinase-Plasminogen Activator (uPA) Transgenic Mouse
The uPA transgenic mouse model was the first to be associated with massive liver repopulation.1,2 In this animal, targeted expression of uPA leads to chronic hepatocyte toxicity. However, it was observed that rare hepatocytes losing the transgene can selectively proliferate and regenerate the entire organ.1 Based on this finding, hepatocytes isolated from a congenic donor were then transplanted into the uPA mouse and tested for their ability to selectively proliferate in the host liver. Four to five weeks later, up to 80% of hepatocytes in the recipient liver were found to be of donor origin,2 thereby confirming that the constitutive expression of the uPA transgene in resident hepatocytes generates a selective environment that favors the growth of cells with a normal (nontransgenic) phenotype. The uPA transgenic mouse is one of the most widely used systems for liver repopulation; in more recent years, human hepatocytes have been transplanted into a double mutant severe combined immune deficient/uPA transgenic mouse, leading to extensive replacement of the mouse liver by human cells.16
As early as 1992, Sandgren et al17 reported the development of liver cancer in the uPA transgenic mouse, with over 70% incidence of adenoma and carcinoma. Interestingly, all cancers were derived from endogenous hepatocytes that had lost the uPA transgene, thereby gaining a growth advantage compared with uPA-expressing neighboring cells. The authors proposed that extended segments of genomic DNA were probably lost in these cells together with the transgene, and this could explain both their growth advantage and their altered phenotype, causing their high propensity to progress to cancer.17 Furthermore, mitogenesis per se was excluded as a driving force for carcinogenesis in this system, in that several regenerative nodules with a normal phenotype were also observed in uPA-transgenic mice.17
Thus, at least two types of rare hepatocytes can be generated in the uPA transgenic livers following loss of the transgene: (1) hepatocytes that are seemingly normal and can participate in the process of liver repopulation, resulting in normal tissue architecture, and (2) phenotypically altered hepatocytes that can represent the site of origin of focal lesions, liver nodules and hepatocellular carcinoma. The important issue here is that both cell types and both processes (ie, liver repopulation with normal histology and cancer development) appear to be driven by the same basic mechanism: the selective emergence of hepatocytes that have lost the uPA transgene and that are stimulated to clonally expand because of the continuous death of uPA-expressing damaged hepatocytes. To our knowledge, no liver tumors have been reported arising from normal transplanted hepatocytes in this system; however, when mice carrying the uPA transgene and lacking recombination activation gene 2 knockout were transplanted with woodchuck hepatitis virus-infected hepatocytes, chronic viral infection and hepatocellular carcinoma were observed after several months18
(ii) The Fumaryl-Acetoacetate Hydrolase (Fah)-null Mouse and Human Hereditary Tyrosinemia
A general principle similar to the one discussed for the uPA transgenic mouse applies to the Fah-null mouse model of liver repopulation.3 The Fah-deficient mouse serves as a model for hereditary tyrosinemia type I in humans. In both humans and mice, the lack of Fah enzyme, which is involved in the tyrosine catabolic pathway, leads to accumulation of its substrate, fumaryl-acetoacetate and its precursor maleyl-acetoacetate. Both fumaryl-acetoacetate and maleyl-acetoacetate are thought to be involved in liver toxicity, which is found in tyrosinemia type I patients and includes progressive liver failure and development of hepatocellular carcinoma (HCC) early in life.19 Using the mouse model of Fah deficiency, Grompe and colleagues3 demonstrated that normal hepatocytes transplanted in the these animals were able to replace >80% of host liver. However, if transplanted Fah-deficient mice were exposed to the drug 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione, which limits the accumulation of toxic metabolites fumaryl-acetoacetate and maleyl-acetoacetate in host cells, no selective growth advantage for donor-derived hepatocytes was observed.3
Nontreated Fah-deficient mice die within 6 weeks from fulminant hepatic failure. However, if the animals were either given 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione or transfected with an adenoviral vector carrying the Fah gene, they could survive for several months.20,21 Interestingly, 50% (3 of 6) of surviving mice developed HCC, whereas no liver cancer was seen in 9 age-matched heterozygous controls,21 indicating that the Fah-null background is conducive to neoplastic development in the liver. Similar to the u-PA transgenic mouse model, no evidence of neoplastic lesions arising from transplanted normal hepatocytes has been reported in the Fah-deficient mouse. In fact, serial transplantation studies conducted in this animal model revealed that up to six serial passages could be performed from donor to recipient, with preservation of repopulating capacity and in the absence of neoplastic transformation.22 The latter finding again points out that mitogenesis per se is not sufficient for cancer development.
