Abstract
Contact hypersensitivity is a T-cell-mediated response to a hapten. Exposing C57BL/6 mice to UV B radiation systemically suppresses both primary and secondary contact hypersensitivity responses. The effects of UVB on in vivo T-cell responses during UVB-induced immunosuppression are unknown. We show here that UVB exposure, before contact sensitization, inhibits the expansion of effector CD4+ and CD8+ T cells in skin-draining lymph nodes and reduces the number of CD4+ and IFN-γ+ CD8+ T cells infiltrating challenged ear skin. In the absence of UVB, at 10 weeks after initial hapten exposure, the ear skin of sensitized mice was infiltrated by dermal effector memory CD8+ T cells at the site of challenge. However, if mice were previously exposed to UVB, this cell population was absent, suggesting an impaired development of peripheral memory T cells. This finding occurred in the absence of UVB-induced regulatory CD4+ T cells and did not involve prostaglandin E2, suggesting that the importance of these two factors in mediating or initiating UVB-induced immunosuppression is dependent on UVB dose. Together these data indicate that in vivo T-cell responses are prone to immunoregulation by UVB, including a novel effect on both the activated T-cell pool size and the development of memory T cells in peripheral compartments.
UVB radiation (290 to 320 nm) represents ∼5% of the total UV radiation present in sunlight. Exposure to UVB triggers a multitude of molecular and cellular changes in skin, the most deleterious consequence of which is skin cancer. In addition to causing DNA damage in the skin, UVB modulates the immune system in distant lymphoid compartments resulting in the suppression of anti-tumor immunity.1 An important feature of UVB exposure is the suppression of both primary and memory recall immune responses resulting in antigen-specific tolerance.2 This study examined the cellular mechanisms of systemic UVB-induced immunosuppression, which is a phenomenon that can be observed when one skin site is exposed to UVB but antigen is applied at a distal, unirradiated site.
Exposure to UVB induces the release of numerous soluble mediators that alter immunity by acting on various cell types in both skin and draining lymph nodes (DLNs). Some of these include interleukin (IL-4), IL-10, prostaglandin-E2 (PGE2), platelet-activating factor, histamine, and cis-urocanic acid (cis-UCA).3 At the cellular level, UVB induces the generation of various regulatory cells,4,5,6 which are central to the concept of transferable UVB-induced tolerance.
UVB can inhibit contact hypersensitivity (CHS) reactions to nonproteineous contact haptens in a systemic and antigen-specific manner. CHS is a cutaneous T-cell-mediated reaction whereby hapten-specific CD4+ and CD8+ T cells are generated in skin DLNs after epicutaneous hapten application. On induction of the efferent phase of CHS, which occurs at a separate site from sensitization, hapten-specific T cells exit lymphoid organs and migrate into the skin site of challenge. The inflammatory response that ensues is primarily thought to involve the cytotoxic killing of hapten-conjugated keratinocytes by infiltrating hapten-specific effector CD8+ T cells.7 How UVB modulates the cellular mechanisms of CHS to reduce the response induced by challenge is not yet known. In particular, it is unknown if UVB has any affect on the magnitude of hapten-specific T-cell responses, which mediate CHS reactions.
Activation of naïve T cells on cognate antigenic stimulation by antigen-presenting cells (APCs) causes changes to adhesion and cytokine receptor cell surface molecules. Stimulated naïve T cells up-regulate the adhesion molecule receptor, CD44 to high levels (CD44hi) from an intermediate to low level of expression (CD44int/lo)8 and down-regulate the lymph node homing receptor, L-selectin or CD62L.9 Activated T cells, therefore, have a CD44hiCD62L− phenotype allowing them to leave lymphoid organs and traverse into peripheral tissues. The life span of differentiated cytotoxic or cytokine-secreting effector T cells from activated T cells is partly regulated by the IL-7 receptor (CD127). Naïve T cells express high levels of CD127, whereas effector T cells down-regulate expression of CD127. Therefore, effector T cells are CD44hiCD62L−CD127− and naive T cells are CD44int/loCD62L+CD127+. Alternatively, if a cell is fated to become a long-lived memory T cell, expression of CD127 is maintained.10 Memory T cells can be further subdivided into populations of central and effector memory. Central memory T cells predominantly circulate through lymphoid organs using CD62L and so are CD44hiCD62L+CD127+. In contrast, effector memory T cells migrate into and monitor peripheral tissues because they lack CD62L and so are CD44hiCD62L−CD127+.11
The effects of UVB on effector and memory T-cell development are unknown. Using a CHS model we investigated the responses of T cells in vivo with and without exposure to UVB before contact sensitization. We examined whether suberythemal low-level UVB exposure, typically acquired during normal daily activities, could modulate T-cell immunity. By monitoring T-cell activation, proliferation, and infiltration during primary CHS and in long-term resting mice, the influence of sensitization and UVB on the development of effector and memory CD4+ and CD8+ T cells was examined. We show that sensitization induces T-cell activation and proliferation in skin DLNs, and that CHS elicitation caused the infiltration of these cells into skin. UVB inhibited the primary T-cell response in skin DLNs during sensitization and decreased T-cell accumulation in challenged skin. Unirradiated mice 10 weeks after sensitization developed dermal effector memory CD8+ T cells at the challenged skin site; however, this was lost in mice that were exposed to UVB. DLN cells from UVB-irradiated mice could not transfer suppression and treatment with a cyclooxygenase (COX) inhibitor to prevent PGE2 production did not reverse the reduction of CD4+ and CD8+ T-cell expansion caused by UVB. These results indicate a novel affect of UVB, in which it can profoundly inhibit in vivo the magnitude of the effector T-cell response and modulate peripheral memory T-cell development. This occurred independently of UVB-induced regulatory T cells and the PGE2 pathway, probably because the UVB dose was not high enough to activate these pathways.
Materials and Methods
Mice
C57BL/6J female mice were used at 8 weeks of age (Animal Resource Centre, Perth, Australia). FVB transgenic GFP (T-GFP) mice on a CD4 promoter were obtained from Ulrich H. von Andrian (The Center for Blood Research, Harvard Medical School, Boston, MA)12 and were backcrossed to C57BL/6J mice. T-GFP mice were used at 8 weeks of age. All experiments were conducted under the approval of the University of Sydney Animal Ethics Committee.
UVB Source
A 1000 W xenon arc lamp solar simulator (Oriel, Stanford, CT) filtered with two 200- to 400-nm dichroic mirrors and a 310-nm narrowband interference filter (CVL Laser, Albuquerque, NM) was used to produce the UVB spectra that had a peak irradiance of 3.69 × 10−2 mW/cm2 at 311-nm wavelength, and a halfband width of ∼11 nm. UVA (more than 320 nm) and UVC (less than 290 nm) contaminated the spectra by ∼16% and 0.31%, respectively. Spectral output and intensity was measured with an OL-754 spectroradiometer (Optronics Laboratories, Orlando, FL) and a broadband radiometer (International Light Technologies, Inc., Peabody, MA) calibrated against the source was used continuously to monitor fluctuations in output. Timing of UVB delivery was accurately maintained using an automated timing device.
UVB Irradiation Protocol
Dorsums were shaved with animal clippers (Oster, McMinnville, TN) and an electric razor (Remington, Braeside, Australia) 24 hours before irradiation. Mice were restrained during irradiation within black Perspex boxes with a quartz lid. Ears and heads were protected from UVB radiation with black Perspex. Mouse dorsums were exposed to 90 mJ/cm2 of UVB daily for 3 consecutive days, which is ∼0.3 of a minimal erythema dose. To examine the effect of COX inhibition, mice were treated with 40 μl of 0.06% (w/v) indomethacin (Sigma, St. Louis, MO) in acetone on their dorsum immediately after irradiation. UVB-irradiated but unsensitized mice were included in every experiment to control against any nonspecific effects caused by irradiation.
