Abstract
Alcoholic liver disease is associated with sustained liver damage and impaired regeneration, as well as significant zinc deficiency. This study was undertaken to examine whether dietary zinc supplementation could improve liver regeneration by increasing the expression of genes involved in hepatic cellular proliferation in a mouse model of alcoholic liver disease. Adult 129S6 mice fed an ethanol-containing liquid diet for 6 months developed alcoholic liver disease as measured by serum alanine transferase activity and histopathological changes. Zinc supplementation to ethanol-exposed mice enhanced liver regeneration as indicated by increased numbers of proliferation cell nuclear antigen (PCNA)-positive and bromodeoxyuridine (BrdU)-labeled hepatocytes. Zinc-enhanced liver regeneration was associated with an increase in hepatocyte nuclear factor-4α (HNF-4α), a liver-enriched, zinc-finger transcription factor. Studies using cultured HepG2 cells showed that zinc deficiency suppressed cell proliferation and cell proliferation-related proteins, including hepatocyte growth factor (HGF), insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 1 (IGFBP1), metallothionein (MT), and cyclin D1, as well as HNF-4α. HNF-4α gene silencing inhibited cell proliferation in association with decreased protein levels of IGF-I, IGFBP1, MT, and cyclin D1. The present study provides evidence that zinc supplementation enhances liver regeneration at least in part by HNF-4α through the up-regulation of cell proliferation-related proteins, suggesting that dietary zinc supplementation may have beneficial effects in alcoholic liver disease.
Zinc deficiency is well documented in alcoholic liver disease.1,2 Clinical studies showed that alcoholic patients have lowered zinc levels in serum and liver, which is associated with decrease in zinc absorption from the intestine and increase in zinc excretion from the urine.3,4 Zinc is well known as one of the most important trace elements in the body. Dietary zinc deficiency is associated with a variety of physiological defects including anorexia, skin lesion, and growth retardation.5 Mechanistic studies demonstrated that zinc deficiency affects a large number of hepatic genes involved in multiple cellular functions. In particular, zinc deficiency has been shown to down-regulate hepatic gene expression of metallothionein (MT), insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 1 (IGFBP1), and cyclin D1, which are involved in cell proliferation.6,7,8 Although the significance of zinc deficiency in the pathogenesis of alcoholic liver disease has not been fully understood, our previous studies demonstrated that zinc supplementation prevents ethanol exposure-induced liver injury.9,10 Zinc supplementation inhibited ethanol-induced oxidative stress in the liver.10 Zinc also suppressed the hepatotoxicity induced by lipopolysaccharide and tumor necrosis factor-α, which are critical mediators in alcoholic liver disease.11,12
The liver has a remarkable capacity to regenerate in response to injury.13 However, experimental investigations have demonstrated that the regeneration capacity of liver is impaired after long-term ethanol intake.14,15,16 Clinical studies demonstrated an imbalance between hepatocyte apoptosis and proliferation in alcoholic patients.17,18,19 Chronic ethanol intake suppresses cell growth factors and cell cycle proteins, such as hepatocyte growth factor (HGF) and cyclin D1.20,21 Data from alcoholic hepatitis patients also demonstrated a positive correlation between HGF and hepatocyte proliferation and survival.22,23 Although zinc deficiency is known to inhibits cell growth, the link between hepatic zinc deficiency and ethanol-impaired liver regeneration response has not been defined.
Increasing evidence from both in vitro and in vivo studies suggest that zinc plays an important role in gene expression through modulating transcription factors.11,12,24,25,26,27,28 Zinc is a critical component in the zinc finger motif of zinc finger transcription factors, and the release of zinc from the zinc finger motif leads to a loss of the DNA binding ability of zinc finger transcription factors.29,30 Hepatocyte nuclear factor-4α (HNF-4α), a zinc finger protein, is a liver-enriched transcription factor,31 and has been reported to bind the promoters of more than 1000 genes involved in most aspects of hepatocyte function including cell proliferation.32 Both human and animal data demonstrate that HNF-4α is essential for maintaining liver gene expression and a decrease in HNF-α correlates with the severity of liver damage.33,34,35,36 Our recent studies demonstrated that preservation of HNF-4α is associated with the protective effect of zinc in TNF-α-induced hepatotoxicity, indicating a link between zinc homeostasis and HNF-4α function.12 The present study was undertaken to determine specifically the role of zinc in liver regeneration and the possible link to HNF-4α in a mouse model of alcoholic liver disease.
