Abstract
Human immunodeficiency virus (HIV) infection is associated with accelerated atherosclerosis and vasculopathy, although the mechanisms underlying these findings have not been determined. Hypotheses for these observations include: 1) an increase in the prevalence of established cardiac risk factors observed in HIV-infected individuals who are currently experiencing longer life expectancies; 2) the dyslipidemia reported with certain HIV anti-retroviral therapies; and/or 3) the proinflammatory effects of infiltrating HIV-infected monocytes/macrophages. An unexplored possibility is whether HIV itself can infect vascular smooth muscle cells (SMCs) and, by doing so, whether SMCs can accelerate vascular disease. Our studies demonstrate that human SMCs can be infected with HIV both in vivo and in vitro. The HIV protein p24 was detected by fluorescence confocal microscopy in SMCs from tissue sections of human atherosclerotic plaques obtained from HIV-infected individuals. Human SMCs could also be infected in vitro with HIV by a mechanism dependent on CD4, the chemokine receptors CXCR4 or CCR5, and endocytosis, resulting in a marked increase in SMC secretion of the chemokine CCL2/MCP-1, which has been previously shown to be a critical mediator of atherosclerosis. In addition, SMC proliferation appeared concentric to the vessel lumen, and minimal inflammation was detected, unlike typical atherosclerosis. Our data suggest that direct infection of human arterial SMCs by HIV represents a potential mechanism in a multifactorial paradigm to explain the exacerbated atherosclerosis and vasculopathy reported in individuals infected with HIV.
In 2006, an estimated 33 million people worldwide are living with human immunodeficiency virus (HIV) infection1 (World Health Organization and United Nations estimates). The advent of more successful antiviral therapies has increased dramatically the life expectancy of HIV-infected individuals. As the HIV-infected population lives longer, an understanding of the impact of the virus on chronic disease processes such as atherosclerosis becomes increasingly relevant. The rates of atherosclerotic lesion development, myocardial infarction, and restenosis after coronary angioplasty are significantly higher in HIV-infected people as compared to uninfected individuals.2,3,4,5 HIV-infected persons show particularly high risk for atherosclerosis for several reasons. Inflammation induced by HIV infection or its associated proteins may promote atherosclerosis and formation of high-risk plaque, thus increasing the risk of myocardial infarction and stroke.6,7,8,9,10,11 HIV infection itself has been associated with an abnormal lipid profile.12 Highly active antiretroviral therapy also may cause lipid abnormalities and insulin resistance, both risk factors for atherosclerosis.13 However, the role of HIV infection itself within the vessel wall in the pathogenesis of atherosclerosis is not well understood. Other studies investigating the vascular effects of HIV have focused on endothelial dysfunction14 and the infiltration of HIV-infected monocyte/macrophages but have not examined the effects of HIV on the smooth muscle cells (SMCs) of the vessel wall.
We demonstrated previously that human arterial SMCs express the three biologically relevant HIV receptors, CD4, CCR5, and CXCR4,15,16,17 that participate in HIV entry into leukocytes.18 SMCs are the predominant cells of the arterial media. The present study underscores the potential role of SMCs in the pathogenesis of HIV-associated vasculopathy by demonstrating that HIV can infect SMCs, both in vivo and in vitro, by using CD4, chemokine coreceptors and endocytosis as a mechanism of entry. HIV infection of SMCs also resulted in an enhanced secretion of the chemokine CCL2.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium, fetal bovine serum, penicillin/streptomycin, and trypsin-EDTA were purchased from GibcoBRL/Invitrogen (Carlsbad, CA). Purified mouse IgG2B and IgG1 myeloma protein were purchased from Cappel Pharmaceuticals Inc. (Westchester, PA). CD4 blocking antibody was purchased from BD Biosciences (San Jose, CA). The MCP-1/CCL2 enzyme-linked immunosorbent assay (ELISA) was purchased from R&D systems (Minneapolis, MN). The in situ cell death detection kit, TMR red terminal deoxynucleotidyl transferase dUTP nick-end labeling, was purchased from Roche (Mannheim, Germany). The HIV isolates, CXCR4 and CCR5 blockers, and blocking antibodies were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health (Germantown, MD). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise designated.
Human Tissue Sections
Coronary artery sections from four individuals without histological evidence of atherosclerosis, designated as HIV-negative/no plaque (HIVneg/no plaque), were obtained from the University of Kentucky Department of Pathology (courtesy of Dr. P. Moreno and Dr. K. Purushothaman). Coronary artery sections from eight HIV-infected persons, 10 uninfected individuals with documented atherosclerosis, and four uninfected individuals without demonstrable atherosclerosis were examined. Established risk factors are listed in Table 1. The sections from atherosclerotic coronaries had plaque classified as either type IV (5 cases) or V (3 cases) (classification based on19) and were obtained from either the Manhattan HIV Brain Bank (R24MH59724) or the University of Kentucky Department of Pathology (courtesy of Dr. P. Moreno and Dr. K. Purushothaman). All specimens were examined by confocal microscopy for p24 antigen and/or α-smooth muscle actin (SMA), a marker for smooth muscle cells. Clinical information was obtained in accordance with HIPAA regulations (Table 1). The investigators were blinded to any patient identifiers.
Table 1.
Sources of Arterial Sections
| Patient | Diagnostic | Age/gender | Viral load/CD4/ARV | ARV drugs used | Smoke/diabetes |
|---|---|---|---|---|---|
| 1 | Normal | 56/F | NA | NA | N/N |
| 2 | Normal | 22/F | NA | NA | N/N |
| 3 | Normal | 48/F | NA | NA | N/Y |
| 4 | Normal | 51/M | NA | NA | N/N |
| 5 | Atherosclerosis | 40/F | NA | NA | Y/N |
| 6 | Atherosclerosis | 58/M | NA | NA | Y/Y |
| 7 | Atherosclerosis | 47/F | NA | NA | Y/N |
| 8 | Atherosclerosis | 47/F | NA | NA | Y/N |
| 9 | Atherosclerosis | 76/M | NA | NA | N/N |
| 10 | Atherosclerosis | 40/F | NA | NA | Y/N |
| 11 | Atherosclerosis | 53/M | NA | NA | Y/Y |
| 12 | Atherosclerosis | 53/M | NA | NA | Y/Y |
| 13 | Atherosclerosis | 46/F | NA | NA | Y/Y |
| 14 | Atherosclerosis | 70/M | NA | NA | Y/Y |
| 15 | HIV/atherosclerosis | 37/M | Undetectable/8/yes | Sustiva, Epivir, Viread | NI/N |
| 16 | HIV/atherosclerosis | 42/M | 230/298/none | None | N/N |
| 17 | HIV/atherosclerosis | 54/M | 111,980/1/yes | Fuseon, Trizivir | N/N |
| 18 | HIV/atherosclerosis | 51/M | 89,138/249/yes | Videx, Viramune, Kaletra | N/N |
| 19 | HIV/atherosclerosis | 43/M | >750,000/53/none | None | N/N |
| 20 | HIV/atherosclerosis | 38/F | 39,184/1/none | None | N/N |
| 21 | HIV/atherosclerosis | 47/M | 3861/13/none | None | Y/N |
| 22 | HIV/atherosclerosis | 45/M | 720,000/75/yes | Tenofovir, abacavir, 3TC, effavirenz | NI/N |
NA, not applicable; NI, no information; N, not present; Y, present; ARV, antiretroviral.