Primary and Secondary CHS Responses
Three days after the last UVB irradiation, mouse abdomens were shaved and sensitized with a 50-μl epicutaneous application of 2% (w/v) oxazolone (Ox, 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one; Sigma) in acetone. Ears were challenged topically 7 days later with 10 μl of 2% (w/v) Ox in acetone per ear. Increases in ear thickness were calculated based on previous and 24 hours after Ox challenge measurements using micrometer calipers (Interapid, Rolle, Switzerland). To determine the CHS, increases in ear thickness of unsensitized but ear-challenged irritant controls were subtracted from sensitized mice. For secondary CHS responses, mice were resensitized with Ox on their abdomens 8 weeks after the initial sensitization and were then rechallenged on the ears 1 week after resensitization. Equivalent Ox concentrations were used as during primary CHS.13 In hapten challenge control experiments, Ox-sensitized mice were challenged with 20 μl of 1% (w/v) 2,4,6-trinitrochlorobenzene (TNCB; Tokyo Kasei, Toyo, Japan) in acetone.
Isolating Cells from Lymph Nodes
The inguinal lymph nodes (ILNs) were disassociated through 70-μm cell strainers (BD Falcon, Bedford, MA) into RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 2% fetal calf serum (Invitrogen). Viable cells were enumerated by trypan blue exclusion on a Vi-CELL counter (Beckman Coulter, Hialeah, FL).
Adoptive Transfer of Cells
Cells (2 × 107 total) from the ILNs and brachial lymph nodes of sensitized UVB-irradiated and unirradiated mice were adoptively transferred intravenously into naïve mice. Twenty-four hours after transfer, mice were sensitized with Ox and 7 days later, challenged on their ears with Ox. The CHS response was measured 24 hours after challenge. Untransferred sensitized and unsensitized control mice were included.
Ear Skin Cell Isolation
Ears were removed from mice, split into dorsal and ventral sides and were incubated in 20 mmol/L ethylenediaminetetraacetic acid (Sigma) in Tris-buffered saline (pH 7.3) for 2 hours at 37°C. Epidermal and dermal layers were first separated before they were finely chopped together into small pieces. Minced skin pieces were then incubated in 2 ml of RPMI 1640 containing 1 mg/ml of collagenase IV (Sigma), 0.02 mg/ml of DNase I (Sigma), and 5% fetal calf serum for 1.5 hours at room temperature with constant agitation. Digestion was stopped by the addition of 200 μl of 0.1 mol/L ethylenediaminetetraacetic acid for 5 minutes. Digested skin pieces were then mashed through a 100-μm steel strainer, washed, and numerated. A skin sample containing spiked lymphoid cells was used as a positive gating control for flow cytometry.
Antibodies
Monoclonal antibodies used for flow cytometry included rat anti-mouse CD3 (145-2C11; fluorescein isothiocyanate, APC), CD4 (RM4-5; PerCP, PE-Cy7), CD8α (53-6.7; PerCP, PE-Cy7), CD16/CD32 (2.4G2; purified), CD25 (PC61; APC, APC-Cy7), CD45 (30-F11; PerCP), CD62L (MEL-14; biotin), CD152 (UC10-4F10-11; PE), and interferon (IFN)-γ (XMG1.2; PE). These were purchased from BD Pharmingen (San Diego, CA). Rat anti-mouse CD62L (MEL-14; APC-Cy7), CD127 (A7R34; APC, APC-Cy7, biotin), and FoxP3 (FJK-16s; PE) were purchased from eBiosciences (San Diego, CA) and rat anti-mouse CD44 (IM7.8.1; PE) from Caltag (Burlingame, CA). Antibodies for immunohistochemistry included purified goat anti-rat/mouse IFN-γ (AF-585-NA; R&D Systems, Minneapolis, MN), rat anti-mouse CD4-biotin (H129.19; BD Pharmingen), rat anti-mouse CD8α-biotin (53-6.7; BD Pharmingen), and donkey anti-goat F(ab′)2 (biotin) (Jackson ImmunoResearch Laboratories, West Grove, PA).
Flow Cytometry
Cells were initially blocked with anti-CD16/CD32 (anti-FcRγIII/II receptor; clone 2.4G2) antibody, before staining with T-cell activation surface antibodies. When required, secondary streptavidin-APC-Cy7 (eBiosciences) was applied. All incubations were performed at 4°C for 30 minutes in fluorescence-activated cell sorting buffer (phosphate-buffered saline, 5% fetal calf serum, and 0.1 mol/L ethylenediaminetetraacetic acid, pH 7.2). Except after anti-CD16/CD32 antibody block, cells were washed three times between incubations. FoxP3 staining was performed following the manufacturer’s instructions. Stained cells were analyzed on a six-color FACSAria (BD Immunocytometry Systems, San Jose, CA) and a minimum of 200,000 events were acquired for every sample. Data analysis was performed using FlowJo software v. 6.4 (Tree Star Inc., Ashland, OR).
Bromodeoxyuridine (BrdU) Incorporation and Flow Cytometry
From the time of sensitization, mice were given fresh 0.8 mg/ml of BrdU (BD Pharmingen) in their drinking water daily. Single-cell suspensions of ILNs and skin were prepared as described above. Labeling for T-cell surface markers and intracellular BrdU was performed using a fluorescein isothiocyanate BrdU Flow kit (BD PharMingen) by following the manufacturer’s instructions. Flow cytometry acquisition and analysis was as described above.
Immunohistochemistry
Mouse ears were snap-frozen in liquid nitrogen in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA). Cryostat sections, 7 μm thick, were cut onto SuperFrost Plus slides (Menzel-Glasser, Braunschweig, Germany), air-dried, fixed in 4% paraformaldehyde (Amresco, Solon, OH), and blocked for endogenous biotin activity using a biotin blocking kit (DAKO, Glostrup, Denmark). Nonspecific antibody labeling was prevented by incubating sections in TNB blocking buffer (Perkin Elmer Life Sciences, Wellesley, MA) supplemented with 5% normal rabbit serum (Hunter Antisera, Jesmond, Australia) for 30 minutes. This blocking buffer was also used to dilute all labeling reagents. Sections were first labeled for T cells with biotinylated anti-CD4 or anti-CD8 antibodies for 1 hour. All subsequent incubations were then performed in the dark. Secondary streptavidin-Alexa Fluor 488 (Molecular Probes, Eugene, OR) was applied for 30 minutes before sections were again blocked to limit biotin and nonspecific antibody labeling. Sections were incubated with anti-IFN-γ antibody for 1 hour, which was followed by biotinylated donkey anti-goat and streptavidin-Alexa Fluor 594 (Molecular Probes) for 30 minutes each. Staining controls included antibody isotype and omission of primary T-cell or IFN-γ antibodies. Between all incubations, except after the nonspecific antibody labeling block, sections were washed three times with Tris-buffered saline and 0.05% (v/v) Tween 20 (Amersco). Sections were counterstained and coverslipped in SlowFade Gold antifade reagent with 4,6-diamidino-2-2phenylindole (DAPI, Molecular Probes). A BX51 fluorescent microscope with a DP70 camera attachment (Olympus, Tokyo, Japan) was used to visualize and photograph the sections.
Data Analysis and Statistics
CHS responses were evaluated by one-way analysis of variance analyses with Tukey post hoc tests. To determine the effect of UVB on Ox-induced T-cell activation, proliferation, and accumulation into skin, background unsensitized (irritant control) groups were subtracted from sensitized groups to enable the direct comparison of sensitization in unirradiated and UVB-irradiated groups. Unpaired Student’s t-tests were applied to compare the parameters of T-cell activation between groups of unsensitized and sensitized, or groups of sensitized unirradiated and UVB-irradiated mice. SPSS v.11 software (SPSS Inc., Chicago, IL) was used to determine significance, where P < 0.05 was considered to be significant.
