Abstract
Complement activation is a crucial early event in Wallerian degeneration. In this study we show that treatment of rats with soluble complement receptor 1 (sCR1), an inhibitor of all complement pathways, blocked both systemic and local complement activation after crush injury of the sciatic nerve. Deposition of membrane attack complex (MAC) in the nerve was inhibited, the nerve was protected from axonal and myelin breakdown at 3 days after injury, and macrophage infiltration and activation was strongly reduced. We show that both classical and alternative complement pathways are activated after acute nerve trauma. Inhibition of the classical pathway by C1 inhibitor (Cetor) diminished, but did not completely block, MAC deposition in the injured nerve, blocked myelin breakdown, inhibited macrophage infiltration, and prevented macrophage activation at 3 days after injury. However, in contrast to sCR1 treatment, early signs of axonal degradation were visible in the nerve, linking MAC deposition to axonal damage. We conclude that sCR1 protects the nerve from early axon loss after injury and propose complement inhibition as a potential therapy for the treatment of diseases in which axon loss is the main cause of disabilities.
The complement system plays a pivotal role in the recognition and processing of pathogens but improper activation is implicated in the pathogenesis, augmentation, and perpetuation of numerous diseases.1 We have previously shown that components of the complement system are locally produced in the healthy peripheral nerve and are activated during Wallerian degeneration (WD).2 WD is the process of axonal and myelin degradation that occurs in the nerve after a mechanical trauma.3 Initial morphological changes are visible as early as 12 hours after injury.4 After degeneration of the axon, the myelin sheath collapses and initially remains within the parent Schwann cell cytoplasm. Early after injury, the resident endoneurial macrophages proliferate, become activated and start to phagocytose myelin. The resident macrophage population is later supplemented by the blood-derived monocytes/macrophages that efficiently participate in myelin phagocytosis and removal.5,6 Although the pathological changes are well characterized, the molecular mechanisms underlying WD are far from clear.
Recently, we demonstrated that WD after peripheral nerve injury is delayed in a C6-deficient rat model, unable to form the cytolytic membrane attack complex (MAC, C5b-9 complex). We proposed that MAC formation triggers a pathway leading to early axon loss.7 Activation of the complement system occurs via three routes: the classical, the lectin, and the alternative pathways. The classical pathway is initiated via the recognition of a foreign antigen by C1q. On binding, C1s and C1r form a complex (C1) with C1q, cleaving C4 and C2 to yield the C3 convertase.8 The lectin pathway is triggered by binding of mannose binding lectin (MBL) to carbohydrates on the pathogen surface, which activates the MBL-associated serine protease (MASP) cleaving C4 and C2.9 The alternative pathway starts by spontaneous low-rate hydrolysis of C3 generating C3(H2O), which binds to factor B, permitting cleavage by factor D to form the fluid-phase C3 convertase C3(H2O)Bb. This enzyme cleaves C3 and deposits C3b on surfaces where, in the absence of complement inhibitors such as factor H, it binds and catalyzes cleavage of factor B to form surface-bound C3 convertase C3bBb. All three pathways converge in the cleavage of C3 and C5. This generates chemoattractants, opsonins, and C5b, which is the anchor for the assembly of the MAC.10
We previously demonstrated activation of the classical pathway during WD.7 Here we show that the alternative pathway is also activated. To determine whether the protective effect seen in the C6-deficient rat model is attainable by systemic treatment with complement inhibitors, we delivered soluble complement receptor 1 (sCR1) to rats and monitored WD after a crush injury of the sciatic nerve. sCR1 is a recombinant soluble form of the human membrane-bound regulatory protein CR1. It inhibits all three pathways of complement activation by dissociating the C3 convertases and targeting C3b and C4b for degradation.11 We also treated rats with C1 inhibitor (C1INH, Cetor) that blocks the classical and lectin complement pathways8,12 to test whether alternative pathway activation is sufficient to cause pathology, and whether low levels of complement activation permit differentiation between the effects of MAC deposition and macrophage infiltration during WD. Insights into the C-mediated events of WD are important because axon loss is the main cause of disabilities in peripheral neuropathies and diseases of the central nervous system, such as multiple sclerosis.13,14 Possible therapeutic targets and strategies may arise from such studies.
Materials and Methods
Animals
This study was approved by the Academic Medical Center Animal Ethics Committee and complies with the guidelines for the care of experimental animals. Male 12-week-old PVG/c rats were obtained from Harlan (Bicester, UK). The animals weighed between 200 g and 250 g and were allowed acclimatization for at least 2 weeks before the beginning of the study. Animals were kept in the same animal facility during the entire course of the experiment and monitored for microbiological status according to the Federation of European Laboratory Animal Science Association recommendations. Animals were housed in pairs in plastic cages. They were given rat chow and water ad libitum and kept at a room temperature of 20°C on a 12-hour:12-hour light:dark cycle.