Materials and Methods
Animals and Treatments
129S6 mice were obtained from Taconic (Germantown, NY). All of the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee. Twelve-week-old male mice (>25 g body weight) were used. Animals were pair-fed with the Lieber-DeCarli liquid diet (Bio-Serv, Frenchtown, NJ), containing either ethanol or isocaloric maltose dextrin. Animals were randomly assigned to 4 groups using a 2 × 2 factorial design, ethanol × zinc. The content of ethanol in the diet (%, w/v) was gradually increased over a 6-month feeding period, starting at 3.1%, increasing 0.3% every month, and reaching 4.6% at the end. Accordingly, the calorie contribution of ethanol started at 22% of total calories, and reached 32% at the end of 6-month feeding. Zinc supplementation was conducted through the 6-month feeding by adding zinc sulfate to the liquid diet at 75 mg elemental zinc/L. Food intake and body weight were recorded daily and weekly, respectively. At the end of the experiment, the mice were anesthetized with Avertin (300 mg/kg), and plasma and liver tissue samples were harvested for assays.
HepG2 Cell Culture
HepG2 cells obtained from American Type Culture Collection (Rockville, MD) were grown in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin (100 U/ml)/streptomycin sulfate (100 μg/ml) (Invitrogen, Carlsbad, CA). To observe the effect of zinc deficiency on cell proliferation and HNF-4α function, zinc deprivation was induced by N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) at 2 μmol/L, and cells were cultured for 4 days. Zinc sulfate was added at 50 and 100 μmol/L elemental zinc 2 hours after TPEN treatment to confirm the specificity of TPEN-induced zinc deprivation. To define the link between zinc and HNF-4α, gene silencing for HNF-4α was performed by siRNA transfection. HepG2 cells were cultured for 3 days with Silencer predesigned human HNF-4α siRNA and Negative Control #1 siRNA (Ambion, Austin, TX) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Zinc sulfate was added at 20 μmol/L elemental zinc 6 hours after HNF-4α siRNA transfection.
Zinc Concentrations in the Liver
Hepatic zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy (Jarrel-Ash, Model 1140, Waltham, MA) after lyophilization and digestion of the tissues with nitric acid and hydrogen peroxide.10
Liver Damage
Alanine aminotransferase (ALT) activity in the plasma was measured following a colorimetric procedure.37 For observing histopathological changes, liver tissues were fixed in 10% formalin, and paraffin sections were stained with H&E.
Assessment of Liver Regeneration
Liver regeneration was assessed by immunohistochemical staining of proliferating cell nuclear antigen (PCNA) and bromodeoxyuridine (BrdU) incorporation. For immunohistochemical staining of PCNA, sections were treated with microwaves to unmask the antigen. Polyclonal rabbit anti-PCNA (Santa Cruz Biotechnologies, Santa Cruz, CA) was used as primary antibody and incubated overnight at 4°C. After rising with PBS, the sections were incubated with DAKO EnVision+ Labeled Polymer-HRP (horseradish peroxidase) anti-rabbit antibody (DAKO, Carpinteria, CA) for 30 minutes. Diaminobenzidine was used as HRP substrate for visualization. For in vivo measurement of hepatocyte proliferation, two doses of BrdU (Roche Applied Science, Indianapolis, IN) were administrated by i.p. injection at 50 mg/kg 24 and 2 hours before sacrificing. The incorporated BrdU in the nuclei of hepatocytes was detected by immunohistochemical staining. In brief, deparaffinized sections were incubated with monoclonal mouse anti-BrdU antibody (Roche Applied Science) overnight at 4°C. Then the sections were incubated with DAKO EnVision+ Labeled Polymer-HRP anti-mouse antibody (DAKO) for 30 minutes. The PCNA- and BrdU-positive cells were accounted under a 20× objective, and the data were expressed as number of positive cells per 1000 hepatocytes. For quantitative assay of PCNA-positive cells, only the cells with intense nuclear staining were accounted because these cells are known to represent S-phase cells.38
Real-time RT-PCR Assay
The gene expression of HNF-4α in the liver was assessed by real-time RT-PCR. In brief, the total RNA was isolated and reverse transcribed with the Moloney murine leukemia virus reverse transcriptase and oligo-dT primers. The forward and reverse primers of HNF-4α were designed using Primer Express Software (Applied Biosystems, Foster City, CA): NM_008261-3203F sequence: 5′-CGGAGCCCCTGCAAAGT-3′, NM_008261-3298R sequence: 5′-CCAGTCTCACAGCCCATTCC-3′. The SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) were used for real-time RT-PCR analysis. The relative differences of gene expression among groups were evaluated using cycle time values and expressed as relative changes, setting the values of control mice as one.