Cell Culture
Human SMCs were prepared as described.20,21 Briefly, SMCs were isolated from donor human thoracic aortas harvested from explanted hearts at the time of cardiac transplantation. SMCs were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and were serially passaged before reaching confluence. Cells were identified as smooth muscle by their typical appearance on light microscopy and by the presence of immunostaining with an antibody to human SMA. In several experiments, primary SMC cultures purchased from Cambrex (Baltimore, MD) were used to compare with the results obtained from primary cultures prepared by our laboratory. We did not detect any differences between the primary cells and those that were purchased commercially.
HIV-1 Infection of Human SMCs
Cell-free viral inocula were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Three isolates were used: the HIVADA, an R5 isolate that infects human monocytes/macrophages; the HIVJR-CSF, an R5 isolate that infects primary human peripheral blood lymphocytes as well as certain mononuclear phagocyte populations, obtained from the cerebrospinal fluid of a patient with Kaposi’s sarcoma and severe acquired immunodeficiency syndrome encephalopathy; and the HIV92UG021, an X4 isolate that infects peripheral blood lymphocytes, that was obtained from an asymptomatic Ugandan patient. Subconfluent SMCs were incubated in Dulbecco’s modified Eagle’s medium with 50 to 90 ng of p24/ml of HIV-1 (ADA, JR-CSF, or 92UG021) for 1 to 2 hours, washed extensively to eliminate unbound virus, resuspended in fresh media, and then maintained in culture for an additional 2, 4, 7, 9, or 12 days. HIV-1 p24 protein levels were determined by an ELISA according to manufacturer’s protocols (PerkinElmer Life Science Inc., Boston, MA).
To examine the mechanism(s) of viral entry into SMCs, specific blocking antibodies for CD4 (2.5 μg/ml), CXCR4 (1, 10, and 30 μg/ml), and CCR5 (1:100, 1:250, 1:500, 1:1000, and 1:3000 dilution), or chemical blockers to the receptors (TAK against CCR5 at 10, 20, 50, and 100 ng/ml; bicyclam against CXCR4 at 10, 100, 300, and 500 ng/ml), were added to the cultured cells to determine whether HIV infection of SMCs was chemokine receptor-mediated. The inhibitors ammonium chloride (NH4Cl) (50 mmol/L), bafilomycin-A1 (50 nmol/L), and chloroquine (100 μg/ml) were used to determine whether HIV infection of SMCs was secondary to an endocytosis-dependent mechanism. Chlorpromazine (50 μmol/L) was used specifically to examine clathrin-mediated endocytosis. SMC cultures were pretreated with either the antibody for 60 minutes or with the inhibitor/chemical blocker for 15 minutes before HIV exposure. One to 2 hours after the addition of HIV, SMCs were washed extensively and placed in fresh medium. The SMC supernatants were then assessed for HIV p24 antigen using an ELISA as described previously.22 Activated human peripheral blood mononuclear cells (PBMCs), incubated at the same time as the SMCs, with high titers of HIV-1, ADA, JR-CSF, or 92UG021 were used as controls for the effectiveness of each respective blocker in reducing HIV infection. PBMC infection by HIV was assessed by measuring p24 production in the supernatants, exactly as was done for the cultured SMCs.
Immunofluorescence and Immunohistochemistry
Tissue sections or SMCs in culture were analyzed by triple or four-color immunostaining with antibodies to p24 antigen, SMA, for nuclear chromatin (4,6-diamidino-2-phenylindole, DAPI) or CD68, as previously described.23,24 Briefly, tissue sections or cultured cells were incubated in a blocking solution for 1 hour and then in diluted primary antibody (anti-p24, 1:20; National Institutes of Health repository, catalog no. 4121, anti-human SMA 1:500; Abcam, catalog no. ab21027 or anti-CD68, 1:200; Santa Cruz, catalog no. sc-7083) overnight at 4°C. Sections or cells were washed and secondary antibody conjugated to fluorescein isothiocyanate, Cy3 (Sigma anti-mouse and anti-rabbit, 1:300), and/or Alexa Fluor 647 (Molecular Probes, anti-goat) were added for 2 hours. Cells and sections were washed and mounted in anti-fade reagent with DAPI (Molecular Probes, Carlsbad, CA) and examined by confocal microscopy (Leica Microsystems GmbH, Germany).
Assessment of CCL2 Secretion
Uninfected or HIV-infected SMCs were grown to confluence in 96-well tissue culture plates and the supernatants were collected at 0, 2, 4, 7, 9, or 12 days postinfection. Supernatants from quadruplicate wells for each treatment group were collected, centrifuged, and analyzed. CCL2/MCP-1 levels were analyzed by quantitative sandwich ELISA, according to the manufacturer’s instructions (R&D Systems Inc, Minneapolis, MN). This ELISA has a 15 pg/ml detection limit. Three independent experiments were performed.
Apoptosis Assay
The percentage of apoptotic SMCs was determined by terminal deoxynucleotidyl transferase dUTP nick-end labeling staining (Roche, Mannheim, Germany) using confocal microscopy. On each coverslip, the total number of SMCs, identified by positive staining with α-actin, and the number of SMCs that were terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive were counted. Ten fields per coverslip were counted. The data were expressed as the percentage of total SMCs that were apoptotic (n = 3).
Statistical Analysis
Student’s one-tailed, paired t-test was used to compare MCP-1/CCL2 and p24 production and to quantify the numbers of p24-positive cells as compared to designated control conditions. A P value <0.05 was considered significant.
Results
SMCs in Arterial Tissue Sections Obtained from Individuals with HIV and Atherosclerosis Are Infected by HIV
To examine whether SMCs are infected in vivo, sections from normal and atherosclerotic arteries were obtained from uninfected and HIV-infected individuals and were analyzed by confocal microscopy for SMA (a marker for SMCs), HIV-p24 (a protein produced by HIV-infected cells), and DAPI (nuclei) staining. As described below, our data demonstrated that, in vivo, a population of SMCs is infected with HIV within the atherosclerotic vessel wall of infected individuals.
