Abstract
The cuprizone model of toxic demyelination in the central nervous system is commonly used to investigate the pathobiology of remyelination in the corpus callosum. However, in human demyelinating diseases such as multiple sclerosis, recent evidence indicates a considerable amount of cortical demyelination in addition to white matter damage. Therefore, we have investigated cortical demyelination in the murine cuprizone model. To induce demyelination, C57BL/6 mice were challenged with 0.2% cuprizone feeding for 6 weeks followed by a recovery phase of 6 weeks with a cuprizone-free diet. In addition to the expected demyelination in the corpus callosum, the cortex of C57BL/6 mice was completely demyelinated after 6 weeks of cuprizone feeding. After withdrawal of cuprizone the cortex showed complete remyelination similar to that in the corpus callosum. When C57BL/6 mice were fed cuprizone for a prolonged period of 12 weeks, cortical remyelination was significantly delayed. Because interstrain differences have been described, we also investigated the effects of cuprizone on cortical demyelination in BALB/cJ mice. In these mice, cortical demyelination was only partial. Moreover, cortical microglia accumulation was markedly increased in BALB/cJ mice, whereas microglia were absent in the cortex of C57BL/6 mice. In summary, our results show that cuprizone feeding is an excellent model in which to study cortical demyelination and remyelination, including contributing genetic factors represented by strain differences.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that leads to focal plaques of the central nervous system white matter and axonal loss.1,2 However, in recent years studies have shown that lesions also arise within gray matter structures, particularly the cortex.3 Cerebral and cerebellar cortical lesions may be characterized by complete demyelination with relative preservation of neurons, axons, and synapses.4 Cortical demyelination and diffuse white matter damage are particularly prominent in primary and secondary progressive MS, but are rare in the acute or relapsing form.5 In contrast, classical active inflammatory plaques predominantly occur in patients with acute or relapsing MS, whereas focal white matter lesions in patients with progressive MS are either inactive or show slow expansion at the edges. Using active sensitization with myelin oligodendrocyte glycoprotein cortical demyelination could be induced only in certain rat strains, whereas all analyzed rat strains developed extensive white matter demyelination.6
Although new aspects of underlying pathomechanisms of demyelination in MS are being discovered continuously, the complex pathophysiological interactions still are far from being completely understood. Therefore rodent models like the cuprizone-induced toxic demyelination have become helpful in exploring the underlying mechanisms. However, all models only partly mimic the processes of MS with every model having its advantages and disadvantages. Cuprizone intoxication is a commonly used model to study experimental remyelination, with the corpus callosum and the superior cerebellar peduncles being the most frequently investigated white matter tracts.7,8 In this model young adult mice are fed with the copper chelator cuprizone (bis-cyclohexanone oxaldihydrazone), which leads to a reproducible central nervous system demyelination within weeks.9 After removal of the toxin spontaneous remyelination occurs.10 Even though cortical demyelination has recently been described,11 there was no detailed description and cortical remyelination has not yet been investigated. Here, we describe that cortical de- and remyelination are a prominent feature in this model and characterize the pathological process in detail.
Materials and Methods
Animals and Induction of Demyelination
C57BL/6 male mice were obtained from Charles River (Sulzfeld, Germany). BALB/cJ male mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals underwent routine cage maintenance once a week and were microbiologically monitored according to Federation of European Laboratory Animal Science Associations recommendations.12 Food and water were available ad libitum. All research and animal care procedures were approved by the Review Board for the Care of Animal Subjects of the district government (Lower Saxony, Germany) and performed according to international guidelines on the use of laboratory animals.
Demyelination was induced by feeding 8-week-old male C57BL/6 mice a diet containing 0.2% cuprizone (bis-cyclohexanone oxaldihydrazone; Sigma-Aldrich Inc., St. Louis, MO) mixed into a ground standard rodent chow. Cuprizone feeding was maintained for 6 weeks, thereafter mice were put on a normal chow for another 6 weeks. At different time points [0 (control), 4, 6, 6.5, 7, 8, and 12 weeks] animals were perfused with 4% paraformaldehyde in phosphate buffer via left cardiac ventricle as previously described.12 A group size of five to six animals was investigated at all time points. The brains were removed, postfixed in 4% paraformaldehyde, and paraffin-embedded. For light microscopy, 7-μm serial paraffin sections were cut and dried at 37°C overnight.
