Abstract
Lactoferrin (LF) is an iron-binding protein found in milk, mucosal secretions, and the secondary granules of neutrophils in which it is considered to be an important factor in the innate immune response against microbial infections. Moreover, LF deficiency in the secondary granules of neutrophils has long been speculated to contribute directly to the hypersusceptibility of specific granule deficiency (SGD) patients to severe, life-threatening bacterial infections. However, the exact physiological significance of LF in neutrophil-mediated host defense mechanisms remains controversial and has not yet been clearly established in vivo using relevant animal models. In this study, we used lactoferrin knockout (LFKO) mice to directly address the selective role of LF in the host defense response of neutrophils and to determine its contribution, if any, to the phenotype of SGD. Neutrophil maturation, migration, phagocytosis, granule release, and antimicrobial response to bacterial challenge were unaffected in LFKO mice. Interestingly, a stimulus-dependent defect in the oxidative burst response of LFKO neutrophils was observed in that normal activation was seen in response to opsonized bacteria whereas an impaired response was evident after phorbol myristate-13-acetate stimulation. Taken together, these results indicate that although LF deficiency alone is not a primary cause of the defects associated with SGD, this protein does play an immunomodulatory role in the oxidative burst response of neutrophils.
The initial level of surveillance and host defense against microbial challenge is provided by the innate immune system. Neutrophils are critical effector cells of this system in which a combination of oxidative and nonoxidative antimicrobial activities responds robustly to kill the invading pathogen.1,2,3 The oxidative arm of the neutrophil defense system is provided by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzyme complex that generates superoxide anion after the catalytic transfer of electrons from NADPH to molecular oxygen. Superoxide is in turn rapidly converted spontaneously and/or catalytically to several downstream reactive oxygen species including hydrogen peroxide and hypochlorous acid.4,5 The nonoxidative arm of the neutrophil defense system is provided by a multitude of proteases, hydrolases, antimicrobial peptides, and protein components present in the neutrophil granules.6,7
The central role of neutrophils in host defense is underscored in a number of human8,9 and mouse models3,10,11,12,13,14,15 in which one or more of the neutrophil proteins is defective resulting in increased susceptibility to microbial infection. Human-specific granule deficiency (SGD) is a rare congenital disease in which the patients have an absence of or severe deficiency in neutrophil secondary and tertiary granules and associated components.8,9,16 Numerous abnormalities exist in the neutrophils from these patients rendering them susceptible to severe, life-threatening microbial infections typically caused by Staphylococcus aureus or Pseudomonas aeruginosa.17,18,19,20 It has been shown that an underlying cause of two cases of SGD is a loss of function mutation in the CCAAT/enhancer-binding protein ε (C/EBPε).21,22 C/EBPε is a basic leucine zipper transcription factor expressed predominantly in myeloid cells that control the developmental expression of several genes associated with late-stage neutrophil differentiation.23 The phenotype of C/EBPε knockout mice closely resembles many aspects of the neutrophil defects observed in human SGD, including abnormal bilobed neutrophil morphology, impaired migration to the site of inflammatory challenge, reduced phagocytosis and bactericidal activity, and an impaired oxidative burst activity.14,24,25 These mice succumb to opportunistic infections, usually caused by P. aeruginosa between 3 to 5 months.14 Like SGD patients, the corresponding mouse model of SGD is deficient in the iron-binding protein, LF, a highly abundant secondary granule component.24,25
In the hematopoietic system, LF is expressed specifically in developing neutrophils during the myelocyte stage of maturation26,27 and the protein is also a prominent component of most exocrine secretions.28 Several lines of evidence suggest that LF may play an integral part of the innate immune response of the neutrophil to microbial infection. Because of its high affinity for iron and low iron saturation, LF acts as an effective bacteriostatic agent, depriving bacteria of this essential growth nutrient.29,30,31 Further, a direct bactericidal activity was shown for LF whereby the protein binds to the outer membrane of Gram-negative bacteria causing the release of lipopolysaccharides with an associated increase in membrane permeability.32,33 An isolated N-terminal cationic peptide of LF (lactoferrin) has been shown to exhibit increased potency of bactericidal activity relative to the intact protein, with efficacy against a wide range of microorganisms including Gram-negative and Gram-positive bacteria.34 It has also been speculated that LF indirectly contributes to the innate immune response of the neutrophil. Various reports indicate that LF may influence neutrophil recruitment to sites of infection by altering aggregation, adhesion, attachment, and/or motility of this cell type.35,36,37,38 In addition, it has been shown that LF stimulates phagocytosis.39,40 Finally, an enhancement of superoxide production by neutrophils was demonstrated in the presence of LF37 whereas contradictory reports in neutrophils and cell-free systems support either an enhancement41,42,43 or no effect44,45 of LF on hydroxyl radical formation.
The host defense properties ascribed to LF suggest that the neutrophil defects and increased susceptibility to microbial infection observed in SGD patients and C/EBPε knockout mice may be attributable, at least in part, to the absence of this normally abundant secondary granule component. However, the contribution of LF deficiency per se to this syndrome has been difficult to decipher because of the array of additional antimicrobial components that are ablated in association with the specific granule neutrophil defect. In the present study, we have used LF knockout (LFKO) mice46 to analyze the specific consequences of LF ablation on the host defense response of the neutrophil. We show that although LF is dispensable for many neutrophil functions, an impairment of phorbol myristate-13-acetate (PMA)-stimulated neutrophil oxidative burst is clearly associated with LF deficiency.
Materials and Methods
Animals
The generation of LFKO mice by targeted disruption of the LF gene in embryonic stem cells has been reported.46 LFKO mice used for these studies were backcrossed at least 10 generations onto the C57BL/6 genetic background. Wild-type (WT) C57BL/6 mice were obtained from Harlan-Sprague Dawley (Indianapolis, IN) and bred in the same facility as the LFKO mice. Mice were maintained in microisolator cages under specific pathogen-free conditions in a 12-hour light/dark cycle and were fed a basal rodent chow ad libitum (LabDiet; PMI, Richmond, IN). Age- and sex-matched adult mice were used for experiments and all animal research complied with National Institutes of Health and Baylor College of Medicine guidelines for research with experimental animals.
Neutrophil Morphology and Blood Cell Analysis
Blood was obtained by cardiac puncture and blood smears were stained with Wright-Giemsa to examine neutrophil morphology. Images were acquired using bright-field microscopy (Zeiss Axioscope; Carl Zeiss, Thornwood, NY). Peripheral blood cells were collected from mice by cardiac puncture and were analyzed using a Bayer Advia 120 hematology analyzer (Bayer Diagnostics, Dallas, TX).
Isolation of Bone Marrow Neutrophils
Mature bone marrow neutrophils were isolated and purified from mice essentially as described previously with minor modifications.47,48 Briefly, bone marrow cells were isolated from femurs and tibias and mature neutrophils were purified using a discontinuous Percoll gradient (81%, 62%, 55%, 50%, 45%). Cells at the interphase of the 81% and 62% layer were isolated and washed in NM buffer (Hanks’ buffered saline solution minus calcium and magnesium (HBSS−/−)/0.1% bovine serum albumin). Contaminating red blood cells were lysed in ACK buffer (BioWhittaker, Walkersville, MD) and the resulting cell population was washed twice in NM buffer. Viable cells were counted with a hemocytometer using trypan blue exclusion and cells were resuspended in HBSS−/−/5 mmol/L glucose.