As already mentioned, the Fah-null mouse is a model for human hereditary tyrosinemia type I, and several analogies are present between the two systems. Patients with this disease survive to early childhood without treatment, but they develop chronic liver disease and liver cancer at a very young age.14 Interestingly, livers from some of these patients were also reported to harbor clones of seemingly normal hepatocytes expressing the Fah enzyme and resulting from self-induced correction of the genetic defect.23 Such clones gradually expand, display a normal histological appearance, and can replace up to 85% of the Fah-deficient human liver. Notably, the extent of repopulation by reverted, Fah-positive hepatocytes was reported to be inversely correlated with the clinical severity of the disease in these patients.19
In summary, the cytotoxic microenvironment of Fah-deficient livers sustains the selective growth of phenotypically normal hepatocytes, leading to extensive repopulation, both in human patients23 and in experimental animals3; on the other hand, the same microenvironment can also foster the expansion of altered hepatocytes resistant to cytotoxicity, leading to the emergence of dysplastic nodules and the development of HCC in young patients,14 as well as in the corresponding experimental animal model.20,21
(iii) The Mouse Model of Progressive Familial Intrahepatic Cholestasis
An interesting variant to the basic paradigm described for the uPA-transgenic and the Fah-null mice is illustrated by the mouse model of human progressive familial intrahepatic cholestasis.9 These animals lack the mdr 2 carrier protein and are therefore unable to secrete phospholipids into bile, leaving unopposed the cytotoxic detergent action of bile salts. Hepatocyte damage is confined to periportal areas, which are most active in bile salt uptake and metabolism. Elegant studies by De Vree et al9 have indicated that the liver of mdr 2-deficient mice can be repopulated via transplantation of normal hepatocytes. However, repopulated areas are confined to periportal regions, where liver toxicity is expressed. The process of repopulation is self-limited and tightly controlled: if animals are fed bile salts in the diet, this causes an expansion in the areas of liver damage and a parallel increase in the extent of repopulation by transplanted, mdr 2-expressing normal cells. However, withdrawal of the bile salt-containing diet results in a relative decrease in repopulated areas.9
Relevant to the present discussion, nontransplanted mdr 2-deficient mouse liver is highly prone to developing hepatocellular carcinoma.24 Most importantly, children affected by progressive familial intrahepatic cholestasis also incur a high risk for the development of liver cancer.15 Thus, the analysis of this model system again reinforces the notion that endogenous conditions in the liver resulting in the selective growth of transplanted normal hepatocytes are also associated with an increased risk for neoplastic disease in the absence of cell transplantation.
(iv) The Retrorsine-Based Rat Model for Hepatocyte Transplantation and Liver Repopulation
Animal models of liver repopulation described thus far rely on specific “genetic” backgrounds of the recipient liver such that the resulting metabolic toxicity on resident hepatocytes translates into a growth advantage for phenotypically normal transplanted cells and/or endogenous altered hepatocytes with a presumptive “resistant” phenotype.12
In our laboratory we have used an alternative approach to develop a more general strategy for effective liver repopulation. In this system, donor hepatocytes are transplanted into a normal host liver in which endogenous growth capacity has been hampered by a preconditioning treatment.5,6 Briefly, recipient animals are pretreated with retrorsine (RS), a naturally occurring pyrrolidizine alkaloid that is able to exert a long-lasting block on the endogenous hepatocyte cell cycle. Under these conditions, normal hepatocytes, transplanted after the alkaloid has been metabolized, can selectively respond to growth stimuli and gradually repopulate the entire liver. In fact, >90% repopulation of the RS-treated liver is observed after 2 to 8 months post-transplantation, depending on the intensity of the growth stimulus applied.5,6 Transplanted cells integrate into the recipient parenchyma, and the repopulated liver appears structurally normal. It is also important to note that no increase in total liver mass is observed at any point in time during the process of liver repopulation; rather, the transplanted cells gradually expand and slowly replace the unresponsive, RS-exposed endogenous cells.5,6 Other models of liver repopulation in the rat are based on a similar preconditioning principle, including preexposure to radiation7 or to monocrotaline,11 a pyrrolizidine alkaloid related to RS.
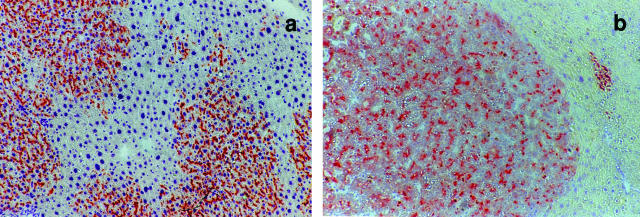
The original observations obtained using normal hepatocyte transplantation have been extended to hepatocytes isolated from preneoplastic liver nodules as the donor cell population.25 Recipient animals pretreated with the same RS-based protocol and then injected with a nodular hepatocyte cell suspension indicate that the growth-constrained microenvironment imposed by RS on endogenous hepatocytes can sustain the selective expansion of transplanted nodular cells, resulting in the emergence of liver nodules and subsequent progression to HCC (Figure 1). By contrast, it is important to note that neither normal nor nodular hepatocytes did proliferate to any significant extent on transplantation into untreated normal hosts.25
Figure 1.
Normal (a) or nodular (b) wild-type rat hepatocytes transplanted into the liver of dipeptidylpeptidase type IV-deficient, syngenic hosts. Tissue samples were stained for the expression of dipeptidylpeptidase type-IV enzyme (rust-orange color). Note the diffuse, infiltrative growth pattern of normal hepatocytes in a, contrasted by the sharp demarcation between nodular and surrounding hepatocytes in b.
Thus, the experimental findings obtained with the RS model provide compelling evidence that the same liver microenvironment that is permissive for the selective growth of normal transplanted hepatocytes also has the capacity to stimulate the clonal expansion of altered/nodular hepatocytes and set the stage for their progression toward neoplasia. Interestingly, animals treated according to the RS protocol for liver repopulation but in the absence of hepatocyte transplantation survive for over 2 years in good health with no overt cancer developing in their livers; however, hepatic nodules were present in 6 of 8 animals (S. Doratiotto and E. Laconi, unpublished observation).
