Abstract
Mutation of the adult hepatocyte keratins K8 and K18 predisposes to liver disease. In contrast, exocrine pancreas K8 and K18 are dispensable and are co-expressed with limited levels of membrane-proximal K19 and K20. Overexpression of mutant K18 or genetic ablation of K8 in mouse pancreas is well tolerated whereas overexpression of K8 causes spontaneous chronic pancreatitis. To better understand the effect of exocrine pancreatic keratin overexpression, we compared transgenic mice that overexpress K18, K8, or K8/K18, associated with minimal, modest, or large increases in keratin expression, respectively, with nontransgenic wild-type (WT) mice. Overexpression of the type-II keratin K8 up-regulated type-I keratins K18, K19, and K20 and generated K19/K20-containing neocytoplasmic typical or short filaments; however, overexpression of K18 had no effect on K8 levels. K8- and K18-overexpressing pancreata were histologically similar to WT, whereas K8/K18 pancreata displayed age-enhanced vacuolization and atrophy of the exocrine pancreas and exhibited keratin hyperphosphorylation. Zymogen granules in K8/K18 pancreata were 50% smaller and more dispersed than their normal apical concentration but were twice as numerous as in WT controls. Therefore, modest keratin overexpression has minor effects on the exocrine pancreas whereas significant keratin overexpression alters zymogen granule organization and causes aging-associated exocrine atrophy. Keratin absence or mutation is well tolerated after pancreatic but not liver injury, whereas excessive overexpression is toxic to the pancreas but not the liver when induced under basal conditions.
Intermediate filaments (IFs) consist of a large group of proteins that are expressed in a tissue-specific manner.1,2 Examples of the cell- or tissue-specific expression of IFs, which is reflected by a broad range of related diseases, includes neurofilaments in neuronal cells, desmin in muscle, and glial fibrillary acidic protein in glial cells.3 Keratins (Ks) are the IFs of epithelial cells and exist as obligate noncovalent heteropolymers with a minimum of one type-I keratin (K9 to K20) and one type-II keratin (K1 to K8).4 Adult hepatocytes are distinct in that they express only K8 and K18, whereas other glandular epithelia such as those of the intestine, pancreatic, or biliary ducts express type-II K8 and K7 and type-I K18, K19, and/or K20.4,5 Pancreatic acinar cells typically include two filamentous keratin compartments and keratin compliments that may vary slightly among species; a network of cytoplasmic keratins that under basal conditions express primarily K8 and K18, and bundles of apicolateral membrane-proximal keratins that include K8/K18/K19 and low levels of K20.6,7,8
An important function of K8/K18 in hepatocytes is protection from mechanical and nonmechanical forms of stress as demonstrated using a variety of transgenic animal models.9,10 Numerous human diseases associate with IF mutations,3,11,12,13 and in the case of K8/K18 several human association studies have provided strong evidence that the KRT8 and KRT18 genes are susceptibility genes for liver disease development.9,14 The human liver disease association studies are supported by an extensive body of animal data involving mice that express mutant K8 or K18 or that are null for K8 or K18.9 The animal data, coupled with ex vivo and in vitro studies, also showed that K8/K18 prevents liver injury by protecting hepatocytes from undergoing apoptosis.9,15,16
In contrast to findings in the liver, keratin function and disease association in the pancreas are less clear although disease-association is unlikely to be significant. For example, K8-null6 and keratin assembly-deficient K18-mutant mice17 have similar susceptibility to pancreatic injury using two experimental pancreatitis models, which may be related to compensatory overexpression of Reg-II.18 However, transgenic mice that overexpress human K8 develop progressive chronic pancreatitis and increased cell proliferation and apoptosis.19 This led to the search and reporting of K8 G61C variants in patients with chronic pancreatitis20 that was not substantiated to associate with chronic pancreatitis in two subsequent large studies.21,22 These latter human association studies in patients with pancreatitis suggest that K8/K18 variants are unlikely to be as significant in pancreatic disease as they are in liver disease. Nevertheless, both mouse pancreatic23 and liver24 injury result in a nearly threefold increase in K8/K18 levels despite their already abundant baseline expression.23 In acute experimental pancreatitis, keratin induction includes the up-regulation of normally apicolaterally distributed K19 and K20 that incorporate into existing and similarly up-regulated K8/K18 cytoplasmic filaments. On recovery from injury, the up-regulated keratins return to their basal levels and cell compartment distribution.6,7,17 The functional significance of keratin overexpression in the pancreas is unknown.
Forced overexpression of several wild-type (WT) IFs has been performed to study their function and potential disease association.4,25 In addition, mice that overexpress IFs have been used as a control for the overexpression of mutant IFs. For example, mice that overexpress WT human (h)K18 have a normal phenotype26,27 whereas those that overexpress mutant R89C hK18 have disrupted cytoplasmic keratin filaments in the liver and pancreas that render hepatocytes fragile27 and susceptible to toxic liver injury28 and apoptosis29 but not to pancreatic injury.17 Similarly, overexpression of WT K8 is well tolerated except for the predisposition to Mallory-Denk body inclusion formation30 (formerly called Mallory bodies),24 whereas overexpression of the liver disease patient-related K8 variant (G61C) predisposes to liver injury and apoptosis.31 The pancreas phenotype of mice that overexpress either hK831 or mouse (m)K830 was unremarkable but has not been studied in great detail. For other IFs, overexpression of WT neurofilaments leads to a phenotype mimicking human amyotrophic lateral sclerosis that in a few cases may be related to neurofilament mutation,32 and overexpression of WT glial fibrillary acidic protein leads to a phenotype that mimics Alexander disease that is caused by glial fibrillary acidic protein mutation.33 For the pancreas, the above-described report of spontaneous pancreatitis in mice that overexpress hK819 led us to hypothesize that the expression levels of WT K8/K18 in the pancreas dictate whether injury takes place. We tested this hypothesis using transgenic mice that overexpress different levels of K8 and K18. We show that increasing keratin expression correlates with the extent of pancreatic injury, whereas the liver appears to be relatively immune from such overexpression effects.