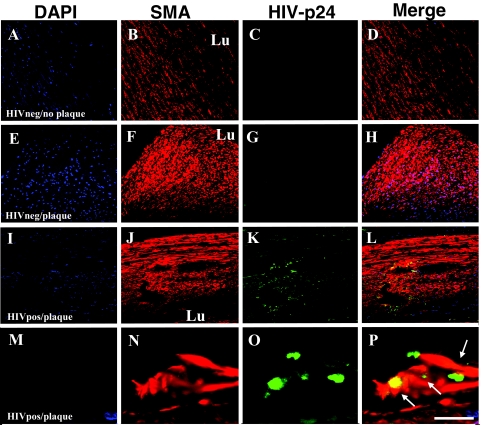
Arterial sections obtained from uninfected individuals, either without (Figure 1, designated as HIVneg/no plaque; A–D) or with detectable atherosclerotic plaque (Figure 1, designated HIVneg/plaque; E–H), had no detectable p24 staining in the vessel wall (Figure 1, C and G). In HIVneg/no plaque sections the SMC distribution, as determined by SMA staining, appeared in an unremarkable configuration with respect to the vessel lumen (Lu) (Figure 1, B and D). In arterial sections obtained from uninfected individuals with atherosclerotic plaque, the SMC staining was intense in the fibrous plaque (Figure 1, F and H) and there was no p24 staining (Figure 1G). In contrast, all arterial specimens obtained from HIV-infected individuals with clearly formed atherosclerotic plaque (Figure 1 designated HIVpos/plaque; panels I–L and M–P) contained a population of SMCs, mainly localized in the intima, that were positive for HIV p24 antigen (Figure 1, K and O). SMCs positive for p24 staining were localized close to areas of cellular fibrous plaque comprised primarily of SMCs (Figure 1, K and L; at higher magnification, O and P). Isotype-matched and preimmune sera controls for p24 and SMA staining were negative (data not shown). These results indicate that, in vivo, SMCs are infected by HIV.
Figure 1.
HIV infects SMCs in vivo. Immunohistochemical analyses of arteries from uninfected individuals without demonstrable plaque (denoted HIVneg/no plaque in A–D) or with atherosclerotic plaque (denoted HIVneg/plaque in E–H) were performed using antibodies to either human smooth muscle α-actin (SMA, red staining), HIV-p24 (green staining, p24), and DAPI (blue staining, nuclei), and were analyzed by confocal microscopy. DAPI staining was used to identify individual cells, blood vessel morphology, and cell accumulation (A, E, I, M). The arterial sections obtained from uninfected individuals without plaque (A–D) had a typical SMA staining (B) pattern with respect to the lumen (Lu) of the blood vessel and no detectable p24 staining (C). In sections obtained from uninfected individuals with plaque (E–H), SMC-rich areas (F) were present without detectable p24 antigen (G). Similar studies were done on atherosclerotic arterial sections from HIV-infected individuals (denoted HIVpos/plaque in I–P). In these samples, HIV p24 staining (K and O) was detected. The distribution of SMA and p24 appeared concentric with respect to the lumen (J) in the arterial sections with plaque from the HIV-infected individuals. At higher magnification, these sections (M–P) demonstrated the colocalization of the SMA-positive cells and p24 (P), as indicated by the arrows. A–L, scale bar = 25 μm; M–P, scale bar = 170 μm.
Tissue sections from HIV-Infected Individuals with Atherosclerosis Have Fewer Monocyte/Macrophages in the Plaque Than Those Specimens Examined from Uninfected Individuals with Atherosclerosis
To demonstrate that HIV-infected SMCs are a distinct cell population from HIV-infected monocyte/macrophages, we performed double staining for SMCs and monocyte/macrophages on sections from normal and atherosclerotic arteries obtained from uninfected and HIV-infected individuals. These sections were analyzed by confocal microscopy for SMA (a marker for SMCs), HIV p24 (a protein produced by HIV-infected cells), CD68 (a monocyte/macrophage marker), and DAPI staining (nuclei).
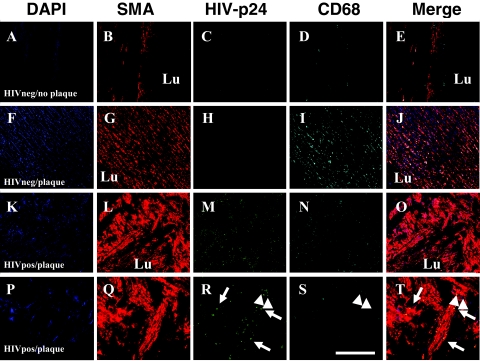
Arterial sections obtained from uninfected individuals, either without (Figure 2, designated as HIVneg/no plaque; A–E) or with detectable atherosclerotic plaque (Figure 2, designated HIVneg/plaque; F–J), had no detectable p24 staining in the vessel wall (Figure 2, C and H). SMC distribution in HIVneg/no plaque were concentric to the lumen (Figure 2, B–E). In sections obtained from uninfected individuals with atherosclerotic plaque, both SMC (Figure 2, G and J) and CD68 (Figure 2, I and J) staining were intense in the fibrous plaque. These two parameters, SMC proliferation and significant monocyte/macrophage infiltration, are characteristic features of atherosclerosis.
Figure 2.
HIV-infected tissue sections of atherosclerotic lesions have fewer monocyte/macrophages that are distinct from SMCs. Immunohistochemical analyses of arterial sections from uninfected individuals without demonstrable plaque (denoted HIVneg/no plaque in A–E) or with atherosclerotic plaque (denoted HIVneg/plaque in F–J) were performed using antibodies to human smooth muscle α-actin (SMA, red staining), HIV-p24 (green staining, p24), CD68 (Cyan staining, monocyte/macrophages), and DAPI (blue staining, nuclei), and then were analyzed by confocal microscopy. DAPI staining was used to identify individual cells, blood vessel morphology, and cell accumulation (A, F, K, P). The arterial sections obtained from uninfected individuals without plaque (A–E) had a typical SMA staining (B) pattern with respect to the lumen (Lu) of the blood vessel and no detectable p24 staining (C) and few macrophages, CD68 staining (D). In sections obtained from uninfected individuals with atherosclerotic plaque (F–J), SMC-rich areas (G) were present without detectable p24 antigen (H) and many monocyte/macrophages (I) were detectable, indicating inflammation. In atherosclerotic arterial sections from HIV-infected individuals (denoted HIVpos/plaque in K–O and P–T), HIV p24 staining (N, S) was detected. The distribution of SMA and p24 appeared concentric with respect to the lumen (L, Q) in the arterial sections with plaque from the HIV-infected individuals. CD68 staining in HIVpos/plaque individuals was minimal (N, S) compared to sections obtained from uninfected individuals (D, I) with atherosclerotic plaque. At higher magnification, these sections (P–T) demonstrated the colocalization of the SMA-positive cells and p24 (P), as indicated by the arrows. Some CD68 staining also colocalized with p24 staining (R, S, T, arrowheads), but the SMA-positive population of cells was distinct from the CD68-positive cells, indicating that HIV-infected SMCs in vivo are distinct from the monocyte/macrophages. A–O, scale bar = 25 μm; P–T, scale bar = 170 μm.
