Abstract
Nerve growth factor (NGF) influences the key pathological events of psoriasis: keratinocyte proliferation, angiogenesis, and T-cell activation. We have systematically examined the kinetics of NGF expression, keratinocyte proliferation, and migration of T lymphocytes in the epidermis in Koebner-induced developing psoriatic plaques. In skin traumatized by the tape-stripping method (n = 12), a marked up-regulation of NGF in Koebner-positive lesions (n = 7) was observed 24 hours after trauma. Synthesis of NGF reached its maximum level in the 2nd week. Furthermore, cultured keratinocytes from nonlesional skin of psoriasis patients produced 10 times higher levels of NGF compared with keratinocytes from healthy individuals. To substantiate the in vivo effect of NGF secreted by keratinocytes in psoriatic plaques, we studied psoriatic plaques and normal human skin in a SCID-human skin xenograft model. The transplanted psoriatic plaques demonstrated marked proliferation of NGF-R (p75)-positive nerve fibers compared with only a few nerves in the transplanted normal human skin. Our results demonstrate that 1) in a developing psoriatic lesion, up-regulation of NGF together with keratinocyte proliferation are early events and precede epidermotropism of T lymphocytes; 2) keratinocytes in patients with psoriasis are primed to produce elevated levels of NGF; and 3) NGF synthesized by these keratinocytes is functionally active.
Nerve growth factor (NGF) is a neurotrophic factor that is expressed both in the nervous system and in peripheral organs. NGF-induced signals are mediated by its high-affinity (tyrosine receptor kinase A, trkA) and low-affinity (p75) receptors (NGF-R). A growing number of studies on inflammatory diseases have demonstrated that the inflammatory state is characterized by up-regulation of NGF synthesis.1 Numerous cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and IL-6 can induce NGF production in fibroblasts, endothelial cells, and glial cells.2,3 In addition, immune cells involved in innate and acquired immunity show a basal level of NGF expression. NGF synthesis in these cells is enhanced after stimulation with specific antigens and cytokines.1,2,3 The immune cells that produce NGF also express the specific NGF receptor TrkA that, on binding to its ligand, activates intracellular pathways and nuclear factors in a manner similar to what happens in neuronal cells. In vitro, the administration of NGF to purified myeloid or lymphoid cell populations influences a wide range of functions: the release of inflammatory mediators, chemotaxis, the production of cytokines and immunoglobulins, proliferation, and survival of cells.1,2,3 Thus, NGF may influence the inflammatory process either directly by regulating immune cell functions, or indirectly, by modulating neuropeptide synthesis, which in turn induces an inflammatory reaction. These evidences have led to the current concept that either de novo synthesis of NGF or up-regulation of induced NGF by proinflammatory cytokines such as TNF-α, IL-1, or IL-6 plays a critical role in initiation, maintenance, and perpetuation of a chronic inflammatory process.
An important role of neurogenic inflammation in the pathogenesis of psoriasis is substantiated by a number of observations4,5,6,7,8,9,10,11,12: exacerbations during periods of stress,4 marked proliferation of terminal cutaneous nerves,6,7 up-regulation of neuropeptides [substance P (SP), vasoactive intestinal peptide, calcitonin gene-related peptide (CGRP)]6,7,8 in the psoriatic plaques, therapeutic response to neuropeptide-modulating agents such as capsaicin,9 somatostatin,11 peptide T,12 and clearance of active plaques of psoriasis at the sites of anesthesia after traumatic denervation of cutaneous nerves.5,10
The unique features of resolution of psoriasis at sites of anesthesia, up-regulation of neuropeptides, and a marked proliferation of terminal cutaneous nerves in psoriatic plaques6,7,8 encouraged us to search for the mechanism of neural influence. NGF augments tissue innervations13 and plays a critical role in regulating certain neuropeptides such as SP and CGRP,14,15 we investigated the role of NGF in psoriasis. Along with other investigators we observed that keratinocytes in lesional and nonlesional psoriatic tissue express high levels of NGF compared to the controls,16,17 and there is a marked up-regulation of NGF receptor (NGF-R) in the terminal cutaneous nerves of psoriatic lesions.18 In a recent study we noticed that transplanted psoriasis plaques on SCID mice treated with NGF receptor (NGF-R)-modulating agent such as K252a and NGF antibody induces significant improvement of psoriasis.19
These observations provide compelling evidence for the role of NGF and its receptor system in the pathogenesis of psoriasis. However, the role of NGF in relation to kinetics of the inflammatory and proliferative cascades of psoriasis is not known and no functional assay has been done to determine whether psoriatic keratinocytes are primed to produce increased level of NGF. Also it is critical to know whether NGF secreted by the psoriatic keratinocytes is functionally active.
In patients with psoriasis, de novo lesional psoriasis often appears at the site of cutaneous trauma. This characteristic clinical feature of psoriasis is known as the Koebner phenomenon or the appearance of isomorphic lesion. We took advantage of this unique clinical feature to create de novo lesions of psoriasis and to study the early pathological events in the pathogenesis of this disease. To determine the regulatory role of NGF/NGF-R in the pathogenesis of psoriasis in this study we performed the following in vivo and in vitro experiments:
1) In developing Koebner-positive lesions induced by tape stripping, we have performed sequential biopsies to investigate the kinetics of expression of NGF and NGF-R in relation to proliferation of keratinocytes and homing of T-cell infiltrates.
2) To evaluate the in vivo effect of NGF, we have studied nerve proliferation and substance P expression in the SCID mouse-psoriasis xenograft model.
3) To determine NGF production by psoriatic keratinocytes, we have cultured keratinocytes from nonlesional psoriatic skin and compared it with that of keratinocytes from healthy individuals.