Materials and Methods
Mice
All transgenic and nontransgenic mice had the same FVB/n genetic background. Transgenic mice that overexpress WT hK18 (hK18 mice) were previously described.26,27,29,34 The mice that overexpress mouse K8 (mK8 mice) and their interbreeding with hK18 mice (to generate K8/K18 mice) and genotype screening were performed as described.30 The hK18 and mK8 transgenes were derived from genomic clones that retain the transcriptional elements necessary for tissue-specific expression that is similar to the corresponding endogenous genes. hK8 overexpressor mice,31 K8-null mice,35 and K19-null mice36 were generated and maintained as previously described.
Mouse and Acute Pancreatitis Experiments
For all of the animal experiments, mice were age- and sex-matched; with young mice being ∼8 to 10 weeks old and older mice being ∼2 years old. Mice were euthanized by CO2 inhalation and blood was collected by intracardiac puncture. Amylase measurement (IU/L) was done using serum from clotted blood. Pancreata were rapidly removed and cut into pieces that were: 1) immediately fixed in 10% formalin for histological analysis, 2) embedded in optimum cutting temperature compound (Miles, Elkhart, IN) for immunofluorescence staining, 3) snap-frozen in liquid N2 for protein analysis, or 4) immediately submerged into RNAlater stabilization reagent (Qiagen, Hilden, Germany) for RNA analysis. Fixed tissues were sectioned then stained using hematoxylin and eosin (H&E). Pancreatic morphology was scored by one of the authors (S.A.M.) for atrophy and areas of fat cells/total pancreas area without knowing the genotype of the mice. Acute pancreatitis was induced in 8-week-old mice by 7 hourly intraperitoneal injections of caerulein (50 μg/kg; Sigma, St. Louis, MO) or saline (controls) as described.6 Mice were allowed to recover from pancreatitis and sacrificed 48 hours after the first injection that is known to represent peak keratin induction.7
Antibodies, Immunofluorescence Staining, and Transmission Electron Microscopy
The antibodies (Abs) used include37: Ab 8592 (anti-m/hK8/K18); Ab 8250 [anti-K18 phospho-(p)S33]; Ab 3055 [anti-K18 phospho-(p)S5238]; Troma I (anti-m/hK8) and Troma III (anti-K19; University of Iowa Hybridoma bank, Ames, Iowa); Troma II (anti-mK18); L2A1 (anti-hK18); LJ4 (anti-mK8 pS79); anti-actin (ACTN05), anti-tubulin (MS-581), and anti-K20 (K20.8) (NeoMarkers, Fremont, CA); and anti-amylase (Sigma).
Immunofluorescence staining was performed as described,37 as was transmission electron microscopy (TEM),17 and 0.5-μm sections were stained for 20 seconds with 0.5% toluidine blue/0.5% sodium borate to help visualize subcellular structures [particularly zymogen granules (ZG)]. Quantification of ZG size was performed using TEM images of WT and K8/K18 pancreata and Volocity software (Improvision, Lexington, MA). Statistical significance was determined using the two-tailed unpaired t-test.
Biochemical Methods
Total protein tissue lysates were prepared by homogenization in 3% sodium dodecyl sulfate (SDS)-containing sample buffer followed by pelleting to remove nonsolubilized debris. Protein concentration was determined using the BCA protein assay (Pierce, Rockford, IL). Equal amounts of protein were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by staining with Coomassie blue. Alternatively, gels were transferred to membranes for immunoblotting then visualized by enhanced chemiluminescence (Perkin Elmer, Boston, MA).
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using an RNeasy midi kit (Qiagen, Chatsworth, CA). The RNA was converted into cDNA using Oligo-dT primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed with an ABI Prism 7900 sequence detection system (PE Biosystems, Foster City, CA) using previously described primers.30 Samples were analyzed in quadruplicates and at least three individual mice were tested for each genotype. L7 ribosomal protein was used as an internal control and cDNA levels were normalized so that L7 expression was approximately equal in all tested mice. After confirming that the amplification efficiency was approximately equal for all genes, the transcript levels relative to L7 were determined and reported as means.
Results
Effect of K18, K8, or K8/K18 Overexpression on Overall Pancreatic Keratin Levels
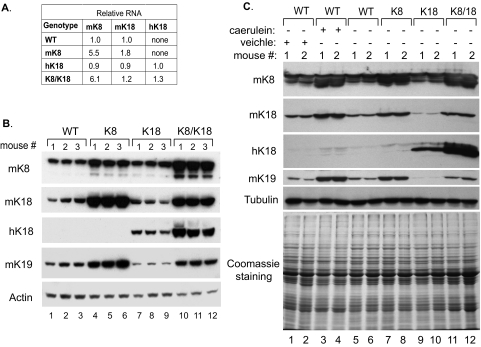
To investigate the role of keratin overexpression on the exocrine pancreas we studied three heterozygous transgenic mouse strains overexpressing: 1) heterozygous mK8, 2) heterozygous hK18, and 3) mK8 and hK18 by interbreeding the mK8 and hK18 heterozygous mouse lines. Keratin mRNA and protein expression in these three transgenic lines were compared to nontransgenic FVB/n mice (henceforth called WT mice). At the RNA level, K18 overexpression had no significant effect on endogenous mouse K8 and K18 RNA whereas K8 overexpression led to up-regulation of mK18 RNA (Figure 1A). The most dramatic effects were seen at the protein level, whereby K8 overexpression led to a modest increase in overall K8 and K18 levels that become even more prominent when K8 and K18 were doubly overexpressed (Figure 1B). K8 overexpression increased not only K18 protein but also the protein expression of the other type-I keratin, K19 (Figure 1B). Such an increase of partner keratins is in line with the established 1:1 type-I to type-II keratin protein ratio that is noted in cells and tissues4,39 and the deregulation of excess keratin subunits40 that also is likely responsible for the observed effect of K18 overexpression on down-regulating K19 protein (Figure 1B, lanes 7 to 9). In addition, K8 and K8/K18 pancreata manifested induction of K20 (Figure 2, compare panels j and l with i and k), but the induced K20 protein was limited and beyond detection by immunoblotting of total pancreas homogenates (not shown).
Figure 1.