(v) Preconditioning with Radiation
Guha et al7 were the first to report that exposure to radiation could prime the liver to massive endogenous hepatocyte replacement via transplantation of normal isolated cells. This finding was later confirmed and extended by Malhi et al,26 who combined radiation with ischemia-reperfusion, followed by hepatocyte transplantation. The above findings suggest that preconditioning with radiation induces critical changes in the liver microenvironment that are conducive to the selective proliferation of normal, transplanted hepatocytes. The precise mechanisms underlying this phenomenon have yet to be elucidated. Radiation is known to inhibit liver growth for extended periods of time;27 thus, its effect would appear to be similar, at least in principle, to that exerted by RS, ie, a persistent block on endogenous hepatocyte cell cycle.
On the other hand, exposure to radiation increases the risk of cancer development in the liver, via a mechanism consistent with a promoting effect,28 although its initiating potential appears to be weaker.29 Interestingly, this very closely parallels similar results that have been reported for pyrrolizidine alkaloids related to RS,30 suggesting that these two types of exposure (ie, radiation and pyrrolizidine alkaloids) may in fact share basic common mechanisms of action, at least in relation to carcinogenesis and liver repopulation.
Why are Liver Repopulation and Carcinogenesis Associated?
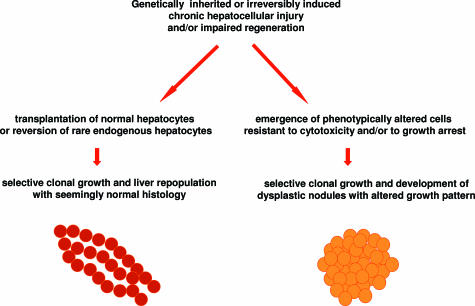
Taken together, the evidence presented indicates that conditions that are permissive for liver repopulation by normal hepatocytes are consistently and inherently associated with an increased risk of cancer development in the liver (Table 1). This association is intriguing and leads one to consider that these two processes may share common basic biological mechanisms; ie, the clonal growth of normal and altered/preneoplastic hepatocytes appears to be sustained by similar driving forces (Figure 2).
Figure 2.
Proposed shared mechanisms for liver repopulation and carcinogenesis.
This concept is best illustrated by data obtained using the RS-based model for cell transplantation. To our knowledge, this is the only experimental system in which the fate of both normal and nodular transplanted hepatocytes has been analyzed and compared, with unequivocal results. The growth behavior of transplanted normal and nodular cells is very similar, in that both cell types can clonally expand when transplanted into RS-treated host liver, whereas neither cell population is able to grow significantly on transplantation into a normal, untreated recipient.5,6,25
These findings, together with those discussed above for other models, suggest a series of important considerations. First, in these systems of liver repopulation, the microenvironment generates specific growth stimuli that are capable of sustaining the clonal expansion of both normal and altered/nodular hepatocytes. Such stimuli result from an altered turnover of endogenous hepatocytes; accordingly, they are very low in the normal liver. Incidentally, this points to a critical role of an altered tissue environment in the emergence of early focal lesions during cancer development.31
Second, altered growth pattern,32,33,34 rather than altered growth per se,35 emerges as the hallmark that better defines the phenotypic behavior of normal versus altered/nodular hepatocytes in these systems. In fact, both undergo selective clonal proliferation, but the outcome is different in either case, with one leading to liver repopulation with normal histology when the cell involved is a normal hepatocyte and the other yielding nodular growth and progression toward cancer when the cell of origin is a phenotypically altered hepatocyte (Figures 1 and 2).
Third, the point above also questions the dominant view that altered/nodular hepatocytes should be endowed with a higher-than-normal growth potential to undergo clonal proliferation. Perfectly normal hepatocytes, with a normal growth potential and a regulated cell cycle, behave very similarly under similar experimental conditions, ie, they also generate clones; conversely, altered/nodular hepatocytes do not grow to any significant extent when transplanted into a normal host liver. This reiterates the concept that parameters related to growth regulation per se need not be altered at the early stages of the carcinogenic process.
What are the possible molecular bases sustaining the different phenotypic behavior during clonal expansion of normal versus altered/nodular hepatocytes? Stated another way, why do repopulating normal hepatocytes integrate into the host liver, resulting in a seemingly normal histology, whereas altered cells display a focal growth pattern and eventually expand and compress the surrounding tissue? Broadly speaking, this leads one to consider that parameters related to cell-to-cell and/or cell-to-extracellular matrix communication might be different in the two cell populations.33 Several studies suggest that, in fact, this might be the case.
Thus, a decreased expression of the gap-junction protein connexin 32 (Cx32) was initially reported during experimental carcinogenesis in rat liver.36,37,38 Later, altered expression and/or distribution of Cx32 was also documented in human liver during chronic hepatitis, cirrhosis, and HCC.39,40 In more recent studies, mice lacking Cx32 protein function were found to be more susceptible to developing liver tumors.41,42 Overall, these data support the hypothesis that altered expression and/or regulation of Cx32 occurs and could play a role at very early stages of cancer development in the liver. However, the issue is not completely settled in that it has been difficult to define whether the reported changes in the regulation of Cx32 regulation are a cause or a consequence of focal growth occurring during carcinogenesis. In fact, when hepatic foci were exposed to phenobarbital, a known tumor promoter, the large majority showed high proliferative rate and decreased expression of Cx32. However, on withdrawal of phenobarbital, a high proportion of foci decreased their growth rate (promoter-dependent) and increased Cx32 expression, whereas a few foci retained a high growth rate (promoter-independent) and showed no increase in Cx32 protein levels.37 It would appear from these results that Cx32 down-regulation in early focal lesions is related to their proliferative state and need not reflect an inherent property of altered hepatocytes. Future studies addressing the pattern of Cx32 expression during clonal expansions of either normal or nodular hepatocytes should provide important insights into this issue.