Materials and Methods
Tape-Stripping Studies to Induce Psoriasis (Koebner Phenomenon) for Evaluation of Kinetics of NGF Expression in a Developing Psoriatic Lesion
The study groups comprised 12 patients with psoriasis vulgaris (10 males, 2 females; 32 to 49 years of age) and 4 normal control patients (two males, two females; 20 to 48 years of age). Patients were screened for disease activity, extent of the disease, and enrolled in the study after getting written consent for study participation. The patients were not on any systemic therapy, PUVA, or UVB for the last 6 months. A 4-mm punch biopsy was taken from uninvolved skin of the anterio-medial side of the right thigh under local anesthesia with lidocaine. A rectangular area of 1 inch × 4 inches was marked at the selected site. Tape stripping was then performed until clinical erythema was produced. Serial biopsies were taken from the traumatized area at 24 hours, 1 week, 2 weeks, 3 weeks, and 4 weeks later. In addition, a biopsy from an existing psoriatic plaque was taken from the patient group. After removal, the biopsies were immediately washed in saline and embedded in OCT compound (Miles Scientific, Naperville, IL) and frozen on the Object Holder (Allegiance Health Care Corporation, McGraw Park, IL) that was immersed in liquid nitrogen. The frozen biopsies were stored at −70°C for further processing.
Evaluation of in Vivo Effect of NGF in the SCID-Psoriasis Xenograft Model
Establishment of SCID Mouse-Human Skin Xenografts
Four- to six-week-old BALB/cByJSmn-Prkdcscid/J SCID mice of either sex were purchased from The Jackson Laboratory, Bar Harbor, ME, and maintained at the Stanford University Research Animal Facility. At 6 to 8 weeks of age mice were used for transplantation. Graft beds of ∼1 cm2 were created on the shaved areas by removing full thickness skin down to fascia. Partial thickness human skin or psoriasis plaques were then orthotropically transferred onto the graft bed. The transplants were held in place by gluing the human skin to mouse skin with Nexaband liquid, a veterinary bandage (Veterinary Products Laboratories, Phoenix, AZ). The normal human skin was obtained from elective plastic surgeries and psoriatic plaques were obtained from shave biopsies from psoriatic volunteers. The medical review board of the Santa Clara Valley Medical Center, Santa Clara, CA, approved the use of such tissues.
Collection of Biopsies from Skin Grafts
The mice were used for experiments 3 to 4 weeks after human skin transplantation. This period was allowed for healing and acceptance of the transplanted grafts. Punch biopsies (2 mm) were collected on days 7 and day 28 after transplantation. Some biopsies were taken at the mouse and human skin junction. Immediately after the biopsies were taken, the skin tissues were embedded in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC) and snap-frozen in liquid nitrogen. Cryosections were prepared from biopsies and evaluated for regeneration of nerve fiber in the transplanted skin.
Measurement of NGF in Cultured Keratinocytes from Nonlesional Psoriasis Skin
Shave biopsies were taken from nonlesional psoriatic skin (n = 5) and normal healthy control (n = 5). Biopsies were taken from the upper thigh. The study patients were age- and sex-matched. Keratinocytes were prepared from fresh biopsies using the method described by Normand and Karasek.20 Keratinocytes were cultured in KGM (Clonetics, San Diego, CA), a serum-free medium with 0.15 mmol/L Ca++.20 All experiments were performed using the third to fifth passage cultures. Ten thousand keratinocytes in 3 ml of KGM from psoriatic patients and normal healthy control were seeded in 35-mm-well tissue-culture plates. Each experiment was done in triplicate. Two hundred μl of culture supernatant was collected every 24 hours from each well and stored at −70°C. NGF concentration was measured by enzyme-linked immunosorbent assay (ELISA) using the NGFEmax Immuno Assay System (Promega, Madison, WI). Total NGF was obtained by acid treatment of the culture supernatant according to Promega’s protocol.
Keratinocyte Proliferation Assay
We have used three different methods, MTT assay, hexosaminidase assay and cell counting method to determine the keratinocyte proliferation. Keratinocytes (10,000/well), were plated in 24-well plates in triplicate wells. Keratinocytes were cultured with KGM medium only, K252a (100 nmol/L), NGF-neutralizing monoclonal antibody (100 ng/ml), p75 monoclonal antibody (100 ng/ml), isotype control (mouse IgG, 100 ng/ml) antibody. Cells were incubated for 2, 4, 6, and 14 days at 37°C in 100% humidity. Then each method was applied to determine cell proliferation. For cell counting method, keratinocytes were harvested after 2, 4, 6, and 14 days of incubation using trypsin/ethylenediaminetetraacetic acid solution and counted by hemocytometer by three independent investigators to avoid bias. For MTT assay, phosphate-buffered saline-diluted MTT (5 mg/ml) was added to each well after 2, 4, 6, and 14 days of incubation and the plate was incubated for 4 hours. Acid-isopropanol (0.04 N HCl in isopropanol,100 μl/well) was added. The plates were maintained at room temperature for 5 minutes and then read on a microtiter plate reader at A570/690 nm. For hexosaminidase assay, after 2, 4, 6, and 14 days of incubation, 60 μl of p-nitrophenol-N-acetyl-β-d-glucosaminide (Sigma, St. Louis, MO), the substrate for hexosaminidase was added to each well. Then cells were incubated for 2 hours at 37°C, 100% humidity. The color reaction was developed and enzyme activity was blocked by the adding 90 μl per well of 50 mmol/L glycine buffer, pH 10.4, containing 5 mmol/L ethylenediaminetetraacetic acid. Absorbances were measured in an ELISA reader at 405 nm. Each test in each assay method was done in triplicate and repeated five times. Results are expressed as mean ± SD and Student’s t-test was performed to compare the effect of k252a, NGF-neutralizing antibody, and p75 antibody on NGF-mediated proliferation of PNKCs using two parameters at a time, eg, comparing keratinocyte proliferation in medium only and with k252a.
Immunohistochemistry
For immunohistochemical staining, 6-μm cryosections were cut and placed on Superfrost Plus slides (VWR Scientific Products, West Chester, PA), serial sections of the same biopsy specimen were fixed in cold acetone for 5 minutes and stained with rabbit anti-NGF (Chemicon International Inc., Temecula, CA) and rabbit anti-SP (Chemicon International Inc.) antibodies at a concentration of 1:1000 and 1:200, respectively. For the conjugation of the peroxidase enzyme, the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used. The peroxidase reaction was visualized by incubating the sections with the DAB substrate kit (Vector Laboratories). Negative control sections were stained with rabbit serum (DAKO Corp., Carpinteria, CA) to substitute anti-NGF and anti-SP antibodies in the same concentrations. The other steps were same with above.