Granulocyte-Specific Antigen (Gr-1) Analysis
Purified bone marrow neutrophils (5 × 105) were incubated with anti Gr-1 (1 μg/ml; clone RB6-8C5; eBioscience, Inc., San Diego, CA) for 30 minutes on ice. Cells were washed and resuspended in 1% paraformaldehyde for flow cytometric analysis using a Coulter EPICS XL-MCL flow cytometer and System 2 version 3.0 software (Beckman Coulter, Hialeah, FL). Cells were gated on the neutrophil population based on characteristic forward and side light scatter.
PMA Stimulation of Neutrophils and Dihydrorhodamine (DHR123)/Rhodamine 123 (R123) Oxidative Burst Assay
Quantitative determination of the oxidative burst of purified bone marrow neutrophils was determined using a DHR123/R123 assay essentially as described.49 DHR123 is the nonfluorescent substrate used in the assay and R123 is the fluorescent oxidation reporter product of this substrate formed during the oxidative burst response of the neutrophil. Briefly, 5 × 105 cells in HBSS−/−/5 mmol/L glucose were incubated with DHR123 (1 μmol/L; Molecular Probes, Eugene, OR) for 5 minutes at 37°C with gentle horizontal agitation. Cells were incubated in PMA (0 to 600 ng/ml; Sigma Chemical Co., St. Louis, MO) at 37°C to stimulate the oxidative burst or incubated with buffer vehicle alone (nonstimulated cells). After 30 minutes, the reactions were stopped by placing the tubes on ice and neutrophil cells were analyzed for conversion of DHR123 to the fluorescent R123 by flow cytometry.
Bacterial Stimulation of Oxidative Burst Response in Purified Neutrophils
S. aureus Wood 46 was opsonized with 50% serum (obtained from WT C57BL6) for 15 minutes at room temperature as described.50 Neutrophils (5 × 105) were incubated with DHR123 (1 μmol/L) for 5 minutes at 37°C followed by an incubation with a 100-fold excess of opsonized bacteria for 1 hour at 37°C. The reaction was stopped by placing tubes on ice and neutrophils were analyzed for conversion of DHR123 to the fluorescent R123 as described above.
Western Immunoblot Analysis
Purified bone marrow neutrophils (2 to 4 × 106 cells/ml) were sonicated in 1× sodium dodecyl sulfate loading buffer and proteins corresponding to 1 to 2 × 105 cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5 to 15%). Immunoblots were probed with antibodies directed against p22phox (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), p40phox, p47phox, p67phox (Millipore, Billerica, MA), GP91phox (a generous gift from Al Jesaitis, Montana State University, Billings, MT), and GAPDH (loading control; Chemicon International, Temecula, CA) following the manufacturer’s recommendations. Signal was detected by ECL Plus detection reagents (GE Health Care, Piscataway, NJ) and relative levels of proteins were determined by using densitometric scanning and ImageQuant software (version 5.2; Amersham Biosciences, Piscataway, NJ).
Thioglycollate-Induced Peritonitis
Analysis of neutrophil migration to the peritoneal cavity after thioglycollate administration was performed as described by Lu and colleagues51 with minor modifications. Briefly, WT and LFKO mice were injected intraperitoneally with 3% thioglycollate media (1 ml) and were euthanized at 0 or 4 hours after injection. Peritoneal exudates were harvested by lavage using 4 ml of cold EB buffer [1× phosphate-buffered saline (PBS) minus calcium and magnesium/40 U/ml heparin] and total cell count was determined using a hemocytometer. The peritoneal extract was washed twice in WB buffer (1× PBS, pH 7.2, minus calcium and magnesium/0.1% bovine serum albumin) and differential cell counts were obtained using CamcoQuik-stained (Fisher Scientific, Pittsburgh, PA) cytospin samples. At least 500 cells were counted per slide under oil immersion microscopy (×100).
Phagocytosis
Phagocytic analysis of blood neutrophils was quantitatively analyzed using a commercially available kit (Phagotest; Orpegen, Heidelberg, Germany). Heparinized whole blood (100 μl) obtained from WT and LFKO mice was cooled on ice and mixed with ice-cold opsonized fluorescein isothiocyanate-labeled bacteria (20 μl). Samples were incubated on ice (control samples) or 37°C (test samples) for 60 minutes. After bacterial incubation, samples were placed on ice and neutralization solution was added to quench the fluorescence of surface-bound bacteria while leaving internalized bacteria unaltered. Erythrocytes were lysed and the remaining cells were resuspended in DNA staining solution for flow cytometric analysis. Neutrophils were distinguished using forward and light scatter patterns and the percentage of cells undergoing phagocytosis and the number of bacteria ingested per cell was determined.
Primary Granule Exocytosis
Purified mature bone marrow neutrophils (5 × 105 cells) were suspended in phenol red-free RPMI 1640 media and stimulated with 5 μg/ml of cytochalsin B (Sigma) and 5 μmol/L fMLP (Sigma) to induce primary granule exocytosis and myeloperoxidase (MPO) secretion.52 MPO in the supernatants was detected using a tetramethylbenzidine assay as described.52,53
CD11b Cell Surface Levels in Resting and Activated Neutrophils
The activation status and secondary/secretory granule release of WT and LFKO neutrophils were assessed by measuring the cell surface expression of CD11b in resting and stimulated neutrophils. Purified bone marrow neutrophils (5 × 105 cells in HBSS−/−/5 mmol/L glucose) were incubated at 37°C with 100 ng/ml of PMA (stimulated cells) or buffer vehicle alone (nonstimulated cells). After 10 minutes, the reactions were stopped by placing the tubes on ice. Cells were analyzed by flow cytometry for cell surface expression of CD11b using phycoerythrin-conjugated anti-CD11b (BD PharMingen, San Diego, CA). Cells were gated on the neutrophil population based on characteristic forward and side light scatter and dead cells were excluded using propidium iodide staining. Isotype-specific antibodies were used to assess nonspecific staining.
Spontaneous Abscess Bacterial Analysis
Swabs of bacterial abscesses were obtained and submitted to Antech Diagnostics (Irvine, CA) or Radil (University of Missouri, Columbia, MO) for bacterial species identification.
S. aureus Bactericidal Assay
The bactericidal response of WT and LFKO neutrophils was assessed using a bactericidal assay with S. aureus (Wood strain) as described by Clemens and colleague54 with minor modifications. Purified bone marrow neutrophils (2 × 106) were incubated with 2 × 105 log phase opsonized bacteria in HBSS/10 mmol/L HEPES/10 mmol/L dextrose/1 mmol/L Ca/3. mmol/L Mg, pH 7.44 containing 10% mouse serum. At 0-, 15-, 30-, and 60-minute time points, 20-μl aliquots were removed and combined with 180 μl of PBS/0.2% Triton X-100 on ice to lyse the neutrophils. Serial dilutions were plated on brain-heart infusion agar and colonies were enumerated after 24 hours. The percent survival is represented as the percentage of colonies remaining at each time point in comparison to the number at time 0 for each condition.