For chronic demyelination the cuprizone diet was maintained for 12 weeks. Thereafter mice were put on a normal chow for another 12 weeks. Animals were investigated at different time points [0 (control), 4, 6, 8, 10, 12, 12.5, 13, 14, 16, 20, and 24 weeks]. A group size of five to six animals was investigated at all time points. To explore strain dependency further experiments using 8-week-old BALB/cJ mice were performed. Demyelination was induced by feeding 0.2% cuprizone. Animals were investigated at different time-points (0, 3, 6, 7, 8, and 12 weeks). A group size of five animals was investigated at each time point.
Histology and Immunohistochemistry
Histology and immunohistochemistry were performed as previously described.13 Briefly, 7-μm serial paraffin sections between bregma −0.94 and bregma −1.8 (according to mouse atlas by Paxinos and Franklin14) were analyzed. Sections were stained for myelin with Luxol-fast blue (LFB)/periodic acid-Schiff (PAS) base. For immunohistochemistry, paraffin-embedded sections were dewaxed, rehydrated, and microwaved for 5 minutes in 10 mmol/L citrate buffer (pH 6.0). Sections were quenched with H2O2, blocked for 1 hour in phosphate-buffered saline (PBS) containing 3% normal goat serum, 0.1% Triton X-100, and then incubated overnight with primary antibody. The following primary antibodies were used: for myelin proteins PLP (mouse IgG; Serotec, Düsseldorf, Germany) and MBP (mouse IgG; Sternberger Monoclonals Inc., Berkeley, CA), for microglia mac-3 (rat IgG; BD Pharmingen, Heidelberg, Germany), for astrocytes GFAP (mouse IgG; Chemicon, Hampshire, UK). After washing, sections were further incubated with biotinylated secondary antibody (Vector Laboratories, Burlingame, UK) for 1 hour, followed by peroxidase-coupled avidin-biotin complex (ABC kit, Vector Laboratories). Reactivity was visualized with diamino-3,3′benzidine (Dako Cytomation, Hamburg, Germany). Isotype controls and stainings without primary antibody were used as controls. There was no significant nonspecific staining of mouse immunoglobulins in mouse tissue.
Determination of Cortical De- and Remyelination
For cortical de- and remyelination myelin protein-stained sections for PLP and MBP were scored by three blinded observer using a scale from 0 (complete demyelination) to 4 (normal myelin). Scoring of myelination was directly done at the light microscope. Camera images of a part of the cortex were drawn and used as representatives for each score-degree as shown in Figure 1, D–I. In addition, cortical myelin was always compared to the most frequently studied corpus callosum myelin. For this, stained sections were scored in a blinded manner by three observers and graded on a scale from 0 (complete demyelination) to 3 (normal myelin) as described previously.13
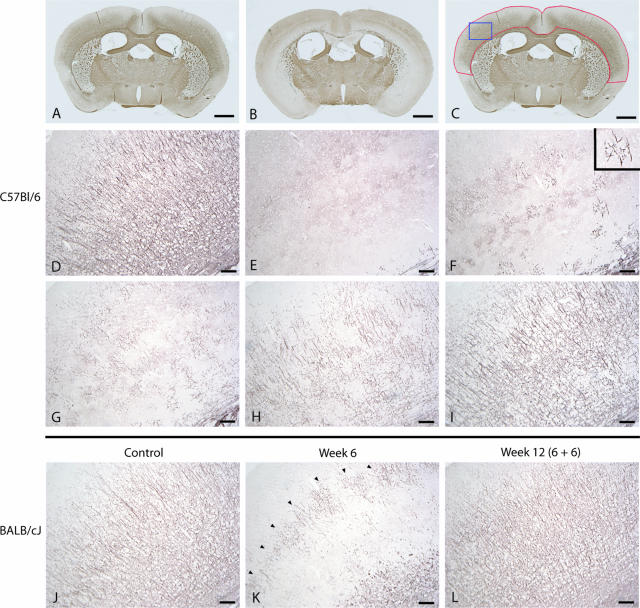
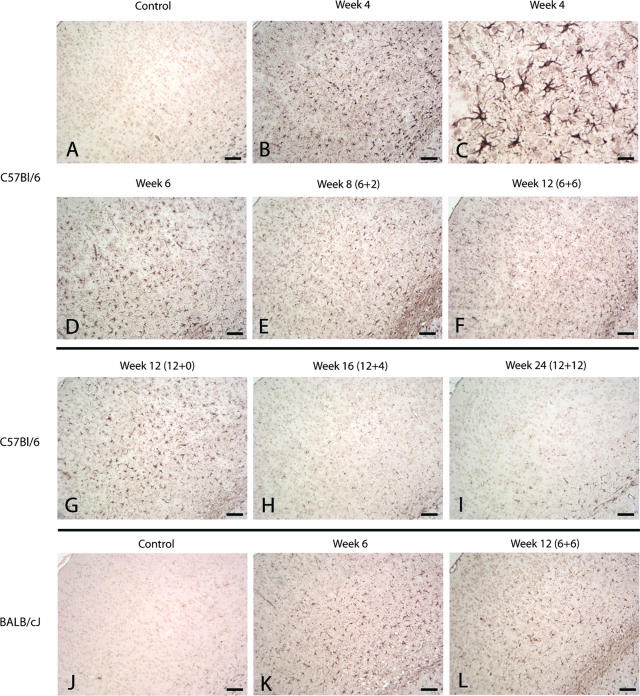
Figure 1.