Results
UVB Inhibits the Expansion of Effector CD4+ and CD8+ T Cells in Skin DLNs after Sensitization
UVB-irradiated mice had a significantly reduced CHS response (19.7 mm−2 ± 2.0, n = 18) compared to unirradiated control mice (28.4 mm−2 ± 1.0, P = 0.002). T-cell reactivity to Ox after sensitization was investigated within the ILNs because this drains both abdominal and dorsal skin with CD44hiCD62L−CD127− effector T cells being numerated 9 days after sensitization. Application of a contact hapten significantly increased the total number of effector CD4+ and CD8+ T cells by more than threefold compared to the number of effector T cells present in the ILNs of unsensitized mice (data not shown). To investigate whether UVB modulates this expansion of ILN effector T cells, mice were irradiated with UVB before sensitization. In contrast to the increases observed in control unirradiated sensitized mice, exposure to UVB significantly reduced the expansion of effector CD4+ and CD8+ T cells by threefold (P = 0.0030) and twofold (P = 0.011), respectively (Figure 1A). A survey of other nondraining lymphoid organs (mesenteric and auricular lymph nodes, spleen), as well as blood and liver in unirradiated and UVB-irradiated sensitized mice showed that these responses to Ox are limited to the skin-draining ILNs (data not shown). This suggests that UVB inhibits the primary activation and expansion of effector CD4+ and CD8+ T cells to contact sensitization locally in the ILNs.
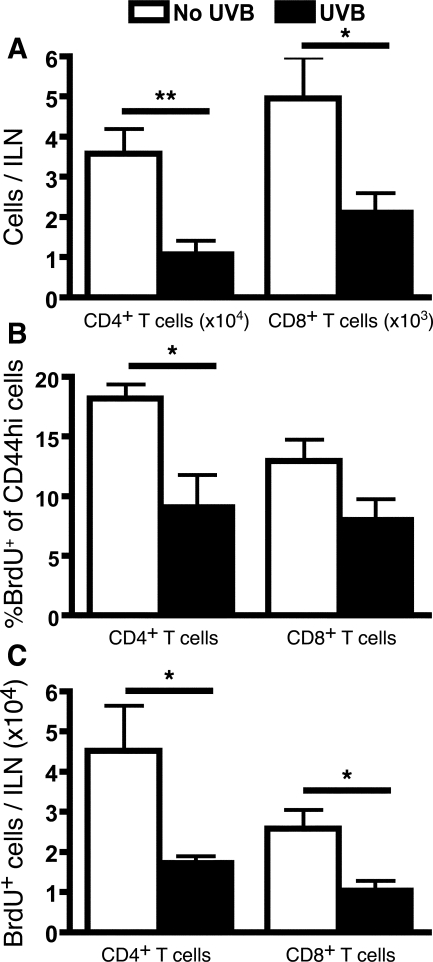
Figure 1.
UVB inhibits the increase in ILN effector T-cell number and proliferation in sensitized mice 9 days after sensitization. Mice were either unirradiated or irradiated with three daily doses of 90 mJ/cm2 UVB on the dorsum. Mice were sensitized on the abdomen with Ox, and 7 days later ears were challenged with Ox. The ILNs were examined 9 days after sensitization (48 hours after challenge). A: The increase in the number of ILN effector T cells attributable to sensitization was determined by subtracting the background number of effector T cells in unsensitized, but Ox ear-challenged mice. B: Mice were given BrdU in their drinking water from the time of sensitization. The increase in the proportion of CD44hi T cells expressing BrdU was determined by subtracting basal proliferation levels in unsensitized mice from that in sensitized mice. C: The number of BrdU+CD44hi T cells in sensitized mice after subtraction of basal proliferation numbers in unsensitized mice. n = 9 mice pooled from three individual experiments, means + SEM are shown. **P < 0.01 and *P < 0.05, comparing no UVB with UVB groups.
To confirm that the T-cell expansion was attributable to antigen-induced proliferation rather than nonspecific lymph node shutdown, BrdU incorporation was assessed 9 days after sensitization. A significantly greater proportion of BrdU+ cells were detected within the activated subset of CD44hi CD4+ and CD8+ T cells in sensitized mice (63% and 45%, respectively) compared to unsensitized mice (45%, P = 0.0004 and 32%, P = 0.0054, respectively), indicating that sensitization does induce active T-cell proliferation leading to an increase in effector CD4+ and CD8+ T-cell populations. In contrast to unirradiated mice, a smaller BrdU+ frequency of CD44hi T cells was detected in the ILNs of UVB-irradiated mice (Figure 1B). This was significantly decreased in the CD4+ subset of T cells (P = 0.0396). Similarly, a reduced number of BrdU+CD44hi CD4+ (P = 0.0355) and CD8+ (P = 0.0231) T cells were found in UVB-irradiated mice (Figure 1C), suggesting that UVB hindered the proliferation of T cells after sensitization.
UVB Irradiation Regime Does Not Generate Regulatory CD4+ T Cells
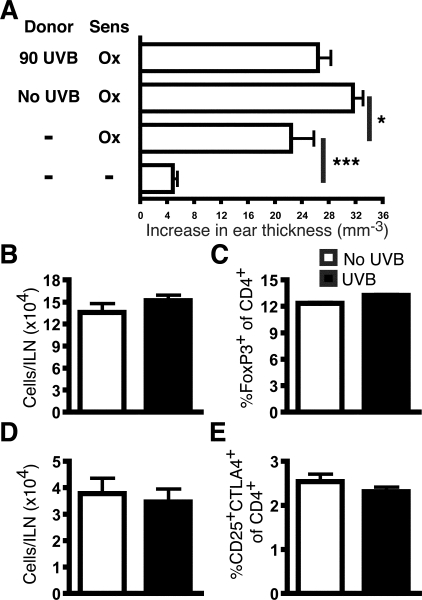
To investigate whether regulatory cells were being generated in the DLNs by the irradiation regime used in this study, total DLN cells from sensitized UVB-irradiated or unirradiated mice were adoptively transferred into naïve mice, which were then sensitized and challenged to elicit a CHS reaction. As expected, recipients of cells from unirradiated donors exhibited an increased CHS response compared to untransferred sensitized mice (P = 0.0307), as these transferred cells included Ox-specific effector T cells (Figure 2A). However, no difference was found between recipients of UVB-irradiated or unirradiated cells, indicating no transfer of suppression.
Figure 2.
Skin DLNs of UVB-irradiated mice do not contain regulatory T cells. A: Lymph node cells from either sensitized UVB-irradiated or unirradiated mice were transferred into naïve recipients, which were then sensitized and challenged with Ox. Controls included sensitized and unsensitized groups that did not receive transferred cells. An experiment representative of two individually performed experiments. The ILNs at 48 hours after challenge were examined for regulatory CD4+ T cells. B: The number of FoxP3+CD4+CD3+ T cells. C: The percentage of FoxP3+ cells of CD4+CD3+ T cells. D: The number of CD4+CD25+CTLA-4+ T cells. E: The percentage of CD25+CTLA-4+ cells of CD4+CD3+ T cells. n = 9 mice pooled from three individual experiments, means + SEM are shown. ***P < 0.001 and *P < 0.05.
UVB-induced regulatory T cells have a CD4+CD25+CTLA-4+(CD152)14 phenotype and are FoxP3+.15 Because recent evidence indicates that UVB increases the number and percentage of these cells in DLNs,15 regulatory CD4+ T cells were examined in the ILNs. UVB-irradiated and unirradiated mice did not differ in the total number of FoxP3+CD4+ (Figure 2B) and CD4+CD25+CTLA-4+ T cells (Figure 2D). The percentages of these cells were also unchanged (Figure 2, D and E). Together, these data suggest that the systemic UVB regime used in this study does not induce the generation of regulatory CD4+ T cells.