In all arterial specimens obtained from HIV-infected individuals with clearly detectable atherosclerotic plaque there was a population of p24-positive SMCs (Figure 2, L, M, O, Q, R, T). In contrast to the inflammatory characteristic of atherosclerosis plaques in uninfected sections, fewer monocytes/macrophages, ie, CD68-positive cells, were detected (Figure 2, N, O, S, T). There was a distinct population of CD68-positive cells that were HIV-infected (Figure 2, N, O, S, T, arrowheads). The HIV-positive SMCs did not colocalize with CD68-positive cells, suggesting that the infected SMCs are a distinct population from the CD68-positive cells (Figure 2, M–O, S, T). These results indicate that, in vivo, SMCs and the few infiltrated monocyte/macrophages are infected by HIV.
HIV-1 R5 and X4 Viruses Infect Human SMCs in Vitro
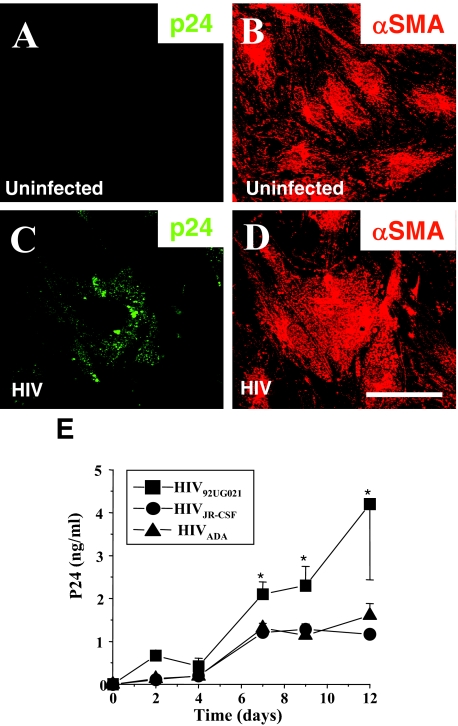
We previously demonstrated the presence and function of CD4, CCR5, and CXCR4 on SMCs by demonstrating HIV glycoprotein envelope (gp120) signaling and gp120 induction of tissue factor activity in SMCs by activation of these receptors.16 As these chemokine receptors are also those responsible for HIV infection in hematopoetic cells, and we had detected HIV infection of SMCs in vivo, we determined whether human SMCs in tissue culture could be infected with HIV. In vitro cultured human SMCs were exposed to R5 or X4 viruses and examined using confocal microscopy and ELISA for p24 at different times following viral exposure. Confocal microscopy was used to quantify the percentage of HIV-infected SMCs in the culture and to examine the intracellular distribution of p24 in these cells. Uninfected SMCs did not show detectable p24 staining (Figure 3A) and evidenced strong SMA staining (Figure 3B). Infection with R5 or X4 isolates, HIVADA (R5), HIVJR-CSF (R5), or HIV92UG021 (X4), resulted in SMCs with intracellular vesicles with p24 staining after 2, 4, 7, 9, and 12 days after viral exposure (Figure 3C). The same cells containing HIV particles were positive for SMA indicating they are SMCs (Figure 3D). The percentages of HIV-infected SMCs in the culture, as determined by immunostaining for p24, were as follows: HIVADA, 19.8 ± 2.6%; HIVJR-CSF, 17.2 ± 4.9%; and HIV92UG021, 23.3 ± 6.5%. Terminal deoxynucleotidyl transferase dUTP nick-end labeling staining of SMCs exposed to virus did not show evidence of apoptotic effects during the time course analyzed (data not shown). The time course of viral production, as measured by ELISA detecting p24 in the culture supernatant, was maximal after 7 days postinfection and was stable until 12 days, the last time point assayed. The p24 values were approximately 2.0 ng/ml after 7 days of infection and remained stable during the time course examined (n = 15, Figure 3E). HIV92UG021 produced higher levels of p24 production (Figure 3, *P < 0.003) compared to HIVADA and HIVJR-CSF.
Figure 3.
HIV infects SMCs in vitro. Human SMCs were stained with antibodies to either human smooth muscle α-actin (αSMA) (B, D) or p24 antigen (p24) (A, C) following infection for 7 days with either HIV92UG021 (C, D) or uninfected (A, B). Control uninfected SMCs did not show detectable p24 staining (A). SMCs exposed to HIV (C) had intracellular vesicular p24 staining, notably with the X4 virus; scale bar = 30 μm. E: HIV-infected SMCs produce increasing amounts of p24. To determine the p24 production following HIV exposure, human SMCs were exposed to either an R5 (HIVADA or HIVJR-CSF) or an X4 (HIV92UG021) virus for 2, 4, 7, 9, and 12 days. Supernatants were analyzed for p24 viral antigen by ELISA. No p24 was detected in uninfected cultures (data not shown). No significant differences were detected between the two R5 viruses and the X4 virus was significantly different from R5 viruses after 7 days postinfection (*P < 0.003, n = 7 independent experiments, mean ± SD).
Entry of HIV into SMCs Is CD4- and Chemokine Receptor-Dependent
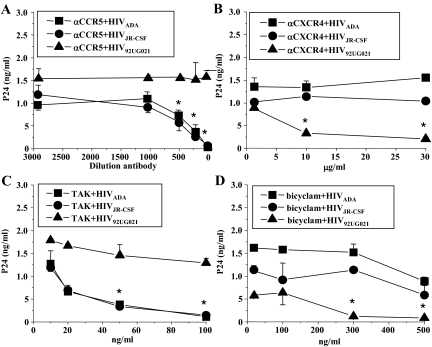
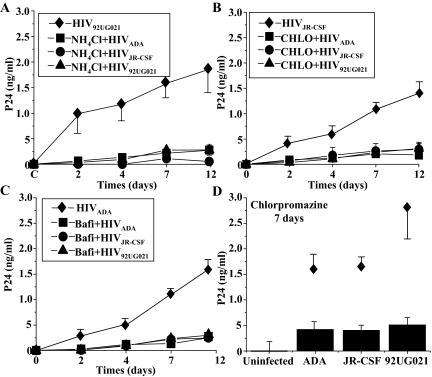
In lymphocytes, CCR5 and CXCR4 are the two chemokine receptors, together with CD4, used by HIV to fuse the viral membrane with the host cell membrane resulting in cellular entry of the virus.25,26,27 To determine the mechanism of viral entry into human SMCs, specific blocking agents were used to inhibit either viral binding mediated through CD4 receptor, specific chemokine receptors, or viral internalization by endocytosis. Human SMC cultures in vitro were treated for 15 minutes before HIV infection with blocking antibodies to CD4 (Figure 4), CCR5 (Figure 5A), or CXCR4 (Figure 5B), or blocking chemokine receptor chemicals, TAK-779, a blocker of CCR5 (Figure 5C) or a bicyclam, known as JM-2987, that blocks CXCR4 (Figure 5D). Cells then were infected and washed, and analyzed for the production of p24 in the SMC supernatants at 1, 2, 4, 5, 7, 9, and 12 days postinfection. Preincubation with blocking antibody for CD4 (bCD4) reduced viral replication of SMCs infected with HIV92UG021 (Figure 4A), HIVJR-CSF (Figure 4B), and HIVADA (Figure 4C) at least for the first 48 to 72 hours postinfection (Figure 4, asterisk). No nonspecific effects of IgG1, the isotype-matched control antibody (control for bCD4), were detected (Figure 4, IgG). These results suggest that HIV entry into SMCs is mediated, in part, by CD4. To determine whether chemokine receptors are involved in SMC infection, blocking antibodies to CCR5 (dilution, 1:100, 1:200, 1:500, 1:1000, or 1:3000) or to CXCR4 (1, 10, or 30 μg/ml) were used. These antibodies significantly decreased SMC infection by R5-specific or X4-specific virus, respectively (Figure 5, A and B, *P ≤ 0.005, n = 4), without altering entry and subsequent replication of the virus with the unrelated tropism (Figure 5, A and B). Data after 7 days postinfection are shown in Figure 5. Similar results were found at days 9 and 12 after HIV infection (data not shown). CCR5 (TAK-779) and CXCR4 (bicyclam) antagonists also blocked HIV entry and subsequent viral replication, resulting in amounts of p24 similar to those observed with the neutralizing antibodies and did so without altering the replication of virus with the alternative tropism (Figure 5, C and D, *P ≤ 0.005).