Immunohistochemical Staining of HNF-4α
Localization of HNF-4α in the liver was performed by an immunohistochemical procedure. Liver sections were incubated overnight at 4°C with a polyclonal rabbit anti-HNF-4α (Santa Cruz Biotechnologies, Santa Cruz, CA) followed by incubation with DAKO EnVision+ Labeled Polymer-HRP anti-rabbit antibody (DAKO) for 30 minutes. Visualization was conducted using diaminobenzidine as HRP substrate.
Cell Proliferation Assay
Cell proliferation was estimated by measuring cell number with a Cell Proliferation Kit I from Roche Applied Science (Indianapolis, IN) following the manufacturer’s instructions. In brief, HepG2 cells were incubated for 4 hours with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide solution. Cells were washed with PBS, followed by the addition of dimethylsulfoxide. Aliquots (200 μl) of the resulting solutions were transferred in 96-well plates, and absorbance at 560 nm, which directly correlates to the cell number according to the instruction, was recorded.
Western Blotting
Nuclear extract of liver tissues was prepared as described previously18 and whole cell extracts from HepG2 cells were prepared using a kit from Active Motif (Carlsbad, CA). Aliquots containing 30 μg protein were loaded on to a 12% SDS-polyacrylamide gel. After electrophoresis, protein was transferred to polyvinylidene fluoride membrane. The membrane was probed with rabbit or goat polyclonal antibodies against HNF-4α, HGF, IGF-I, IGFBP-1, and cyclin D1 (Santa Cruz Biotechnologies), and a monoclonal mouse anti-MT-I (Invitrogen) antibody. The membrane was then processed with HRP-conjugated donkey anti-rabbit IgG, donkey anti-goat IgG, or sheep anti-mouse IgG (GE Healthcare, Piscataway, NJ) antibodies. The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare) and quantified by densitometry analysis.
Assessment of HNF-4α Function
Nuclear extracts from HepG2 were prepared using a kit from Active Motif. The HNF-4α function was assessed by measuring the DNA binding ability with a Trans AM HNF Family Transcription Factor ELISA Kit from Active Motif. The kit consists of a 96-well plate into which oligonucleotide containing the HNF-4α consensus site (5′-TGGACTTAG-3′) is immobilized. The HNF-4α bound to this consensus site is recognized by a rabbit polyclonal antibody against HNF-4α, followed by a horseradish peroxidase-conjugated secondary antibody for the colorimetric quantification. Results were expressed as percentages of the control.
Statistics
All data are expressed as mean ± SE. The data were analyzed by analysis of variance (ANOVA) and Newman-Keuls’ Multiple-Comparison Test. Differences between groups were considered significant at P < 0.05.