Twenty-μm tissue sections were used to stain the cutaneous nerves. Sections were incubated with the following antibodies: monoclonal anti-MAP2 antibody (1:200, Sigma), anti-NGF-R (p75) monoclonal antibody (1:40 dilution; Boehringer Mannheim, Mannheim, Germany), and anti-PGP 9.5 monoclonal antibody (1:800; Ultraclone, Cambridge, UK) for 18 hours at 4°C. After washing, the sections were incubated with fluorescein isothiocyanate-conjugated horse anti-mouse IgG (1:100, Sigma). For tarkA, polyclonal antibodies from two sources (Chemicon International; Santa Cruz Biotechnology, Santa Cruz, CA) were used in various dilutions. Tissues were also stained for arrays of inflammatory and proliferative markers using monoclonal antibodies against Ki-67, CD3, CD4, CD8, TNF-α, IL-8, and RANTES (Pharmingen, La Jolla, CA).
Sections stained with the same protocol except the primary antibody and sections stained with isotype antibody served as controls. In each section the entire tissue was scanned for the presence of positive staining within the epidermis and dermis. Sections were independently examined by two investigators. Only structures with strong, continuous linear staining were counted as positive nerve fibers. With adjustment to the fine tuning mechanism of the microscope, it was easy to detect the linear nature of the structures, given the thickness of the 20-μm cryosections.
Institutional Review Board and Animal Facility Protocol Approval
The Administrative Panel on Laboratory Animal Care at Stanford University approved the protocol for animal experiments. The protocol for tape-stripping studies and human skin shave biopsies were approved by the Medical Review Board at Santa Clara Valley Medical Center, Santa Clara, CA.
Statistics
Statistical analyses of the histological changes and cell culture studies were assessed by Student’s t-test. The differences between the study and the control groups were tested by Fisher’s exact test.
Results
Tape-Stripping Studies: Kinetics of NGF Expression in a Developing Psoriatic Lesion and Its Relationship with Proliferation of Keratinocytes, Cytokine Induction, and Migration of T Lymphocytes in the Epidermis
Seven of twelve patients developed psoriatic lesions at the site of trauma after tape-stripping injury. Clinically lesions could be identified as fine papular scaly lesions 2 to 3 weeks after the tape-stripping injury. Sequential biopsies were taken from the traumatized area irrespective of appearance of lesions. In Koebner-positive patients, once lesions were visible, biopsies were taken from the developing scattered papules restricted to the area traumatized by tape stripping.
Positive staining was observed in the tissues stained with the primary antibodies. Sections stained with the mouse IgG, normal rabbit serum, and the sections that were stained with the NGF antibody preabsorbed with NGF did not show any positive staining for NGF. The stratum corneum stained positively in both psoriasis and control skin, but this is a well known nonspecific event secondary to autofluorescence. All sections were examined by one investigator (W.Y.J.) and independent confirmation of the numerical counting was performed by another investigator (S.R.). Only cells in which staining could be appreciated without doubt were considered to be positively stained. NGF was detected only in keratinocytes. Surface area of the epidermis was determined with the help of a reticle/grid (10 × 10 mm with 1-mm2 boxes; Microscoptics, Inc., Milford, MI) placed in the eye piece of the microscope. The number of cells positive for NGF per mm2 of epidermis was calculated by dividing the total number of NGF-positive cells with the surface area. The data are described in Table 1.
Table 1.
Expression of NGF in Keratinocytes in Tape Stripping Experiment
| Subjects | 0 Hours | 24 Hours | 1st Week | 2nd Week | 3rd Week | 4th Week |
|---|---|---|---|---|---|---|
| Psoriasis Koebner+ (n = 7) | 99.12 ± 10.97 | 215.67 ± 22.45 | 156.89 ± 22.91 | 194.66 ± 30.18 | 204.42 ± 35.69 | 190 ± 37.07 |
| Psoriasis Koebner− (n = 5) | 110.60 ± 17.21 | 69.81 ± 27.07 | 86.33 ± 22.98 | 181.15 ± 37.70 | 132.05 ± 32.74 | 119.81 ± 32.07 |
| Normal (n = 4) | 42.30 ± 13.16 | 31.72 ± 19.67 | 30.61 ± 14.60 | 33.90 ± 14.97 | 49.80 ± 21.70 | 39.81 ± 17.07 |
| Chronic plaque (n = 12) | 194.44 ± 38.41 |
Results are expressed as positive cell number (±SD) for NGF in per square mm of epidermis at different time points.
Compared to the nonlesional skin in the keratinocytes of the developing Koebner-positive lesions marked up-regulation of NGF (P < 0.01) was evident within 24 hours of traumatization (Figure 1, A and B; Table 1). Increased levels of NGF were maintained while these lesions clinically and histologically progressed to form a psoriatic plaque (Figure 1, D–F; and Table 1). It is worth noting that in parallel with the increased expressions of NGF in the keratinocytes, the NGF-R-positive nerve fibers increased in size and numbers (Figure 2, A–C) in the developing psoriatic plaques. We used antibodies against several markers of nerve fibers, which included PGP9.5, MAP-2, and NGF-R-p75. All these antibodies could positively recognize nerve fibers. We also used anti-trkA antibody from several sources but did not observe good staining for nerves fibers. Compared to anti-PGP9.5 and anti-MAP-2 antibody, anti-p75 antibody stained more nerve fibers in the psoriatic tissues. Anti-p75-stained nerves fibers were also very prominent and had a higher degree of fluorescence and linear measurement. Whereas in the traumatized skin of the normal controls and in psoriatics who did not develop Koebner lesions, NGF synthesis in the keratinocytes did not increase compared to the basal level (Figure 1C and Table 1).
Figure 1.