Inhibition of Local T-Cell Activation Does Not Involve the PGE2 Pathway
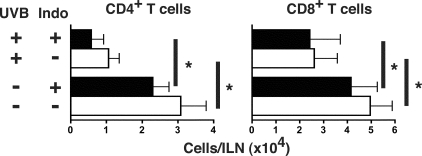
Prostaglandin production was inhibited to investigate whether it is a mediator of UVB inhibition of T-cell activation and expansion. Immediately after each UVB irradiation, indomethacin, a COX inhibitor, was applied to the UVB-irradiated skin. Mice were then sensitized and challenged. Indomethacin administration had no effect on the expansion of ILN effector T cells in unirradiated mice, and it did not prevent UVB-induced reduction in effector CD4+ (absence of indomethacin, P = 0.0392, and presence of indomethacin, P = 0.0233) and CD8+ T cells (absence of indomethacin, P = 0.0309, and presence of indomethacin, P = 0.0234) (Figure 3). This suggests that UVB-induced PGE2 production in irradiated skin is not responsible for suppressing local T-cell activation in DLNs.
Figure 3.
COX inhibition does not prevent UVB from reducing T-cell expansion. Immediately after each UVB irradiation, mice were treated with topical indomethacin. Control mice were administered base acetone solution only. After irradiation, sensitization, and challenge, effector T cells in the ILNs 48 hours after challenge were quantified. UVB irradiation with or without indomethacin significantly decreased the number of effector CD4+ and CD8+ T cells in the ILNs compared to unirradiated mice. n = 9 mice pooled from three individual experiments, means + SEM are shown, *P < 0.05.
CHS Elicitation Induces Leukocyte Recruitment into Ear Skin, Including Activated and Proliferating T Cells
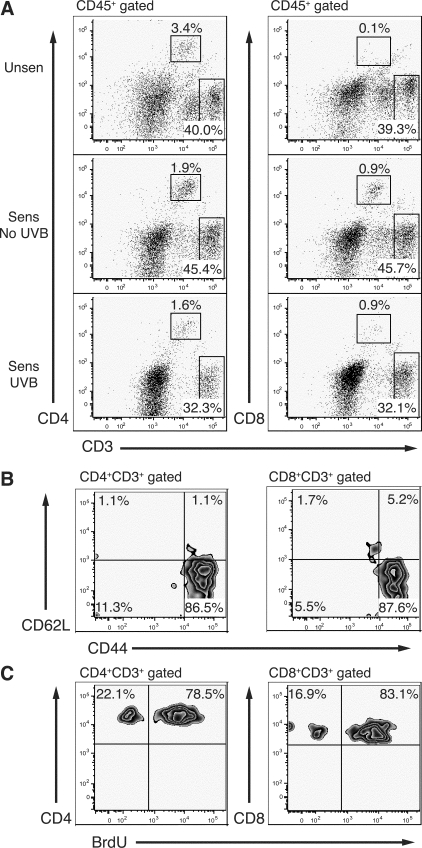
To determine whether UVB influences CD45+ leukocyte and T-cell recruitment into the challenge site, we examined ear skin by flow cytometry 48 hours after challenge because this was determined to be the time of maximal leukocyte infiltrate. Single-cell suspensions of total ear skin were prepared from epidermal and dermal skin layers and then stained with antibodies against CD45, CD44, CD62L, CD4, CD8, and CD3. Representative dot plots of CD4+CD3+ and CD8+CD3+ labeling in ear skin are shown in Figure 4A. Uninflamed ear skin from unsensitized mice contained a small resident population of CD4+ T cells, representing ∼3% of the CD45+ population. Few to no CD8+ T cells were detected in unsensitized mice. Consistently, a sizeable population of CD3hi, CD4−CD8− cells were found in the ear skin (∼40% of the CD45+ population) that were also γδTCR+, suggesting the presence of dendritic epidermal T cells (data not shown). Sensitization followed by ear challenge induced an influx of CD45+ cells into ear skin, including CD4+ and CD8+ T cells. Both unirradiated and UVB-irradiated sensitized mice showed a similar recruitment frequency of CD4+ and CD8+ T cells (of total CD45+) into ear skin, suggesting that UVB does not affect the proportional recruitment of T cells against other inflammatory cell types. The majority of T cells were CD44hiCD62L− (Figure 4B) and were BrdU+ (Figure 4C), indicating that these cells are activated and proliferating T cells.
Figure 4.
The activated and proliferating T-cell infiltrate in ear skin at 48 hours after challenge. Single-cell suspensions were made from ear skin 48 hours after challenge from unsensitized (Unsen) and sensitized (Sens) mice. A: Gated CD45+ cells were analyzed for CD4+CD3+ and CD8+CD3+ T-cell expression. Unsensitized mice contain a resident population of CD4+ T cells and CD3high CD4−CD8− cells, but no CD8+ T cells in skin. Percentages of gated CD45+ cells are shown. Skin-derived T cells are predominately activated CD44hiCD62L− cells (B) and are BrdU+ (C) in unirradiated sensitized mice. Percentages of gated CD4+CD3+ or CD8+CD3+ are shown.
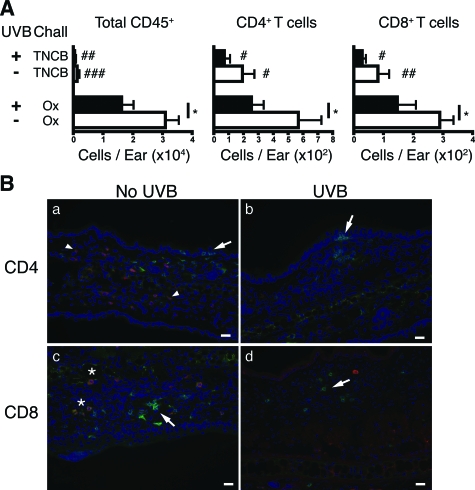
UVB Reduces the Leukocyte Infiltrate into Ear Skin
UVB significantly decreased the total number of recruited CD45+ leukocytes (P = 0.0248), CD4+ T cells (P = 0.0448), and CD8+ T cells (P = 0.0415) by ∼50% compared to unirradiated sensitized mice (Figure 5A). To determine the contribution of nonspecific cells migrating into irritated skin, Ox-sensitized mice were challenged with a different unrelated hapten, TNCB. Neither Ox sensitization nor UVB irradiation significantly altered the CHS response compared to the reaction measured in unsensitized TNCB-challenged mice (7.9 mm−2 ± 0.9). Moreover, the ears of these TNCB-challenged mice contained significantly fewer CD45+ cells, CD4+ T cells, and CD8+ T cells compared to Ox-sensitized and challenged mice. These results confirm that the cellular infiltrate detected in the ear skin of Ox-sensitized and Ox-challenged mice were recruited in a hapten-specific manner.
Figure 5.
IFN-γ producing CD8+ T cells infiltrate ear skin 48 hours after Ox challenge in sensitized mice. A: Sensitization increased the total number of leukocytes (total CD45+) and T cells in the ear skin of Ox-challenged mice (determined by subtracting the number of CD45+ or T cells in unsensitized from sensitized skin). Challenging ears of Ox-sensitized mice with TNCB did not cause a significant increase in CD45+ or T cells. n = 9 mice pooled from three individual experiments, means + SEM shown. *P < 0.05 comparing Ox-challenged groups. ###P < 0.001, ##P < 0.01, and #P < 0.05 comparing Ox with TNCB-challenged groups. B: Ear sections were stained for CD4, CD8, and IFN-γ. CD4+ cells (green, arrows) were detected in the epidermis and dermis of unirradiated (a) and UVB-irradiated (b) ear skin. However, there was no co-expression of IFN-γ (red, arrowheads) with CD4+ cells. CD8+ cells were mostly localized in the dermis of unirradiated (c) and UVB-irradiated (d) mice (green, arrows). A proportion of CD8+ cells were co-expressed with IFN-γ (yellow, asterisk). Scale bars = 20 μm.
CD8+ but Not CD4+ Cells in the Ear Skin of Sensitized and Challenged Mice Produce IFN-γ
We examined the functional capacity of skin-localized T cells by assessing their in situ IFN-γ expression 48 hours after challenge (Figure 5B). CD4+ cells in sensitized mice were localized to both the epidermal and dermal layers (Figure 5Ba, arrows). In contrast, CD8+ cells were mostly observed in the dermis (Figure 5Bc, arrows). IFN-γ+ cells were detected in the dermis of mice, although, none were co-labeled with CD4+ cells (Figure 5Ba, arrowheads). Rather, a proportion of CD8+ cells expressed IFN-γ (Figure 5Bc, asterisk), indicating that production of this cytokine is limited to CD8+ T cells in ear skin. No IFN-γ reactivity was observed in the ear skin of unsensitized mice (data not shown).