Keratin protein and mRNA levels in pancreata of keratin-overexpressing transgenic mice and in mice recovering from acute pancreatitis. mRNA (A) and protein (B) levels of normal nontransgenic mice (WT) and mice overexpressing WT mouse K8 (K8), human K18 (K18), and both K8 and K18 (K8/K18) are shown. A: mRNA was determined by real-time RT-PCR using previously described primers30 to the overexpressed keratins. Expression levels are shown as a ratio to WT mice for mK8, mK18, and to K18 mice for hK18 (n = 3 mice/genotype), and normalized to ribosomal L7 RNA. B: Equal amounts of total pancreas lysates from the mice analyzed in A were separated by SDS-PAGE and then immunoblotted with antibodies to the indicated keratins and actin. Three individual mice (nos. 1 to 3) were used for each genotype. C: Keratin protein levels in pancreata of nontransgenic (WT) mice and mice overexpressing mK8 (K8), hK18 (K18), and mK8 and hK18 (K8/K18) were compared to those in nontransgenic mice 2 days after acute pancreatitis injury. The nontransgenic mice were administered 7 hourly injections of caerulein (50 μg/kg of mouse weight) or of vehicle (saline) followed by isolation of the pancreas 48 hours after the first injection. Equal amounts of total pancreas lysates were separated by SDS-PAGE then immunoblotted with antibodies to the indicated proteins. Coomassie brilliant blue staining and the tubulin blot are included to show equal protein loading. Two age- and sex-matched individual mice (nos. 1 and 2) were used for each genotype.
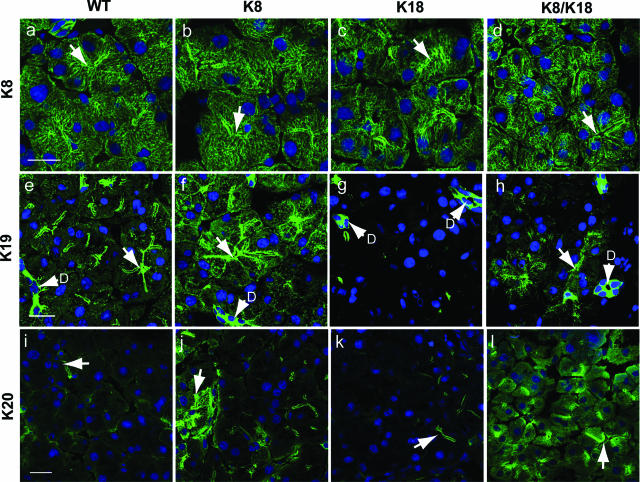
Figure 2.
Keratin immunofluorescence staining of control and keratin-overexpressor pancreata. Keratins from WT (a, e, i), K8 (b, f, j), K18 (c, g, k), and K8/K18 (d, h, l) mouse pancreata were visualized by double-immunofluorescence staining using antibodies to K8 (a–d), K19 (e–h), and K20 (i–l) and counterstained for nuclei (blue). Arrows point to examples of lumens; D, ducts. Scale bars = 20 μm.
Keratin overexpression occurs in the mouse pancreas on recovery from acute pancreatitis, and in the case of caerulein-mediated pancreatitis K8/K18 levels peak 2 days after recovery.7,17 To assess the level of transgenic keratin overexpression compared to a physiological situation, we compared keratin protein levels in the K8, K18, and K8/K18 genotypes with WT FVB/n before and 2 days after caerulein pancreatitis. Notably, K8-overexpressor mice expressed similar keratin levels to the postpancreatitis nontransgenic mice whereas the K8/K18 mice manifested somewhat higher levels (Figure 1C).
Effect of K8, K18, and K8/K18 Overexpression on Keratin Filament Organization in the Exocrine Pancreas
We examined the consequence of keratin overexpression on keratin filament organization, particularly because previous work showed that K19 and K20 overexpression in the context of pancreatic injury results in redistribution of these keratins from a membrane-proximal apicolateral compartment to a cytoplasmic filamentous distribution.7 All three transgenic lines (K8, K18, and K8/K18) had a similar overall pancreatic keratin distribution as visualized by K8 staining (Figure 2, a–d). Morphologically, the acini in all three strains were comparable with respect to a defined central lumen and a basally positioned nucleus in each cell. K8/K18 acini appeared slightly rounded up with somewhat dilated lumens (Figure 2d). The most dramatic keratin filament organizational change was in K19 and K20, which assumed a cytoplasmic short filament and punctuate distribution in K8 and K8/K18 pancreata (Figure 2, e–l), in addition to their normal membrane-proximal distribution. The cytoplasmic K19 and K20 staining was patchy, and the changes in K19 were most prominent in acinar cells, with ductal cells manifesting bright intense staining in all of the genotypes (Figure 2, e–h). Taken together, the total level of keratins in the pancreata we examined was K8/K18 > K8 > K18 ≅ WT.
Keratin Overexpression and Associated Keratin Phosphorylation Changes in K8 and K8/K18 Pancreata
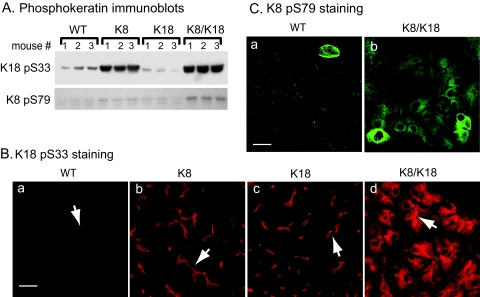
The function and filament organization of keratins are regulated by phosphorylation that also serves as a cellular stress marker.41 This led us to assess keratin phosphorylation at K18 S33 and K8 S79 in the keratin-overexpressing pancreata using phospho-epitope-specific keratin antibodies (Figure 3). K8 and K8/K18 pancreata had a marked increase in K18 pS33 (Figure 3A), a site that regulates keratin/14-3-3 binding.42 No effect on 14-3-3 isoforms staining or distribution was noted (not shown). The increase in K18 pS33 phosphorylation (Figure 3A) paralleled the increase in overall K18 levels (Figure 1B). The constitutive K18 pS33 phosphorylation in WT acini localized preferentially in the apicolateral domain, where the increase in K8 acini was observed (Figure 3B). However, K8/K18 pancreata manifested extension of K18 S33 phosphorylation into the cytoplasmic filaments (Figure 3B) that was not seen in the K18 or K8 pancreata. K8 S79 that is phosphorylated during stress, mitosis, and apoptosis by Jun and p38 kinases41 was minimally phosphorylated except in pancreata from double-overexpressor mice (Figure 3, A and C). The increase in K8 S79 phosphorylation was likely related to a stress response because there was no apparent increase in proliferating nuclear antigen as tested by immunoblotting (used as a proliferation marker, not shown) or of caspase-cleaved K18 or K19 (tested by immunoblotting and immune staining using a keratin fragment-specific antibody, not shown). Therefore, keratin overexpression, particularly in the K8/K18 mice, is accompanied by keratin hyperphosphorylation at K8 S79 and extension of the K18 pS33 species into the cytoplasmic compartment.