Figure 4.
CD4 participation in HIV infection of SMCs in vitro. Human SMCs were exposed to either an X4 (HIV92UG021) or an R5 (HIVADA or HIVJR-CSF) virus in the presence of blocking antibodies to CD4 (bCD4, 2.5 μg/ml). Blocking antibodies to CD4 were added to the SMC cultures 15 minutes before viral exposure. After 2 hours of viral exposure, the cells were washed and the supernatants were analyzed for p24 viral antigen by ELISA at 24, 48, 72, 96, and 120 hours after treatment. Significant differences were detected at 24, 48, and 72 hours postinfection with HIV92UG021 strain (A), HIVJR-CSF (B), and HIVADA (C) with the exception that HIVJR-CSF was not significantly different, P < 0.32, n = 3, at 72 hours, P < 0.32, n = 3. Significance was P < 0.005, n = 3. and further differences were in p24 accumulation were not detected at 168 and 288 hours postinfection (data not shown). Nonspecific effects were not detectable by using the nonimmune isotype IgG1 (IgG) (results expressed as means ± SD, n = 3).
Figure 5.
HIV infection of SMCs in vitro is mediated by chemokine receptors. Human SMCs were exposed to either an R5 (HIVADA or HIVJR-CSF) or an X4 (HIV92UG021) virus. Blocking agents or antibodies were added to the SMC cultures 15 minutes before viral exposure. After 2 hours of viral exposure, the cells were washed and the supernatants were analyzed for p24 viral antigen by ELISA at 7 and 12 days after treatment. No significant differences were detected at 7 and 14 days. Representative data from 7 days are presented. Neutralizing antibodies to either human CCR5 (A, αCCR5; at concentrations 1:100, 1:200; 1:500, 1:1000, and 1:3000 dilution) or to human CXCR4 (B, αCXCR4; at concentrations 1, 10, and 30 μg/ml) resulted in an abrogation of p24 protein accumulation in the supernatants of SMCs treated with the R5 and X4 viruses, respectively. An inhibitor of binding to CCR5 (C), TAK-779 (TAK), at concentrations of 10, 20, 50, and 100 ng/ml, or an inhibitor of CXCR4 (D), the bicyclam (JM-2987), at concentrations of 10, 100, 300, and 500 ng/ml, also reduced the p24 production in the supernatants by R5 or X4 viruses, respectively (results expressed as means ± SD).
As controls for HIV infection and for the efficiency of CD4 neutralizing antibody and the chemokine receptor blockers, human PBMCs were used (data not shown). The CD4 neutralizing antibody reduced HIV92UG021-replication in PBMCs at days 2 and 3 postinfection by 42 ± 3.7 and 32 ± 6.8% as compared to PBMCs just exposed to the virus. CCR5 and CXCR4 blocking agents were effective in reducing viral entry and subsequent replication after 2 days following viral exposure. The percentage of reduction in viral replication in PBMCs using the higher concentrations of chemokine receptor blockers that were used in SMC cultures were HIVADA, 78 ± 9.1%; HIVJR-CSF, 75 ± 7.5%; or HIV92UG021, 81 ± 10.7%, as compared to HIV-infected PBMCs without blockers. Thus, our data indicate that HIV infection of human SMCs is CD4- and chemokine receptor-dependent.
HIV Entry into SMCs Is Mediated by Endocytosis
To determine whether endocytosis contributes to viral infection of SMCs, in vitro HIV infection of SMCs was assessed in the presence of agents that are known to disrupt endocytosis, including NH4Cl, chloroquine, bafilomycin-A1, or chlorpromazine. Concentrations of these reagents were selected as those that had been optimized to inhibit maximally the entry of avian leukosis virus (ALV-B) into cells, a virus that requires endocytosis for infection. HIV infection of PBMCs was used as a negative control because HIV entry into leukocytes is not mediated by endocytosis.18,28 NH4Cl (50 mmol/L) and chloroquine (100 μg/ml) are lysosomotropic agents that selectively accumulate in endocytic compartments and increase the endosomal pH. Bafilomycin-A1 (50 nmol/L) is a specific blocker of v-type H+-ATPase, and chlorpromazine (50 μmol/L) disrupts clathrin mediated uptake. Blocking endocytosis reduced the infectivity of the SMCs to both R5 and X4 HIV strains and the subsequent viral production, as assessed by p24 production in the SMC supernatant, at 2, 4, 7, and 12 days after viral exposure (Figure 6). NH4Cl (Figure 6A), chloroquine (Chlo, Figure 6B), bafilomycin-A1 (Bafi, Figure 6C), and chlorpromazine (Figure 6D), all significantly reduced p24 production (*P < 0.0005 compared to HIV-infected SMCs treated with the respective diluents, n = 4) and abolished intracellular p24 staining as determined by confocal microscopy (data not shown). These data support the hypothesis that viral entry into SMCs requires an active endocytic process. None of these inhibitors of endocytosis altered viral entry of HIV into PBMCs (data not shown).
Figure 6.