For determination of cortical de- and remyelination, brain sections were immunohistochemically stained for PLP (A–I: C57BL/6 mice; J–L: BALB/cJ mice). In A brains of control animals are shown, whereas B represents complete demyelination after 6 weeks of cuprizone diet. All brain sections were directly scanned using a light microscope for the complete cortex that is marked with a red line in C. The blue line marked area of the cortex in C represents the demonstrated cortex area of which camera images were drawn. Camera images were used as representatives for each score-degree as shown in D–I. Images are representative for five to six animals per time point. D: a normal myelination structure in the cerebral cortex of control animals is shown (score 4), whereas E represents complete cortical demyelination (score 0). First indicators of beginning remyelination are several separately radiating myelin structures as shown in F (score 1). G: In the course of further remyelination a beginning crosslinking between the several myelination areas could be identified (score 2). In H there is almost normal myelination in the cortex but several demyelinated areas indicate cortical pathology (score 3). I: Complete remyelination after demyelination. J: Normal cortical myelination pattern of BALB/cJ mice is shown. K: After 6 weeks of cuprizone feeding a band-like residual myelin pattern remained in the middle of the cortex (arrowheads) showing that cortical demyelination was still incomplete. L: After 6 weeks of cuprizone-free diet, the cortical myelination looked complete again. Scale bars: 1 cm (A–C); 100 μm (D–L).
Quantification of Cells
Immunopositive cells for mac-3 and with identified nucleus (counterstaining with hematoxylin) were counted left and right of the midline within the corpus callosum at least within an area of 0.125 mm2 using a magnification of ×40 (Leica DMLB, Wetzlar, Germany). Counted cells were given as cell number per mm2.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance with the factor “time/week” followed by the Fisher-PLSD test for post hoc comparison if appropriate. All data are given as arithmetic means ± SEM. P values of the different analyses of variance are given in the Results, and group comparisons derived from post hoc analysis are provided in the figures. In the latter case, significant effects are indicated by asterisks or rhombs (*#P < 0.05; **##P < 0.01; ***###P < 0.001).
Results
Cortical De- and Remyelination Is Prominent in the Cuprizone Model
To investigate whether mice show cortical myelin damage after cuprizone treatment, brain sections were immunohistochemically stained for the myelin proteins MBP and PLP (Figure 1, A–I). A marked demyelination after exposure of C57BL/6 mice to 0.2% cuprizone was evident as determined by a significant loss of myelin already after 4 weeks compared to control animals (Figure 2A, P < 0.001). After 6 weeks of cuprizone treatment no cortical myelin was detectable. After removal of cuprizone from the diet after 6 weeks a time-dependent increase of remyelination was observed. Normal myelin structures were seen already 6 weeks after withdrawal of the toxin in all animals. Because there was no significant difference between the analyzed PLP- and MBP-stained sections, only PLP results are shown. The sensitivity of the LFB staining was not sufficient to uncover cortical myelination.