Results
Zinc Supplementation Normalized Hepatic Zinc Level and Suppressed Ethanol Exposure-Induced Liver Injury
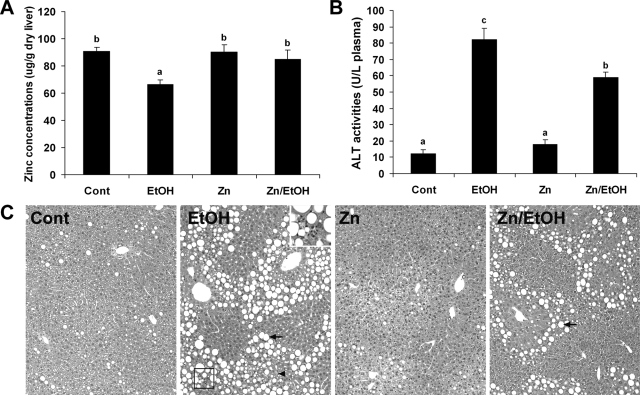
Ethanol exposure for 6 months caused a significant decrease in zinc concentrations in the liver (Figure 1A). Dietary zinc supplementation did not elevate the basal levels, but prevented ethanol-induced decrease in hepatic zinc concentration. Liver injury was assessed by measuring serum ALT activity and histopathological changes. Ethanol exposure significantly elevated serum ALT activity, which was partially inhibited by zinc supplementation (Figure 1B). Corresponding to the serum ALT changes, light microscopy showed that ethanol exposure induced hepatic tissue damage including macrovesicular steatosis, hepatocyte necrosis, and leukocytes infiltration (Figure 1C). All these histopathological changes were attenuated by dietary zinc supplementation.
Figure 1.
Effect of zinc supplementation on hepatic zinc concentrations and liver injury in 129S6 mice chronically fed ethanol for 6 months. A: Normalization by zinc supplementation of ethanol-induced zinc deficiency in the liver. Zinc concentrations in the liver were measured by inductively coupled argon plasma emission spectroscopy. B: Inhibition by zinc supplementation of ethanol-induced elevation of serum ALT activities. C: Attenuation by zinc supplementation of ethanol-induced histopathological changes. Arrows: lipid droplets. Arrowhead: necrosis. Framed area: leukocytes infiltration. Inset is higher magnification of the framed area. H&E staining. Original magnification, ×130; inset, ×280. Results in A and B are means ± SD (n = 6). Significant differences (P < 0.05) among a, b, and c are determined by ANOVA.
Zinc Supplementation Enhanced Hepatocyte Proliferation
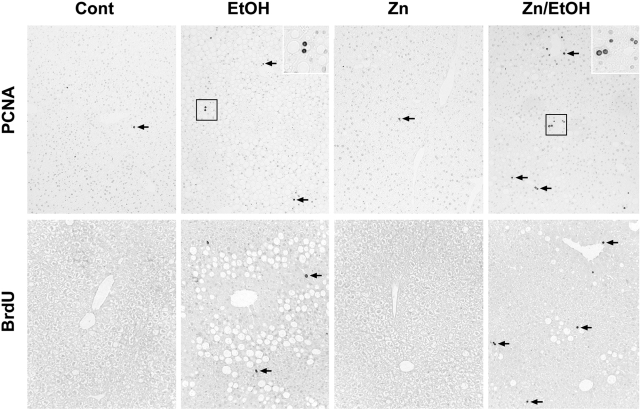
To determine whether zinc supplementation stimulates liver regeneration under ethanol exposure, expression of PCNA and BrdU incorporation were examined by immunohistochemistry. As shown in Figure 2, PCNA immunohistochemistry showed positive cells in the liver of all treatments, and the majority of PCNA-positive cells are hepatocytes. In comparison with the control (0.52 ± 0.38 ‰, positive cells per 1000 hepatocytes) and zinc (0.59 ± 0.4‰), ethanol exposure increased the number of PCNA-positive cells in the liver (2.58 ± 0.71‰), but zinc supplementation further increased the number of PCNA-positive hepatocytes (3.78 ± 1.21‰). Examination of S-phase hepatocytes by BrdU incorporation showed that BrdU-labeled hepatocytes were extremely rare in groups of control (0.24 ± 0.23‰) and zinc (0.29 ± 0.31‰), and ethanol exposure increased the BrdU-labeled hepatocytes (1.02 ± 0.49‰). However, zinc supplementation to ethanol-fed mice significantly increased the number of BrdU-labeled hepatocytes (1.60 ± 0.31‰).