S. aureus in Vivo Models of Infection
To compare the antimicrobial response of WT and LFKO mice to S. aureus challenge, both an airpouch55,56 and an intraperitoneal50 model of bacterial infection were performed. For the airpouch study, a subcutaneous pouch was created in anesthetized WT and LFKO mice on day 0 using 5 ml of sterile air followed by reinflation on day 3 with 2.5 ml of sterile air. Anesthetized mice were inoculated with 5 × 106 CFUs or 5 × 107 CFUs of early exponential phase S. aureus (8325-4) in 100 μl of 0.9% saline. At 24 hours after infection, mice were euthanized. The airpouch was lavaged using 5 ml of ice-cold PBS and the lavage fluid was centrifuged and resuspended in PBS/0.05% Triton X-100. Bacterial suspensions were sonicated, serially diluted in tryptic soy broth (TSB) and plated onto TSB agar. Plates were incubated at 37°C and bacterial counts were enumerated 24 hours later. For the intraperitoneal infection study, mice were inoculated with 5 × 107 CFUs of logarithmic growth phase S. aureus (LS-1) in 200 μl of PBS. Four hours after infection, mice were euthanized, the peritoneal cavity was flushed with 6 ml of ice-cold PBS, 5 mmol/L ethylenediaminetetraacetic acid, and 5 U/ml heparin. Peritoneal lavages were sonicated, serially diluted, and plated on TSB agar for bacterial enumeration.50
Experimental Infection with P. aeruginosa
P. aeruginosa acute infection of adult WT and LFKO mice was performed using an intranasal challenge.57,58 Briefly, anesthetized WT and LFKO mice were infected intranasally with a clinical blood isolate of P. aeruginosa (105 or 106 CFUs in 13 μl). Mice were sacrificed 6 hours later and lungs were aseptically removed. Lungs were weighed, homogenized, and strained through a nylon mesh. The homogenate was centrifuged at 10,000 × g for 5 minutes and incubated in TSB/0.1% Triton X-100. Serial dilutions of the homogenate were plated onto TSB agar. Plates were incubated at 37°C and bacterial colonies were enumerated 24 hours later.
Statistical Analysis
Statistical analysis was performed using SPSS (version 10) software (SPSS, Chicago, IL). Differences between groups were examined using the Student’s t-test for equality of means with a P value of ≤0.05 considered statistically significant. When the F values for Levene’s test for the equality of variance were significant, the Mann-Whitney U-test was used to determine the level of significance.
Results
LF Is Not Required for Neutrophil Differentiation
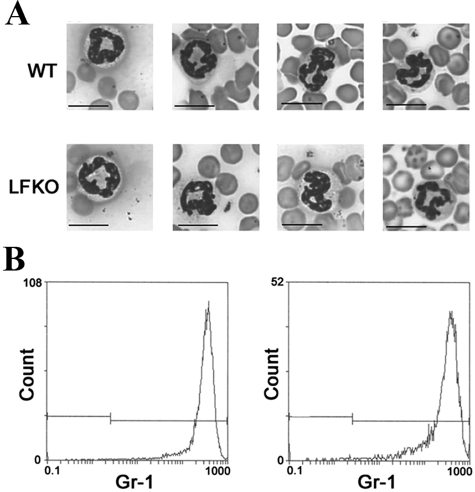
C/EBPε is essential for proper differentiation and functional activity of the neutrophil as evidenced by the pleiotropic defects in C/EBPε-deficient neutrophils.9,14,24,25 To determine whether ablation of the prominent secondary granule component LF contributes to any of these defects, we examined the differentiation and function of neutrophils in LFKO mice. One of the first defects noted on specific granule deficiency was a failure of the neutrophil to terminally differentiate as evidenced by the presence of bilobed nuclei and their deficient granularity.8,9 To determine whether LF is required for neutrophil differentiation, Wright-Giemsa staining of peripheral blood smears from LFKO and WT mice was performed. Mature WT neutrophils were identified by their pale staining of abundant cytoplasm and segmented ring-shaped nuclei. Neutrophils from LFKO mice displayed a similar morphology and were indistinguishable from WT neutrophils (Figure 1A). Further, a similar pattern of surface staining for the granulocyte differentiation antigen, Gr-1, a neutrophil maturation marker,59 was observed in mature WT [mean fluorescent intensity (MFI), 355.15 ± 21.7] and LFKO (MFI, 329.82 ± 21.2) neutrophils (Figure 1B). Finally, flow cytometric analysis of mature LFKO neutrophils exhibited an indistinguishable forward and side scatter profile to WT neutrophils (Figure 2, A and E) indicating that the size and granularity of the neutrophil populations from the two genotype groups were similar. Collectively, these data demonstrate that LF is not required for neutrophil differentiation.
Figure 1.
Normal maturation of neutrophils in LFKO mice. A: Peripheral blood neutrophils from LFKO mice display normal mature morphology. Representative neutrophils from blood smears stained with Wright-Giemsa from WT (top) and LFKO (bottom) mice. Neutrophils from both genotypes have ring-shaped segmented nucleus characteristic of mature morphology in mouse blood. B: Expression of Gr-1 in WT and LFKO neutrophils. Purified bone marrow cells from WT and LFKO mice (n = 8 per genotype) were stained with anti-Gr-1 as described in the Material and Methods. Left: Histogram of representative fluorescence staining for Gr-1 in WT neutrophils. Right: Histogram of representative fluorescence staining for Gr-1 in LFKO neutrophils. Scale bars = 10 μm.
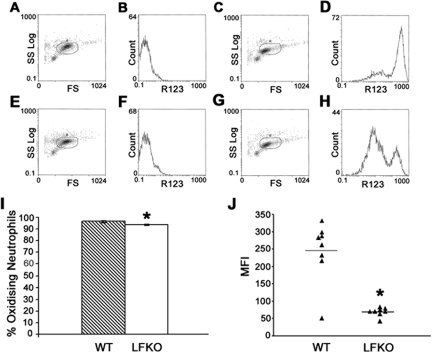
Figure 2.