Administration of sCR1 or Cetor for Inhibition Studies
Recombinant soluble complement receptor 1 (sCR1) was obtained as previously described.15 Human plasma-derived C1 inhibitor (Cetor) was kindly provided by Sanquin (Amsterdam, The Netherlands). sCR1 was administered intraperitoneally in 15 rats at a dose of 15 mg/kg/day. Cetor was administered intravenously in 15 rats at a dose of 50 U/rat/day. Thirteen rats were treated with equal volumes of vehicle (phosphate-buffered saline, PBS) alone. One group of animals (sCR1-treated, n = 9; Cetor-treated, n = 9; PBS-treated, n = 9) received treatment 1 day before the crush injury (day −1) and every 24 hours (days 0, 1, 2) until the nerves were removed at 3 days after injury. A second group of animals (sCR1-treated, n = 6; Cetor-treated, n = 6; PBS-treated, n = 4) were treated up to 6 days after injury (day −1, 0, 1, 2, 3, 4, 5, 6) and the nerves were removed 1 day after the end of the treatment (day 7).
Hemolytic Assay and Enzyme-Linked Immunosorbent Assay
Blood samples from PBS- and sCR1-treated rats were collected from the tail vein 1 day before the crush injury (day −1) and every following day (days 0, 1, 2) until the animals were sacrificed at 3 days after the injury. In the group treated up to 6 days, additional blood samples were collected at days 3, 5, and 7 after injury. All samples were collected immediately before each injection of treatment. Plasma was separated and stored at −80°C until used to monitor sCR1 inhibitory activity via standard complement hemolytic assay (%CH50, the reciprocal of the dilution of serum to lyse 50% of antibody-coated sheep red blood cells).16 Plasma levels of sCR1 were measured using enzyme-linked immunosorbent assay as previously described17 using serial dilutions assayed in duplicates.
Nerve Crush Injury and Tissue Processing
All of the surgical procedures were performed aseptically under deep isoflurane anesthesia (2.5% vol isoflurane, 1 L/minute O2, and 1 L/minute N2O). The right thigh was shaved and the sciatic nerve was exposed via an incision in the upper thigh. The nerve was crushed for three 10-second periods at the level of the sciatic notch using smooth, curved forceps (no.7). The crush site was marked by a suture through the epineurium that did not constrict the nerve. On the left contralateral side, a sham surgery was performed to expose the sciatic nerve but not disturb it. A suture was also placed. The muscle and the skin were closed with stitches. After the crush, the rats were allowed to recover for 2 days (untreated, n = 3), 3 days (PBS-treated, n = 9; sCR1-treated, n = 9; Cetor-treated, n = 9) or 7 days (PBS-treated, n = 4; sCR1-treated, n = 6; Cetor-treated, n = 6).
Three untreated rats were euthanized by CO2 inhalation at 2 days after injury. The nerves were immediately frozen in liquid nitrogen and stored at −80°C until they were processed for Western blot analysis. All of the remaining animals were intracardially perfused with 4% paraformaldehyde in piperazine-N-N′-bis (2-ethane sulfonic acid) (PIPES) buffer (pH 7.6). Injured and contralateral uninjured sciatic nerves from nine rats (PBS-treated, n = 3; sCR1-treated, n = 3; Cetor-treated, n = 3) removed 3 days after injury were conventionally processed into paraffin wax for immunohistochemistry. Injured and contralateral uninjured sciatic nerves from nine rats (PBS-treated, n = 3; sCR1-treated, n = 3; Cetor-treated, n = 3) removed 3 days after injury were postfixed with 1% glutaraldehyde, 1% paraformaldehyde, and 1% dextran (MW 20,000) in 0.1 mol/L piperazine-N-N′-bis (2-ethane sulfonic acid) (pH 7.6) and conventionally processed into epoxy resin. Resin sections of 0.5 μm were stained with thionine and acridine orange to assess degenerative morphological changes. In all cases, one segment of 5-mm length distal from the crush site was removed from the injured sciatic nerve. An equivalent piece of nerve was removed from the contralateral uninjured side.
Electron Microscopy
Electron microscopy was performed on ultrathin sections of sciatic nerve (surgically removed as described above) from PBS- (n = 3), sCR1- (n = 3), and Cetor-treated (n = 3) rats at 3 days after the crush injury. Sections were contrasted with uranyl acetate and lead citrate as previously described.18 Images were captured with a digital camera attached to an electron microscope (FEO 10; Philips, Eindhoven, The Netherlands).
Immunohistochemistry
Paraffin wax sections were stained using a two-step immunofluorescence method. All of the incubations were performed at room temperature. In all cases, microwave antigen retrieval was used (800 W for 3 minutes followed by 10 minutes at 440 W in 10 mmol/L Tris/1 mmol/L ethylenediaminetetraacetic acid, pH 6.5). To block the nonspecific binding sites, slides were incubated with 10% normal goat serum in 50 mmol l−1 Tris-HCl, 137 mmol l−1 NaCl, pH 7.6 (Tris-buffered saline) for 20 minutes. After incubation with the appropriate primary antibody diluted in 1% bovine serum albumin (Table 1) for 90 minutes, sections were incubated for 30 minutes with either goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated or sheep anti-mouse Cy3-conjugated IgG (or both for co-localization analysis) from Sigma-Aldrich (St. Louis, MO) diluted 1:200 in 1% bovine serum albumin. When indicated, slides were counterstained with 4,6-diaminodine-2-phenylindole (DAPI) (Sigma-Aldrich) and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Sections immunostained with secondary conjugate alone were included with every experiment and showed no immunoreactivity in all cases. Sections of rat spinal cord and lymph nodes served as positive controls. Images were captured with a digital camera (DP12; Olympus, Zoeterwoude, The Netherlands) attached to a fluorescent microscope (Vanox, AHBT3; Olympus, The Netherlands).