A–G: NGF is increased in the keratinocytes of Koebner-positive lesions. Compared to nonlesional psoriasis skin (A), marked up-regulation of NGF is demonstrated in the keratinocytes within 24 hours of traumatization in the developing Koebner-positive lesion (B). C: Whereas after 24 hours of traumatization in a Koebner-negative lesion no significant up-regulation of NGF is noticed. D (2nd week), E (3rd week), and F (4th week) in a developing Koebner-positive psoriasis lesion demonstrate that intracellular expression of NGF remained high as the lesion continued to develop into a mature psoriasis plaque. Representative positively stained NGF cells are marked with the arrows. G: Immunocytochemical control for the primary antibody: section from the same tissue of F stained without primary anti-NGF antibody does not show any positively stained NGF cell.
Figure 2.
A–C: Increased NGF-R-positive nerve fibers in Koebner-positive lesions. In parallel with the up-regulation of lesional NGF (Figure 1) marked proliferation of terminal cutaneous nerves with increased expression of NGF-R is noticed in the developing lesions of psoriasis. Figure 1B demonstrates sprouting of nerve fibers in the 2nd week whereas proliferation of these fibers along the side of the rete ridges a unique feature of psoriatic plaques is demonstrated in C.
A major focus of this report is to understand the kinetics of expression of NGF and NGF-R in a developing psoriasis lesion. However, tissues were also stained for arrays of proliferative and inflammatory markers using monoclonal antibodies against Ki-67, CD3, CD4, CD8, TNF-α, IL-8, and RANTES. In developing lesions induced by the Koebner phenomenon there were marked up-regulation of NGF and TNF-α in the keratinocytes within 24 hours of trauma. Whereas IL-8 and RANTES, the other two regulatory cytokines of keratinocyte origin could not be identified at this time point. Significant expression of IL-8 and RANTES in the keratinocytes of developing lesions of psoriasis was noticed in the 3rd and 4th week, respectively.
In the developing lesions of psoriasis, early rete peg formation and increased Ki-67-positive keratinocytes in the basal layer were evident within the 1st week of Koebnerization. No CD3+, CD4+, and CD8+ T lymphocytes could be identified in the dermis or epidermis of traumatized nonlesional psoriatic skin within 24 hours of traumatization. In Figure 3 it can be clearly seen that the developing psoriasis lesion in the 1st week had epidermal thickening, early rete peg formation, and only few CD3-positive T lymphocytes were present perivascularly in the mid-dermis. In the 2nd week CD3 infiltrates migrated to the dermo-epidermal junction, whereas significant epidermotropisim of CD3+ lymphocytes could be seen in the 3rd week.
Figure 3.
Kinetics of CD3+ T-lymphocyte infiltrates in Koebner-positive psoriatic lesion. Immunoperoxidase staining for CD3 infiltrates in developing lesions of psoriasis. Developing psoriasis lesion in the 1st week demonstrates epidermal thickening, early rete peg formation whereas scattered trafficking of CD3-positive T lymphocytes are localized perivascularly in the dermis. In the 2nd week CD3 infiltrates migrate to dermo-epidermal junction, whereas significant epidermotropisim of CD3+ lymphocytes could be seen in the 3rd week.
Evaluation of in Vivo Effect of NGF in the SCID Mouse-Psoriasis Xenograft Model
Clinical Features of the Transplant
After 3 to 4 weeks of transplantation, successful skin grafts were examined by punch biopsy. The psoriatic plaques displayed typical clinical and morphological features of psoriatic lesions (Figure 4). Transplanted plaques were scaly, showed remarkable hyperplasia, erythema, and in some cases hyperpigmentation.
Figure 4.
Clinical features of transplanted psoriatic plaque in SCID mouse. Representative transplanted psoriatic plaque in SCID mouse, 4 weeks after transplantation. This plaque shows typical features of psoriasis-like scaling, thickening of the skin, and erythema.
Morphology of Transplanted Psoriatic Plaque and Normal Skin
Four weeks after transplantation psoriatic plaques maintained the characteristic features of psoriasis (Figure 5A). The biopsy from the plaque shows marked acanthosis, infiltration of mononuclear cells in the papillary dermis, and parakeratosis. The transplanted psoriatic plaques had epidermotropism of CD8+ T cells, a unique immunohistological feature of psoriasis (Figure 5B, white arrowhead).
Figure 5.
Histological features of transplanted psoriatic plaque. A: H&E staining of a biopsy from a 4-week-old psoriatic plaque transplant. The figure shows typical histopathological features of psoriasis with marked acanthosis, infiltration of mononuclear cells in papillary dermis, and parakeratosis. B: A biopsy from transplanted plaque was stained for CD8+ T cells. White arrows indicate intraepidermal CD8+ T cells, an immunohistopathological hallmark of a psoriasis plaque.
Regeneration of Sensory Nerve Fiber in the Transplanted Skin
Because mouse and human NGF are 97% homologous it is likely that mouse nerves will promptly proliferate into the NGF-rich transplanted psoriasis plaques on a SCID mouse. We observed a marked proliferation of NGF-R (p75)-positive nerve fibers in the transplanted psoriatic plaque compared to a few nerves in the transplanted normal human skin (Figure 6). Because up-regulation of p75 receptor is a marker of an in vivo effect of NGF, for quantification of nerve regeneration we used the data from p75 antibody staining.19 The numbers of regenerated terminal cutaneous nerves positive for NGF-R were significantly higher (P < 0.01) in the transplanted psoriatic plaques (Figure 6, C–E) compared to the normal human skin (Figure 6A). Table 2 summarizes the quantitative differences in number of nerve fibers in transplanted plaques and transplanted normal skin. We also observed that, at the 4th day after transplantation, all of the nerve fibers initially present in the human skin were degenerated (Figure 6B). The biopsy sections were also stained for substance P and a significant number of nerve fibers were strongly positive for substance P.
Figure 6.
Regeneration of nerve fiber in the transplanted normal human skin and psoriatic plaque. A: No significant regeneration of cutaneous nerves in the transplanted normal human skin. B: A biopsy from 4-day-old plaque transplant was stained for NGF-R with anti-NGF-R antibody. Note that there is no NGF-R-positive fiber in this section. C–E: Biopsies from psoriatic plaques after 4 weeks of transplantation show strongly positive staining for NGF-R-positive nerve fibers.