Figure 3.
Keratin phosphorylation is increased in K8 and K8/K18 mice. The level of keratin phosphorylation in WT, K8, K18, and K8/K18 mice was investigated by immunoblotting (A) as described in Figure 1 and by immunofluorescence staining using Abs to K18 pS33 and K8 pS79 (B and C, respectively). Arrows point to examples of lumens. In A, three separate mice (nos. 1 to 3) were used per genotype. Scale bars = 20 μm.
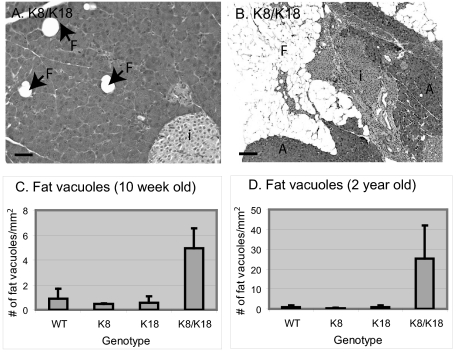
High Keratin Overexpression Leads to Pancreatic Atrophy
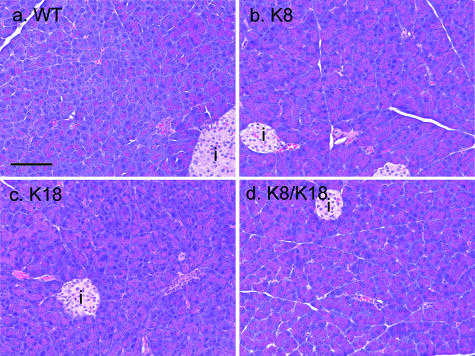
We examined the effect of keratin overexpression on pancreatic histopathology using H&E staining. Keratin overexpression did not cause inflammation, edema, or other hallmarks of pancreatitis (Figure 4). However, K8 and K8/K18 pancreata (which had the highest keratin protein levels and the most prominent phosphorylation changes) manifested increased eosinophilic staining in their acinar cytoplasm (Figure 4, compare panels b and d with panels a and c). K8/K18 pancreata also had scattered vacuoles (Figure 5A) that were rarely if ever seen in the other genotypes (not shown). As the mice increased in age (particularly mice older than 18 months), marked atrophic changes became evident with near complete replacement of the parenchyma by fatty vacuoles (eg, Figure 5B). This was supported by quantification of the fat vacuoles in young (Figure 5C) versus old mice (Figure 5D). No apparent histological changes were seen in the islets. Similar eosinophilic acini with mild fatty changes was also noted in mice overexpressing hK8 (not shown), thereby indicating that these changes are independent of whether mouse or human K8 are overexpressed. In this regard, the keratin level in the hK8 overexpressor mice31 was similar or slightly higher than in the K8 mice overexpressing mK8 (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Figure 4.
Pancreatic histology of WT and keratin-overexpressing mice. Pancreata from WT (a), K8 (b), K18 (c), and K8/K18 (d) mice were fixed, sectioned, and stained with H&E. i, islets. Scale bar = 100 μm.
Figure 5.
Age-related pancreatic atrophy in K8/K18-overexpressing mice. Pancreata from K8/K18 (A, 10-week-old; B, 2-year-old) mice were stained with H&E as in Figure 4. Fat vacuoles (F), as seen in the 10-week-old mice increased to large atrophic areas in the older mice. The number of pancreatic vacuoles in 10-week-old (C) and 2-year-old (D) overexpressor strains was determined using sections obtained from three to five mice/genotype and are shown as the number of total fat vacuoles/mm2 ± SD. i, islets. Scale bars: 50 μm (A); 250 μm (B).
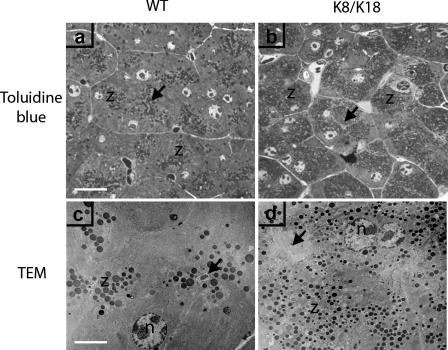
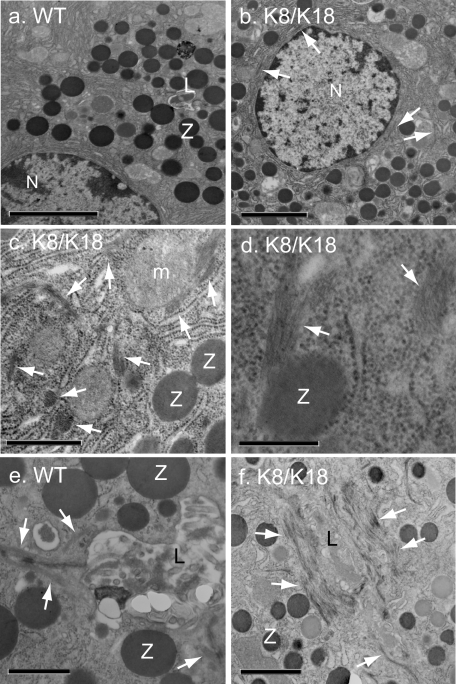
Effect of K8/K18 Overexpression on Pancreatic ZGs
The increased eosinophilic morphology of K8/K18 mice led us to closely compare their ZG morphology to those of WT pancreata. Pancreas sections stained with toluidine blue and examined by light microscopy or TEM revealed that K8/K18 pancreata had an increased number of acinar ZG as compared to WT pancreata (Figure 6). Morphometric quantification from TEM images showed that K8/K18 pancreata had twice the number of ZG compared to WT (86 ± 16 and 43 ± 15 ZG/130 μm2, respectively; Table 1) but that were half the size of WT ZG (Table 1). Both the toluidine blue staining and the TEM images indicated that the ZG in WT mice were located at the apical lumen of each acinus whereas in the K8/K18 pancreata they were spread evenly throughout the cytoplasm (Figure 6). No major differences in the ZG of K8-null and K19-null pancreata compared to WT mice were observed (not shown). Detailed EM studies showed that K8/K18 pancreata had thicker keratin IF bundles around the apical lumen compared to WT (Figure 7, e and f). In addition, the IF bundles were rarely seen in perinuclear or cytoplasmic locations in WT acinar cells but were frequently observed in these areas in K8/K18 acini (Figure 7; b, c, and d). Cytoplasmic IF bundles could also be seen in close contact with ZG (Figure 7d).