Table 1.
Antibodies, Source, and Dilutions for Immunohistochemistry
| Antibodies | Source | Dilutions |
|---|---|---|
| Monoclonal mouse anti-human phosphorylated neurofilament (SMI31 clone) | Stemberger (Lutherville, UK) | 1:1000 |
| Polyclonal rabbit anti-human MBP | DakoCytomation (Glostrup, Denmark) | 1:100 |
| Monoclonal mouse anti-rat CD68 (ED1 clone) | Serotec (Oxford, UK) | 1:100 |
| Polyclonal rabbit anti-rat C9 | B.P. Morgan | 1:300 |
| Polyclonal rabbit anti-human C3c | DakoCytomation | 1:750 |
| Polyclonal rabbit anti-human C4c | DakoCytomation | 1:100 |
Quantitative Analysis of Immunohistochemistry
All analyses were performed with the Image Pro Plus version 5.02 (Media Cybernetics Europe, Marlow, UK). CD68 (ED1 clone)-immunoreactive (-ir) cells were scored positive when the CD68-positive signal was associated with nuclei. Thirty nonconsecutive sections of sciatic nerve per rat were scored. An average of three nonoverlapping fields of view including >90% of the entire nerve area was taken for each section. Determination of size distribution of the CD68-ir cells was performed on 11 cells in the uninjured nerves, 778 cells in the PBS-treated nerves, 294 cells in the sCR1-treated nerves, and 218 cells in the Cetor-treated nerves. Quantification of the MAC and MBP immunostaining was performed at ×40 magnification on two nonoverlapping fields per section examined. Ten sections per rat were scored. The surface area stained is expressed as percentage of total area examined. The MBP-ir surface area is normalized to control levels.
Protein Extraction and Western Blot Analysis
Injured and contralateral uninjured sciatic nerves (surgically removed as described above) from three untreated rats sacrificed at 2 days after the crush injury were homogenized using a pestle and mortar in liquid nitrogen in 20 mmol/l−1 Tris (pH 7.4), 5 mmol l−1 1,4-dithio-dl-threitol and 0.4% sodium dodecyl sulfate and 6% glycerol. The homogenates were centrifuged at 10,000 × g, at 2°C for 10 minutes. The supernatant fraction was collected and used for protein analysis. Protein concentrations were determined with a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA), using bovine serum albumin as a standard.
Protein extracts (20 μg/sample) were boiled for 5 minutes, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane overnight at 4°C. Before blotting, the nitrocellulose membranes were stained with Ponceau red for 30 seconds to verify protein load. The membranes were preincubated in Tris-buffered saline containing 0.05% Tween20 (TBST) and 5% nonfat dried milk for 1 hour at room temperature. Blots were incubated for 2 hours in the polyclonal goat anti-factor Bb (fBb) (Quidel, San Diego, CA) diluted in TBST containing 5% nonfat dried milk. After washing in TBST, the membranes were incubated for 1 hour in polyclonal rabbit anti-goat horseradish peroxidase-conjugated secondary antibody diluted 1:2000 in TBST containing 5% nonfat dried milk. Membranes were washed in TBST for 3 × 10 minutes and immunoreactive bands were detected using enhanced chemiluminescence (Roche Diagnostics, Mannheim, Germany). Quantification of the immunoreactive bands was performed using Advanced Image Data Analyzer software version 3.4 (Raytest, Straubenhardt, Germany).
Statistical Analysis
Two-way analysis of variance with Bonferroni’s correction was performed to determine statistically significant differences (P ≤ 0.001). Statistical analysis of the immunoblotting quantification was determined by unpaired t-test (P ≤ 0.05).
Results
Activation of the Alternative Complement Pathway after Acute Nerve Trauma
We have previously shown that the classical pathway of the complement system is activated after acute nerve trauma.7 To determine whether the alternative pathway is also triggered by a crush injury of the sciatic nerve, we measured the expression level of Bb, the 60-kDa protein fragment that results from the cleavage of factor B. Low levels of Bb immunoreactivity were detected in protein extracts of uninjured rat nerves, whereas a near twofold increase (1.8 ± 0.1) was seen at 2 days after the crush injury (see Supplementary Figure S1, A and B, at http://ajp.amjpathol.org). These results indicate that the alternative pathway loop is triggered after acute nerve trauma, generating more cleaved fB.
Inhibition of Complement Activation after Acute Nerve Trauma
To determine the effects of inhibition of all complement activation pathways on WD, we treated animals with sCR1. Treatment was started 1 day before a crush injury of the sciatic nerve. We measured plasma sCR1 levels and CH50 after daily intraperitoneal injections of either sCR1 at a dose of 15 mg/kg/day or equal volume of vehicle. sCR1 levels increased after the first day of injection and hemolytic complement activity was reduced to ∼30% of controls (Figure 1, A and B). Cetor dosage was extrapolated from the work of de Smet and colleagues.19
Figure 1.