Table 2.
NGF-R-Positive Fibers in Transplanted Plaque and Normal Skin
| Type of transplant | Number of biopsies | Duration | Number of NGF-R-positive fibers (mean ± SD in 2-mm biopsy) |
|---|---|---|---|
| Plaque | 4 | 4 days | 0.75 ± 0.95 |
| Plaque | 12 | 4 weeks | 14.13 ± 5.31* |
| Normal skin | 8 | 4 weeks | 2.37 ± 1.4 |
P < 0.01 compared NGF-R-positive fibers in the normal skin.
Measurement of NGF in Cultured Keratinocytes from Nonlesional Psoriasis Skin
In both cultured nonlesional psoriatic keratinocytes (PNKCs) and keratinocytes of healthy patients NGF could be detected in the supernatant within 24 hours of incubation. NGF levels increased from days 1 to 6 from 5 pg/ml to 70 pg/ml in the keratinocytes of healthy patients and then started decreasing (Figure 7). In the keratinocytes from nonlesional psoriasis NGF level continued to rise with time (Figure 7) and plateaued on day 14. In the keratinocytes from healthy individuals the highest level of NGF (70pg/ml) in the supernatants was noticed on day 6, whereas in the supernatants from nonlesional psoriatic keratinocytes, levels of NGF compared to the healthy patients were higher from day 1 and reached it is peak levels on day 14 (305 pg/ml). NGF was undetectable in the KGM. NGF was undetectable in culture supernatant of murine fibroblasts (3T3).
Figure 7.
Psoriatic keratinocytes from nonlesional skin (PNKCs) produce more NGF than normal keratinocytes (NKCs). Ten thousand keratinocytes from psoriatic patients (n = 5) and normal controls (n = 5) were seeded into 35-mm-well tissue-culture plates. Two hundred μl of supernatant was collected every 24 hour from each culture. NGF level was detected by ELISA. Culture medium alone was taken as background control for ELISA. Each point represents the mean ± SD of five experiments and each experiment is done in triplicate.
NGF Secreted by Nonlesional Psoriasis Skin Keratinocytes Induces an Autocrine Loop for Keratinocyte Proliferation
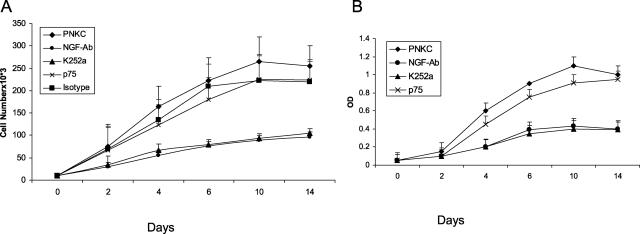
NGF is mitogenic to keratinocytes. In the above described experiment (Figure 7) we noticed that PNKCs produced large amount of NGF. We designed specific studies to investigate whether NGF secreted by the PNKCs through its autocrine effect induce proliferation of keratinocytes. Keratinocyte proliferation assays were performed by three different methods, MTT assay, hexosaminidase assay, and cell counting method. Results of proliferation assays by hexosaminidase and cell counting are shown in Figure 8, A and B. We observed that keratinocyte cell numbers increased (Figure 8, A and B) with duration of the culture period. The specificity for effect of NGF on keratinocyte proliferation was assessed by the addition of an excess of anti-NGF neutralizing monoclonal antibody (Figure 8). Addition of anti-NGF neutralizing monoclonal antibody caused significant reduction of keratinocyte proliferation. The addition of isotype-control (mouse IgG) did not affect proliferation. To examine the role of p75 and trkA, the anti-p75 monoclonal antibody or the natural alkaloid K252a were added to the culture medium. K252a inhibits NGF-induced trk proto-oncogene trkA, whereas anti-p75 monoclonal antibody inhibits NGF binding to low-affinity NGF-R. Figure 8 shows that K252a significantly (P < 0.01, Student’s t-test) inhibited NGF-induced PNKC proliferation. K252a did not affect cell viability assessed by the Trypan blue dye exclusion method, indicating that inhibition is not attributable to cytotoxic effects. Anti-p75 antibody did not affect NGF-induced proliferation (P = 0.1, Student’s t-test) (Figure 8). Marked inhibition of keratinocyte proliferation by both anti-NGF neutralizing antibody and K252a (P < 0.01) suggests that secreted NGF is acting through its high-affinity receptor, trkA, inducing an autocrine process for keratinocyte proliferation in the cultured PNKCs. It is important to note that NGF-neutralizing antibody and K252a did not completely stop the keratinocyte proliferation, suggesting that in addition to NGF, other growth factors in the KGM are also contributing to the proliferation of the keratinocytes.
Figure 8.
A and B: Secreted NGF acting through its high-affinity receptor trkA induces an autocrine loop for keratinocyte proliferation in the cultured psoriatic keratinocytes from nonlesional skin (PNKCs). Ten thousand keratinocytes from psoriatic patients (n = 5) were seeded in 35-mm-well tissue-culture plates. Keratinocytes were cultured with medium only, K252a (100 nmol/L), NGF-neutralizing monoclonal antibody (100 ng/ml), p75 monoclonal antibody (100 ng/ml), isotype control (mouse IgG, 100 ng/ml) antibody. Keratinocyte proliferation at different time points was performed by the cell-counting method (A) and hexosaminidase assay (B). Keratinocyte cell numbers increased with duration of the culture period. Keratinocytes cultured in the presence of the NGF neutralizing antibody or K252a, the trkA inhibitor had significant growth inhibition; compared to no effect in presence of the anti-p75 monoclonal antibody that binds with the low-affinity NGF-R. Marked inhibition of keratinocyte proliferation by the anti-NGF neutralizing antibody and K252a suggests that secreted NGF acting through its high-affinity receptor trkA induces an autocrine loop for keratinocyte proliferation in the cultured PNKCs. Each test in each assay method was done in triplicate and repeated five times. Each point represents the mean ± SD.