Figure 6.
Imaging of ZGs in WT and K8/18-overexpressor pancreata. WT (a, c) and K8/K18 (b, d) pancreas sections were stained using toluidine blue (a, b) to visualize ZGs (z) or were processed for TEM (c, d). In the TEM images, the ZG appear as rounded electron-dense structures. In c and d, arrows point to apical lumens, which in WT pancreata appear filled with dark-staining material from released zymogens (c) but is less electron-dense in the K8/K18 pancreas (d). Scale bars: 15 μm (a, for a and b); 5 μm (c, for c and d).
Table 1.
K8/K18 Overexpressor Pancreata Display Smaller and More Numerous Zymogen Granules per Cell
| WT | K8/K18 | P value | |
|---|---|---|---|
| Number of ZG/130 (μm2) | 43 ± 15 | 86 ± 16 | 0.002 |
| Average ZG area (μm2) | 0.39 ± 0.04 | 0.20 ± 0.03 | 0.00001 |
Two mice per genotype and a total of five to six acinar cells/genotype were photographed at ×6300 magnification after TEM. Images were scanned and an area of 13 × 10 μm was analyzed for the number of zymogen granules (ZG) and for ZG sectional areas (μm2) as described in the Materials and Methods. Values are given as averages ± SD and P values were determined using the two-tailed unpaired Student’s t-test.
Figure 7.
Distribution of ZG-associated keratin bundles in pancreata of K8/K18 mice. Pancreata from WT (a, e) and K8/K18 (b–d, f) mice were excised and fixed for subsequent TEM. Perinuclear and cytoplasmic IF bundles occur frequently in K8/K18-overexpressor (b, c) but rarely in WT pancreata (a). Keratin IF bundles surrounding the lumen in WT mice (e) are much more prominent in K8/K18 mice (f). The cytoplasmic IF bundles in K8/K18 were frequently associated with ZG (d). Z, zymogen granule; L, lumen (arrows point to keratin bundles); N, nuclei. Scale bars: 3.8 μm (a); 2.7 μm (b); 0.8 μm (c); 0.4 μm (d); 0.9 μm (e); 1.6 μm (f).
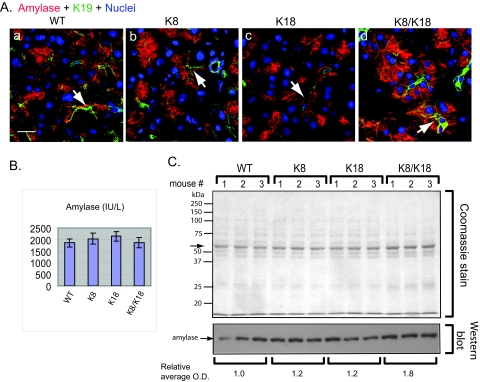
Given the change in the quality and quantity of the ZG in K8/K18 pancreata, we examined serum amylase and pancreatic amylase content and distribution in the four mouse genotypes. Staining for amylase confirmed the TEM data in that ZG-containing amylase was located preferentially near the lumen in WT whereas it was more dispersed within the cytoplasm of K8/K18 acinar cells (Figure 8A). K8-overexpressor mice, which had intermediate keratin levels, also showed an intermediate redistribution of amylase-staining when comparing WT or K18 with K8/K18 pancreata (Figure 8A, compare panels b and d with panels a and c). Serum amylase levels were similar in all four genotypes (Figure 8B), consistent with their primarily normal pancreas histology (Figure 4). When analyzed biochemically in pancreatic tissues, a small but significant increase of amylase was noted in total pancreas homogenates in K8/K18 pancreata (Figure 8C). Collectively, our results show that medium to high-level keratin overexpression leads to an increase in the number of ZG concomitant with a decrease in their size and an alteration in their distribution from apically based to diffuse cytoplasmic.
Figure 8.
Comparison of pancreatic amylase distribution and levels in control and keratin-overexpressor mice. A: Pancreata from WT (a), K8 (b), K18 (c), and K8/K18 (d) mice were analyzed by triple-immunofluorescence staining using Abs to amylase (red), K19 (green), and nuclear staining (blue). Arrows point to examples of lumens. B: Serum amylase ± SD of 10-week-old mice (n = 3/genotype). C: Total pancreatic lysates from WT, K8, K18, and K8/K18 mice were analyzed by SDS-PAGE then Coomassie staining (equal amount of protein was loaded per lane, arrow highlights the most abundant protein band that likely represents amylase primarily). Duplicate pancreatic lysates were also analyzed by immunoblotting using anti-amylase Ab followed by densitometric scanning of the amylase band (mean o.d. is shown). Scale bar = 20 μm.