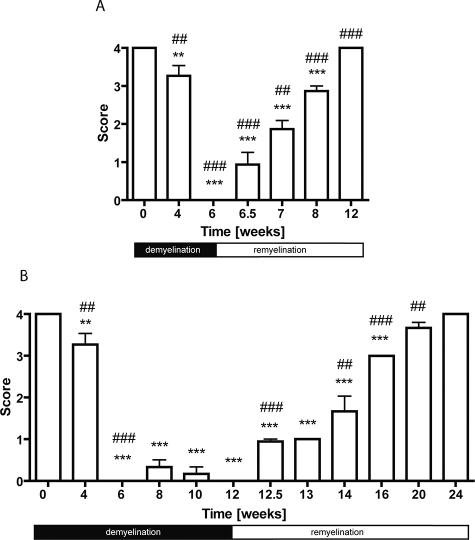
Figure 2.
Time course of acute (A) and chronic (B) cortical de- and remyelination induced by 0.2% cuprizone in C57BL/6 mice. The extent of cortical de- and remyelination was assessed by scoring PLP-stained sections. A score of 4 represents complete myelination, whereas a score of 0 represents complete demyelination (compare to Figure 1). Significant effects versus controls are indicated by asterisks (*P < 0.05, **P < 0.01, and ***P < 0.001) and significant effects versus the preceding time point are indicated by rhombs (#P < 0.05, ##P < 0.01, and ###P < 0.001).
Cortical Remyelination Depends on the Duration of Cuprizone Treatment
When C57BL/6 mice were maintained on a cuprizone diet beyond 6 weeks, cortical demyelination persisted (Figure 2B). Significant remyelination in the cortex began only after withdrawal of the toxin at week 12. Thereupon a time-dependent increase of remyelination was seen (P < 0.001). Cortical remyelination was significantly delayed in the chronic 12-week cuprizone feeding as compared to the acute demyelination for 6 weeks (P < 0.001). Time to reappearance of normal myelin was approximately twice as long.
De- and Remyelination in the Cortex Are Similar to Changes in the Corpus Callosum
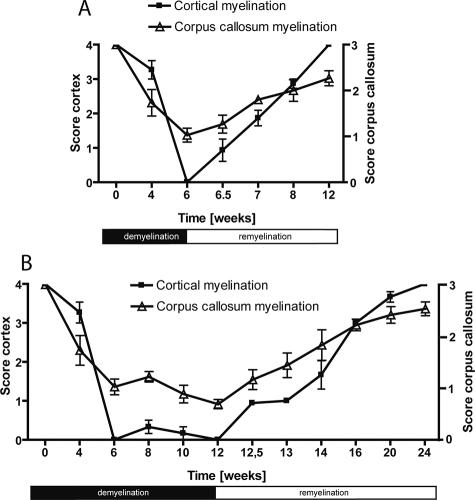
To further characterize the model for cortical de- and remyelination we compared for each time point the changes of the cortical myelin structure with the well characterized processes in the corpus callosum. As seen in Figure 3 in the acute demyelination model (Figure 3A) as well as in the chronic demyelination model (Figure 3B) complete cortical demyelination can be induced (acute model: lowest value at week 6, score 0 ± 0; chronic model: lowest value at week 12, score 0 ± 0). This demyelination was even more prominent than in the corpus callosum where a severe but not complete demyelination was observed. Nonetheless, the time course of de- and remyelination was nearly identical in both short- and long-term demyelination.
Figure 3.
Comparison of the course of de- and remyelination in the cortex as well as in the more commonly investigated corpus callosum in the acute (A) and chronic (B) cuprizone model. For cortical myelination changes: a score of 4 represents complete myelination, whereas a score of 0 represents complete demyelination. For myelination changes in the corpus callosum: a score of 3 represents complete myelination, whereas a score of 0 represents complete demyelination.