Inhibition of complement activation. A: Plasma sCR1 levels in sCR1-treated rats, showing concentration of sCR1 throughout time with daily treatment. B: Plasma hemolytic activity of PBS- and sCR1-treated rats, showing decreased activity in the sCR1-treated rats compared to the PBS-treated controls. A and B: Day 0 is the day of the crush injury. Rats received intraperitoneal injections of sCR1 (15 mg/kg/day) or PBS (equal volume) at days −1, 0, 1, 2, 3, 4, 5, and 6. Blood was collected immediately before each treatment. C–F: Representative immunostaining for MAC in cross-sections of PBS-, sCR1-, and Cetor-treated rat sciatic nerves at 3 days after injury. The uninjured nerve is shown as control (C) and no MAC immunoreactivity is detected. The PBS-treated nerve shows an abundant and diffuse MAC immunoreactivity (D, green color), demonstrating activation of the complement system, whereas no MAC deposition is detected in the sCR1-treated nerve (E), demonstrating blockade of complement activation. A low amount of MAC immunoreactivity is still detected in the Cetor-treated nerve (F, arrows), demonstrating low level of complement activation. Sections are stained with a two-step immunofluorescence method with a FITC-conjugated secondary antibody. Scale bar = 50 μm.
Local inhibition of complement activation in the crushed nerve was virtually complete in the sCR1-treated animals (0.8 ± 0.9% of total area examined) whereas significantly higher amounts of MAC immunoreactivity were visible in the nerves of Cetor-treated animals at 3 days after injury (7.3 ± 2.7% of total area examined, P ≤ 0.001) but in both cases the levels of MAC immunoreactivity were significantly lower than the level detected in the PBS-treated nerves (31.4 ± 7.8% of the total area, P ≤ 0.001). MAC immunoreactivity was undetectable in the uninjured control nerves (Figure 1C). Deposition of C4c, the activation product of the classical pathway, was also prevented in both the sCR1- and Cetor-treated nerves, demonstrating blockade of the classical complement pathway whereas deposition of C3c, the activation product common to all complement pathways, was detected in the Cetor-treated but not in the sCR1-treated nerves, indicating activation of the alternative complement pathway. High amounts of C4c and C3c immunoreactivity were detected in the PBS-treated nerves (not shown). These results demonstrate that sCR1 and, to a lesser extent, Cetor are effective inhibitors of complement activation after acute nerve trauma.
Effect of Complement Inhibition on WD
To determine the effects of sCR1- and Cetor-mediated complement inhibition on WD, we analyzed morphological changes of axons and myelin at 3 days after injury (Figure 2, A–J). Neurofilament (SMI31) and myelin (MBP) stainings of cross (Figure 2C) and longitudinal (Figure 2D) sections of the sciatic nerve of PBS-treated rats showed loss of axons as well as collapsed and degraded myelin. In contrast, cross-sections of the sCR1-treated rat nerves still showed the typical punctuated appearance of axons and annulated myelin morphology (Figure 2E, arrowhead), similarly to the uninjured control nerve (Figure 2A, arrowhead). A high amount of neurofilament and myelin staining was also evident in the longitudinal sections of the sCR1-treated nerves (Figure 2F), demonstrating rescued axonal and myelin breakdown at 3 days after injury. Electron microscope analysis showed persistent axonal content (Figure 2I, asterisk) within the intact myelin sheath (Figure 2I, arrowhead). In the Cetor-treated nerves, the neurofilament staining was generally lost (Figure 2G, asterisk) but the myelin staining produced the normal annulated morphology (Figure 2G, arrowhead). This was further proven by ultrastructural studies that showed loss of axonal contents (Figure 2I, asterisk) with axonal remnants along the intact myelin (Figure 2I, arrows), demonstrating that Cetor rescues the myelin but not the axons at 3 days after injury. Taken together, these observations demonstrate that sCR1 protects nerves from axonal degradation and myelin breakdown and that activation of the alternative complement pathway is sufficient to determine axonal damage at 3 days after injury.
Figure 2.
Analysis of WD at 3 days after injury. Representative immunostaining for phosphorylated neurofilament (SMI31 clone, orange color) and myelin basic protein (MBP, green color) in cross (A, C, E, G) and longitudinal (B, D, F, H) sections of PBS- (C, D), sCR1- (E, F) and Cetor-treated (G, H) rat sciatic nerves at 3 days after injury. The PBS-treated nerve shows loss of phosphorylated neurofilament staining (C, D) and broken down myelin (C, inset, arrow) whereas the sCR1-treated nerve shows a high amount of phosphorylated neurofilament immunoreactivity (F), typical punctuated neurofilament and annulated myelin staining (E, insets, open arrowhead) similarly to the uninjured nerve (A, insets, open arrowhead), sign of preserved axon and myelin morphology. In the Cetor-treated nerves, the typical annulated myelin staining is maintained (G, inset, open arrowhead) but the phosphorylated neurofilament staining is mostly lost (G, and inset, asterisk; and H). Normal punctuate axon staining is occasionally visible in the Cetor-treated nerves (G, inset, arrow). Sections are stained with a two-step immunofluorescence method. MBP is detected with a FITC-conjugated secondary antibody and SMI31 is detected with a Cy3-conjugated secondary antibody. The nuclei are stained with DAPI (blue color). Electron microscopy of complement inhibitor-treated nerves, showing preserved annulated myelin structure in both nerves (I, J; arrowheads). In the sCR1-treated nerves, the axons retain their axonal contents (I, asterisk) whereas in Cetor-treated nerves, the axonal content is generally lost (J, asterisk). Some remnants of axonal content remain visible in the Cetor-treated nerve (J, arrows). Scale bars: 50 μm (A, B); 5 μm (I, J).