Another membrane component possibly involved in the altered phenotypic behavior of nodular hepatocytes are the integrins, a class of transmembrane proteins that function as major adhesion receptors with extracellular matrix.43 These molecules play key roles in several basic biological processes, including carcinogenesis. Integrins are heterodimers of α and β subunits. The heterodimer α-5-β-1 (IG-5-1), which binds to fibronectin (FN) in the extracellular matrix, is one of the major integrins expressed in the liver, being involved in cell proliferation and cell death. For example, epidermal growth factor receptor signaling is mediated through the uPA receptor, which is linked to IG-5-1/FN and the extracellular regulated kinase pathway.44 Additional data suggest that IG-5-1 ligation by fibronectin might translate into a cell survival signal, whereas up-regulation of IG-5-1 in the absence of fibronectin leads to cell death.45
Expression of IG-5-1 is decreased in human HCC and is further reduced in less differentiated cancers.46 These changes are related to the acquisition of an invasive and metastatic phenotype by cancer cells. Data on the experimental system are limited: however, they do suggest that reduced expression of IG-5-1 might be an early event during cancer development in the liver.47 Interestingly, a recent report has indicated that integrin and extracellular matrix are important regulators of cell engraftment following normal hepatocyte transplantation in the liver,48 supporting the concept that these proteins are indeed key players in cell-to-cell and cell-to-extracellular matrix interactions in the liver.
Within this context, one should also consider the potential role of matrix metalloproteinases (MMPs), a family of enzymes that collectively degrade all components of the extracellular matrix.49 They play fundamental roles in tissue remodeling, inflammation, wound healing, fibrosis, cell migration, and metastasis, among others.50 MMP2, also known as gelatinase A, is expressed in many epithelial tissues, including liver; it is synthesized as a pro-enzyme that becomes activated on proteolytic cleavage. Unlike other MMPs, the activation of MMP2 occurs at the cell surface,51 a feature that confers to this enzyme a central role in cell migration. In the liver, the source of MMP2 is mainly the hepatic stellate cell (HSC).52 However, when activated HSCs are cultured in vitro, only the native pro-MMP2 is secreted in the culture medium. The presence of active MMP2 becomes evident only when HSCs are cocultured with hepatocytes.53 Moreover, fragments of hepatocyte membrane can activate pro-MMP2 secreted by HSCs.53 These findings highlight the complexity and the specificity of MMP2 activation during various processes associated with liver physiology and pathology.54 MMP2 activity is also up-regulated at early time points (within 6 to 12 hours) following partial hepatectomy in rat liver, indicating involvement of this enzyme in matrix remodeling in preparation for hepatocyte cell division.55,56 Furthermore, Koenig et al57 have demonstrated increased MMP2 protein expression at the leading edge of proliferating clusters of normal hepatocytes transplanted into RS-treated rat livers. This finding is strongly suggestive of a role of MMP2 in normal hepatocyte migration and, as an inference, in the process of integration of transplanted hepatocyte clones in the host liver. If this hypothesis is correct, it is possible to envision how altered regulation of MMP2 activation could hamper the capacity of nodular hepatocytes to integrate into the host liver architecture.
The latter proposition could appear as paradoxical, in view of the fact that increased activity of MMPs, including MMP2, has been associated with cancer and metastasis.50 For example, human HCCs reportedly express higher MMP2 levels compared with surrounding tissue, and this parameter correlates with tumor recurrence.58,59 However, it should be noted that acquisition of invasiveness and metastatic potential are late events in cancer progression, and nodular hepatocytes are not endowed with these phenotypic properties. Interestingly, it has been reported that HSCs, the main source of MMP2, are fewer in number in human dysplastic nodules compared with cirrhotic nodules and HCC,60 suggesting that MMP2 availability could be decreased in early cancer precursor lesions. Thus, altered regulation of MMP2 protein is yet another potential component contributing to the focal growth pattern of nodular hepatocytes.
Significance and Future Perspectives
Is the general paradigm derived from the analysis of liver repopulation of broader significance in the context of other experimental or clinical settings? In a recent review, Rudnick and Perlmutter61 suggest that this might be the case for α-1-antitrypsin (α-1AT) deficiency and, possibly, other liver diseases. In α-1AT deficiency, globules of unsecreted protein accumulate in hepatocytes, leading to cell injury, chronic toxicity, and a higher risk for the development of HCC.62 Studies conducted in a mouse model of this disease indicate that hepatocytes loaded with globules are relatively growth suppressed, providing a proliferative advantage for a population of hepatocytes that is relatively devoid of protein globules.63 In fact, the latter population occupies an increasing proportion of the liver as the animal ages, ultimately becoming the site of origin for liver cancer.62 It is noteworthy that these findings have recently been matched with a clinical counterpart: Hadzic et al64 reported that hepatocellular carcinoma arising in a patient with α-1AT deficiency was also devoid of globules, whereas surrounding hepatocytes were loaded with protein deposits. Interestingly, Rudnick et al63 have further suggested that α-1AT deficiency could possibly benefit from therapeutic strategies based on normal hepatocyte transplantation, given that endogenous parenchymal cells are relatively refractory to growth stimuli. If proven, the latter possibility would extend the analogies between α-1AT deficiency and other conditions associated with both liver repopulation and carcinogenesis discussed in the preceding paragraphs.