Figure 2.
Effect of zinc supplementation on liver regeneration in 129S6 mice chronically fed ethanol for 6 months. PCNA: Immunohistochemical staining of PCNA-positive cells in the liver. Zinc supplementation enhanced ethanol-induced increase in the number of PCNA-positive cells (Arrows). Original magnification, ×130. Insets are higher magnification (×280) of the framed areas. BrdU: Immunohistochemical detection of BrdU incorporation in the hepatocyte nuclei after BrdU injection. Zinc supplementation increased the number of BrdU-labeled hepatocytes (Arrows) in ethanol-fed mice. Original magnification, ×130.
Zinc Supplementation Preserved HNF-4α in the Liver
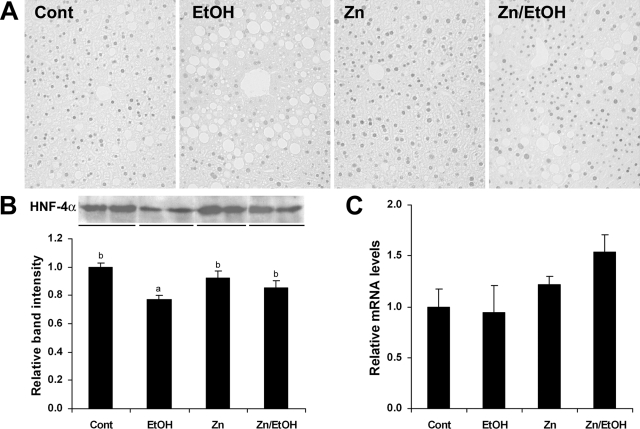
To understand how zinc enhances liver regeneration, the protein and mRNA levels of HNF-4α in the liver were determined. As shown in Figure 3, ethanol exposure induced a marked decrease in HNF-4α staining in some hepatocyte nuclei, which was inhibited by zinc supplementation (Figure 3A). Western blotting analysis demonstrated that ethanol exposure decreased the protein level of HNF-4α in the nuclear extract of liver, and zinc pretreatment attenuated ethanol-induced decrease in HNF-4α protein (Figure 3B). The mRNA level of HNF-4α was not significantly affected by ethanol or zinc supplementation, determined by real-time RT-PCR (Figure 3C).
Figure 3.
Effect of zinc supplementation on HNF-4α in the liver of 129S6 mice chronically fed ethanol for 6 months. A: Immunohistochemical staining of HNF-4α. B: Western blotting of HNF-4α protein (Upper panel). The bands were quantified by densitometry analysis (Lower panel). Significant differences (P < 0.05) between a and b are determined by ANOVA. C: Real-time RT-PCR assay of the mRNA levels of HNF-4α. Results in B and C are means ± SD (n = 3 in B, n = 4 in C).