Analysis of sciatic nerves of PBS-, sCR1-, and Cetor-treated rats at 7 days after injury showed loss of annulated myelin morphology in all groups of animals (Figure 3, A–F), demonstrating that WD is delayed but not prevented in complement inhibitor-treated nerves after the crush injury. To determine the effect of complement inhibition on myelin clearance we quantified the MBP staining at 7 days after injury. As expected, the amount of MBP immunoreactivity was significantly lower in the crushed nerves than in the uninjured nerves (Figure 3G). The amount of MBP immunoreactivity differed between crushed nerves of PBS- and complement inhibitor-treated rats. The PBS-treated nerves showed significantly lower percentage of MBP immunoreactivity (11.2 ± 4.7%) compared to the sCR1-treated (30.8 ± 5.0%, P ≤ 0.001) and Cetor-treated nerves (35.5 ± 13.0%, P ≤ 0.001). This demonstrates that clearance of myelin debris is delayed in the complement inhibitor-treated nerves.
Figure 3.
Analysis of WD and myelin clearance at 7 days after injury. Representative immunostaining for myelin basic protein (MBP) (green color) in cross (A, C, E) and longitudinal (B, D, F) sections of PBS- (A, B), sCR1- (C, D) and Cetor-treated (E, F) rat sciatic nerves at 7 days after injury, showing myelin degradation in all nerves, demonstrating that WD occurs with delay in the complement inhibitor-treated nerves. Sections are stained with a two-step immunofluorescence method with a FITC-conjugated secondary antibody. G: Quantification of MBP immunoreactivity normalized to uninjured control levels, showing low amount of MBP immunoreactivity in the PBS-treated nerve whereas high levels are detected in the sCR1- and Cetor-treated nerves, demonstrating delayed clearance of myelin in the complement inhibitor-treated nerves compared to the PBS-treated controls. Data represent mean ± SD. Statistical significance is determined by two-way analysis of variance with Bonferroni’s correction. Scale bars = 50 μm.
Effect of Complement Inhibition on Macrophage Accumulation and Activation
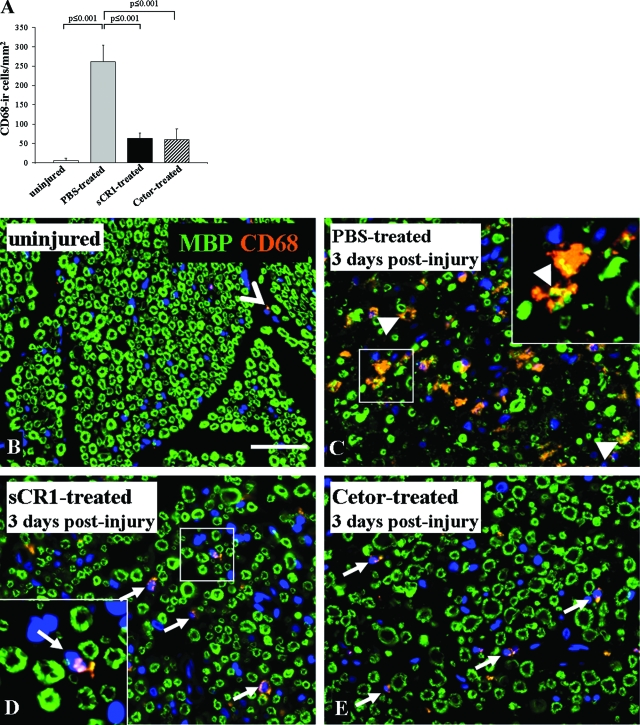
We monitored accumulation and morphological changes of macrophages because complement activation mediates macrophages recruitment20 and activation.21 We used the CD68 antibody (ED1 clone), a lysosomal marker, as marker for their metabolic state. A few CD68-immunoreactive cells were found in the control uninjured nerve (5.3 ± 1.7 cells/mm2). The number increased to 261.2 ± 10.7 cells/mm2 in the nerves of the PBS-treated rats at 3 days after injury whereas the nerves from the sCR1-treated (63.1 ± 4.7 cells/mm2) and Cetor-treated rats (59.8 ± 28.3 cells/mm2) showed a milder increase (Figure 4A).
Figure 4.
Analysis of macrophages. A: Quantification of CD68-ir cells in nonconsecutive sections of sciatic nerves, showing a significantly higher number of cells in the PBS-treated compared to the uninjured nerves and a significant decrease in the sCR1- and Cetor-treated nerves compared to the PBS-treated control. Data represent mean ± SD. Statistical significance is determined by two-way analysis of variance with Bonferroni’s correction. B–E: Representative double staining for MBP (green color) and CD68 (orange color) in cross-sections of PBS-, sCR1-, and Cetor-treated rat sciatic nerves at 3 days after injury. Note the irregular morphology of CD68-ir cells engulfing myelin debris in the PBS-treated nerve (C, arrowhead and yellow color) whereas sCR1- (D) and Cetor-treated (E) nerves show small and round CD68-ir cells (arrows) resting between the morphologically normal myelin similarly to the uninjured nerve (B, open arrowhead), suggesting activated macrophages in the PBS-treated but not in the sCR1- or Cetor-treated nerves. Sections are stained with a two-step immunofluorescence method. MBP is detected with a FITC-conjugated secondary antibody and CD68 is detected with a Cy3-conjugated secondary antibody. The nuclei are stained with DAPI (blue color). Scale bar = 50 μm.