Along these lines, is any of the above applicable to the clinical condition most commonly associated with the development of liver cancer in humans, ie, liver cirrhosis? Obviously, the complexity of cirrhosis and its different etiologies defy any attempts to make general statements regarding its basic biology. However, one cannot overlook the fact that cirrhotic liver also harbors different types of nodular growths, including those referred to as regenerative nodules, composed of bona fide normal hepatocytes, and those displaying various degrees of dysplastic changes.65,66,67,68 The issue regarding the clonal nature of these lesions is still highly debated.65,66,67,68,69,70 Various authors67,69,70,71 have reported that a significant proportion of bona fide regenerative nodules and most, if not all, dysplastic nodules are clonal in origin. This would indicate that the microenvironment of the cirrhotic liver is able to stimulate the clonal expansion of both seemingly normal and altered/dysplastic hepatocytes, thereby suggesting a basic pathogenetic analogy with the systems described above. Pertinent to this point, it is worth mentioning that cirrhosis is associated with impaired liver regeneration both in humans72,73 and in experimental animals.74,75 Thus, it is reasonable to hypothesize that such a growth-impaired background could foster the selective emergence of rare hepatocytes,76 with either normal or altered phenotype. This is in agreement with the hypothesis of Park et al,60 who proposed that dysplastic nodules arise as clonal expansions of altered hepatocytes in a process that runs in parallel with the development of liver cirrhosis.
The liver has enormous regenerative capacity.77 However, it has been shown that even a single episode of massive regeneration (such as that elicited by two-thirds partial hepatectomy) attenuates hepatocyte replicative potential and activates events related to cell aging.78 Interestingly, hepatocytes isolated from a normal untreated donor and transplanted in a regenerated liver display a relative growth advantage compared with the resident counterparts and are able to form clonal clusters.78 Furthermore, it has been reported that repeated partial hepatectomy can exert a promoting stimulus on chemically induced hepatic nodules in experimental animals.79 These findings reinforce the concept that the selective growth of normal and altered/nodular hepatocytes appears to be elicited under similar experimental conditions.
As a final note, one should consider the possibility that a better understanding of the interplay between normal and altered cells in a chronically injured tissue may help in the design of novel therapeutic approaches. If in fact carcinogenesis and liver repopulation do represent two sides of the same coin, as suggested by the above considerations, would it be possible to devise strategies to increase the likelihood of one side of the coin (repopulation) prevailing over the other (carcinogenesis)? In simpler terms, would it be feasible to establish a competition between the two cell types (normal versus altered hepatocytes), thereby modulating their respective rate of growth? There are no data to directly answer this question at the present time. However, some relevant evidence is beginning to emerge. As already mentioned, Demers et al19 have reported that in Fah-deficient patients the extent of liver replacement by endogenous, seemingly normal, hepatocytes generated through mutation reversion was inversely correlated with the clinical severity of the disease; the authors suggest that the reverted hepatocytes play a protective role in the evolution of chronic liver disease in these patients. It is tempting to speculate that normal transplanted hepatocytes could be equally (or possibly more) efficient in positively modulating the clinical progression of this disease, as it is observed in the Fah-deficient mouse.3 Another case in point is the Long Evans Cinnamon rat, a model for human Wilson’s disease.80 In these animals, normal hepatocyte transplantation performed at a young age yields repopulation as high as 80% of the liver, resulting in the correction of the associated metabolic defects and chronic liver disease.80 As a corollary to these findings, it is important to note that experimental liver repopulation per se does not result in any increased risk of donor cell-derived neoplastic disease.22,81
In summary, the analysis of available models for liver repopulation provides compelling evidence to indicate that any cytotoxic microenvironment (the same coin) sustaining the selective growth of normal transplanted hepatocytes (side one) can also select for the emergence of rare resistant cells with an altered phenotype (the other side of the coin). This establishes a close mechanistic link between liver repopulation on one hand and early phases of cancer development on the other. Such a link requires further investigation and can possibly be exploited for devising novel therapeutic strategies.
Footnotes
Address reprint requests to Ezio Laconi, MD, PhD, Dipartimento di Scienze e Tecnologie Biomediche, Sezione di Patologia Sperimentale, Università di Cagliari, 09125 Cagliari, Italy. E-mail: elaconi@unica.it.
Work discussed in this paper was supported in part by grants from Ministero dell’Istruzione, dell’Università e della Ricerca, the Italian Ministry of Universities and Research (to E.L. and to P.P.) and from Associazione Italiana Per La Ricerca Sul Cancro, the Italian Association for Cancer Research (to E.L.).