Oxidative burst is impaired in LFKO neutrophils. The oxidative burst activity of purified bone marrow neutrophils isolated from WT and LFKO mice was determined using a DHR123/R123 assay and flow cytometric analysis. A–D: Representative WT cells. A: WT unstimulated neutrophils showing gated cells based on forward scatter (FS) and side scatter (SS) profiles. B: WT unstimulated neutrophils showing mean fluorescence intensity (MFI) of rhodamine 123 (R123). C: WT neutrophils stimulated with PMA showing gated cells. D: MFI of R123 in WT stimulated cells. E–H: Representative LFKO cells. E: LFKO unstimulated neutrophils showing gated cells. F: MFI of R123 in LFKO unstimulated neutrophils. G: LFKO neutrophils stimulated with PMA showing gated cells. H: MFI of R123 in LFKO stimulated cells. I: Data represent the mean ± SEM percentage of stimulated neutrophils undergoing oxidative burst for WT and LFKO mice (n = 8 per genotype). J: Data represent the MFI of R123 from stimulated neutrophils undergoing oxidative burst for WT and LFKO mice (n = 8 per genotype). The mean values are indicated by horizontal lines. *Significantly different (P = 0.007) when compared to WT mice. Oxidative burst experiments were performed six times with slight variations using three to eight animals per genotype, and in all cases the mean MFI of LFKO neutrophils was lower (1.4- to-3.9-fold) than WT controls, reaching statistical significance in four of the six experiments.
Oxidative Burst Is Impaired in LFKO Neutrophils in Response to PMA Stimulation but Is Normal in Response to Stimulation with Opsonized Bacteria
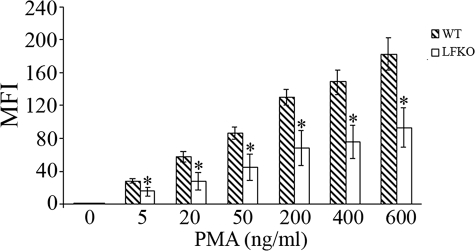
C/EBPε-deficient mouse neutrophils display a defective oxidative burst activity in response to stimulation with PMA, a potent stimulator of the NADPH oxidase-dependent respiratory burst.14,24 To establish if the oxidative arm of the neutrophil host defense response was altered in the absence of LF, purified bone marrow neutrophils were stimulated with PMA and the production of reactive oxygen metabolites was determined by measuring the conversion of the fluorigenic substrate, DHR 123, to fluorescent R123.49 A dramatic impairment in oxidative burst activity was observed in stimulated LFKO neutrophils as compared to WT controls (Figure 2). Minimal differences were observed in the total number of neutrophils undergoing burst activity in LFKO versus WT neutrophils (Figure 2I). However, the MFI, corresponding to the mean oxidative burst activity per cell, was greatly reduced in stimulated LFKO neutrophils versus stimulated WT neutrophils (LFKO, 70.0 ± 4.4 versus WT, 245.6 ± 30.6; P = 0.007) (Figure 2J). To determine whether the significant impairment in the oxidative burst response of LFKO neutrophils is observed throughout a range of PMA concentrations, we analyzed the oxidative burst response in LFKO neutrophils in response to a PMA dose curve (Figure 3). A similar significant defect in the oxidative burst response of the LFKO neutrophils remained throughout a range of PMA concentrations tested (5 to 600 ng/ml). These results suggest that LF is required for optimal oxidative burst activity in neutrophils in response to PMA stimulation. Finally, we characterized the oxidative burst in WT and LFKO neutrophils in response to opsonized bacteria. Interestingly, using this stimulus, no difference was detected in the oxidative burst response of WT and LFKO neutrophils (Figure 4).
Figure 3.
Dose response of PMA activation of oxidative burst in LFKO neutrophils. The dose response oxidative burst activity of purified bone marrow neutrophils isolated from WT and LFKO mice was determined using a DHR123/R123 assay and flow cytometric analysis. Data represent the mean MFI (±SEM) of R123 from stimulated neutrophils undergoing oxidative burst for WT and LFKO mice (n = 6 per genotype). *Significantly different (P ≤ 0.05) when compared to WT mice.
Figure 4.
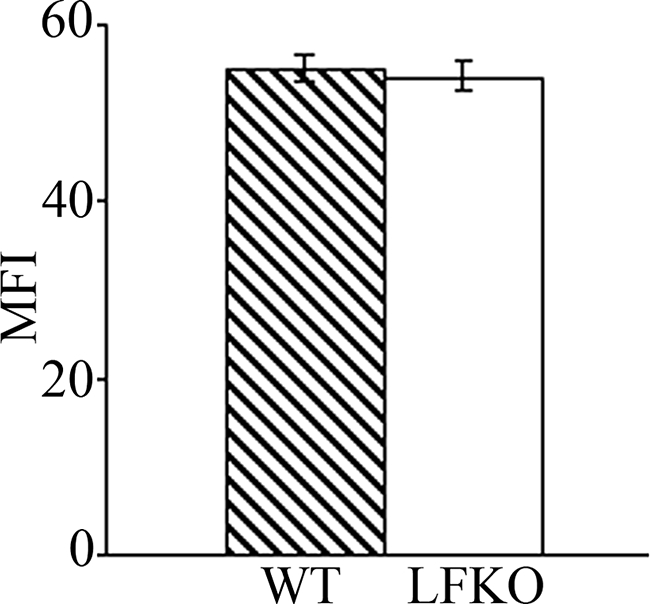
Oxidative burst in LFKO neutrophils in response to bacterial stimulation. Purified bone marrow neutrophils from WT and LFKO mice were stimulated with a 100-fold excess of opsonized bacteria. The oxidative burst response was determined using a DHR123/R123 assay and flow cytometric analysis. Data represent the MFI (±SEM) of R123 from stimulated neutrophils undergoing oxidative burst for WT and LFKO mice (n = 6 per genotype).
Normal Expression of NADPH Oxidase Components in LFKO Neutrophils
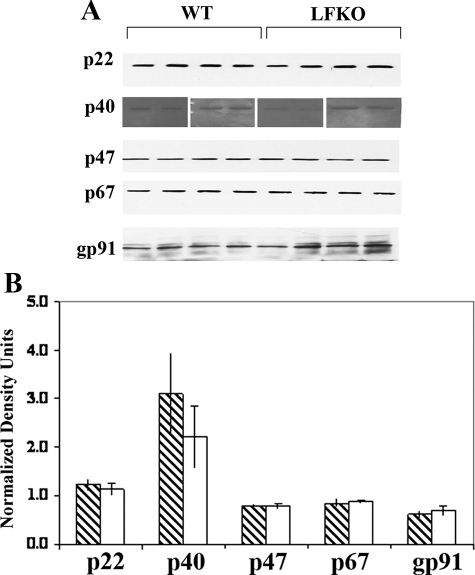
The NADPH oxidase is a multisubunit enzyme complex consisting of gp91phox and p22phox membrane-associated catalytic core components (cytochrome b558) and four cytosolic components, p40phox, p47phox, and p67phox and a small GTPase (Rac1/2). The cytosolic components translocate to the membrane on neutrophil activation to form a functional oxidase complex with cytochrome b558.60,61 To determine the level of expression of the NADPH oxidase subunits, quantitative Western immunoblot analysis was performed in WT and LFKO neutrophils. No significant difference in the levels of p22phox, p40phox, p47phox, p67phox, or gp91phox were detected in the two groups (Figure 5) indicating that the abnormal PMA-stimulated oxidative burst response observed in LFKO neutrophils is not because of altered expression of the NADPH oxidase subunits in these mice.
Figure 5.