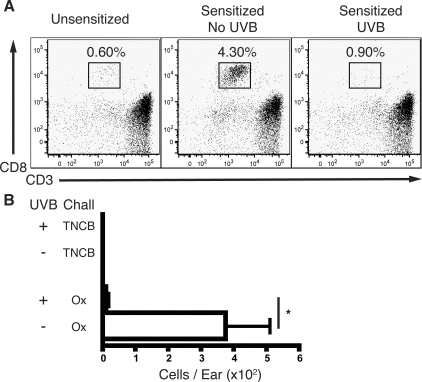
UVB Blocks the Development of Effector Memory CD8+ T Cells in the Ear Skin of Sensitized Mice
UVB-irradiated mice maintained a suppressed secondary memory response to Ox resensitization and rechallenge compared to unirradiated mice (22.9 mm−2 ± 1.9 versus 29.6 mm−2 ± 1.5, P = 0.0102, n = 14). To determine whether UVB inhibited these reactions by affecting the development of memory T cells, UVB-irradiated, sensitized, and challenged mice were rested for 10 weeks after sensitization (memory mice). As mice were not resensitized or rechallenged during this period, T cells with an activated phenotype were defined as memory T cells. A comparable number of central memory T cells (CD44hiCD62L+CD127+) in draining and nondraining lymphoid organs, blood, and liver were detected in sensitized and unsensitized mice, indicating that the lymphoid system was in a resting and homeostatic state (data not shown).
The ear skin of memory mice was examined to determine whether previous hapten exposure or UVB had any effect on the resident T-cell population in skin. The number of total CD45+ leukocytes and CD4+ T cells in ear skin was similar between unirradiated and UVB-irradiated mice, suggesting that UVB does not play a role in regulating these cells in memory mice (data not shown). In contrast, the ears of unirradiated memory mice were populated by a significant number of effector memory (CD44hiCD62L−) CD8+ T cells that represented ∼4% of skin CD45+ cells, compared to 0.60% in unsensitized mice (Figure 6A). UVB-irradiated, sensitized mice had a CD8+ T-cell frequency similar to unsensitized mice. Moreover, compared to unirradiated memory mice, the total number of memory CD8+ T cells in the ears of mice previously exposed to UVB was significantly decreased by more than 30-fold (P = 0.0121) (Figure 6B). Hence, UVB inhibited the development of peripheral effector memory CD8+ T cells that would normally localize to the skin of sensitized mice. No CD8+ T cells were detected in either unirradiated or UVB-irradiated Ox-sensitized and TNCB-challenged mice, demonstrating that these memory T cells only localize to the skin when mice are sensitized and challenged with the original primary CHS hapten.
Figure 6.
UVB inhibits the development of CD8+ T cells in the ear skin of resting mice 10 weeks after sensitization. Ox-sensitized unirradiated and UVB-irradiated mice were challenged with either Ox or TNCB before being rested. T cells were examined in the ear skin of resting mice at 10 weeks after sensitization. A: Representative dot plots of skin-derived cells gated on CD45+ expression. A prominent CD8+ T-cell population is present in the ear skin of unirradiated sensitized mice, but is absent in UVB-irradiated sensitized or unsensitized mice. Percentages of gated CD45+ cells are shown. B: The number of CD8+ T cells recovered from the ear skin of sensitized mice. No CD8+ T cells were detected in Ox-sensitized but TNCB-challenged mice. n = 9 mice pooled from three individual experiments, means + SEM are shown. *P < 0.05 comparing no UVB with UVB mice.
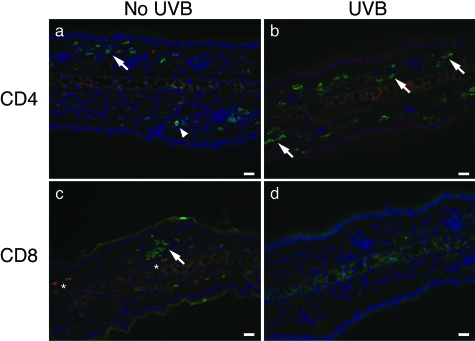
A hallmark of memory T cells is their rapid response to cognate antigen. To test this response, IFN-γ production by skin-resident effector memory CD4+ and CD8+ T cells was examined by immunohistochemistry. Memory mice were rechallenged with Ox and the ears analyzed 24 hours later. To limit the response to skin-resident effector memory T cells, mice were not resensitized. The dermis of unirradiated and UVB-irradiated memory mice contained CD4+ cells (Figure 7, a and b; arrows), whereas detection of CD8+ cells was limited to unirradiated mice (Figure 7c, arrow), which is consistent with our flow cytometry findings (Figure 6B). Only CD8+ T cells in the skin of unirradiated mice expressed IFN-γ (Figure 7c, asterisk). Hence, CD8+ cells in the dermis of memory unirradiated mice are the major source of IFN-γ. These results are similar to the responses observed by skin CD8+ cells during primary CHS reactivity (Figure 5B).
Figure 7.
CD8+ cells in the ear skin of memory mice produce IFN-γ 24 hours after rechallenge. Resting memory mice at 10 weeks after sensitization were rechallenged with Ox and 24 hours later, ear sections were stained for CD4, CD8, and IFN-γ. Prominent populations of CD4+ (green, arrows) cells are present in both unirradiated (a) and UVB-irradiated mice (b). CD4+ and IFN-γ+ (red, arrowheads) cells are not co-labeled. CD8+ cells (green) are detectable in the ear skin of unirradiated mice (c, arrow) but not in UVB-irradiated mice (d). IFN-γ+ cells are co-labeled with CD8+ cells (yellow, asterisk) in the ear skin of unirradiated mice. Scale bars = 20 μm.
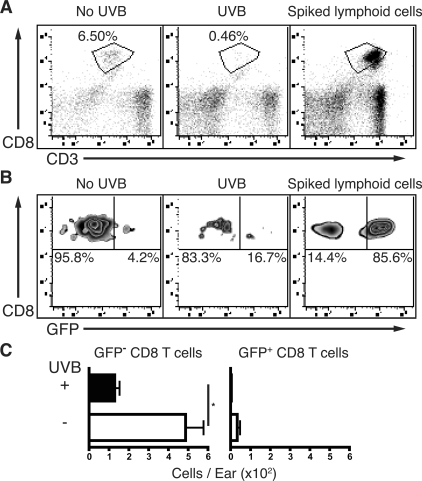
CD8+ T Cells in the Skin of T-GFP Mice Maintain an Effector Memory GFP− Phenotype
Naïve T cells in T-GFP mice are GFP+ until they become activated in an antigen-specific manner, wherein they then down-regulate GFP to become cytotoxic effector-like GFP− cells.12 Effector memory T cells maintain a GFP− phenotype, whereas memory T cells that have bypassed the effector phase retain their GFP expression.16 Using this model, we examined whether CD8+ T cells in the ear skin of memory mice had experienced an effector phase before progressing into memory T cells. T-GFP mice were sensitized and challenged with Ox, and were then rested for 5 weeks after sensitization. There was no difference between memory mice at 5 or 10 weeks after sensitization, and an assessment of draining and nondraining lymphoid organs indicated a homeostatic lymphoid system (data not shown). Similar to nontransgenic wild-type C57BL/6J mice, unirradiated and sensitized T-GFP mice showed an increased population of CD8+ T cells in ear skin, which was absent in UVB-irradiated mice (Figure 8A). Skin-derived CD8+ T cells from both unirradiated and UVB-irradiated memory mice were predominately GFP− (Figure 8B), but the total number of GFP− CD8+ T cells recovered from UVB-irradiated mice was again significantly decreased compared to unirradiated mice (P = 0.0255) (Figure 8C). Importantly, only a few GFP+ CD8+ T cells were isolated from the skin of sensitized mice. These GFP+ CD8+ T cells are expected to be nonspecific circulating naïve or memory CD8+ T cells derived from the dermal microvasculature, isolated during skin preparation. Their relative low frequency compared to GFP− CD8+ T cells highlights that very little contamination of blood derived T cells is present in the observed effector memory GFP− CD8+ pool of T cells.