References
- Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou C-N, Finegold M, Grompe M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tryosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- Laconi E, Sarma DSR, Pani P. Transplantation of normal hepatocytes modulates the development of chronic liver lesions induced by a pyrrolizidine alkaloid, lasiocarpine. Carcinogenesis. 1995;16:139–142. doi: 10.1093/carcin/16.1.139. [DOI] [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long term, near total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;158:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771–777. doi: 10.1016/s0002-9440(10)64019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, Rana S, Roy-Chowdhury N, Tanka KE, Vikram B, Roy-Chowdhury J. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–5874. [PubMed] [Google Scholar]
- Mignon A, Guidotti J-E, Mitchell C, Fabre M, Wernet A, De LaCoste A, Soubrane O, Gilgenkrantz H, Kahn A. Selective repopulation of a normal mouse liver by Fas/CD95-resistant hepatocytes. Nat Med. 1998;4:1185–1188. doi: 10.1038/2681. [DOI] [PubMed] [Google Scholar]
- De Vree JML, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude-Elferink RPJ. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology. 2000;119:1720–1730. doi: 10.1053/gast.2000.20222. [DOI] [PubMed] [Google Scholar]
- Karnezis AN, Dorokhov M, Grompe M, Zhu L. Loss of p27Kip1 enhances the transplantation efficiency of hepatocytes transferred into diseased livers. J Clin Invest. 2001;108:383–390. doi: 10.1172/JCI11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Kumaran V, Berishvili E, Barghava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44:1411–20. doi: 10.1002/hep.21416. [DOI] [PubMed] [Google Scholar]
- Laconi S, Laconi E. Principles of hepatocyte transplantation. Semin Cell Dev Biol. 2002;13:433–438. doi: 10.1016/s1084952102001313. [DOI] [PubMed] [Google Scholar]
- Laconi E. Differential growth: from carcinogenesis to liver repopulation. Am J Pathol. 2000;156:389–392. doi: 10.1016/S0002-9440(10)64741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieles LA, Esquivel CO, Van Thiel DH, Konery B, Makowka L, Tzakis AG, Starzl TE. Liver transplantation for tyrosinemia. A review of 10 cases from the University of Pittsburgh. Dig Dis Sci. 1990;35:153–157. doi: 10.1007/BF01537237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, László A, Tiszlavicz L, Moore L, Raftos J, Arnell H, Fischler B, Németh A, Papadogiannakis N, Cielecka-Kuszyk J, Jankowska I, Pawłowska J, Melín-Aldana H, Emerick KM, Whitington PF, Mieli-Vergani G, Thompson RJ. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–86. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Palmiter RD, Heckel JL, Brinster RL, Degen JL. DNA rearrangement causes hepatocarcinogenesis in albumin-plasminogen activator transgenic mice. Proc Natl Acad Sci USA. 1992;89:11523–11527. doi: 10.1073/pnas.89.23.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Dandri M, Gupta S, Rogler CE. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1999;95:310–315. doi: 10.1073/pnas.95.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers SI, Russo P, Lettre F, Tanguay RM. Frequent mutation reversion inversely correlates with clinical severity in a genetic liver disease, hereditary tyrosinemia. Hum Pathol. 2003;34:1313–20. doi: 10.1016/s0046-8177(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Grompe M, Lindstedt S, al-Dhalimy M, Kennaway MG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- Grompe M, Overturf K, al-Dhalimy M, Finegold M. Therapeutic trials in the murine model of hereditary tyrosinaemia type I: a progress report. J Inherit Metab Dis. 1998;21:518–31. doi: 10.1023/a:1005462804271. [DOI] [PubMed] [Google Scholar]
- Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–80. [PMC free article] [PubMed] [Google Scholar]
- Kvittingen EA, Rootwelt H, Berger R, Brandtzaeg P. Self-induced correction of the genetic defect of tyrosinemia type I. J Clin Invest. 1994;94:1657–1661. doi: 10.1172/JCI117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CMJ, Dingemans KP, Smit JJM, Schinkel AH, Notenboom RGE, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RPJ, van der Valk MA, Borst P, Offerhaus GJA. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–45. [PMC free article] [PubMed] [Google Scholar]
- Laconi S, Pani P, Pillai S, Pasciu D, Sarma DSR, Laconi E. A growth constrained environment drives tumor progression in vivo. Proc Natl Acad Sci USA. 2001;98:7806–7811. doi: 10.1073/pnas.131210498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gorla GR, Irani AN, Annamaneni P, Gupta S. Cell transplantation after oxidative hepatic preconditioning with radiation and ischemia-reperfusion leads to extensive liver repopulation. Proc Natl Acad Sci USA. 2002;99:13114–13119. doi: 10.1073/pnas.192365499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci JP, Mariano MS. Radiation hepatology of the rat: parenchymal and nonparenchymal cell injury. Radiat Res. 1993;136:205–213. [PubMed] [Google Scholar]
- Mori H, Iwata H, Morishita Y, Mori Y, Ohno T, Tanaka T, Sasaki S. Synergistic effect of radiation on N-2-fluorenylacetamide-induced hepatocarcinogenesis in male ACI/N rats. Jpn J Cancer Res. 1990;81:975–978. doi: 10.1111/j.1349-7006.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T, Nomura K, Sasaki S. Induction by X-irradiation of adenosine triphosphatase-deficient islands in the rat liver and their characterization. Cancer Res. 1985;45:6078–6082. [PubMed] [Google Scholar]