Discussion
Differential Protective Roles of Keratins in the Liver and Pancreas
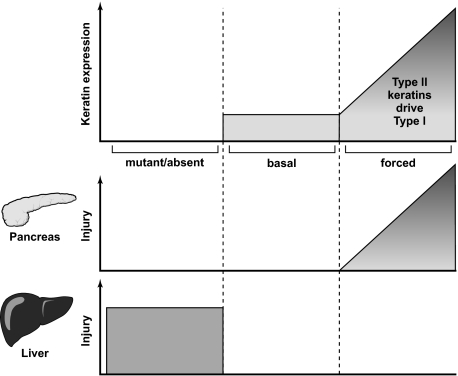
One major impetus for us to perform the study herein is the previous observation19 that hK8 overexpression in mice leads to chronic pancreatitis with acinar changes beginning at 2 weeks and adipose conversion starting at 2 to 4 months of age yet no liver injury was observed. This result was unexpected given that perturbations in keratins, such as their absence or mutation, spare the pancreas but have a dramatic effect on the liver particularly in terms of predisposition to liver injury.9 Potential explanations for this observation include the overexpression of human rather than mouse K8 (hK8 sequence NP_002264.1 versus mK8 sequence NP_112447.2 have 89.2% sequence identity) or that K8 overexpression, if at high enough levels, causes a toxic or gain-of-function effect. Our findings addressed both potential variables and showed that mouse K8 overexpression resulted in features of chronic pancreatitis (atrophy) in a manner that depended on the overall level of keratin expression (Figure 9). Hence, mK8 overexpression that leads to a modest increase in K8/K18 protein promotes mild changes in acinar cell amylase distribution (Figure 8A), whereas interbreeding mK8 and hK18 overexpressors leads to a more dramatic increase in K8/K18 pancreatic protein and prominent changes in acinar cell ZG (Figures 6 and 7) with ultimate fatty replacement of the pancreas in old mice (Figure 5). Notably, modest overexpression of hK831 using the identical human K8 transgene construct used by Casanova and colleagues19 did not cause any histological changes in the pancreas in mice as old as 6 months. Although we are unable to compare the keratin expression level in the two hK8 strains side-by-side, we reason, based on findings of both studies, that the more severe phenotype in the pancreata described by Casanova and colleagues19 are related to much higher keratin levels than we have in our K8 overexpressors. The similar keratin overexpression in the hK8- and mK8-overexpressor mice we generated (see Supplemental Figure S1 at http://ajp.amjpathol.org), coupled with their lack of a significant pancreatic phenotype, lends support to the conclusion that the previously described pancreatic phenotype in the hK8-overexpressing mice19 is unlikely to be related to the overexpression of human rather than mouse K8. Therefore, keratin absence or mutation is well tolerated after pancreatic, but not liver, injury, but excessive overexpression is toxic to the pancreas but not the liver when enforced under basal conditions (Figure 9).
Figure 9.
Comparison of the effect of keratin overexpression or mutation in the pancreas and liver. The schematic contrasts the effect of mutant/absent, normal (basal), and forced keratin expression with the effects of such expression on pancreatic and liver injury. Keratin mutation or absence (schematic, left) is well tolerated in the pancreas but predisposes to significant injury in the liver.9 As would be expected, normal keratin expression does not lead to pancreas/liver injury (schematic, middle). In contrast, forced K8/K18 expression as we observed in the graded keratin overexpression in the K8 and K8/K18 transgenic mice promotes progressive parallel injury in the pancreas without having a clearly discernible effect in the liver (schematic, right). The most severe form of pancreatic injury is reflected by the likely overwhelming pancreatic keratin overexpression reported by Casanova and colleagues.19
The reason for the toxic effect of keratin overexpression is likely to be multifactorial. Pancreatic keratin overexpression is associated with several keratin-related molecular events including keratin hyperphosphorylation and keratin filament reorganization (Figures 2 and 3). Although keratin phosphorylation and filament reorganization changes may be secondary rather than primary to the toxic effects of keratin overexpression, such alterations nonetheless serve as markers of injury for several IFs in multiple tissues.41 In addition, the preferential toxic effect of keratin overexpression in the pancreas versus the liver may be related to the level of keratin induction in each organ. For example, K8 and K8/K18 pancreata have relatively higher keratin mRNA and protein levels as compared to livers of the corresponding mice. In quantitative terms, the K8 mRNA fold increase compared to WT mice is: K8 pancreas, 5.5-fold increase; K8 liver, 3.2-fold increase; K8/K18 pancreas, 6.1-fold increase; and K8/K18 liver, 2.5-fold increase (Figure 1A30).
In contrast to the liver in which transgenic overexpression of K8 alone predisposes to spontaneous formation of Mallory-Denk bodies in older mice and after toxin exposure in young mice,30 we did not observe keratin aggregates in pancreata of the K8 overexpressor mice. This is despite the higher K8 levels in the pancreas versus the liver in the K8-overexpressor mice. A higher K8 than K18 ratio is essential for Mallory-Denk body formation in the liver, in part because of the suitability of K8 as a transglutaminase substrate.43 Keratin aggregates have been described rarely in patients with pancreatic cancer44,45 but it is not known if such aggregates are similar to Mallory-Denk bodies in terms of including ubiquitin and p62 proteins. Interestingly, the liver, brain, and muscle are the major organs where cytoplasmic IF-containing inclusions are typically found that likely reflects organ-selective inclusion-promoting features such as protein turnover and oxidative exposure.24
Regulation of Type-I Keratins by Type-II Keratins
Another regulatory aspect to our findings is the observation that type-II keratins have a more profound effect on modulating type-I keratins than vice versa, both at the RNA and protein levels. This is based on the observation that K8 overexpression results in increased K18, K19, and K20 in the pancreas, whereas K18 overexpression had no appreciable effect on K8 expression (Figures 1 and 2). Similarly, overexpression of K20 in transgenic mouse intestine had no significant effect on K8 levels although it did decrease K19 levels.5 The reason for the increase in overall keratin protein in K8/K18 mice, as compared with K8 mice (without a significant change in K8 mRNA), is the ability of overexpressed K8 and K18 protein to heteropolymerize and stabilize each other and hence raise the overall keratin protein level. Several previous studies have demonstrated that expression of a single keratin alone without its partner leads to rapid degradation40 likely via ubiquitination.46 In contrast, overexpression of a given type-I keratin also leads to down-regulation of other type-I keratin(s) in the same cell as noted in the hK18 overexpressor mice that show down-regulation of the endogenous mK18 as well as K19 (Figure 1B). Similarly, overexpression of the type-I K16 caused decreased levels of type-I K10 in mouse skin, although K17 was also induced.47 This likely reflects the competition between excess keratin subunits for limiting amounts of a compatible complementary subunit.