Western immunoblot analysis of NADPH oxidase components. Purified mature bone marrow neutrophils were sonicated into loading buffer, and protein samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5 to 15%). Immunoblots were probed with antibodies directed against NADPH phox components. Blots were reprobed with GAPDH as a loading control, and relative levels of proteins was determined by using densitometric scanning. Data show the Western immunoblot results from four WT and four LFKO mice. The graph shows the quantitation of the relative amounts of the proteins (±SEM) in WT and LFKO samples after normalization against GAPDH. Note: For the p40phox Western, two gels were run with the first gel containing duplicate samples from two WT and two LFKO mice and the second gel containing duplicate samples from another two WT and two LFKO mice. The transferred blots were split in half and one half probed with p40phox and the other half probed with GAPDH because these two proteins are similar in size.
Normal Neutrophil Migration and Phagocytosis in LFKO Neutrophils
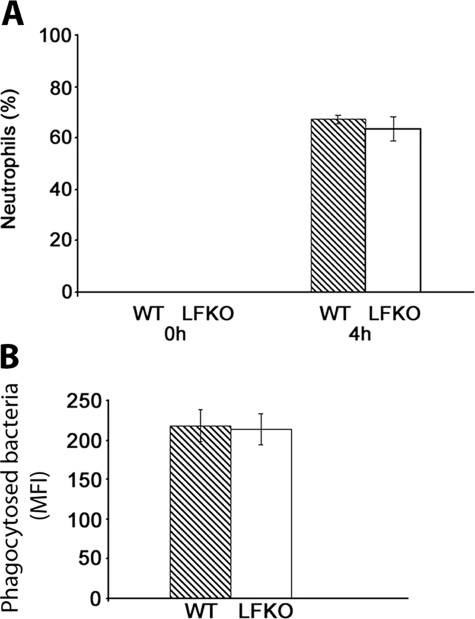
To determine whether the recruitment of neutrophils is altered in the absence of LF, WT and LFKO mice were challenged with 3% thioglycollate and peritoneal exudate cells were analyzed immediately and at 4 hours after challenge. No neutrophils were detected in the peritoneal cavity in unstimulated WT or LFKO mice. On thioglycollate challenge, similar neutrophil percentages (Figure 6A) and absolute neutrophil counts (data not shown) was observed in WT and LFKO animals indicating that LF is not required for neutrophil recruitment.
Figure 6.
Normal migration and phagocytosis of LFKO neutrophils. A: Inflammatory influx of neutrophils into the peritoneal cavity after thioglycollate administration. WT and LFKO mice were injected with thioglycollate and euthanized at the time points indicated. The percentage of neutrophils was obtained from cytospin differentials. Data are represented as mean ± SEM. Six WT and five LFKO mice were used for the 0-hour time point and 10 WT and 7 LFKO mice were used for the 4-hour time point. B: Phagocytosis of peripheral blood neutrophils. Heparinized blood from WT and LFKO mice was incubated with fluorescein isothiocyanate-labeled bacteria at 37°C for 60 minutes and analyzed by flow cytometric analysis. Neutrophils were distinguished based on light scatter properties. Data show the MFI, corresponding to the number of ingested bacteria per neutrophil, from WT and LFKO mice (n = 12 per genotype). Data are represented as mean ± SEM.
We next determined if phagocytosis was normal in LFKO neutrophils. Heparinized blood obtained from WT and LFKO mice was incubated with fluorescein isothiocyanate-labeled opsonized bacteria and uptake was assessed by flow cytometric analysis. The MFI, corresponding to the number of ingested bacteria per neutrophil, was similar for both WT and LFKO mice (Figure 6B). In addition, no difference in the number of neutrophils undergoing bacterial phagocytosis at 37°C were observed between the two genotype groups (data not shown) demonstrating that LF is not required for the uptake of opsonized bacteria by neutrophils.
LFKO Neutrophils Show Normal Granule Mobilization
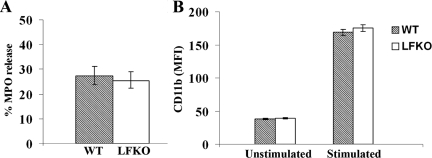
On stimulation with cytochalsin B and the chemoattractant, fMLP, neutrophils release the contents of their primary granules into the extracellular space.62 To determine whether LF was required for primary granule exocytosis, we assessed the levels of the major primary granule protein, MPO, in the supernatants of cytochalsin B/fMLP-stimulated neutrophils. No differences were observed in MPO levels in the supernatants of stimulated neutrophils from WT and LFKO mice (Figure 7A) indicating that the loss of LF does not affect primary granule release.
Figure 7.
Primary granule myeloperoxidase secretion and CD11b up-regulation is normal in LFKO neutrophils. A: Purified bone marrow neutrophils (5 × 106/ml) from WT and LFKO mice were incubated with 5 μg/ml cytochalasin B and 5 μmol/L fMLP for 15 minutes, and supernatants were collected and analyzed for secretion of (MPO). Data represent the mean percentage of MPO released (±SEM) relative to total cellular MPO. Neutrophils from six WT and six LFKO mice were used for this analysis. B: Expression of CD11b on the surface of resting and PMA-activated neutrophils was detected by staining with phycoerythrin-conjugated anti-CD11b followed by flow cytometric analysis. Data represent the mean (±SEM) of the MFI for CD11b on WT and LFKO neutrophils (n = 6 per genotype).
CD11b is an integrin that is stored primarily in the secondary and secretory granules of unstimulated neutrophils with lower levels located at the cell surface. On neutrophil activation, the granule CD11b stores are mobilized resulting in an up-regulation of CD11b at the cell surface. To determine whether there was any alteration in the activation status or mobilization of the secondary and secretory granules of LFKO neutrophils, we compared the expression of CD11b, on resting and PMA-activated WT and LFKO neutrophils. Under resting conditions, a similar level of cell surface CD11b was detected in WT and LFKO neutrophils (Figure 7B). On PMA stimulation, a dramatic increase (4.4-fold) in surface expression of CD11b was detected in WT neutrophils reflecting the translocation of neutrophil intracellular granule reserves of this protein to the cell surface.63 A similar increase (4.48-fold) of cell surface expression of CD11b was also detected in LFKO neutrophils indicating that secondary/secretory granule mobilization in PMA-stimulated LFKO neutrophils is indistinguishable from WT neutrophils.
LFKO Mice Display No Increased Susceptibility to Acute S. aureus and P. aeruginosa Challenge
A hallmark of specific granule deficiency is the increased incidence of opportunistic bacterial infections with S. aureus and P. aeruginosa being two of the most prevalent pathogens identified.14,17,18,19,20 LFKO mice displayed an approximately twofold higher incidence of opportunistic infections than WT mice, with 8 of 99 LFKO mice infected between 3 to 15 months as compared to 4 of 101 mice infected in this age group for the WT group that were housed under similar conditions. The abscesses presented around the eye or mouth and in some cases, more than one site of abscess formations were present in the same animal. In the majority of cases, microbiological analysis demonstrated that the bacterium responsible for the abscess was the Gram-positive opportunistic pathogen, Staphylococcus, and in which further conclusive identification was performed, S. aureus was the identified species. Although the increased incidence of infection in LFKO mice did not reach statistical significance, these findings are noteworthy considering the fact that LFKO mice displayed this increased susceptibility to infection even while housed in an SPF facility.