- Hayes MA, Robert E, Farber E. Initiation and selection of resistant hepatocyte nodules in rats given the pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res. 1985;45:3726–3734. [PubMed] [Google Scholar]
- Laconi E. The evolving concept of tumor microenvironments. BioEssays. 2007;29:1–7. doi: 10.1002/bies.20606. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Young KA, DeLellis RA. Incorporating pathologists’ criteria of malignancy into the evolutionary model for cancer development. J Cell Biochem. 2004;93:28–36. doi: 10.1002/jcb.20105. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: stem cells and cell-cell communication. Ann NY Acad Sci. 2004;1028:192–201. doi: 10.1196/annals.1322.023. [DOI] [PubMed] [Google Scholar]
- Steinbeck RG. Dysplasia in view of the cell cycle. Eur J Histochem. 2004;48:203–211. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Oyamada M, Enomoto K, Mori M. Differential changes in expression of gap junction proteins connexin 26 and 32 during hepatocarcinogenesis in rats. Jpn J Cancer Res. 1992;83:1210–1215. doi: 10.1111/j.1349-7006.1992.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu MJ, Hully JR, Babcock KL, Hertzberg EL, Nicholson BJ, Paul DL, Pitot HC. Multiple mechanisms are responsible for altered expression of gap junction genes during oncogenesis in rat liver. J Cell Sci. 1994;107:83–95. doi: 10.1242/jcs.107.1.83. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Asamoto M, Baba H, Iwahori Y, Matsumoto K, Iwase T, Nishida Y, Nagao S, Hakoi K, Yamaguchi S, Okazi K, Yamasaki H. Cell proliferation and advancement of hepatocarcinogenesis in the rat are associated with a decrease in connexin 32 expression. Carcinogenesis. 1995;16:101–105. doi: 10.1093/carcin/16.1.101. [DOI] [PubMed] [Google Scholar]
- Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon AM, Mesnil M, Berger F, Partensky C, Yamasaki H. Altered homologous and heterologous gap junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer. 1994;56:87–94. doi: 10.1002/ijc.2910560116. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Ono T, Yamanoi A, El-Assal ON, Kohno H, Nagasue N. Expression of gap junction protein connexin 32 in chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Gastroenterol. 2004;39:763–68. doi: 10.1007/s00535-003-1386-2. [DOI] [PubMed] [Google Scholar]
- Moennikes O, Buchmann A, Ott T, Willecke K, Schwarz M. The effect of connexin 32 null mutation on hepatocarcinogenesis in different mouse strains. Carcinogenesis. 1999;20:1379–1382. doi: 10.1093/carcin/20.7.1379. [DOI] [PubMed] [Google Scholar]
- Zaidan-Dagli ML, Yamasaki H, Krutovskikh V, Omori Y. Delayed liver regeneration and increased susceptibility to chemical hepatocarcinogenesis in transgenic mice expressing a dominant-negative mutant of connexin 32 only in the liver. Carcinogenesis. 2004;25:483–492. doi: 10.1093/carcin/bgh050. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Plath T, Detjen K, Welzel M, von Marchall Z, Murphy D, Schirner M, Wiedenmann B, Rosewicz S. A novel function for the tumor suppressor p16(INK4a): induction of anoikis via upregulation of the alpha(5)beta(1) fibronectin receptor. J Cell Biol. 2000;150:1467–1478. doi: 10.1083/jcb.150.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Zhou XD, Zha XL, Shi DR, Fu J, He JY, Lu HF, Tang ZY. Expression of the integrin alpha5 subunit and its mediated cell adhesion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 1997;123:435–440. doi: 10.1007/BF01372547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoglou SC, Manson MM, Green JA, Mayol X, Hughes RC. Distribution of fibronectin and fibronectin-binding proteins. AGp110 and integrin alpha 5 beta 1, during chemically induced hepatocarcinogenesis in adult rats. J Cell Sci. 1991;100:599–604. doi: 10.1242/jcs.100.3.599. [DOI] [PubMed] [Google Scholar]
- Kumaran V, Joseph B, Benten D, Gupta S. Integrin and extracellular matrix interactions regulate engraftment of transplanted hepatocytes in the rat liver. Gastroenterology. 2005;129:1643–1653. doi: 10.1053/j.gastro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodelling. FASEB J. 1991;5:2145–2151. [PubMed] [Google Scholar]
- Ellerbroek SM, Stack MS. Membrane associated matrix metalloproteinases in metastasis. BioEssays. 1999;21:940–949. doi: 10.1002/(SICI)1521-1878(199911)21:11<940::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lehti K, Lohi J, Valtalen H, Keski-Oja J. Proteolytic processing of membrane-type-1 matrix metalloproteinase is associated with gelatinase A activation at the cell surface. Biochem J. 1998;334:345–353. doi: 10.1042/bj3340345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Paradis V. Liver extracellular matrix in health and disease. J Pathol. 2003;200:504–515. doi: 10.1002/path.1397. [DOI] [PubMed] [Google Scholar]
- Theret N, Musso O, L’Helgoualc’h A, Clement B. Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interaction with hepatocytes. Am J Pathol. 1997;150:51–58. [PMC free article] [PubMed] [Google Scholar]