HNF-4α Partially Mediated Zinc Action on Regulation of Cell Proliferation and Cell Proliferation-Related Proteins in HepG2 Cells
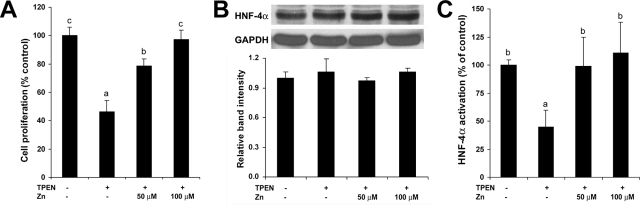
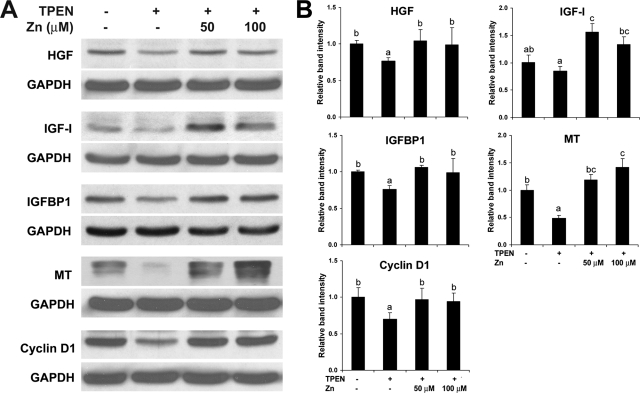
To explore the possible link between zinc deficiency and HNF-4α suppression in hepatocyte proliferation, HepG2 cell cultures were used. As shown in Figure 4A, zinc deprivation by TPEN suppressed cell proliferation, which was reversed by zinc supplementation. Western blotting showed that the protein level of HNF-4α was not affected by TPEN (Figure 4B). However, zinc deprivation by TPEN remarkably inhibited the DNA binding ability of HNF-4α, which was fully recovered by zinc supplementation (Figure 4C). Western blotting of cell proliferation-related proteins demonstrated that zinc deprivation by TPEN decreased HGF, IGF-I, IGFBP1, MT, and cyclin D1, and zinc supplementation reversed the effect of TPEN (Figure 5).
Figure 4.
Effects of zinc deprivation on cell proliferation and HNF-4α function in HepG2 cells. TPEN was added at 2 μmol/L with or without zinc supplementation at 50 and 100 μmol/L elemental zinc. Cells were incubated for 4 days. A: Cell proliferation assay. Cell proliferation was measured using a Cell Proliferation Kit I from Roche Applied Science as described in the Materials and Methods. B: Western blotting of HNF-4α. The bands were quantified by densitometry analysis (Lower panel). C: HNF-4α function assay. The DNA binding ability of HNF-4α was assessed by a Trans-AM HNF Family Transcription Factor ELISA Kit. Results are means ± SD (n = 12 in A, n = 2–3 in B, n = 6 in C). Significant differences (P < 0.05) among a, b, and c are determined by ANOVA.
Figure 5.
Effects of zinc deprivation on cell proliferation-related proteins in HepG2 cells. TPEN was added at 2 μmol/L with or without zinc supplementation at 50 and 100 μmol/L elemental zinc. Cells were incubated for 4 days. A: Western blotting. B: Densitometry analysis of Western blotting bands. Results are means ± SD (n = 2–3). Significant differences (P < 0.05) among a, b, and c are determined by ANOVA.
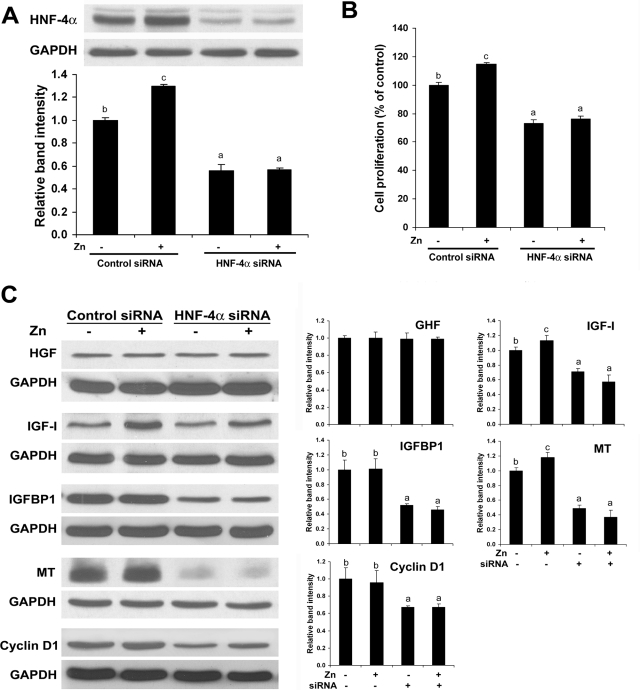
To assess the role HNF-4α in gene regulation and the link to zinc, gene silencing for HNF-4α by siRNA transfection was conducted with or without zinc supplementation. Western blotting demonstrated that HNF-4α siRNA transfection produced a decrease in HNF-4α protein level, and zinc supplementation increased HNF-4α protein level only in control cells (Figure 6A). Importantly, HNF-4α siRNA transfection remarkably suppressed cell proliferation, and zinc supplementation stimulated cell proliferation in control cells but not in HNF-4α siRNA-transfected cells (Figure 6B). HNF-4α siRNA transfection decreased the protein levels of IGF-I, IGFBP1, MT, and cyclin D1 (Figure 6C). Zinc supplementation increased the protein levels of IGF-I and MT, which was abrogated by HNF-4α siRΝΑ transfection.