Keratin Levels Contribute to ZG Organization and Pancreatic Homeostasis
One of the consequences of keratin overexpression in the pancreas is a decrease in the size of ZGs but an increase in their number (Figure 6 and Table 1). Similar but more profound ZG abnormalities were noted in the previously described hK8-overexpressor mice that develop severe atrophy of the pancreas coupled with dwarfism, fibrosis, and inflammation.19 The ZG findings in our keratin-overexpressing pancreata may be related to a limited secretion block. Interestingly, a similar increase in ZG was observed in VAMP8/endobrevin-null mice48 and syncollin-null mice.49 Syncollin is a small protein that interacts with GP-2 on the ZG and is required for efficient ZG exocytosis,50 whereas VAMP8 is a major v-SNARE for the exocrine system.48 The syncollin-null mice have an exocrine pancreatic secretory defect, because delivery of newly synthesized protein to ZG was delayed,49 whereas GP-2-null mice were not reported to have a ZG defect.51 It would be relevant to determine in future studies whether keratin levels are elevated in patients with the various entities that are associated with chronic pancreatitis such as alcohol-related injury or genetic mutations related to trypsinogen activation or the cystic fibrosis conductance regulator.52 Notably, K8/K18 levels increase approximately threefold during mouse experimental acute pancreatitis23 but whether this also occurs in humans is unknown. These pancreatitis-associated increases are similar to what is observed in the keratin-overexpression mouse models described herein (Figure 1C), which renders these mice useful models to study the significance of keratin overexpression after pancreatic injury.
Acknowledgments
We thank Evelyn Resurreccion for assistance with tissue sectioning and immunofluorescence staining, Pauline Chu for H&E staining, and Dr. Anson Lowe for fruitful discussions.
Footnotes
Supported by the National Institutes of Health (grant DK47918 to M.B.O., and Digestive Disease Center grant DK56339 to Stanford University), the Department of Veterans Affairs (to M.B.O.), and the European Molecular Biology Organization (postdoctoral fellowship to P.S.).
D.M.T. and I.N. contributed equally to this study.
Supplemental material for this article can be found on http://ajp. amjpathol.org.
Current addresses: D.M.T., Abo Akademi University, Biosciences, Department of Biology, Turku, Finland; I.N., Department of Internal Medicine, Kyusyu Dental College, Kitakyushu, Japan; P.S., Department of Internal Medicine I, University of Ulm, Ulm, Germany; M.H., Department of Medicine, Kurume University School of Medicine, Kurume, Japan.
References
- Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Toivola DM, Feng N, Greenberg HB, Franke WW, Omary MB. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell. 2003;14:2959–2971. doi: 10.1091/mbc.E03-02-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola DM, Baribault H, Magin T, Michie SA, Omary MB. Simple epithelial keratins are dispensable for cytoprotection in two pancreatitis models. Am J Physiol. 2000;279:G1343–G1354. doi: 10.1152/ajpgi.2000.279.6.G1343. [DOI] [PubMed] [Google Scholar]
- Zhong B, Zhou Q, Toivola DM, Tao GZ, Resurreccion EZ, Omary MB. Organ-specific stress induces mouse pancreatic keratin overexpression in association with NF-kappaB activation. J Cell Sci. 2004;117:1709–1719. doi: 10.1242/jcs.01016. [DOI] [PubMed] [Google Scholar]
- Bouwens L. Cytokeratins and cell differentiation in the pancreas. J Pathol. 1998;184:234–239. doi: 10.1002/(SICI)1096-9896(199803)184:3<234::AID-PATH28>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB. Keratins let liver live: mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–1649. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- Vijayaraj P, Sohl G, Magin TM. Keratin transgenic and knockout mice: functional analysis and validation of disease-causing mutations. Methods Mol Biol. 2007;360:203–251. doi: 10.1385/1-59745-165-7:203. [DOI] [PubMed] [Google Scholar]
- Uitto J, Richard G, McGrath JA. Diseases of epidermal keratins and their linker proteins. Exp Cell Res. 2007;313:1995–2009. doi: 10.1016/j.yexcr.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele RA, Oshima J. Phenomics and lamins: from disease to therapy. Exp Cell Res. 2007;313:2134–2143. doi: 10.1016/j.yexcr.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, Stumptner C, Fuchsbichler A, Fickert P, Lackner C, Trauner M, Denk H. The keratin cytoskeleton in liver diseases. J Pathol. 2004;204:367–376. doi: 10.1002/path.1649. [DOI] [PubMed] [Google Scholar]
- Oshima RG. Apoptosis and keratin intermediate filaments. Cell Death Differ. 2002;9:486–492. doi: 10.1038/sj.cdd.4400988. [DOI] [PubMed] [Google Scholar]
- Galarneau L, Loranger A, Gilbert S, Marceau N. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp Cell Res. 2007;313:179–194. doi: 10.1016/j.yexcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Ku NO, Ghori N, Lowe AW, Michie SA, Omary MB. Effects of keratin filament disruption on exocrine pancreas-stimulated secretion and susceptibility to injury. Exp Cell Res. 2000;255:156–170. doi: 10.1006/excr.1999.4787. [DOI] [PubMed] [Google Scholar]
- Zhong B, Strnad P, Toivola DM, Tao GZ, Ji X, Greenberg HB, Omary MB. Reg-II Is an exocrine pancreas injury-response product that is up-regulated by keratin absence or mutation. Mol Biol Cell. 2007;18:4969–4978. doi: 10.1091/mbc.E07-02-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Bravo A, Ramirez A, Morreale de Escobar G, Were F, Merlino G, Vidal M, Jorcano JL. Exocrine pancreatic disorders in transgenic mice expressing human keratin 8. J Clin Invest. 1999;103:1587–1595. doi: 10.1172/JCI5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavestro GM, Frulloni L, Nouvenne A, Neri TM, Calore B, Ferri B, Bovo P, Okolicsanyi L, Di Mario F, Cavallini G. Association of keratin 8 gene mutation with chronic pancreatitis. Dig Liver Dis. 2003;35:416–420. doi: 10.1016/s1590-8658(03)00159-2. [DOI] [PubMed] [Google Scholar]