HIV infection of SMCs in vitro is mediated by endocytosis and clathrin-mediated endocytosis. Human SMCs were exposed for 2 hours to either an R5 (HIVADA or HIVJR-CSF) or an X4 (HIV92UG021) virus in the presence of three general inhibitors of endocytosis, NH4Cl (50 mmol/L) (A), chloroquine (Chlo, 100 μg/ml) (B), bafilomycin-A (Bafi, 50 nmol/L) (C), and chlorpromazine, a clathrin-specific inhibitor of endocytosis (D). All inhibitors were added to SMCs 15 minutes before viral exposure. Viral replication was determined by assaying p24 antigen in the tissue culture media at 2, 4, 7, 9, and 12 days after HIV exposure. Inhibitors of endocytosis abolished the entry of all viruses tested (A–D) (n = 4 independent experiments). A representative experiment illustrating SMCs infected with HIV92UG021 (A), HIVJR-CSF (B), or HIVADA (C) in the absence of inhibitors (♦) is shown. These inhibitors had no effect on p24 levels in supernatants from human PBMCs that had been exposed to these isolates (data not shown) (results expressed as means ± SD).
HIV Infection Increases SMC Production of the Chemokine CCL2/MCP-1
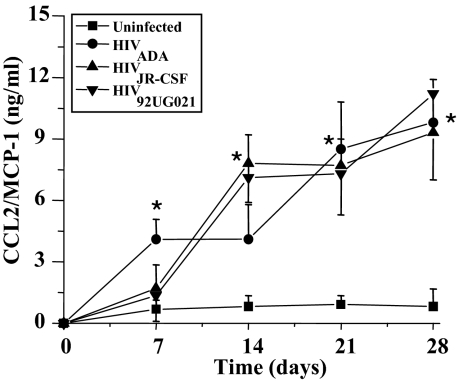
CCL2 is a potent chemoattractant for monocytes and plays an important role in both the pathogenesis of HIV infection22 and of atherosclerosis.29 We previously demonstrated that CCL2 induces SMCs to express tissue factor, a procoagulant protein that may participate in plaque progression and rupture.21 It was unknown whether infection by HIV alters SMC production of CCL2. Our hypothesis was that HIV infection would serve as a proinflammatory and/or atherogenic stimulus, leading to augmentation of CCL2 secretion by SMCs. Supernatants from SMC cultures infected with different viral strains were collected at the designated time points and assayed using an ELISA for CCL2. SMC infection by all three viral isolates significantly increased SMC production of CCL2 (*P < 0.005) at 14 days postinfection compared to uninfected cells (Figure 7). CCL2 levels remained significantly elevated through day 28, the last time point assayed, as compared to control, uninfected SMCs (Figure 7). The time course of CCL2 production was similar regardless of which strain of viral isolate was used to infect the SMCs.
Figure 7.
HIV infection of SMCs increases CCL2/MCP-1 production. SMCs in vitro were exposed to one of the three strains HIVADA, HIVJR-CSF, or HIV92UG021 for 1 to 2 hours. Cells were washed extensively and then fresh medium placed. Media was collected and assayed by quantitative sandwich ELISA for CCL2 after 7, 14, 21, and 28 days postinfection. Significant amounts of CCL2 (*) were detected in the SMC supernatants of HIV-infected cultures at 14 days and from the ADA isolate beginning at 7 days postinfection. No differences in the amount of CCL2 production were noted among the three HIV isolates tested. A P value <0.05 is considered significant (*) as compared to untreated SMCs (n = 3 independent experiments, mean ± SD).
Discussion
The results indicate that SMCs can be infected by HIV, in vivo and in vitro, by a CD4, chemokine receptor-, and endocytosis-dependent mechanism. This infection also results in increased CCL2 production by SMCs. Based on these data, we propose that HIV infection of SMCs may contribute to the pathogenesis of atherosclerosis observed in HIV-infected individuals.
The advent of highly active antiretroviral therapy coupled with effective prophylaxis for opportunistic infections has resulted in a dramatic increase in survival in individuals with HIV infection.30,31 This population of individuals represents one of the most rapidly growing groups with cardiovascular disease globally.32,33,34,35 The importance of cardiovascular disease in HIV-infected people receiving highly active antiretroviral therapy has been underscored by the reported increased risk of ischemic heart disease in this group as compared with the general uninfected population.36
A number of mechanisms mediating atherosclerosis in this HIV-infected population include dyslipidemia, hypertriglyceridemia, and insulin resistance, all of which are potent risk factors for coronary artery and vascular disease.8,35,37,38,39 However, an unexplored process that also can contribute to atherosclerosis is HIV infection of cells in the vasculature, including SMCs. Two previous studies using vessels from HIV-infected individuals demonstrated p24 antigen-positive cells in the apparently nondiseased intima as well as in atherosclerotic lesions.40,41 These authors concluded that the p24 staining was associated with dendritic cells (S100/p55-positive cells) rather than with SMCs. Another report9 documented a single case of HIV-associated coronary arteritis in an infected individual who had died of myocardial infarction and was found to have HIV sequences, as detected by in situ hybridization, in the intima and media of the left anterior descending artery.6 In agreement with the human studies, a transgenic mouse model carrying a replication-defective HIV-1 provirus develops an extensive vasculopathy characterized by intimal thickening and luminal narrowing.42,43
Our finding of p24 antigen in SMCs in arterial sections from individuals infected with HIV with vascular disease indicate that HIV directly can infect SMCs and therefore may contribute to the pathogenesis of atherosclerosis and the vasculopathy that has been reported in individuals infected with HIV.44 In all of the tissue sections from different HIV-infected individuals with atherosclerosis, a population of HIV-infected SMCs was detected by p24 staining, suggesting active HIV replication. A striking difference between the sections obtained from atherosclerotic vessels from uninfected people compared to those from HIV-infected individuals was the symmetry of the SMC accumulation in the intima of tissues from HIV-infected people. In the latter sections, the SMC proliferation was concentric to the vessel lumen, and minimal inflammation was detected. These histological findings are reminiscent of allograft vasculopathy as opposed to the more eccentric, focal, lipid-rich plaques that are characteristic of atherosclerosis.45 In addition, in uninfected individuals with atherosclerosis, more inflammation was detected as evidenced by the infiltration and accumulation of macrophages and SMCs forming a more classic asymmetric atherosclerotic plaque.46,47
SMCs, as a target for HIV infection, had not yet been examined in depth. We previously demonstrated that human SMCs express the CD4 receptor at low levels, as well as the two biologically relevant chemokine receptors for HIV entry, CCR5 and CXCR4. The presence of these receptors on SMCs supports the possibility that SMCs may be a target for HIV infection.
Our data demonstrate that anti-CD4 blocking antibodies, anti-CCR5 antibodies, and TAK-779, as well as anti-CXCR4 antibodies and bicyclam JM-2987, block infection and subsequent viral replication of SMCs as determined by p24 ELISA, indicating that these receptors are essential for viral entry into these cells. HIV-p24 immunostaining of these infected cultures pretreated with chemokine receptor inhibitors were also negative, underscoring that entry is mediated by these receptors. The tropism of the virus also was important, because blockers of CCR5 did not alter activity of CXCR4-dependent viruses, suggesting an entry effect dependent on the gp120 expressed on the surface of the virus. To determine whether endocytosis was required for HIV infection of SMCs, various blockers of endocytosis were used. All of the blockers used reduced infection and subsequent viral production to a similar degree. Unlike the chemokine receptor blocking experiments for which viral tropism was important, the endocytosis blockers reduced infectivity of all three viral isolates tested (R5 and X4) equivalently as measured by p24 ELISA and immunostaining. In contrast, our positive control of PBMCs, for which infection is mostly mediated by virus fusion with the cellular membrane, was not affected by endocytosis blockers.