- Mazzocca A, Cappadona Sciammetta S, Carloni V, Cosmi L, Annunziato F, Harada T, Abrignani S, Pinzani M. Binding of hepatitis C virus envelope protein E2 to CD81 up-regulates matrix metalloproteinase 2 in human hepatic stellate cells. J Biol Chem. 2005;280:11329–11339. doi: 10.1074/jbc.M410161200. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Trautwein C, Kubicka S, Rakemann T, Bahr MJ, Sedlaczek N, Schuppan D, Manns MP. Differential regulation of extracellular matrix synthesis during liver regeneration after partial hepatectomy in rats. Hepatology. 1999;30:1159–1166. doi: 10.1002/hep.510300502. [DOI] [PubMed] [Google Scholar]
- Kim T-H, Mars WM, Stolz D, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;30:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- Koenig S, Stoesser C, Krause P, Becker H, Markus PM. Liver repopulation after hepatocellular transplantation: integration and interaction of transplanted hepatocytes in the host. Cell Transplant. 2005;14:31–40. doi: 10.3727/000000005783983322. [DOI] [PubMed] [Google Scholar]
- Ogata R, Torimura T, Kim M, Ueno T, Tateishi Y, Kuromatsu R, Shimauchi Y, Sakamoto M, Tamaki S, Sata M, Tanikawa K. Increased expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 with tumor dedifferentiation in hepatocellular carcinomas. Hum Pathol. 1999;30:443–450. doi: 10.1016/s0046-8177(99)90121-1. [DOI] [PubMed] [Google Scholar]
- Theret N, Musso O, Turlin B, Lotrian D, Bioulac-Sage P, Campion J-P, Boudjema K, Clement B. Increased extracellular matrixremodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology. 2001;34:82–88. doi: 10.1053/jhep.2001.25758. [DOI] [PubMed] [Google Scholar]
- Park YN, Yang CP, Cubukcu O, Thung SN, Theise ND. Hepatic stellate cell activation in dysplastic nodules: evidence for an alternate hypothesis concerning human hepatocarcinogenesis. Liver. 1997;17:271–274. doi: 10.1111/j.1600-0676.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology. 2005;42:514–521. doi: 10.1002/hep.20815. [DOI] [PubMed] [Google Scholar]
- Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res. 2006;60:233–238. doi: 10.1203/01.pdr.0000228350.61496.90. [DOI] [PubMed] [Google Scholar]
- Rudnick DA, Liao Y, An JK, Muglia LJ, Perlmutter DH, Teckman JH. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology. 2004;39:1048–1055. doi: 10.1002/hep.20118. [DOI] [PubMed] [Google Scholar]
- Hadzic N, Quaglia A, Mieli-Vergani G. Hepatocellular carcinoma in a 12-year-old child with PiZZ alpha1-antitrypsin deficiency. Hepatology. 2006;43:194. doi: 10.1002/hep.21009. [DOI] [PubMed] [Google Scholar]
- Wada K, Kondo F, Kondo Y. Large regenerative nodules and dysplastic nodules in cirrhotic livers: a histopathologic study. Hepatology. 1988;8:1684–1688. doi: 10.1002/hep.1840080636. [DOI] [PubMed] [Google Scholar]
- Wanless IR, International Working Party Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Paradis V, Laurendeau I, Vidaud M, Bedossa P. Clonal analysis of macronodules in cirrhosis. Hepatology. 1998;28:953–958. doi: 10.1002/hep.510280409. [DOI] [PubMed] [Google Scholar]
- Park YN, Roncalli M. Large liver cell dysplasia: a controversial entity. J Hepatol. 2006;45:734–743. doi: 10.1016/j.jhep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Britto Garcia S, Novelli M, Wright NA. The clonal origin and clonal evolution of epithelial tumors. Int J Exp Pathol. 2000;81:89–116. doi: 10.1046/j.1365-2613.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 2005;25:16–27. doi: 10.1111/j.1478-3231.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Gaffey MJ, Iezzoni JC, Weiss LM. Clonal analysis of focal nodular hyperplasia of the liver. Am J Pathol. 1996;148:1089–1096. [PMC free article] [PubMed] [Google Scholar]
- Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30–39. doi: 10.1097/00000658-198707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MA, Kneteman NM, Minuk GY. Research toward safer resection of the cirrhotic liver. HPB Surg. 2000;11:285–297. doi: 10.1155/2000/31945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiiwa K, Nakano K, Kameoka N, Nagai E, Tanaka M. Proliferating cell nuclear antigen, plasma fibronectin, and liver regeneration rate after seventy percent hepatectomy in normal and cirrhotic rats. Surgery. 1994;116:544–549. [PubMed] [Google Scholar]
- Kaido T, Yoshikawa A, Seto S, Yamaoka S, Sato M, Ishii T, Imamura M. Portal branch ligation with a continuous hepatocyte growth factor supply makes extensive hepatectomy possible in cirrhotic rats. Hepatology. 1998;28:756–769. doi: 10.1002/hep.510280323. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR, Jr, Reid LM, Gupta S. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol. 1999;276:G1260–G1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- Pound AW, McGuire LJ. Repeated partial hepatectomy as a promoting stimulus for carcinogenic response of liver to nitrosamines in rats. Br J Cancer. 1978;37:585–594. doi: 10.1038/bjc.1978.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Irani AN, Volemberg I, Schilsky ML, Gupta S. Early cell transplantation in LEC rat modeling Wilson’s disease eliminates hepatic copper with reversal of liver disease. Gastroenterology. 2002;122:438–447. doi: 10.1053/gast.2002.31086. [DOI] [PubMed] [Google Scholar]
- Laconi S, Montisci S, Doratiotto S, Greco M, Pasciu D, Pillai S, Pani P, Laconi E. Liver repopulation by transplanted hepatocytes and risk of hepatocellular carcinoma. Transplantation. 2006;82:1319–1323. doi: 10.1097/01.tp.0000228239.78290.13. [DOI] [PubMed] [Google Scholar]