The nerves of the PBS-treated rats showed large and asymmetrical CD68-immunoreactive cells (average size, 103.6 ± 71.8 μm2), which contain myelin debris as shown by the co-localization with the MBP staining at 3 days after injury (Figure 4C, arrowheads; and yellow in inset). In contrast, small and round cells, which did not contain degraded myelin, were detected in the nerves of the sCR1-treated (average size, 22.8 ± 14.1 μm2) (Figure 4D, arrows) and Cetor-treated (average size, 19.1 ± 10.5 μm2) rats (Figure 4E, arrows), a size and shape similar to that seen in the uninjured control nerves (average size, 18.8 ± 6.6 μm2) (Figure 4B, open arrowhead).
Because activated macrophages change their shape from a small and round to an enlarged and asymmetrical morphology, we determined the CD68-immunoreactive cell size distribution as indication of macrophage activation. Cell size distribution showed high variability in the PBS-treated nerves with cell dimension ranging from 20 to more than 400 μm2 with a large population of cells of ∼60 μm2. In contrast, the sCR1-treated nerves showed cell dimension lower than 40 μm2, similar to the size of cells found in the uninjured control nerves (Supplementary Figure 2, see http://ajp.amjpathol.org). These results show that macrophages are activated in the PBS-treated nerves but not in the sCR1- and Cetor-treated ones.
Discussion
This study demonstrates that systemic treatment with sCR1, an inhibitor of classical, lectin, and alternative pathways of complement activation, protects from early axon loss and myelin breakdown after peripheral nerve injury. Daily administration of sCR1 to injured rats prevented both systemic and local complement activation, resulting in blockade of MAC deposition in the nerve. In untreated animals, crush injury led to a rapid increase of CD68-positive cells that enlarged and phagocytosed myelin. In the inhibitor-treated nerves only a slight increase of CD68-positive cells was detectable but they failed to enlarge. This could be attributable to the proliferation and differentiation of the endoneurial macrophage population that occurs already at 2 days after injury.5 Both long-term and short-term resident macrophages newly express the lysosomal ED1 antigen and have the potential to phagocytose myelin.22 However, this is a complement-mediated event.23 Because complement activation is inhibited in the sCR1-treated nerves, complement opsonins are not deposited on the nervous tissue hampering target recognition and preventing myelin phagocytosis. In addition, complement inhibition also results in inefficient chemotaxis, preventing the recruitment of blood-derived macrophages, which probably accounts for the additional fourfold increase observed in the PBS-treated nerve.
Despite the diminished recruitment and activation of macrophages, sCR1 cannot protect the nerve from axonal degradation and myelin breakdown at 7 days after injury even when hemolytic complement activity is maintained low. Therefore we conclude that inhibition of complement activation only affects the early events of WD. We observed the same effect on rats deficient in the complement protein C6,7 demonstrating that lack of effect at 7 days after injury is not attributable to incomplete inhibition of complement activation but points to an early effect of complement activation during WD that can be rescued by complement inhibition. Lack of C4c deposition in the sCR1-treated nerves is a noteworthy finding because sCR1 inhibits the C3 convertase that is downstream of C4 cleavage, thus little effect on C4c deposition would be expected. However, as also noted in previous studies,15 blockade of complement-mediated damage by sCR1 will also inhibit overall complement deposition on damaged tissue, also resulting in undetectable C4c levels.
We demonstrated that, beside the classical pathway, also the alternative pathway is activated after a crush injury of the peripheral nerve. Blockade of the classical (and lectin) complement pathway with C1 inhibitor (Cetor), a serine protease inhibitor that blocks activation of the C1q-C1r-C1s (and MBL-MASP) complex,6,10 diminished but did not ablate MAC deposition in the nerve. Because low-rate activation of the alternative pathway occurs under physiological conditions and is negatively regulated by complement inhibitors, disruption of membrane bound complement regulatory components at the site of injury could set the alternative pathway out of control, generating more C3 convertase and leading to MAC deposition. In addition, we cannot rule out that low levels of C3b, which would accumulate during activation of the classical pathway, could escape inhibition by Cetor forming low levels of C5 convertase and acting as substrate for the alternative pathway to further amplify activation. Partial blockade of complement activation results in reduced C3 deposition, which reduces macrophage accumulation and prevents their activation while low amounts of MAC are still deposited in the nerve. Interestingly, this is sufficient to cause marked axonal injury (but not much myelin degradation), emphasizing the sensitivity of the axons to MAC-induced damage. This also suggests that myelin loss is an indirect effect of axon loss and it requires macrophages to target the opsonized surface, become activated, strip, and degrade the myelin.