Figure 8.
CD8+ T cells in ear skin maintain a GFP− phenotype 5 weeks after sensitization. Unirradiated and UVB-irradiated T-GFP mice were sensitized with Ox and 7 days later, ears were challenged with Ox. Mice were rested for 5 weeks after sensitization before T cells were examined in the ear skin. A: Representative dot plots of skin-derived cells gated on CD45+ showing CD3+CD8+ T cells isolated from T-GFP ear skin in unirradiated and UVB-irradiated sensitized mice. Percentages of gated CD45+ cells are shown. A skin sample spiked with lymphoid cells is presented as a positive gating control. B: The GFP expression of gated CD3+CD8+ skin-derived T cells is predominately GFP− compared to lymphoid-derived GFP+ T cells. C: The number of GFP− and GFP+ CD8+ T cells in unirradiated and UVB-irradiated mice. n = 5 to 9 mice pooled from two experiments, means + SEM are shown. *P < 0.05 comparing unirradiated with UVB mice.
Discussion
This study demonstrates for the first time the effect of systemic low-level exposure to UVB on the suppression of primary and memory T cells in vivo during CHS. Sensitization of mice after UVB exposure decreased the expansion of effector CD4+ and CD8+ T-cell populations in DLNs, reducing the size of the effector T-cell pool. During CHS elicitation, which is characterized by T-cell and other leukocyte migration into challenged skin, UVB inhibited the accumulation of inflammatory cells in skin. Activated and proliferating CD8+ T cells were shown to produce IFN-γ in the skin, underlining their role as an effector cell during elicitation. More importantly, these effector CD8+ T cells were retained in the skin of memory mice, wherein they formed an effector memory population that rapidly produced IFN-γ on rechallenge. UVB, however, prevented this peripheral development of memory CD8+ T cells in skin. The UVB dose used was too low to activate regulatory CD4+ T cells, and these results occurred independently of COX activation, which is a key mediator of immunosuppression attributable to high doses of UVB. Therefore, for the first time we have shown that systemic UVB designed to mimic the lower levels of exposure during normal daily activities suppresses CHS by subverting the generation of anti-hapten effector and memory T cells.
The reduced expansion of effector CD4+ and CD8+ T cells in UVB-irradiated mice is consistent with previous investigations reporting decreased production of IFN-γ17 and reduced cytotoxic activity18 by T cells derived from the DLNs of UVB-irradiated mice. Because it is still unclear how haptens interact with MHC molecules to stimulate naïve T cells, it is currently impossible to detect hapten-specific T cells by tetramer technology. Therefore, we restricted our definition of an effector T cell to very late activated T cells, but we cannot exclude the possibility that nonspecific bystander activation contributed to our expanded pool of effector T cells. Because of the water insoluble nature of Ox, in vitro stimulation for IFN-γ as a measure of hapten specificity was also not possible. However, studies performed by Ghoreishi and Dutz19 that also showed a reduced enumeration and proliferation of OVA-specific tetramer+ CD8+ T cells in the skin DLNs of mice immunized through UV-irradiated skin lends support to our findings here in mice sensitized at a distal site to UVB irradiation. However, these investigators attributed the decreased activation of antigen-specific T cells to the presence of CD4+CD25+Foxp3+ regulatory T cells that were not detectable in this study.
UVB-induced regulatory T cells have been demonstrated in other systemic immunosuppression studies with single high doses of UVB (800 to 1500 mJ/cm2, 3 to 4 minimal erythema dose15,20). It is widely considered within such systems that regulatory T cells mediate UVB-induced suppression by inhibiting effector T cells in DLNs.21 However, the lack of transferable suppression and the unchanged numbers of FoxP3+ regulatory CD4+ T cells in our study suggest that low levels of systemic UVB does not activate regulatory CD4+ T cells to reduce effector T-cell expansion. Our UVB dose (0.3 minimal erythema dose, 90mJ/cm2 daily for 3 days) is several fold lower than those previously found to activate regulatory T cells. This investigation also found that UVB-induced COX activity was not required for this inhibition of T-cell activation. PGE2, a product of COX, is thought to be central to UVB-induced systemic immunosuppression.22 It is likely that our UVB regime would have up-regulated COX-1/2 mRNA expression and PGE2 production within irradiated skin because this has been shown with a single dose of 220 mJ/cm2 UVB, 1 minimal erythema dose.23 However, significant induction of plasma PGE2 requires doses higher than 300 mJ/cm2.24 Thus, it is possible that our low UVB dose was insufficient to cause systemic PGE2 production, such that it could be a mediator of UVB-induced immunosuppression. PGE2 can stimulate in an autocrine or paracrine manner, the formation of adaptive regulatory FoxP3+CD4+ T cells from CD4+CD25− cells,25 which could suggest that PGE2 production and the presence of regulatory T cells after UVB may be a closely linked relationship. Each 90 mJ/cm2 UVB dose administered here was equivalent to just ∼5 minutes of Sydney summer sunlight and it did not cause tanning or burning of exposed skin. Interestingly, severe tissue injury such as burns, triggers excessive inflammation, including prostaglandin production, and this has been associated with enhanced regulatory T-cell activity in skin DLNs. Burn patients often exhibit decreased immunity, which is reminiscent of a UVB-irradiated state.26 Instead, the reduced DLN T-cell response could be a consequence of suppressor B cells or serum cis-UCA that are known to be generated by low UVB doses (100 mJ/cm2 daily for 3 days, single 216 mJ/cm2, respectively27,28). IL-10-producing suppressor B cells impinging on Ox-presenting dendritic cells and cis-UCA inhibition of Th1 (IL-2 and IFN-γ) cells29 would lead to impaired antigen presentation and reduced IL-2 production. Such interference of T-cell activation and proliferation was shown by the decreased percentage and number of BrdU+ cells in the DLNs of UVB-irradiated mice.
Uninflamed normal skin contained resident CD4+ T cells supporting the findings of Clark and colleagues30,31 that human skin is populated by various memory and regulatory CD4+ T cells. CHS elicitation via the reapplication of hapten induced leukocyte infiltration, including CD4+ and CD8+ T cells into the ear skin of unirradiated sensitized mice. Flow cytometry on cells isolated directly ex vivo showed that the majority of the CD4+ and CD8+ T cells were activated (CD44hiCD62L−) and were proliferating (BrdU+), suggesting that these T cells may be daughter cells derived from the primary T-cell expansion that occurred in the ILNs. Challenging Ox-sensitized mice with TNCB did not result in a discernable inflammatory reaction, as shown by the insignificant CHS response and the low number of skin-infiltrating T cells that were detected in these mice. This is consistent with the notion that only hapten-specific T cells accumulate at reaction sites32 and it supports the view that CD8+ T cells must specifically recognize hapten-conjugated keratinocytes to initiate a fully fledged CHS reaction.7 Thereby providing evidence that the T cells that were recruited into the ear skin of Ox-sensitized and challenged mice are likely to be Ox-specific.