An additional mechanism of blood vessel activation and HIV infection in vivo may be mediated by viral proteins, such as gp120 or tat, that can activate SMCs or endothelial cells and compromise vessel wall integrity. These viral proteins have been shown to activate the vasculature in other organ systems.10,48,49,50
In HIV infection of T cells, the delivery of its genome into the cytoplasm is by fusion of the envelope with the plasma membrane, mediated by viral interaction with CD4 and CCR5 and/or CXCR4.25 Viral fusion is a pH-independent mechanism and only requires binding of the virus to CD4 and its coreceptors, CCR5 and/or CXCR4. In contrast, in some other cell types, HIV enters into the cell by endocytosis.27,51 For example, in macrophages and dendritic cells, HIV particles bind to surface receptors, including CD4, CCR5, and/or CXCR4, and are taken up by macropinocytosis/endocytosis, suggesting that active infection and subsequent viral release into the cytoplasm can be effectively mediated by vesicular trafficking.52,53,54 Our current studies of HIV entry into SMCs demonstrate the participation of the chemokine receptors CCR5 and CXCR4 in binding of the virus to the cell, but also show that endocytosis is essential to trigger infection and subsequent viral replication in SMCs. When the gp120 of the virus binds to these chemokine receptors on SMCs, we propose that HIV is retained on the surface of SMCs and then uses the normal recycling/trafficking of these receptors,55 mediated by endocytosis, to enter into SMCs.
Whether small populations of HIV-infected cells with low viral replication contribute to the pathogenesis of HIV is still in debate. Nevertheless, it is clear that even low numbers of HIV-infected dendritic cells56 and astrocytes57,58 can play an important role as potential viral reservoirs. These reservoirs may be silent and persistent for long periods and, under specific inflammatory conditions, can be reactivated and transmit the virus to cells that support high viral replication, such as T cells and monocyte/macrophages. This mechanism of cross-infection is termed dissemination in trans.59,60 We propose that HIV-infected SMCs may participate in this trans process. One of the potential signals that recruit these inflammatory cells that support high HIV replication is CCL2. CCL2 is, to date, the most potent monocyte chemoattractant and is also chemotactic for activated T cells.61,62,63,64 Thus, the high levels of CCL2 expressed by HIV-infected SMCs may recruit monocytes and T cells into the vessel wall, facilitating their subsequent infection. Surprisingly, we observed minimal mononuclear cell infiltration in areas with SMC infection. Although significant inflammation in the HIV-infected atherosclerotic sections was not observed, it is possible that at earlier stages in the atherosclerotic process, a more significant infiltration of HIV-infected monocyte/macrophages and T cells could exist39 and potentially play a role in HIV infection of the arterial wall in vivo. In addition, CCL2 has other functions in that it promotes a thrombotic potential in the vessel wall by inducing tissue factor, the initiator of the coagulation cascade.20,21 CCL2 also has also been shown to increase SMC proliferation in vivo.65
Our findings demonstrate HIV infection of SMCs in vivo and in vitro. HIV infection of SMCs in the atherosclerotic plaque and in vitro suggests that direct infection of human arterial SMCs by the virus may be a potential mechanism in a multifactorial paradigm to explain the atherosclerosis and vasculopathy reported in individuals infected with HIV.
Acknowledgments
We thank Dr. P. Moreno and Dr. K. Purushothaman for their generosity in providing tissue samples. We also thank the staff of the Analytical Imaging Facilities at the Albert Einstein College of Medicine.
Footnotes
Address reprint requests to Alison D. Schecter, M.D., Mount Sinai School of Medicine, Box 1030, One Gustave L. Levy Place, New York, NY 10029. E-mail: alison.schecter@mssm.edu.
Supported by National Institute of Mental Health grants MH052974, MH070297, and MH075679 (to E.A.E. and J.W.B) and R24MH59724 (to S.M.); National Institutes of Health grant NS11920 (to E.A.E. and J.W.B.); National Institutes of Health Centers for AIDS Research grant AI-051519; the CFAR Immunology/Pathology Core (Albert Einstein School of Medicine); K01 grant MH076679 from the National Institute of Mental Health (to E.A.E); and R01HL7458 (to A.D.S).
References
- Bokazhanova A, Rutherford GW. The epidemiology of HIV and AIDS in the world. Coll Antropol. 2006;30(Suppl 2):3–10. [PubMed] [Google Scholar]
- de Saint Martin L, Vandhuick O, Guillo P, Bellein V, Bressollette L, Roudaut N, Amaral A, Pasquier E. Premature atherosclerosis in HIV positive patients and cumulated time of exposure to antiretroviral therapy (SHIVA study). Atherosclerosis. 2006;185:361–367. doi: 10.1016/j.atherosclerosis.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, Waters DD. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–319. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- Segev A, Cantor WJ, Strauss BH. Outcome of percutaneous coronary intervention in HIV-infected patients. Catheter Cardiovasc Interv. 2006;68:879–881. doi: 10.1002/ccd.20774. [DOI] [PubMed] [Google Scholar]
- van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- Barbaro G, Barbarini G, Pellicelli AM. HIV-associated coronary arteritis in a patient with fatal myocardial infarction. N Engl J Med. 2001;344:1799–1800. doi: 10.1056/NEJM200106073442316. [DOI] [PubMed] [Google Scholar]
- Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185:1–11. doi: 10.1016/j.atherosclerosis.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Galli M, Ridolfo AL, Gervasoni C. Cardiovascular disease risk factors in HIV-infected patients in the HAART era. Ann NY Acad Sci. 2001;946:200–213. doi: 10.1111/j.1749-6632.2001.tb03913.x. [DOI] [PubMed] [Google Scholar]
- Grody WW, Cheng L, Lewis W. Infection of the heart by the human immunodeficiency virus. Am J Cardiol. 1990;66:203–206. doi: 10.1016/0002-9149(90)90589-s. [DOI] [PubMed] [Google Scholar]
- Kan H, Xie Z, Finkel MS. HIV gp120 enhances NO production by cardiac myocytes through p38 MAP kinase-mediated NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2000;279:H3138–H3143. doi: 10.1152/ajpheart.2000.279.6.H3138. [DOI] [PubMed] [Google Scholar]
- Twu C, Liu NQ, Popik W, Bukrinsky M, Sayre J, Roberts J, Rania S, Bramhandam V, Roos KP, MacLellan WR, Fiala M. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways, Proc Natl Acad Sci USA. 2002;99:14386–14391. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor-Posner G, Basit A, Lu Y, Cabrejos C, Chang J, Fletcher M, Mantero-Atienza E. Hypocholesterolemia is associated with immune dysfunction in early human immunodeficiency infection, Am J Med. 1993;94:515–519. doi: 10.1016/0002-9343(93)90087-6. [DOI] [PubMed] [Google Scholar]
- Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. Am J Med. 2005;118(Suppl 2):23S–28S. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Cotter BR. Endothelial dysfunction in HIV infection. Curr HIV/AIDS Rep. 2006;3:126–131. doi: 10.1007/BF02696656. [DOI] [PubMed] [Google Scholar]
- Schecter AD, Berman AB, Taubman MB. Chemokine receptors in vascular smooth muscle. Microcirculation. 2003;10:265–272. doi: 10.1038/sj.mn.7800192. [DOI] [PubMed] [Google Scholar]
- Schecter AD, Berman AB, Yi L, Mosoian A, McManus CM, Berman JW, Klotman ME, Taubman MB. HIV envelope gp120 activates human arterial smooth muscle cells. Proc Natl Acad Sci USA. 2001;98:10142–10147. doi: 10.1073/pnas.181328798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter AD, Calderon TM, Berman AB, McManus CM, Fallon JT, Rossikhina M, Zhao W, Christ G, Berman JW, Taubman MB. Human vascular smooth muscle cells possess functional CCR5. J Biol Chem. 2000;275:5466–5471. doi: 10.1074/jbc.275.8.5466. [DOI] [PubMed] [Google Scholar]
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- Schecter AD, Giesen PL, Taby O, Rosenfield CL, Rossikhina M, Fyfe BS, Kohtz DS, Fallon JT, Nemerson Y, Taubman MB. Tissue factor expression in human arterial smooth muscle cells. TF is present in three cellular pools after growth factor stimulation. J Clin Invest. 1997;100:2276–2285. doi: 10.1172/JCI119765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter AD, Rollins BJ, Zhang YJ, Charo IF, Fallon JT, Rossikhina M, Giesen PL, Nemerson Y, Taubman MB. Tissue factor is induced by monocyte chemoattractant protein-1 in human aortic smooth muscle and THP-1 cells. J Biol Chem. 1997;272:28568–28573. doi: 10.1074/jbc.272.45.28568. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–361. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Gamss R, Buckner C, Buono D, Klein RS, Schoenbaum EE, Calderon TM, Berman JW. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukoc Biol. 2006;79:444–452. doi: 10.1189/jlb.0405215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Doms RW. HIV-1 entry and its inhibition. Curr Top Microbiol Immunol. 2003;281:1–27. doi: 10.1007/978-3-642-19012-4_1. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Doms RW. Evaluation of current approaches to inhibit HIV entry. Curr Drug Targets Infect Disord. 2002;2:9–16. doi: 10.2174/1568005024605864. [DOI] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Viral entry. Curr Top Microbiol Immunol. 2005;285:1–23. doi: 10.1007/3-540-26764-6_1. [DOI] [PubMed] [Google Scholar]
- Diaz-Griffero F, Hoschander SA, Brojatsch J. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J Virol. 2002;76:12866–12876. doi: 10.1128/JVI.76.24.12866-12876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Currier JS. Management of antiretroviral treatment-related complications. Infect Dis Clin North Am. 2007;21:103–132. doi: 10.1016/j.idc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Holtgrave DR. Causes of the decline in AIDS deaths, United States, 1995–2002: prevention, treatment or both? Int J STD AIDS. 2005;16:777–781. doi: 10.1258/095646205774988109. [DOI] [PubMed] [Google Scholar]
- Highleyman L. Mortality trends: toward a new definition of AIDS? BETA. 2005;17:18–28. [PubMed] [Google Scholar]
- Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- Stewart S, Wilkinson D, Becker A, Askew D, Ntyintyane L, McMurray JJ, Sliwa K. Mapping the emergence of heart disease in a black, urban population in Africa: the Heart of Soweto Study. Int J Cardiol. 2006;108:101–108. doi: 10.1016/j.ijcard.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- Paton P, Tabib A, Loire R, Tete R. Coronary artery lesions and human immunodeficiency virus infection. Res Virol. 1993;144:225–231. doi: 10.1016/s0923-2516(06)80033-6. [DOI] [PubMed] [Google Scholar]
- Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors: the Swiss HIV Cohort Study. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–46. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV. Identification of HIV-1 in the aortic wall of AIDS patients. Atherosclerosis. 2000;152:529–530. doi: 10.1016/s0021-9150(00)00547-5. [DOI] [PubMed] [Google Scholar]
- Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle BT, Ngo L, Luciw PA, Maciag T, Jay G. Human immunodeficiency virus-associated vasculopathy in transgenic mice. J Virol. 1997;71:4809–4814. doi: 10.1128/jvi.71.6.4809-4814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle BT, Ueda H, Ngo L, Luciw PA, Shaw K, Rosen CA, Jay G. Transgenic dissection of HIV genes involved in lymphoid depletion. J Clin Invest. 1997;100:32–39. doi: 10.1172/JCI119518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga RS, Chaves AA, Jing L, Ayers LW, Bauer JA. AIDS-related vasculopathy: evidence for oxidative and inflammatory pathways in murine and human AIDS. Am J Physiol Heart Circ Physiol. 2005;289:H1373–H1380. doi: 10.1152/ajpheart.00304.2005. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Bobardt MD, Salmon P, Wang L, Esko JD, Gabuzda D, Fiala M, Trono D, Van der Schueren B, David G, Gallay PA. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol. 2004;78:6567–6584. doi: 10.1128/JVI.78.12.6567-6584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P. Syndecans and HIV-1 pathogenesis. Microbes Infect. 2004;6:617–622. doi: 10.1016/j.micinf.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–2959. [PubMed] [Google Scholar]
- Kielian M, Jungerwirth S. Mechanisms of enveloped virus entry into cells. Mol Biol Med. 1990;7:17–31. [PubMed] [Google Scholar]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003;162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S, Rose JJ, Lodge R, Murphy PM, Foley JF. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol Biol Cell. 2003;14:3305–3324. doi: 10.1091/mbc.E02-11-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A, Shimeliovich I, Pack M, Trumpfheller C, Steinman RM. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J Immunol. 2006;176:991–998. doi: 10.4049/jimmunol.176.2.991. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: hIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger PM, Terkeltaub R, Lotz M. Monocyte chemoattractant protein-1 (MCP-1) expression in human articular cartilage. Induction by peptide regulatory factors and differential effects of dexamethasone and retinoic acid, J Clin Invest. 1992;90:488–496. doi: 10.1172/JCI115885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiger PM, Terkeltaub R, Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992;149:722–727. [PubMed] [Google Scholar]
- Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]