Extent of Cortical Demyelination in Mice Is Strain-Dependent
Because strain dependency could play a role in cortical demyelination we performed further investigations in BALB/cJ mice, which were fed with 0.2% cuprizone for 6 weeks. Control BALB/cJ animals (Figure 1J) showed a similar myelin structure before cuprizone treatment compared to C57BL/6 mice (Figure 1D). Loss of myelin in the cortex was not seen before 6 weeks of cuprizone feeding. Furthermore the BALB/cJ mice showed only incomplete cortical demyelination as compared with C57BL/6 mice. A band-like residual myelin structure remained in the middle of the cortex (Figure 1K). Time course of remyelination after withdrawal of the toxin showed no differences between the two strains (data not shown). De- and remyelination in the corpus callosum followed the same patterns in both mouse strains (data not shown).
Cortical Microglia Response in De- and Remyelination Is Strain-Dependent
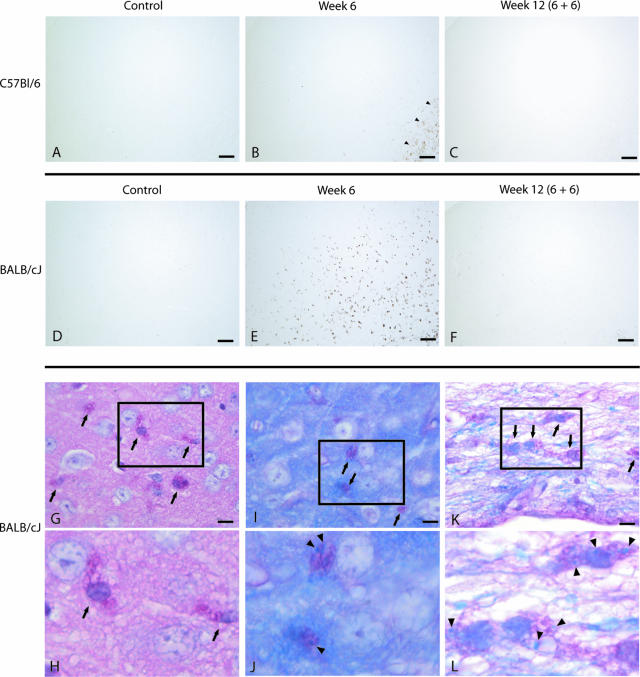
Accumulation of microglia/macrophages was investigated by mac-3 staining in both mouse strains. In contrast to the corpus callosum only a few microglia were seen in the cortex after 6 weeks of cuprizone diet in the C57BL/6 strain (Figure 4, A–C). At all other time points of investigation microglia were absent and only sporadically seen in the cortex. In the BALB/cJ strain cortical microglia accumulation began after 3 weeks of cuprizone diet and peaked in a pronounced manner after 6 weeks of cuprizone (Figure 4, D–F). After withdrawal of the toxin the number of cortical microglia decreased and microglia were only sporadically seen at week 12.
Figure 4.
For determination of cortical microglia/macrophages brain sections of control animals (A: C57BL/6; D: BALB/cJ), animals after 6 weeks of cuprizone treatment (B: C57BL/6; E: BALB/cJ), and animals after 6 weeks of cuprizone free-diet (C: C57BL/6; F: BALB/cJ) were immunohistochemically stained for mac-3. B: In C57BL/6 mice microglia were seen in the area of the corpus callosum after 6 weeks of cuprizone diet (arrowheads), but were almost absent in the cortex. E: In contrast a marked cortical microglia accumulation occurs in BALB/cJ mice after 6 weeks of cuprizone treatment. G–H: In the cortex of BALB/cJ mice accumulation of PAS-positive microglia/macrophages was prominent after 6 weeks of cuprizone (arrows). As seen in the LFB/PAS-stained sections many of the PAS-positive microglia (indicated by arrows in the cortex, I; and in the area of the corpus callosum, K) contained myelin debris (indicated by arrowheads in the cortex, J; and in the area of the corpus callosum, L). Scale bars: 100 μm (A–F); 10 μm (G, I, K).