We propose that epitopes exposed by the mechanical damage trigger both the classical and alternative complement activation pathways leading to abundant MAC assembly and deposition. MAC creates nonspecific pores on the nerve fibers allowing uncontrolled calcium influx into the axon.24 Calcium activates calcium-dependent proteases that breakdown cytoskeletal proteins including neurofilament. This results in structural disorganization of the nerve. Our data show that even low levels of MAC deposition, occurring with C1 inhibitor treatment, are sufficient to cause marked axonal damage. MAC deposits have been found on damaged nerve terminal axons and surrounding perisynaptic Schwann cells in a mouse model of neuropathy. Furthermore, inhibition of MAC formation resulted in both, axonal and perisynaptic Schwann cell integrity.25
The protective effects of sCR1 and C1 inhibitor on tissue injury have been described in a variety of animal models of human diseases, including brain,19,20,21 myocardial,11,26,27,28 skeletal muscle,29 intestinal,30 and pancreatic31 ischemia/reperfusion injury, transplant rejection,32,33,34,35 and in autoimmune disease models including experimental autoimmune encephalomyelitis, a model of multiple sclerosis,15,36 and experimental autoimmune neuritis, a model of Guillane-Barrè syndrome.37,38
Here we demonstrated that inhibition of activation of all complement pathways protects the peripheral nerve from early WD. WD is common to many injury and noninjury related disorders of the PNS and CNS.39 It leads to axon loss, which is the major determinant of disability in such disorders. Complement inhibition directly prevents axonal damage and indirectly inhibits macrophage accumulation in the nerve, possibly ameliorating the disease outcome.
Traumatic brain and spinal cord injuries are characterized by complement activation and secondary axonal damage that occurs hours after the initial insult.40 Inhibition of complement activation could prevent spreading of secondary axon loss that is a major determinant of clinical outcome.41 In some neurodegenerative diseases such as multiple sclerosis, axonal damage is a substantial determinant of pathology.14 Delaying axonal degeneration could give a chance for more axons to survive a period of demyelination, arresting the decline from the relapsing-remitting to the progressive phase of the disease.
The immune system plays also a role in the pathogenesis of certain type of inherited peripheral neuropathies. Macrophages actively contribute to pathology and inhibition of their activation ameliorates the effects of the primary genetic defect.42 We observed that the complement system is activated in inherited peripheral neuropathies43 and propose inhibition of complement activation as a potential treatment to ameliorate pathology.
Footnotes
Address reprint requests Frank Baas, Academic Medical Center, Neurogenetics Laboratory, Meibergdreef 9, 1105 AZ Amsterdam Zuidoost, The Netherlands. E-mail: f.baas@amc.uva.nl.
Supported by The Netherlands Organization for Scientific Research (grant 050-10-010).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Morgan BP, Harris CL. Complement therapeutics: history and current progress. Mol Immunol. 2003;40:159–170. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- de Jonge RR, van Schaik IN, Vreijling JP, Troost D, Baas F. Expression of complement components in the peripheral nervous system. Hum Mol Genet. 2004;13:295–302. doi: 10.1093/hmg/ddh029. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations on the alterations produced thereby in the structure of their primitive fibers. Philosophy Trans Royal Society London B Bulletin. 1850;140:423–429. [Google Scholar]
- Ballin RH, Thomas PK. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis. I. Demyelination. J Neurol Sci. 1969;8:1–18. doi: 10.1016/0022-510x(69)90037-9. [DOI] [PubMed] [Google Scholar]
- Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol. 2001;159:2187–2197. doi: 10.1016/S0002-9440(10)63070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kawabuchi M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc Res Tech. 2002;57:541–547. doi: 10.1002/jemt.10108. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, King RH, Nourallah M, Wolterman R, de Jonge R, Ramkema M, Vigar MA, van der WS, Morgan BP, Troost D, Baas F. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J Neurosci. 2007;27:7663–7672. doi: 10.1523/JNEUROSCI.5623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel PC, Cooper NR. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975;55:593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Roos A, Daha MR. A regulatory role for complement in innate immunity and autoimmunity. Int Arch Allergy Immunol. 2004;134:310–323. doi: 10.1159/000079261. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Boyle MP, Marsh HC, Jr, Carson GR, Roux KH, Weisfeldt ML, Fearon DT. Recombinant soluble CR1 suppressed complement activation, inflammation, and necrosis associated with reperfusion of ischemic myocardium. Trans Assoc Am Physicians. 1990;103:64–72. [PubMed] [Google Scholar]
- Sim RB, Arlaud GJ, Colomb MG. C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J. 1979;179:449–457. doi: 10.1042/bj1790449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A. Neuroprotection in the peripheral nervous system: rationale for more effective therapies. Arch Neurol. 2006;63:1681–1685. doi: 10.1001/archneur.63.12.1681. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Pham-Dinh D, Reynolds R. Axon loss is responsible for chronic neurological deficit following inflammatory demyelination in the rat. Exp Neurol. 2006;197:373–385. doi: 10.1016/j.expneurol.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. J Immunol. 1994;152:5477–5484. [PubMed] [Google Scholar]