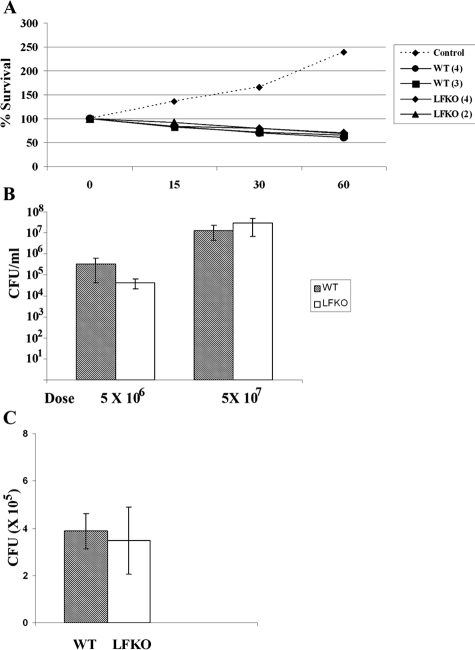
We next assessed the ability of LFKO neutrophils to kill S. aureus using both in vitro and in vivo models of S. aureus infection. The results of this analysis are shown in Figure 8. The bactericidal activity of LFKO neutrophils against opsonized S. aureus in an in vitro assay was similar to that of WT neutrophils (Figure 8A). Further, no difference in bacterial CFUs was observed between WT and LFKO mice using either an airpouch (Figure 8B) or intraperitoneal model (Figure 8C) of acute S. aureus infection that are highly contingent on neutrophil-dependent clearance of the bacteria.
Figure 8.
Antimicrobial response to acute S. aureus infection is similar in WT and LFKO mice. A: In vitro bactericidal assay. Log-phase growth S. aureus cells were incubated alone (control) or with purified WT or LFKO neutrophils. Aliquots were removed at 0, 15, 30, and 60 minutes, serially diluted, and plated on brain-heart infusion agar plates. Bacterial colonies were enumerated 24 hours later. Neutrophils used in this assay came from pooled samples obtained from two to four mice (number of mice indicated in brackets after genotype). The assay was repeated twice with similar results. B: In vivo S. aureus infection. WT and LFKO mice were infected in an airpouch with S. aureus at the doses indicated. Results are represented as the mean CFUs per ml of lavage fluid ± SEM. Eight WT and seven LFKO mice were used for the 5 × 106 CFU dose and eight mice per genotype were used for the 5 × 107 CFU dose. C: WT and LFKO mice were infected intraperitoneally with S. aureus (5 × 107 CFUs). Peritoneal fluid was extracted 4 hours after infection and bacterial CFUs were recorded. Results represent mean CFUs per mouse ± SEM. Six WT and six LFKO mice were used for the analysis.
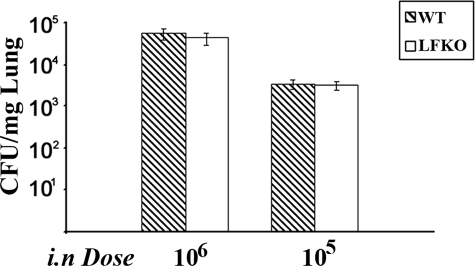
Finally, we determined the effect of LF deficiency on susceptibility to an acute bacterial infection with the Gram-negative pathogen, P. aeruginosa. WT and LFKO mice were challenged intranasally with P. aeruginosa (∼105 or 106 CFUs) and bacterial counts were assessed 6 hours later. No difference in bacterial killing was observed between the two groups with a similar number of CFUs observed in lung homogenates from WT and LFKO mice (Figure 9). Taken together, these results suggests that the antimicrobial function of LF may be more responsible for surveillance against opportunistic infections in the exocrine secretions rather than contributing to the host defense response of the neutrophil against infections with high doses of bacteria.
Figure 9.
Acute P. aeruginosa lung challenge. WT and LFKO mice were infected intranasally with P. aeruginosa at the doses indicated. Results are represented as the mean CFUs per mg of lung tissue ± SEM. Eight mice per genotype were used for the 106 CFU dose and 14 WT and 16 LFKO mice were used for the 105 CFU dose.
Alterations in Peripheral White Blood Cells in LFKO Mice
To determine whether LF was required for normal neutrophil cell distributions, we examined the percentages of white blood cells in the peripheral blood of WT and LFKO mice. The total white blood cell (WBC) counts were slightly decreased in LFKO mice versus WT controls (WT,7.7 ± 0.4 versus LFKO, 6.5 ± 0.4; P = 0.046). Interestingly, a decrease in lymphocyte percentages and a more marked elevation in neutrophil and eosinophil percentages were also observed in response to LF ablation (Table 1) suggesting that LF may play an immunomodulatory role in these leukocyte cell populations.
Table 1.
White Blood Cell Indices in WT and LFKO Mice
| Percent | WT | LFKO |
|---|---|---|
| Lymphocytes | 84.8 ± 1.0* | 79.6 ± 1.4 |
| Neutrophils | 8.7 ± 0.8* | 12.7 ± 1.1 |
| Monocytes | 1.5 ± 0.1 | 1.7 ± 0.2 |
| Eosinophils | 1.8 ± 0.2* | 3.4 ± 0.4 |
| Basophils | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Large unstained cells | 2.7 ± 0.3 | 2.0 ± 0.2 |
Mice (WT, n = 46; LFKO, n = 50) were between 7 and 11 weeks of age at the time of analysis. Values are presented as mean of percent cells ± SEM.
Significant difference (P < 0.05) between WT and LFKO.
Discussion
In the present study, we have used LFKO mice to decipher the role of LF in the host defense response of the neutrophil, with particular emphasis on determining its contribution to the phenotype of SGD syndrome. Previous studies have shown that C/EPBε is required for terminal differentiation of neutrophils and for elaboration of a variety of neutrophil functions including migration, phagocytosis, oxidative burst, and antimicrobial activity.9,14,24,25 Of these activities, we show here that the major secondary granule protein, LF, is a stimulus-dependent regulator of the oxidative burst response of the differentiated neutrophil, but it is not required for neutrophil maturation, migration, phagocytosis, granule mobilization and release, or for antimicrobial activity against acute S. aureus or P. aeruginosa bacterial challenges.