To determine the activation state of microglia after cuprizone diet, images with higher magnification of brain sections stained for PAS and for LFB/PAS are shown (Figure 4, G–L). In the cortex of BALB/cJ mice accumulation of PAS-positive microglia/macrophages began after 3 weeks of cuprizone diet and peaked in a pronounced manner after 6 weeks of cuprizone, similar to the mac-3 staining. Many of the PAS-positive microglia contained myelin debris. PAS-positive microglia were only sporadically seen in the cortex of C57BL/6 mice after cuprizone treatment (data not shown). Microglia infiltration in the corpus callosum was also strain-dependent (P < 0.001). In controls only a few cells were seen without significant strain differences (C57BL/6, 0 ± 0; BALB/cJ, 42.7 ± 30.0). Interestingly, after 6 weeks of cuprizone diet microglia numbers were higher in C57BL/6 mice (1541.3 ± 123.4) as compared to BALB/cJ mice (467.2 ± 21.6).
Cortical Astrocyte Response during De- and Remyelination
There was a marked increase in GFAP-positive astrocytes in the cortex already after 4 weeks of cuprizone treatment in C57BL/6 mice (Figure 5, B and C). Furthermore the bodies of the astrocytes were hypertrophic and the processes were thick. The decrease of astrogliosis in the cortex began slowly after withdrawal of the toxin. Thereupon a time-dependent decline was seen, but astrocytes were still present at week 16 in the chronic demyelination model (Figure 5H) and at week 8 in the acute demyelination model (Figure 5E). At the last investigated time points (week 24 in the chronic model, Figure 5I; week 12 in the acute model, Figure 5F) only a few astrocytes were still seen in the cortex. There was no significant difference between the mice strains. Brain sections of control BALB/cJ mice and of BALB/cJ animals after 6 weeks and 12 weeks of cuprizone diet are shown in Figure 5, J–L.
Figure 5.
Brain sections of C57BL/6 (A–I) and BALB/cJ (J–L) mice that were immunohistochemically stained for GFAP. In A and J only some occasional GFAP-positive astrocytes are seen in the cortex. As seen in B and C (higher magnification) after 4 weeks of cuprizone feeding a marked cortical astrogliosis with hypertrophied bodies and thick processes were seen. Thereupon a time-dependent decline was seen. Scale bars: 100 μm (A, B, D–L); 25 μm (C).
Discussion
Here we show that feeding of the neurotoxicant cuprizone represents an appropriate model to study cortical de- and remyelination. Although it has long been recognized that de- and remyelination can occur in the corpus callosum and in the superior cerebellar peduncles7,8 the extent of cortical damage has not been determined to date in detail. The present study showed that in young adult male C57BL/6 mice both demyelination as well as the consecutive remyelination in the cortex were almost complete. Exposure of mice to dietary cuprizone for 6 weeks induced loss of cortical myelin. When animals were allowed to recover on a regular diet, the myelin loss was completely compensated within another 6 weeks. If exposure to cuprizone was extended to 12 weeks, demyelination persisted. Only after removal of cuprizone from the diet remyelination started. Interestingly the time for recovery was delayed by a factor of ∼2 compared to the short demyelination phase of 6 weeks. When we compared the changes of cortical myelin to those in the corpus callosum, de- and remyelination processes occurred in the same temporal pattern. Surprisingly, demyelination in the cortex was more prominent than in the corpus callosum where a severe but not complete demyelination was observed. This may suggest that cortical myelin changes are even more sensitive in the cuprizone model.
Because the LFB staining offers excellent results investigating myelin changes in the corpus callosum after cuprizone treatment it is a widely and the most often used myelin staining method. However, its sensitivity is not sufficient to uncover cortical myelination, which might explain why cortical changes were not described in detail to date using this method. Therefore for analysis of cortical de- and remyelination immunohistochemical stainings for myelin protein should be preferred. In contrast to C57BL/6 mice, BALB/cJ mice challenged with 0.2% cuprizone exhibited less cortical demyelination. It is well known that the extent of cuprizone-induced damage to corpus callosum myelin primarily depends on the cuprizone dose, and the age and the strain of mice.15,16 This seems to hold true also for the cortical changes described here.