Discussion
Although clinical and laboratory studies suggest a critical role for NGF and NGF-R system in the inflammatory process of psoriasis, a regulatory role of NGF with respect to its in vivo effect, and a time course relationship for NGF synthesis by the nonlesional psoriatic keratinocytes to the disease process of psoriasis have not been investigated. In this study we performed a series of investigations to determine the kinetics of NGF expression in a developing psoriatic lesion and its relationship to the proliferation of keratinocytes and T-lymphocyte migration to the epidermis. We have also demonstrated that keratinocytes in patients with psoriasis are primed to produce a higher level of NGF and in in vivo and in vitro studies we have observed that NGF synthesized by these keratinocytes is functionally active.
NGF is a 118-amino acid hormone involved in the development and maintenance of sensory and sympathetic nerve fibers. After injury to the cutaneous nerves, denervated skin is reinnervated by two mechanisms: axonal regeneration and collateral reinnervation.21 NGF has a regulating role on both of these processes.22,23,24
Skin is densely innervated, and it is not surprising that NGF is synthesized in the skin. Several studies have shown that keratinocytes are a source of NGF and it has been recovered from the supernatants of cultured normal keratinocytes.25,26 As in other tissues, NGF in the skin has been shown to correlate directly with the density of nerve fibers.27 NGF-R mRNA correlates with NGF mRNA expression.28 It has also been reported that keratinocytes are an important source of NGF, and topical administration of NGF significantly accelerates regeneration of nerve fibers.26,27,29,30 Thus, in conditions with the increased epidermal expression of NGF, such as in psoriasis16,17 it is expected that there would be increased nerve fibers and abundant expression of NGF-R in the cutaneous nerves. Up-regulation of the p75 receptor along with sprouting of terminal cutaneous nerve fibers are an unique in vivo effect of NGF.31 In our earlier studies we have substantiated this hypothesis by demonstrating that the psoriasis plaques are characterized by marked proliferation of terminal cutaneous nerves in association with up-regulation of NGF-R (p75) and increased density of neuropeptergic nerve fibers expressing SP and CGRP.18,29,32 In this study we observed a marked proliferation of NGF-R (p75)-positive nerve fibers in the transplanted psoriatic plaque compared to a few nerves in the transplanted normal human skin (Figure 6). These observations firmly demonstrate that NGF released from the keratinocytes of psoriatic plaques is functionally active.
We also performed in vivo and in vitro experiments to assess the function of nonlesional keratinocytes from psoriasis patients with respect to NGF production. In the in vivo study we induced an isomorphic lesion (Koebner phenomenon) by the tape-stripping technique. Increased mitotic index determined by the positive Ki-67 in the basal layer keratinocytes and rete peg formation demonstrate that hyperproliferation of keratinocytes is an early event and it is demonstrated by immunohistochemistry and histological staining within the 1st week of the development process of psoriasis. We noticed that key regulatory molecules for proliferation and inflammation such as NGF and TNF-α are up-regulated within 24 hours of traumatization of skin and the up-regulation was maintained throughout the developing phase. In Koebner-negative patients and in healthy individuals traumatized skin did not have the similar response. We noticed these epidermal changes before the migration of intraepidermal T cells (Figure 3). These observations suggest that although activated T lymphocytes have an undisputed role in the pathogenesis of psoriasis, there are other regulatory systems that contribute to the inflammatory and proliferative processes of psoriasis. Thus, a complete understanding of the pathogenesis of psoriasis is still not established yet.
Evidence suggests that both T lymphocytes and keratinocytes contribute to the pathogenesis of psoriasis.4,33 The uniqueness of psoriatic keratinocytes has yet to be established. In this study as well as in earlier studies higher levels of NGF has been reported in keratinocytes of lesion-free psoriatic skin compared to normal skin and inflammatory skin disease such as lichen planus.17,18Increased synthesis of NGF by the nonlesional psoriatic keratinocytes is a novel observation. Our results suggest that psoriatic keratinocytes are primed to make increased levels of NGF. Here we have demonstrated that keratinocytes from nonlesional skin of psoriatic patients in in vitro culture on day 14 produce almost 10 times higher levels of NGF than that produced by keratinocytes from healthy individual (Figure 7). This observation was also replicated in the in vivo setting after cutaneous trauma. Synthesis of NGF reached its peak level in the 2nd week and remained persistently high thereafter (Figure 1, Table 1). It has been reported that keratinocytes in healing wounds express increased levels of NGF.30 We are seeing a similar up-regulation of NGF in the microwounds induced by the tape-stripping injuries. It is worth noting that the clinical appearance of psoriasis 2 weeks after trauma coincided with the peak level of lesional NGF and the appearance of SP-positive nerve fibers along with significant proliferation of terminal cutaneous nerves. Increased expression of NGF-R, in sequential biopsies from the developing psoriatic plaques (Figure 2) substantiates the in vivo effects of NGF. This suggests that the NGF/NGF-R system is functionally active at a very early phase of the inflammatory and proliferative processes of psoriasis. After skin injury there is increased expression of IL-1 and TNF-α.34,35 In this study we also noticed there was increased expression of TNF-α within 24 hours of skin trauma. Marked up-regulation of NGF in the Koebner-positive lesions could be induced by IL-1 and TNF-α.
In addition to being a growth factor for the nervous system, NGF has an array of biological actions on inflammation, the immune system, and cell proliferation. NGF is mitogenic to keratinocytes.26 NGF recruits mast cells and promotes their degranulation.36,37 Both of these are early events in a developing lesion of psoriasis. In addition, NGF activates T lymphocytes, recruits inflammatory cellular infiltrates,37,38,39,40 is mitogenic to endothelial cells, and induces ICAM on endothelial cells.41 NGF is also known to up-regulate the expression of SP.42 Thus, high expression of NGF as an early event in the developing lesions of psoriasis is likely to have a regulatory role on several salient pathological events of psoriasis such as proliferation of keratinocytes, angiogenesis, T-cell activation, expression of adhesion molecules, proliferation of cutaneous nerves, and up-regulation of neuropeptides.