- Treiber M, Schulz HU, Landt O, Drenth JP, Castellani C, Real FX, Akar N, Ammann RW, Bargetzi M, Bhatia E, Demaine AG, Battagia C, Kingsnorth A, O’Reilly D, Truninger K, Koudova M, Spicak J, Cerny M, Menzel HJ, Moral P, Pignatti PF, Romanelli MG, Rickards O, De Stefano GF, Zarnescu NO, Choudhuri G, Sikora SS, Jansen JB, Weiss FU, Pietschmann M, Teich N, Gress TM, Ockenga J, Schmidt H, Kage A, Halangk J, Rosendahl J, Groneberg DA, Nickel R, Witt H. Keratin 8 sequence variants in patients with pancreatitis and pancreatic cancer. J Mol Med. 2006;84:1015–1022. doi: 10.1007/s00109-006-0096-7. [DOI] [PubMed] [Google Scholar]
- Schneider A, Lamb J, Barmada MM, Cuneo A, Money ME, Whitcomb DC. Keratin 8 mutations are not associated with familial, sporadic and alcoholic pancreatitis in a population from the United States. Pancreatology. 2006;6:103–108. doi: 10.1159/000090029. [DOI] [PubMed] [Google Scholar]
- Zhong B, Omary MB. Actin overexpression parallels severity of pancreatic injury. Exp Cell Res. 2004;299:404–414. doi: 10.1016/j.yexcr.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Abe M, Oshima RG. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J Cell Biol. 1990;111:1197–1206. doi: 10.1083/jcb.111.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Michie S, Oshima RG, Omary MB. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995;131:1303–1314. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Michie SA, Soetikno RM, Resurreccion EZ, Broome RL, Oshima RG, Omary MB. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest. 1996;98:1034–1046. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Soetikno RM, Omary MB. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology. 2003;37:1006–1014. doi: 10.1053/jhep.2003.50181. [DOI] [PubMed] [Google Scholar]
- Nakamichi I, Toivola DM, Strnad P, Michie SA, Oshima RG, Baribault H, Omary MB. Keratin 8 overexpression promotes mouse Mallory body formation. J Cell Biol. 2005;171:931–937. doi: 10.1083/jcb.200507093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, Millecamps S, Julien JP, Garcia ML. New movements in neurofilament transport, turnover and disease. Exp Cell Res. 2007;313:2110–2120. doi: 10.1016/j.yexcr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Quinlan RA, Brenner M, Goldman JE, Messing A. GFAP and its role in Alexander disease. Exp Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Ji X, Chen L, Greenberg HB, Lu SC, Omary MB. Keratin mutation primes mouse liver to oxidative injury. Hepatology. 2005;41:517–525. doi: 10.1002/hep.20578. [DOI] [PubMed] [Google Scholar]
- Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- Tamai Y, Ishikawa T, Bosl MR, Mori M, Nozaki M, Baribault H, Oshima RG, Taketo MM. Cytokeratins 8 and 19 in the mouse placental development. J Cell Biol. 2000;151:563–572. doi: 10.1083/jcb.151.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Toivola DM, Zhou Q, Tao GZ, Zhong B, Omary MB. Studying simple epithelial keratins in cells and tissues. Methods Cell Biol. 2004;78:489–517. doi: 10.1016/s0091-679x(04)78017-6. [DOI] [PubMed] [Google Scholar]
- Liao J, Lowthert LA, Ku NO, Fernandez R, Omary MB. Dynamics of human keratin 18 phosphorylation: polarized distribution of phosphorylated keratins in simple epithelial tissues. J Cell Biol. 1995;131:1291–1301. doi: 10.1083/jcb.131.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RA, Cohlberg JA, Schiller DL, Hatzfeld M, Franke WW. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984;178:365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Kulesh DA, Cecena G, Darmon YM, Vasseur M, Oshima RG. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989;9:1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;133:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P, Harada M, Siegel M, Terkeltaub RA, Graham RM, Khosla C, Omary MB. Transglutaminase 2 regulates Mallory body inclusion formation and injury-associated liver enlargement. Gastroenterology. 2007;132:1515–1526. doi: 10.1053/j.gastro.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Chetty R, Asa SL. Pancreatic endocrine tumour with cytoplasmic keratin whorls. Is the term “rhabdoid” appropriate? J Clin Pathol. 2004;57:1106–1110. doi: 10.1136/jcp.2004.018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli G, Preda P, Martinelli GN, Galassi A, Santini D, Venza E. Filamentous inclusions in nonneoplastic and neoplastic pancreas: an ultrastructural and immunogold labeling study. Ultrastruct Pathol. 1995;19:495–500. doi: 10.3109/01913129509014625. [DOI] [PubMed] [Google Scholar]
- Ku NO, Omary MB. Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J Cell Biol. 2000;149:547–552. doi: 10.1083/jcb.149.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Folmer J, Coulombe PA. Increased expression of keratin 16 causes anomalies in cytoarchitecture and keratinization in transgenic mouse skin. J Cell Biol. 1994;127:505–520. doi: 10.1083/jcb.127.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wäsle B, Turvey M, Larina O, Thorn P, Skepper J, Morton AJ, Edwardson JM. Syncollin is required for efficient zymogen granule exocytosis. Biochem J. 2005;385:721–727. doi: 10.1042/BJ20041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus I, Hodel A, Koch A, Kleene R, Edwardson JM, Schrader M. Interaction of syncollin with GP-2, the major membrane protein of pancreatic zymogen granules, and association with lipid microdomains. Biochem J. 2002;362:433–442. doi: 10.1042/0264-6021:3620433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Michie SA, Lowe AW. Absence of the major zymogen granule membrane protein. GP2, does not affect pancreatic morphology or secretion. J Biol Chem. 2004;279:50274–50279. doi: 10.1074/jbc.M410599200. [DOI] [PubMed] [Google Scholar]
- Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–1573. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]