Microglia were absent or only sporadically seen in the cortex of C57BL/6 mice. As previously described most of the microglia were seen in the area of the corpus callosum.16 In contrast, BALB/cJ mice showed a marked cortical microglia recruitment after cuprizone treatment that was associated with a less pronounced cortical demyelination. These observations indicate different pathomechanisms between the investigated mice strains leading to various cortical myelin damages possibly via microglia responses. Storch and colleagues6 demonstrated that the incidence and extent of cortical demyelination in rat experimental autoimmune encephalomyelitis is regulated by genetic influences from the MHC I and II isotypes and alleles. Cortical demyelination could be induced only in certain rat strains, whereas all analyzed rat strains developed extensive white matter demyelination. These rat strains showed minimal macrophage recruitment in active lesions. Because the C57BL/6 mice and the BALB/cJ mice have different MHC haplotypes,17 it may be an influencing factor for the strain-dependent demyelination after cuprizone treatment.
The specific role of microglia in disease is still controversial because there is evidence that microglia have both neuroprotective and neurodegenerative functions. Some studies reported increased neuronal damage in vitro by activated cultured microglia.18,19 In experimental autoimmune encephalomyelitis persistent microglial activation has been associated with cortical neuropathology.20 However, other reports support the concept that proinflammatory cytokines produced by microglia are neuroprotective as shown by increased excitotoxicity in tumor necrosis factor-α/interleukin-1β knockout mice.21 Simard and Rivest22 have shown that microglia activation and nuclear factor-κB signaling are potent neuroprotective mechanisms after acute excitotoxicity. Moreover, there are several lines of evidence that microglia are also supportive for remyelination. Depletion of macrophages impairs remyelination after lysolecithin-induced demyelination.23 In particular, macrophages are beneficial during early phases of remyelination. Macrophage reduction corresponded with delayed recruitment of oligodendrocyte progenitor cells. Because oligodendrocyte progenitor cell recruitment precedes myelin phagocytosis, this result indicated a macrophage effect on oligodendrocyte progenitor cells independent of myelin debris clearance.24 In addition, a reparative role for inflammatory cytokines such as tumor necrosis factor-α in the central nervous system is also known.25 In the cuprizone model the lack of tumor necrosis factor-α in mice led to a significant delay in remyelination by means of reduction in the number of mature oligodendrocytes. In our work a marked cortical microglia accumulation in BALB/cJ mice was associated with a less pronounced cortical demyelination indicating protective effects for myelin-laden microglia in demyelination. Microglia/macrophages containing myelin are already known to confer anti-inflammatory functions. In MS lesions myelin-containing foam cells consistently express a series of anti-inflammatory molecules while lacking proinflammatory cytokines.26
Analysis of astrocytes showed a marked increase in cortical astrogliosis after cuprizone treatment in both mice strains. After withdrawal of the toxin a time-dependent slow decrease of the astrogliosis occurred. The role of this astrocytosis is currently not clear. It can only be speculated if this represents an unspecific activation or if these astrocytes actively interfere with the de- or remyelination. Further experiments need to clarify a possible role of astrocytes in the cortical myelination changes after the cuprizone diet.
In recent years, the presence of cortical lesions has come into the focus of MS research.3,5,27 The pathological mechanisms leading to cortical demyelination and the functional clinical consequences of the cortical lesions have not yet been completely understood. Cortical lesions may lead to sensory and motor deficits, as well as to cognitive impairment, which is found in MS patients.28 Kutzelnigg and colleagues5 could show that cortical demyelination and diffuse white matter damage are prominent in primary and secondary progressive MS, but are sparse in the acute or relapsing form.5 Active cortical lesions in progressive MS show only very mild lymphocytic infiltrates in the lesion parenchyma.5,29 Because the pathomechanisms of cortical demyelination and possible repair processes have not yet been studied in detail the cuprizone model may help to understand the molecular mechanisms in the future and serve as an complementary model to fill gaps not represented by other animal models of cortical demyelination.30 In contrast to cortical lesions induced by focal injections of proinflammatory mediators30 in the cuprizone model there is no breakdown of the blood-brain-barrier.31,32 Furthermore, the cuprizone model offers the advantage of consistent, anatomically reproducible and well detectable de- and remyelination processes.8,13,33
In conclusion, the present work demonstrates that cuprizone feeding is an excellent model to study cortical pathology during de- and remyelination. Further experiments should follow to clarify the pathophysiology and the functional consequences of cortical demyelination.