Intriguingly, we show that the oxidative burst is impaired in LFKO neutrophils in response to treatment with PMA, supporting an essential role for this protein in the production and/or metabolism of reactive oxygen species after stimulation with this potent protein kinase C agonist.64 Interestingly, despite the latter defect, we show that the oxidative burst of neutrophils in response to opsonized S. aureus does not require LF. The reason for this selective stimulus-dependent defect is currently unknown but it has been reported previously that activation of the oxidative burst in neutrophils can result in quantitative, qualitative, and subcellular differences in the pattern of reactive oxygen species formed depending on the stimulus used.50,64,65,66 Future work is warranted to determine the exact cellular and molecular mechanism by which LF regulates the production and/or metabolic fate of reactive oxygen species and to determine whether its ability to bind iron and/or impinge on cellular signaling pathways67,68,69,70,71 plays a role in this regulation. The negative effect of LF ablation on the oxidative burst activity is at variance with a study by Hock and colleagues72 in which expression of neutrophil secondary granule proteins, including LF, was ablated secondary to disruption of the transcription factor, Gfi-1, and normal oxidative burst activity was reported in response to PMA stimulation. This discrepancy may be explained by technical differences in the measurement of the reactive oxygen species and/or may be more reflective of the fact that Gfi-1-deficient atypical myeloid cells have acquired traits of the monocyte/macrophage lineage, which may compensate for the lack of LF.
LFKO mice displayed an approximately twofold increased incidence of spontaneous abscess formation compared to WT control mice housed in the same SPF facility. The bacterium identified in the majority of the abscesses was Staphylococcus and in which species determination was performed, S. aureus was the species identified. Staphylococcus infections have also been observed in SGD patients17,18,20 and a decreased intracellular bactericidal activity against this pathogen was reported in vitro using neutrophils from C/EPBε knockout mice.25 However, the incidence of opportunistic infection in LFKO mice was not statistically significant and was lower (∼8% of mice aged 3 to 15 months) than that reported for C/EPBε knockout mice (60% of mice by 2 to 5 months) housed under similar SPF conditions14 or SGD patients of which all patients are infected early in life.8,9,17 In addition, the neutrophil oxidative burst response to opsonized S. aureus was similar in WT and LFKO mice and neutrophils from both genotype groups exerted a similar bactericidal response against S. aureus in vitro. Further, no increased susceptibility to acute challenges with S. aureus in LFKO mice was observed using either airpouch or intraperitoneal models of infection that are highly dependent on neutrophils for killing. These results, together with the likely oral and ocular origins of the spontaneous S. aureus abscesses observed in LFKO mice, suggest that LF may be redundant for the antimicrobial response of the neutrophil against high doses of this pathogen whereas LF may play a more nonredundant role in the sentinel antimicrobial defenses at the mucosal surface. Future work is warranted to examine the protective role of LF by monitoring spontaneous infection rates in larger groups of LFKO and WT mice housed in nonbarrier facilities.
LF has previously been postulated to play a role in lymphocyte and neutrophil development and function67,68,69,70,71 and in eosinophil activation.73 Interestingly, peripheral blood analysis uncovered a significant decrease in the white blood cells of LFKO mice. Increases in the percentages of neutrophils and eosinophils (∼1.5-fold and 2-fold elevation, respectively, versus WT controls) and slighter decreases in the percentage of lymphocytes were also observed on LF ablation. Although the physiological significance of these findings remains to be established, these results suggest that LF may play an immunomodulatory role in these leukocyte subtypes.
In sum, the results presented here demonstrate that LF is a positive modulator of the neutrophil oxidative burst in response to PMA stimulation. However, LF is dispensable for many neutrophil functions and LF ablation alone is not sufficient to recapitulate the disease severity of SGD syndrome. This suggests that the diverse array of antimicrobial defense mechanisms present in the neutrophil provides considerable redundancy in the host response to infection. The observation that LFKO mice trend toward increased incidence of spontaneous infections suggest that LF may play an important role in the antimicrobial surveillance at the mucosal barrier, protecting against opportunistic infections, in particular, those caused by S. aureus. Finally, the alterations observed in the leukocytes in the peripheral blood of LFKO mice suggest that LF might play a broader regulatory role in the immune system.
Acknowledgments
We thank Eugene Paz and Sheila Duncan for technical assistance, Dr. Dorothy Lewis and Dr. William Hirt for helpful suggestions on the mouse phagotest and oxidative burst assays, Dr. Hattie Gresham for advice on the S. aureus airpouch experiments, and the Study Design and Clinical Specimen Core at the Texas Gulf Coast Digestive Diseases Center for help with the analysis of the incidence of infection in LFKO mice.
Footnotes
Address reprint requests to Orla M. Conneely, Ph.D., Department of Molecular and Cellular Biology, Baylor College of Medicine, One Baylor Plaza, Houston, TX, 77030. E-mail: orlac@bcm.tmc.edu.
Supported by the United States Department of Agriculture/Agricultural Research Service (grant 6250 5100 048 to O.M.C.) and the Cystic Fibrosis Foundation (grant WARD03G0 to P.P.W.).
Present address of P.W.P.: Department of Pediatrics, Children’s Hospital at Harvard Medical School, Boston, Massachusetts.
References
- Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–1315. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- Gallin JI. Neutrophil specific granule deficiency. Annu Rev Med. 1985;36:263–274. doi: 10.1146/annurev.me.36.020185.001403. [DOI] [PubMed] [Google Scholar]
- Dinauer MC, Lekstrom-Himes JA, Dale DC. Inherited neutrophil disorders: molecular basis and new therapies. Hematology (Am Soc Hematol Educ Program) 2000:303–318. doi: 10.1182/asheducation-2000.1.303. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka R, Barlow C, Lekstrom-Himes J, Castilla LH, Liu PP, Eckhaus M, Decker T, Wynshaw-Boris A, Xanthopoulos KG. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein epsilon-deficient mice. Proc Natl Acad Sci USA. 1997;94:13187–13192. doi: 10.1073/pnas.94.24.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehrer RI. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and “specific” granule deficiency. J Clin Invest. 1988;82:552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Gorius J, Mason DY, Buriot D, Vilde JL, Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am J Pathol. 1980;99:413–428. [PMC free article] [PubMed] [Google Scholar]
- Komiyama A, Morosawa H, Nakahata T, Miyagawa Y, Akabane T. Abnormal neutrophil maturation in a neutrophil defect with morphologic abnormality and impaired function. J Pediatr. 1979;94:19–25. doi: 10.1016/s0022-3476(79)80343-1. [DOI] [PubMed] [Google Scholar]
- Parmley RT, Ogawa M, Darby CP, Jr, Spicer SS. Congenital neutropenia: neutrophil proliferation with abnormal maturation. Blood. 1975;46:723–734. [PubMed] [Google Scholar]