UVB-irradiated mice, on the other hand, presented a reduced influx of leukocytes, as well as CD4+ and CD8+ T cells into ear-challenged skin. Besides T cells, the decreased total leukocyte infiltrate may consist of macrophages,33 NK cells,34 and NKT cells35 that are also involved in mediating the efferent phase of CHS. This is the first demonstration that systemic UVB irradiation that causes reduced CHS responses is associated with a decreased leukocyte infiltrate in ear skin. It also indicates a new mechanism as to how UVB irradiation modulates CHS elicitation independent of regulatory T cells that cannot migrate into challenged skin.4 Impaired CHS responses occur when factors such as chemokine and cytokine production,36,37 adhesion molecule expression,38 or the presence of CD8+ T cells39 are disrupted. Cytotoxic CD8+ T cells are the primary cell type required to initiate and amplify CHS reactivity via the production of chemokines and IFN-γ.7,40,41 We showed that IFN-γ production is an effector function restricted to the CD8+, but not the CD4+ T-cell population in skin, which has been suggested by other investigations.42 IFN-γ stimulates chemokine secretion, such as IP-10,43 and it promotes the expression of molecules involved in antigen presentation and adhesion that help sustain CHS reactions.44 However, not all of the CD8+ cells in inflamed skin were IFN-γ+, reflecting the heterogeneous nature of the infiltrating CD8+ T-cell pool. The remaining CD8+IFN-γ− population may include IL-17-secreting CD8+ T cells also critical for CHS elicitation.45 Given the importance of CD8+ T cells in mediating CHS, it may be surmised that a reduced circulation of effector CD8+ T cells able to migrate into challenged ear skin and kill hapten-conjugated keratinocytes, would certainly inhibit the magnitude of the inflammatory response. Thus, the reduced CHS response and twofold reduction of CD8+ T cells in ear skin exhibited by UVB-irradiated mice is likely to be a direct corollary of the twofold to threefold diminished effector CD4+ and CD8+ T-cell pool size being generated in the ILNs. Interestingly, with respect to CD45+ cells, the proportion of T cells migrating into ear skin was unaffected by UVB, suggesting that the molecular signals responsible for recruiting T cells into skin was primarily intact, albeit reduced.
Flare-up responses in humans or repeat CHS reactivity in mice has previously been attributed to the retention of hapten-specific T cells within skin to form local skin memory at the site of primary challenge.46 There is some evidence showing that CD4+ T cells preferentially accumulate in human skin 21 days after patch testing;47 however, no direct evidence of T cell retention exists in murine models of CHS. In this study, unirradiated memory mice at 10 weeks after sensitization exhibited a population of dermal CD8+ T cells similar to the number recovered during the initial period of CHS elicitation, and that were absent in unsensitized mice. Long-term studies performed in 2,4-dinitrofluorobenzene-challenged mice indicate that immediate CHS reactivity can be demonstrated on rechallenge even 1 year after the time of initial challenge,48 suggesting that CD8+ T-cell persistence may be sustained past the time frame investigated here. Only hapten-specific T cells were retained, because mice that were challenged with TNCB did not exhibit any CD8+ T cells in memory ear skin. The GFP− phenotype of the CD8+ T cells strongly supports the view that these are effector memory T cells that have undergone an effector T-cell phase on activation. Indeed, 24 hours after rechallenge in memory mice, CD8+ cells could produce IFN-γ akin to a memory T-cell phenotype. Memory T cells require IL-7 and IL-15 for maintenance, in that IL-7 promotes survival whereas IL-15 supports cell turnover.49 Both of these T-cell homeostatic growth cytokines can be produced locally in skin by keratinocytes50 and dermal fibroblasts,51 respectively. Thus, it can be seen that skin can provide an environment conducive for memory T-cell development in the absence of antigen.
Mice exposed to UVB, however, failed to develop effector memory CD8+ T cells in the ear skin of sensitized memory mice. This demonstrates a novel systemic effect of UVB to suppress the normal development of CD8+ memory T cells within challenged skin. This property of UVB occurred after only a short duration of UVB at low levels before epicutaneous application of hapten at distal sites. Because UVB is known to have a dose-dependent effect on CHS responses,13 we can speculate that higher doses of UVB, for example at erythemal levels, would be expected to have a more pronounced effect on effector and memory T cells than what was observed in this investigation. The lack of peripheral memory CD8+ T-cell development in ear skin reflects the reduced number of effector CD8+ T cells that were localized at this site during CHS elicitation in UVB-irradiated mice. Memory T-cell pool sizes and recall qualities are determined during the primary T-cell response against an antigen. Factors such as the duration and signaling strength of antigen presentation52,53 and primary effector T-cell burst size54 are important in establishing the development of functional memory T cells. In accordance with this, we have shown in this study that UVB limited the primary burst size of effector T cells in the ILNs during sensitization, whereas other investigations have demonstrated that UVB inhibits the capacity for antigen presentation within lymph nodes draining the site of irradiation.5 Thus, UVB may have inhibited memory T-cell development by interfering with T-cell activation during sensitization, to have reduced the migration of CD8+ T cells into skin, thereby suppressing their development into memory CD8+ T cells at this site.
From our findings we propose that in the absence of UVB-induced regulatory T cells to enforce tolerance against Ox, the apparent nonresponsiveness to hapten rechallenge in UVB-irradiated mice is associated with the drastically reduced or absent population of dermal effector memory CD8+ T cells in these mice. Under these circumstances, mice previously exposed with UVB will continue to exhibit reduced CHS reactions compared to unirradiated sensitized mice because they lack memory T cells able to respond rapidly to resensitization and rechallenge. Further studies are required to determine whether UVB influences central memory T-cell development within lymphoid organs.
In conclusion, our studies provide evidence that UVB at levels low enough for humans to encounter during routine daily activities interferes systemically with normal effector T-cell activation to a contact sensitizer in lymph nodes. Importantly, because of the low UVB doses used, this modulation of T cells occurred without the involvement of regulatory CD4+ T cells and PGE2, which suggests that the importance of these two factors in mediating or initiating UVB-induced immunosuppression is dependent on UVB dose. Other molecular mechanisms must be involved in UVB-induced immunosuppression and in the inhibition of effector and memory T cells described here. Accumulation of T cells, including IFN-γ-secreting CD8+ T cells, at challenged skin sites was also inhibited by UVB. This had a concomitant affect on the normal development of memory T cells localized in peripheral skin. Because T cells play a prominent role in CHS, these factors explain to a large extent how UVB reduces primary and secondary CHS responses at these UVB doses. The ramifications of this study further suggest that excessive sunlight exposure could potentially inhibit or challenge the efficacy of vaccination protocols. Moreover, our findings underline a possible dual role of UVB in its involvement in skin tumor development. Although directly causing the mutagenic changes which leads to the formation of cancerous cells, the downstream effects of UVB irradiation may act in parallel to stifle anti-tumor T-cell immunity.
Footnotes
Address reprint requests to Prof. Gary Halliday, Dermatology, Blackburn Bldg. D06, The University of Sydney, NSW 2006, Australia. E-mail: garyh@med.usyd.edu.au.
Supported by the National Health and Medical Research Council (grant 302021 and C.J. Martin fellowship 307726 to S.N.B.) of Australia and Cure Cancer Australia.