Acknowledgments
We thank I. Cierpka-Leja for excellent technical assistance.
Footnotes
Address reprint requests to Prof. Dr. Martin Stangel, Department of Neurology, Medical School of Hannover, Carl-Neuberg-Str-1, 30625 Hanover, Germany. E-mail: stangel.martin@mh-hannover.de.
Supported by the German Research Foundation (grant SFB566, project A11) and the Medical School of Hannover, Hochschulinterne Leistungsförderung.
T.S. and M.L. contributed equally to this study.
References
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Noseworthy J. Multiple sclerosis: recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment. Curr Opin Neurol. 2001;14:259–269. doi: 10.1097/00019052-200106000-00002. [DOI] [PubMed] [Google Scholar]
- Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Faber-Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Storch MK, Bauer J, Linington C, Olsson T, Weissert R, Lassmann H. Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatability complex (MHC) haplotype-related. J Neuropathol Exp Neurol. 2006;65:1137–1142. doi: 10.1097/01.jnen.0000248547.13176.9d. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF. Remyelination of the superior cerebellar peduncle in old mice following demyelination induced by cuprizone. J Neurol Sci. 1974;22:121–126. doi: 10.1016/0022-510x(74)90059-8. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. J Neuropathol Exp Neurol. 2006;65:245–256. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, Li X, Dyck LE, Devon RM, Deng Y, Li XM: Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry 2007, [Epub ahead of print]; doi: 10. 1038/sj.mp.4002064 [DOI] [PubMed] [Google Scholar]
- Rehbinder C, Baneux P, Forbes D, van Herck H, Nicklas W, Rugaya Z, Winkler G. FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Animal Health accepted by the FELASA Board of Management, November 1995. Lab Anim. 1996;30:193–208. doi: 10.1258/002367796780684881. [DOI] [PubMed] [Google Scholar]
- Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F, Grothe C, Baumgaertner, Stangel M: Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol 2007, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. New York: Academic Press,; The Mouse Brain in Stereotaxic Coordinates. 2001 [Google Scholar]
- Stidworthy MF, Genoud S, Suter U, Mantei N, Franklin RJ. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 2003;13:329–339. doi: 10.1111/j.1750-3639.2003.tb00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 1998;92:38–49. doi: 10.1016/s0165-5728(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Hancock GE, Tebbey PW, Scheuer CA, Pryharski KS, Heers KM, LaPierre NA. Immune responses to the nonglycosylated ectodomain of respiratory syncytial virus attachment glycoprotein mediate pulmonary eosinophilia in inbred strains of mice with different MHC haplotypes. J Med Virol. 2003;70:301–308. doi: 10.1002/jmv.10395. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue LF. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. J Neurosci Res. 2005;81:412–425. doi: 10.1002/jnr.20484. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Wang Y, Kivisäkk P, Bronson RT, Meyer M, Imitola J, Khoury SJ. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing-remitting experimental autoimmune encephalomyelitis. Brain. 2007;130:2816–2829. doi: 10.1093/brain/awm219. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26:143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J Comp Neurol. 2007;504:716–229. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–175. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–526. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Bø L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Bø L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain. 2006;129:972–983. doi: 10.1093/brain/awl135. [DOI] [PubMed] [Google Scholar]
- Bakker DA, Ludwin SK. Blood-brain barrier permeability during cuprizone-induced demyelination. Implications for the pathogenesis of immune-mediated demyelinating diseases. J Neurol Sci. 1987;78:125–137. doi: 10.1016/0022-510x(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Kondo A, Nakano T, Suzuki K. Blood-brain barrier permeability to horseradish peroxidase in twitcher and cuprizone-intoxicated mice. Brain Res. 1987;425:186–190. doi: 10.1016/0006-8993(87)90499-9. [DOI] [PubMed] [Google Scholar]
- Mason JL, Langaman C, Morell P, Suzuki K, Matsushima GK. Episodic demyelination and subsequent remyelination within the murine central nervous system: changes in axonal caliber. Neuropathol Appl Neurobiol. 2001;27:50–58. doi: 10.1046/j.0305-1846.2001.00301.x. [DOI] [PubMed] [Google Scholar]