Earlier it has been reported that NGF being mitogenic for keratinocytes plays a contributing role in wound healing.26,30 We have further substantiated a critical role for NGF in wound healing by providing evidence that in addition to its effect on keratinocytes NGF promotes angiogenesis and regeneration of cutaneous nerves, the two other essential components of wound healing.19,41 A wound induces a reaction characterized by proliferation of keratinocytes, fibroblasts, vascular elements, nerves, and accumulation of inflammatory cells. In nonpsoriatics, healing stops after a finite time depending on the nature of the wound. In psoriatic patients, wound healing is dysregulated. A wound frequently results in papulosquamous lesions, also known as the Koebner phenomenon. As evidenced in this study, keratinocytes, after mechanical trauma such as tape-stripping injury, produce significantly higher levels of NGF in lesion-free psoriasis skin compared to healthy patients. Also, we have noticed that cultured keratinocytes of psoriatic patients are capable of making almost 10 times more NGF compared to normal patients. Increased expression of NGF in nonlesional skin is likely to play a key role in the development of a Koebner reaction in a psoriatic patient. Elevated levels of NGF will induce an inflammatory response, proliferation of nerves, and up-regulation of neuropeptides such as SP and CGRP. Neuropeptides and NGF will also promote keratinocyte proliferation.4,26 Mitogenic keratinocytes will result in a cumulative rise in NGF levels. In this study we estimated NGF levels in the culture supernatants and keratinocyte proliferation at different time points in cultured keratinocytes from nonlesional psoriatic skin. We observed that with time both NGF level and keratinocyte cell numbers increased (Figures 7 and 8). When the keratinocytes were cultured in presence of NGF-neutralizing antibody or K252a, a trkA inhibitor, there was significant reduction of the proliferation of the keratinocytes (Figure 8). These observations substantiate for a NGF-induced potential autocrine loop contributing to keratinocyte proliferation in psoriasis. Thus, an optimal level of NGF is likely to lead to a vicious cycle of proliferative and inflammatory cascades required for initiation/perpetuation for the pathophysiology of psoriasis in Koebner-positive patients. In contrast, we observed that the healing events in nonpsoriatic skin or in Koebner-negative patients failed to generate or maintain higher levels of NGF. Involvement of NGF in other hyperproliferative conditions has also been documented. Particularly in brain, ovarian, prostatic, pancreatic, lung, and breast cancers increased NGF production has been correlated with tumor cell growth.43,44,45,46,47,48 In breast cancer, NGF is overexpressed and acts as an autocrine factor to enhance tumor cell growth and survival.48,49
The Koebner reaction is a salient feature of psoriasis. Pathomechanism for this unique dermatological phenomenon has remained an unsolved puzzle. In this study we demonstrated that in a developing psoriasis lesion after cutaneous trauma, keratinocyte proliferation and up-regulation of NGF in basal keratinocytes are early events and preceded epidermotropism of T lymphocytes. Further, we demonstrated that NGF secreted by the psoriatic keratinocytes is functionally active and keratinocytes of psoriatic patients produces higher levels of NGF than in normal individuals. NGF influences all key pathological events of psoriasis; it is mitogenic to keratinocytes, promotes angiogenesis, and activates T cells.26,38,41 Antagonism of NGF by a neutralizing antibody or blocking of trkA by an alkaloid K252a is therapeutically effective in psoriasis.19 Putting all these evidences together, it is suggestive that NGF plays a critical role in the pathophysiology of psoriasis, and the regulatory role of NGF and its receptor system is functionally active in the early stage of developing lesions of psoriasis.
Acknowledgments
We thank Drs. Brian Nickoloff and Marvin Karasek for their advice. This work is dedicated to Dr. Eugene M Farber, President (deceased), Psoriasis Research Institute, Palo Alto, CA.
Footnotes
Address reprint requests to Siba P Raychaudhuri, M.D., 1911 Geneva Pl., Davis, CA 95618. E-mail: sraychaudhuri@ucdavis.edu.
See related Commentary on page 865
Supported by the Psoriasis Research Institute.
References
- Aloe L. Nerve growth factor and neuroimmune responses: basic and clinical observations. Arch Physiol Biochem. 2001;109:354–356. doi: 10.1076/apab.109.4.354.4235. [DOI] [PubMed] [Google Scholar]
- Hattori A, Iwasaki S, Murase K, Tsujiimoto M, Sato M, Hyashi K, Kohno M. Tumor necrosis factor is markedly synergistic with interleukin1 and interferon gamma in stimulating the production of nerve growth factor in fibroblasts. FEBS Lett. 1994;340:177–180. doi: 10.1016/0014-5793(94)80132-0. [DOI] [PubMed] [Google Scholar]
- Heese K, Hock C, Otten U. Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem. 1998;70:699–707. doi: 10.1046/j.1471-4159.1998.70020699.x. [DOI] [PubMed] [Google Scholar]
- Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriasis lesions? J Am Acad Dermatol. 1993;28:488–489. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- Naukkarinen A, Nickoloff BJ, Farber EM. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J Invest Dermatol. 1989;92:126–129. doi: 10.1111/1523-1747.ep13071340. [DOI] [PubMed] [Google Scholar]
- Al’Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384–389. doi: 10.1111/j.1365-2230.1995.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Ekman R, Sunder F. Occurrence and distribution of neuropeptides in human skin. An immunocytochemical and immunohistochemical study on normal skin and blister fluid from inflamed skin. Acta Derm Venereol. 1987;67:185–192. [PubMed] [Google Scholar]
- Bernstein JE, Parish LC, Rapaport M, Rosenbaum MM, Roenigk HH. Effects of topically applied capsaicin on moderate and severe psoriasis vulgaris. J Am Acad Dermatol. 1986;15:504–507. doi: 10.1016/s0190-9622(86)70201-6. [DOI] [PubMed] [Google Scholar]
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;6:418–420. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Camisa C, O’Dorisio TM, Maceyko RF, Schacht GE, Mekhjian HS, Howe BA. Treatment of psoriasis with chronic subcutaneous administration of somatostatin analog 201–295 (sandostatin). I. An open-label pilot study. Clev Clin J Med. 1990;57:71–76. doi: 10.3949/ccjm.57.1.71. [DOI] [PubMed] [Google Scholar]