References
- Halliday GM, Rana S. Waveband and dose dependency of sunlight-induced immunomodulation and cellular changes. Photochem Photobiol. 2008;84:35–46. doi: 10.1111/j.1751-1097.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- Poon TSC, Barnetson RSC, Halliday GM. Sunlight-induced immunosuppression in humans is initially because of UVB, then UVA, followed by interactive effects. J Invest Dermatol. 2005;125:840–846. doi: 10.1111/j.0022-202X.2005.23894.x. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D, Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol. 2005;124:570–578. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- Akiba H, Kehren J, Ducluzeau M-T, Krasteva M, Horand F, Kaiserlian D, Kaneko F, Nicolas J-F. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168:3079–3087. doi: 10.4049/jimmunol.168.6.3079. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Van Seventer GA, Siraganian R, Wahl L, Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989;143:2457–2463. [PubMed] [Google Scholar]
- Hamann A, Jablonski-Westrich D, Scholz KU, Duijvestijn A, Butcher EC, Thiele HG. Regulation of lymphocyte homing. I. Alterations in homing receptor expression and organ-specific high endothelial venule binding of lymphocytes upon activation. J Immunol. 1988;140:737–743. [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Manjunath N, Shankar P, Stockton B, Dubey PD, Lieberman J, von Andrian UH. A transgenic mouse model to analyze CD8+ effector T cell differentiation in vivo. Proc Natl Acad Sci USA. 1999;96:13932–13937. doi: 10.1073/pnas.96.24.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SN, Spinks N, Halliday GM. Ultraviolet A irradiation of C57BL/6 mice suppresses systemic contact hypersensitivity or enhances secondary immunity depending on dose. J Invest Dermatol. 2002;119:858–864. doi: 10.1046/j.1523-1747.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Beissert S, Grosse-Heitmeyer K, Gunzer M, Bluestone JA, Grabbe S, Schwarz T. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J Immunol. 2000;165:1824–1831. doi: 10.4049/jimmunol.165.4.1824. [DOI] [PubMed] [Google Scholar]
- Gorman S, Tan JWY, Yerkovich ST, Finlay-Jones JJ, Hart PH. CD4+ T cells in lymph nodes of UVB-irradiated mice suppress immune responses to new antigens both in vitro and in vivo. J Invest Dermatol. 2007;127:915–924. doi: 10.1038/sj.jid.5700600. [DOI] [PubMed] [Google Scholar]
- Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garssen J, Vandebriel RJ, De Gruijl R F, Wolvers DA, Van Dijk M, Fluitman A, Van Loveren H. UVB exposure-induced systemic modulation of Th1- and Th2-mediated immune responses. Immunology. 1999;97:506–514. doi: 10.1046/j.1365-2567.1999.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ. The involvement of antigen-presenting cells and suppressor cells in the ultraviolet radiation-induced inhibition of secondary cytotoxic T cell sensitization. J Immunol. 1983;130:2071–2074. [PubMed] [Google Scholar]
- Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- Kripke ML, Morison WL. Studies on the mechanism of systemic suppression of contact hypersensitivity by ultraviolet B radiation. Photodermatology. 1986;3:4–14. [PubMed] [Google Scholar]
- Hill LL, Shreedhar VK, Kripke ML, Owen-Schaub LB. A critical role for Fas ligand in the active suppression of systemic immune responses by ultraviolet radiation. J Exp Med. 1999;189:1285–1294. doi: 10.1084/jem.189.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreedhar V, Giese T, Sung VW, Ullrich SE. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, Conti CJ. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–240. [PubMed] [Google Scholar]
- Chung HT, Burnham DK, Robertson B, Roberts LK, Daynes RA. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478–2484. [PubMed] [Google Scholar]
- Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- Choileain NN, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Ahmed J, Halliday GM. Ultraviolet B but not A radiation activates suppressor B cells in draining lymph nodes. Photochem Photobiol. 2005;81:1366–1370. doi: 10.1562/2005-04-20-RA-495. [DOI] [PubMed] [Google Scholar]
- Moodycliffe AM, Norval M, Kimber I, Simpson TJ. Characterization of a monoclonal antibody to cis-urocanic acid: detection of cis-urocanic acid in the serum of irradiated mice by immunoassay. Immunology. 1993;79:667–672. [PMC free article] [PubMed] [Google Scholar]
- Holán V, Kuffova L, Zajicova A, Krulova M, Filipec M, Holler P, Jancarek A. Urocanic acid enhances IL-10 production in activated CD4+ T cells. J Immunol. 1998;161:3237–3241. [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper RJ, van Dinther-Janssen AC, Polak L. Specific accumulation of hapten-reactive T cells in contact sensitivity reaction sites. J Immunol. 1985;134:1333–1336. [PubMed] [Google Scholar]
- Szczepanik M, Bryniarski K, Pryjma J, Ptak W. Distinct populations of antigen-presenting macrophages are required for induction of effector and regulatory cells in contact sensitivity response in mice. J Leukoc Biol. 1993;53:320–326. doi: 10.1002/jlb.53.3.320. [DOI] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis EES, Gillessen S, Scheper RJ, Exley MA, Taniguchi M, Balk SP, Strominger JL, Dranoff G, Blumberg RS, Wilson SB. CD1d and CD1d-restricted iNKT-cells play a pivotal role in contact hypersensitivity. Exp Dermatol. 2005;14:250–258. doi: 10.1111/j.0906-6705.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Narumi S, Sudo K, Horai R, Tagawa Y-I, Sekikawa K, Matsushima K, Asano M, Iwakura Y. IL-1-induced tumor necrosis factor-alpha elicits inflammatory cell infiltration in the skin by inducing IFN-gamma-inducible protein 10 in the elicitation phase of the contact hypersensitivity response. Int Immunol. 2003;15:251–260. doi: 10.1093/intimm/dxg028. [DOI] [PubMed] [Google Scholar]
- Erdmann I, Scheidegger EP, Koch FK, Heinzerling L, Odermatt B, Burg G, Lowe JB, Kundig TM. Fucosyltransferase VII-deficient mice with effective E-, P-, and L-selectin ligands show impaired CD4+ and CD8+ T cell migration into the skin, but normal extravasation into visceral organs. J Immunol. 2002;168:2139–2146. doi: 10.4049/jimmunol.168.5.2139. [DOI] [PubMed] [Google Scholar]
- Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- Abe M, Kondo T, Xu H, Fairchild RL. Interferon-gamma inducible protein (IP-10) expression is mediated by CD8+ T cells and is regulated by CD4+ T cells during the elicitation of contact hypersensitivity. J Invest Dermatol. 1996;107:360–366. doi: 10.1111/1523-1747.ep12363337. [DOI] [PubMed] [Google Scholar]
- Kehren J, Desvignes C, Krasteva M, Ducluzeau M-T, Assossou O, Horand F, Hahne M, Kagi D, Kaiserlian D, Nicolas J-F. Cytotoxicity is mandatory for CD8+ T cell-mediated contact hypersensitivity. J Exp Med. 1999;189:779–786. doi: 10.1084/jem.189.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki F, Kanzaki H, Fujii K, Arata J, Akiba H, Tsujii K, Iwatsuki K. Initial recruitment of interferon-gamma-producing CD8+ effector cells, followed by infiltration of CD4+ cells in 2,4,6-trinitro-1-chlorobenzene (TNCB)-induced murine contact hypersensitivity reactions. J Dermatol. 2002;29:699–708. doi: 10.1111/j.1346-8138.2002.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Tokuriki A, Seo N, Ito T, Kumakiri M, Takigawa M, Tokura Y. Dominant expression of CXCR3 is associated with induced expression of IP-10 at hapten-challenged sites of murine contact hypersensitivity: a possible role for interferon-gamma-producing CD8+ T cells in IP-10 expression. J Dermatol Sci. 2002;28:234–241. doi: 10.1016/s0923-1811(01)00172-4. [DOI] [PubMed] [Google Scholar]
- Saulnier M, Huang S, Aguet M, Ryffel B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receptor-deficient mice. Toxicology. 1995;102:301–312. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper RJ, von Blomberg M, Boerrigter GH, Bruynzeel D, van Dinther A, Vos A. Induction of immunological memory in the skin. Role of local T cell retention. Clin Exp Immunol. 1983;51:141–148. [PMC free article] [PubMed] [Google Scholar]
- Moed H, Boorsma DM, Tensen CP, Flier J, Jonker MJ, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Rustemeyer T, Gibbs S. Increased CCL27-CCR10 expression in allergic contact dermatitis: implications for local skin memory. J Pathol. 2004;204:39–46. doi: 10.1002/path.1619. [DOI] [PubMed] [Google Scholar]
- Natsuaki M, Yamashita N, Sagami S. Reactivity and persistence of local immunological memory on murine contact hypersensitivity. J Dermatol. 1993;20:138–143. doi: 10.1111/j.1346-8138.1993.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Matsue H, Bergstresser PR, Takashima A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J Immunol. 1993;151:6012–6019. [PubMed] [Google Scholar]
- Rappl G, Kapsokefalou A, Heuser C, Roler M, Ugurel S, Tilgen W, Reinhold U, Abken H. Dermal fibroblasts sustain proliferation of activated T cells via membrane-bound interleukin-15 upon long-term stimulation with tumor necrosis factor-alpha. J Invest Dermatol. 2001;116:102–109. doi: 10.1046/j.1523-1747.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]