- Morgan BP. Measurement of complement hemolytic activity, generation of complement-depleted sera, and production of hemolytic intermediates. Methods Mol Biol. 2000;150:61–71. doi: 10.1385/1-59259-056-X:61. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Yeh CG, Rudolph AR, Ward PA. Protective effects of soluble CR1 in complement- and neutrophil-mediated tissue injury. J Immunol. 1992;148:1479–1485. [PubMed] [Google Scholar]
- King RHM. New York: Oxford University Press Inc.,; Atlas of Peripheral Nerve Pathology. 1999 [Google Scholar]
- de Smet BJ, de Boer JP, Agterberg J, Rigter G, Bleeker WK, Hack CE. Clearance of human native, proteinase-complexed, and proteolytically inactivated C1-inhibitor in rats. Blood. 1993;81:56–61. [PubMed] [Google Scholar]
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Reichert F, Rotshenker S. Complement-receptor-3 and scavenger-receptor-AI/II mediated myelin phagocytosis in microglia and macrophages. Neurobiol Dis. 2003;12:65–72. doi: 10.1016/s0969-9961(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Leonhard C, Muller M, Hickey WF, Ringelstein EB, Kiefer R. Lesion response of long-term and recently immigrated resident endoneurial macrophages in peripheral nerve explant cultures from bone marrow chimeric mice. Eur J Neurosci. 2002;16:1654–1660. doi: 10.1046/j.1460-9568.2002.02236.x. [DOI] [PubMed] [Google Scholar]
- Brück W, Friede RL. The role of complement in myelin phagocytosis during PNS Wallerian degeneration. J Neurol Sci. 1991;103:182–187. doi: 10.1016/0022-510x(91)90162-z. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW, Bunge RP. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol. 1973;59:456–470. doi: 10.1083/jcb.59.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith RA, Willison HJ. Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann Neurol. 2005;58:203–210. doi: 10.1002/ana.20546. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Satoh PS, Kilgore KS, Lucchesi BR. Soluble complement receptor type 1 prevents human complement-mediated damage of the rabbit isolated heart. J Immunol. 1993;150:1055–1064. [PubMed] [Google Scholar]
- Smith EF, III, Griswold DE, Egan JW, Hillegass LM, Smith RA, Hibbs MJ, Gagnon RC. Reduction of myocardial reperfusion injury with human soluble complement receptor type 1 (BRL 55730). Eur J Pharmacol. 1993;236:477–481. doi: 10.1016/0014-2999(93)90487-3. [DOI] [PubMed] [Google Scholar]
- Fu J, Lin G, Wu Z, Ceng B, Wu Y, Liang G, Qin G, Li J, Chiu I, Liu D. Anti-apoptotic role for C1 inhibitor in ischemia/reperfusion-induced myocardial cell injury. Biochem Biophys Res Commun. 2006;349:504–512. doi: 10.1016/j.bbrc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150:5104–5113. [PubMed] [Google Scholar]
- Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- von Dobschuetz E, Bleiziffer O, Pahernik S, Dellian M, Hoffmann T, Messmer K. Soluble complement receptor 1 preserves endothelial barrier function and microcirculation in postischemic pancreatitis in the rat. Am J Physiol. 2004;286:G791–G796. doi: 10.1152/ajpgi.00407.2003. [DOI] [PubMed] [Google Scholar]
- Pruitt SK, Kirk AD, Bollinger RR, Marsh HC, Jr, Collins BH, Levin JL, Mault JR, Heinle JS, Ibrahim S, Rudolph AR. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994;57:363–370. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- Pierre AF, Xavier AM, Liu M, Cassivi SD, Lindsay TF, Marsh HC, Slutsky AS, Keshavjee SH. Effect of complement inhibition with soluble complement receptor 1 on pig allotransplant lung function. Transplantation. 1998;66:723–732. doi: 10.1097/00007890-199809270-00006. [DOI] [PubMed] [Google Scholar]
- Kallio EA, Lemstrom KB, Hayry PJ, Ryan US, Koskinen PK. Blockade of complement inhibits obliterative bronchiolitis in rat tracheal allografts. Am J Respir Crit Care Med. 2000;161:1332–1339. doi: 10.1164/ajrccm.161.4.9901114. [DOI] [PubMed] [Google Scholar]
- Chai PJ, Nassar R, Oakeley AE, Craig DM, Quick G, Jr, Jaggers J, Sanders SP, Ungerleider RM, Anderson PA. Soluble complement receptor-1 protects heart, lung, and cardiac myofilament function from cardiopulmonary bypass damage. Circulation. 2000;101:541–546. doi: 10.1161/01.cir.101.5.541. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Jiang S, Levin JL, Vincent A, Morgan BP. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol. 1996;71:173–177. doi: 10.1016/s0165-5728(96)00144-0. [DOI] [PubMed] [Google Scholar]
- Jung S, Toyka KV, Hartung HP. Soluble complement receptor type 1 inhibits experimental autoimmune neuritis in Lewis rats. Neurosci Lett. 1995;200:167–170. doi: 10.1016/0304-3940(95)12115-k. [DOI] [PubMed] [Google Scholar]
- Vriesendorp FJ, Flynn RE, Pappolla MA, Koski CL. Soluble complement receptor 1 (sCR1) is not as effective as cobra venom factor in the treatment of experimental allergic neuritis. Int J Neurosci. 1997;92:287–298. doi: 10.3109/00207459708986406. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Leinhase I, Schmidt OI, Thurman JM, Hossini AM, Rozanski M, Taha ME, Scheffler A, John T, Smith WR, Holers VM, Stahel PF. Pharmacological complement inhibition at the C3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Exp Neurol. 2006;199:454–464. doi: 10.1016/j.expneurol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- Martini R, Toyka KV. Immune-mediated components of hereditary demyelinating neuropathies: lessons from animal models and patients. Lancet Neurol. 2004;3:457–465. doi: 10.1016/S1474-4422(04)00822-1. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, de Jonge R, Wolterman R, King RHM, Baas F. Delayed Wallerian degeneration and faster regeneration in complement-deficient rat sciatic nerve following acute axonal injury (abstract). J Periph Nerv System. 2005;10(Suppl 1):S76. [Google Scholar]