- Farber EM, Cohen EN, Trozak DJ, Wilkinson DI. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658–664. doi: 10.1016/0190-9622(91)70249-2. [DOI] [PubMed] [Google Scholar]
- Wyatt S, Shooeter EM, Davies AM. Expression of the NGF receptor gene in sensory neurons and their cutaneous targets prior to and during innervation. Neuron. 1990;2:421–427. doi: 10.1016/0896-6273(90)90054-j. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptides genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Pearson J, Johnson E. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982;244:378–381. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Fantini F, Magnoni C, Brauci-Laudeis L, Pincelli C. Nerve growth factor is increased in psoriatic skin. J Invest Dermatol. 1995;105:854–855. doi: 10.1111/1523-1747.ep12326689. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Jiang W-Y, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84–86. doi: 10.1080/000155598433368. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Jiang W-Y, Smoller BR, Farber EM. Nerve growth factor and its receptor system in psoriasis. Br J Dermatol. 2000;143:198–200. doi: 10.1046/j.1365-2133.2000.03617.x. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Sanyal M, Weltman H, Raychaudhuri SK. K252a, a high affinity NGF receptor blocker improves psoriasis: an in vivo study using the SCID mouse-human skin model. J Invest Dermatol. 2004;122:812–819. doi: 10.1111/j.0022-202X.2003.12602.x. [DOI] [PubMed] [Google Scholar]
- Devor M, Schonfeld D, Seltzer Z, Wall P. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979;185:211–220. doi: 10.1002/cne.901850113. [DOI] [PubMed] [Google Scholar]
- Normand J, Karasek MA. A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In Vitro Cell Dev Biol Anim. 1995;6:447–455. doi: 10.1007/BF02634257. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Farber EM, Raychaudhuri SK. Role of nerve growth factor in RANTES expression by keratinocytes. Acta Derm Venereol. 2000;80:247–250. doi: 10.1080/000155500750012108. [DOI] [PubMed] [Google Scholar]
- Owen D, Logan A, Robinson P. A role of nerve growth factor in collateral reinnervation by cutaneous C-fibers in the rat. Brain Res. 1989;476:156–160. doi: 10.1016/0006-8993(89)91245-6. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Clark H, Johnson E. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci USA. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer H, Heumann R, Thoenen H. The synthesis of nerve growth factor (NGF) in developing skin is independent of innervation. Dev Biol. 1988;128:240–244. doi: 10.1016/0012-1606(88)90286-2. [DOI] [PubMed] [Google Scholar]
- Pincelli C, Sevignani C, Manfredini R, Grande A, Fantini F, Bracci-Laudiero L, Aloe L, Ferrari S, Cossarizza A, Giannetti A. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol. 1994;103:13–18. doi: 10.1111/1523-1747.ep12388914. [DOI] [PubMed] [Google Scholar]
- Harper S, Davies AM. NGF mRNA expression in developing cutaneous epithelium related to innervation density. Development. 1990;110:515–519. doi: 10.1242/dev.110.2.515. [DOI] [PubMed] [Google Scholar]
- Wyatt S, Shooter EM, Davies AM. Expression of the NGF receptor gene in sensory neurons and their cutaneous targets prior to and during innervation. Neuron. 1990;4:421–427. doi: 10.1016/0896-6273(90)90054-j. [DOI] [PubMed] [Google Scholar]
- Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch Dermatol Res. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S, Davies AM. Regulation of expression of mRNAs encoding the nerve growth factor receptors p75 and trkA in developing sensory neurons. Development. 1993;119:635–648. doi: 10.1242/dev.119.3.635. [DOI] [PubMed] [Google Scholar]
- Jiang WY, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572–574. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- Grellner W. Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. 2002;130:90–96. doi: 10.1016/s0379-0738(02)00342-0. [DOI] [PubMed] [Google Scholar]
- Reilly DM, Green MR. Eicosanoid and cytokine levels in acute skin irritation in response to tape stripping and capsaicin. Acta Derm Venereol. 1999;79:187–190. doi: 10.1080/000155599750010931. [DOI] [PubMed] [Google Scholar]
- Aloe L, Levi-Mantalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Pearce FL, Thompson HL. Some characteristics of histamine secretion from rat peritoneal mast cells stimulated with nerve growth factor. J Physiol. 1986;372:379–393. doi: 10.1113/jphysiol.1986.sp016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe LW, Werrbach-Perez K, Perez-Polo JR. Effects of nerve growth factor on the expression of IL-2 receptors on cultured human lymphocytes. Ann NY Acad Sci. 1987;496:310–311. doi: 10.1111/j.1749-6632.1987.tb35781.x. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662–2669. [PubMed] [Google Scholar]
- Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–414. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SK, Raychaudhuri SP, Weltman H, Farber EM. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dermatol Res. 2001;293:291–295. doi: 10.1007/s004030100224. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Adler JE. Nerve growth factor regulates substance P in adult sensory neurons through both TrkA and p75 receptors. Exp Neurol. 2006;197:430–436. doi: 10.1016/j.expneurol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, Trope CG, Florenes VA. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 2003;9:2248–2259. [PubMed] [Google Scholar]
- Geldof AA, Van Haarst EP, Newling DW. Neurotrophic factors in prostate and prostatic cancer. Prostate Cancer Prostatic Dis. 1998;1:236–241. doi: 10.1038/sj.pcan.4500247. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Kleeff J, Kayed H, Wang L, Korc M, Buchler MW, Friess H. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- Ricci A, Graziano P, Mariotta S, Cardillo G, Sposato B, Terzano C, Bronzetti E. Neurotrophin system expression in human pulmonary carcinoid tumors. Growth Factors. 2005;23:303–312. doi: 10.1080/08977190500233813. [DOI] [PubMed] [Google Scholar]
- Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–17870. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- Dollé L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene. 2003;22:5592–5601. doi: 10.1038/sj.onc.1206805. [DOI] [PubMed] [Google Scholar]