- Strauss RG, Bove KE, Jones JF, Mauer AM, Fulginiti VA. An anomaly of neutrophil morphology with impaired function. N Engl J Med. 1974;290:478–484. doi: 10.1056/NEJM197402282900903. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes JA, Dorman SE, Kopar P, Holland SM, Gallin JI. Neutrophil-specific granule deficiency results from a novel mutation with loss of function of the transcription factor CCAAT/enhancer binding protein epsilon. J Exp Med. 1999;189:1847–1852. doi: 10.1084/jem.189.11.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Shiohara M, Kwok SH, Agematsu K, Komiyama A, Koeffler HP. Neutrophil-specific granule deficiency: homozygous recessive inheritance of a frameshift mutation in the gene encoding transcription factor CCAAT/enhancer binding protein-epsilon. Blood. 2001;97:2561–2567. doi: 10.1182/blood.v97.9.2561. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes JA. The role of C/EBP(epsilon) in the terminal stages of granulocyte differentiation. Stem Cells. 2001;19:125–133. doi: 10.1634/stemcells.19-2-125. [DOI] [PubMed] [Google Scholar]
- Verbeek W, Lekstrom-Himes J, Park DJ, Dang PM, Vuong PT, Kawano S, Babior BM, Xanthopoulos K, Koeffler HP. Myeloid transcription factor C/EBPepsilon is involved in the positive regulation of lactoferrin gene expression in neutrophils. Blood. 1999;94:3141–3150. [PubMed] [Google Scholar]
- Lekstrom-Himes J, Xanthopoulos KG. CCAAT/enhancer binding protein epsilon is critical for effective neutrophil-mediated response to inflammatory challenge. Blood. 1999;93:3096–3105. [PubMed] [Google Scholar]
- Baggiolini M, De Duve C, Masson PL, Heremans JF. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970;131:559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rado TA, Bollekens J, St Laurent G, Parker L, Benz EJ., Jr Lactoferrin biosynthesis during granulocytopoiesis. Blood. 1984;64:1103–1109. [PubMed] [Google Scholar]
- Masson PL, Heremans JF, Dive C. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966;14:735–739. [Google Scholar]
- Molloy AL, Winterbourn CC. Release of iron from phagocytosed Escherichia coli and uptake by neutrophil lactoferrin. Blood. 1990;75:984–989. [PubMed] [Google Scholar]
- Bullen JJ, Rogers HJ, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Aguila A, Herrera AG, Morrison D, Cosgrove B, Perojo A, Montesinos I, Perez J, Sierra G, Gemmell CG, Brock JH. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol Med Microbiol. 2001;31:145–152. doi: 10.1111/j.1574-695X.2001.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Ellison RT, III, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56:2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RR, Cole MF, McGhee JR. A bactericidal effect for human lactoferrin. Science. 1977;197:263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- Oseas R, Yang HH, Baehner RL, Boxer LA. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981;57:939–945. [PubMed] [Google Scholar]
- Boxer LA, Bjorksten B, Bjork J, Yang HH, Allen JM, Baehner RL. Neutropenia induced by systemic infusion of lactoferrin. J Lab Clin Med. 1982;99:866–872. [PubMed] [Google Scholar]
- Gahr M, Speer CP, Damerau B, Sawatzki G. Influence of lactoferrin on the function of human polymorphonuclear leukocytes and monocytes. J Leukoc Biol. 1991;49:427–433. doi: 10.1002/jlb.49.5.427. [DOI] [PubMed] [Google Scholar]
- Boxer LA, Haak RA, Yang HH, Wolach JB, Whitcomb JA, Butterick CJ, Baehner RL. Membrane-bound lactoferrin alters the surface properties of polymorphonuclear leukocytes. J Clin Invest. 1982;70:1049–1057. doi: 10.1172/JCI110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai K, Komine K, Komine Y, Kuroishi T, Kozutsumi T, Kobayashi J, Ohta M, Kitamura H, Kumagai K. Lactoferrin stimulates A Staphylococcus aureus killing activity of bovine phagocytes in the mammary gland. Microbiol Immunol. 2002;46:187–194. doi: 10.1111/j.1348-0421.2002.tb02685.x. [DOI] [PubMed] [Google Scholar]
- Miyauchi H, Hashimoto S, Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H. Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell Immunol. 1998;187:34–37. doi: 10.1006/cimm.1997.1246. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, van Asbeck BS, Jacob HS. Oxygen radical-induced erythrocyte hemolysis by neutrophils. Critical role of iron and lactoferrin. J Clin Invest. 1985;76:956–962. doi: 10.1172/JCI112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister JV, Bannister WH, Hill HA, Thornalley PJ. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982;715:116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Ambruso DR, Johnston RB., JR Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981;67:352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille JC, Doi K, Aisen P. Hydroxyl radical formation and iron-binding proteins. Stimulation by the purple acid phosphatases. J Biol Chem. 1987;262:59–62. [PubMed] [Google Scholar]
- Baldwin DA, Jenny ER, Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984;259:13391–13394. [PubMed] [Google Scholar]
- Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Spencer LK, Nulsen MF, McDonald PJ, Finlay-Jones JJ. Neutrophil activity in abscess-bearing mice: comparative studies with neutrophils isolated from peripheral blood, elicited peritoneal exudates, and abscesses. Infect Immun. 1986;51:936–941. doi: 10.1128/iai.51.3.936-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Weidemann MJ. Further characterization of the neutrophil oxidative burst by flow cytometry. J Immunol Methods. 1993;162:261–268. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- Ellson CD, Davidson K, Ferguson GJ, O’Connor R, Stephens LR, Hawkins PT. Neutrophils from p40phox−/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- Clemens RA, Newbrough SA, Chung EY, Gheith S, Singer AL, Koretzky GA, Peterson EJ. PRAM-1 is required for optimal integrin-dependent neutrophil function. Mol Cell Biol. 2004;24:10923–10932. doi: 10.1128/MCB.24.24.10923-10932.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Mocsai A, Zhang H, Ding RX, Morisaki JH, White M, Rothfork JM, Heiser P, Colucci-Guyon E, Lowell CA, Gresham HD, Allen PM, Brown EJ. Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity. 2003;19:95–104. doi: 10.1016/s1074-7613(03)00172-9. [DOI] [PubMed] [Google Scholar]
- Rothfork JM, Dessus-Babus S, Van Wamel WJ, Cheung AL, Gresham HD. Fibrinogen depletion attenuates Staphylococcus aureus infection by preventing density-dependent virulence gene up-regulation. J Immunol. 2003;171:5389–5395. doi: 10.4049/jimmunol.171.10.5389. [DOI] [PubMed] [Google Scholar]
- Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect Immun. 2000;68:3998–4004. doi: 10.1128/iai.68.7.3998-4004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Bentwood BJ, Henson PM. The sequential release of granule constituents from human neutrophils. J Immunol. 1980;124:855–862. [PubMed] [Google Scholar]
- Bainton DF, Miller LJ, Kishimoto TK, Springer TA. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987;166:1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Edashige K, Sato EF, Inoue M, Matsuno T, Utsumi K. Luminol chemiluminescence and active oxygen generation by activated neutrophils. Arch Biochem Biophys. 1991;285:325–330. doi: 10.1016/0003-9861(91)90367-r. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–145. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- Mayer-Scholl A, Averhoff P, Zychlinsky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7:62–66. doi: 10.1016/j.mib.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62:2540–2548. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem Cell Biol. 2002;80:95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Traver D, Bronson RT, Cameron S, Orkin SH. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity. 2003;18:109–120. doi: 10.1016/s1074-7613(02)00501-0. [DOI] [PubMed] [Google Scholar]
- Thomas LL, Xu W, Ardon TT. Immobilized lactoferrin is a stimulus for eosinophil activation. J Immunol. 2002;169:993–999. doi: 10.4049/jimmunol.169.2.993. [DOI] [PubMed] [Google Scholar]