Figure 6.
Effects of HNF-4α siRNA transfection on cell proliferation and cell proliferation-related proteins in HepG2 cells. HepG2 cells were transfected with human HNF-4α siRNA and control siRNA and incubated for 3 days with or without zinc supplementation at 20 μmol/L. A: Western blotting of HNF-4α. The bands were quantified by densitometry analysis (Lower panel). B: Cell proliferation assay. Cell proliferation was measured using a Cell Proliferation Kit I from Roche Applied Science as described in the Materials and Methods. C: Western blotting of cell proliferation-related proteins. The bands were quantified by densitometry analysis (Right panel). Results are means ± SD (n = 2–3 in A and C, n = 12 in B). Significant differences (P < 0.05) among a, b, and c are determined by ANOVA.
Discussion
The results obtained from the present study demonstrated that alcohol-induced liver injury is associated with zinc deficiency in the liver, and dietary zinc supplementation not only replenishes liver zinc concentrations, but also enhances liver regeneration. The enhanced liver regeneration is associated with a preservation of HNF-4α in the liver. Studies in HepG2 cells further demonstrated that zinc deficiency remarkably suppresses cell proliferation and cell proliferation-related proteins including HGF, IGF-I, IGFBP1, MT, and cyclin D1, which is associated with HNF-4α dysfunction. Furthermore, gene silencing for HNF-4α in HepG2 cells leads to inhibition of IGF-I, IGFBP1, MT, and cyclin D1. These results thus suggest that zinc supplementation enhances liver generation under the condition of a long-term ethanol exposure through up-regulating cell proliferation-related proteins by preserving the activity of HNF-4α.
Chronic ethanol intake is well known to impair liver regeneration. Several models have been used to define the effect of ethanol on liver regeneration. Partial hepatectomy or toxic chemicals such as D-galactosamine has been used as models of injury-initiated liver regeneration.14,15,16 Primary hepatocyte cultures were used to investigate the effect of ethanol on hepatocyte proliferation stimulated by growth factor such as epidemic growth factor, insulin, and HGF, and cytokines such as TNF and IL-6.39,40,41,42,43 All of the studies demonstrated that chronic ethanol intake inhibits liver regeneration and/or hepatocyte proliferation in response to different stimuli. However, the status of liver regeneration after a long term ethanol exposure without second stimuli is poorly understood. Clinical studies demonstrated that both the apoptotic index and proliferating cell numbers were higher in patients with chronic alcoholic disease than controls, indicating the induction of hepatocyte proliferation by liver damage.17,18,19 However, the number of proliferating cells was less than the apoptotic cells, suggesting an imbalance between hepatocyte death and proliferation. Experimental data showed that rats exposed to ethanol for 5 weeks had higher numbers of PCNA-positive hepatocytes at weeks 1, 2, and 3, in comparison with controls, followed by a mild decrease in ALT activities at 4 and 5 weeks.44 The present study also showed that the number of proliferating hepatocytes in the livers of ethanol-fed mice is greater than that of controls. These findings suggest that chronic ethanol intake indeed induces hepatocyte proliferation, but it is not sufficient to recover ethanol-induced liver damage. Dietary zinc supplementation to ethanol-fed mice further increased the number of proliferating hepatocytes. We also observed that long-term ethanol exposure induces hepatocyte apoptosis and zinc supplementation reduced the number of apoptotic hepatocytes.45 Overall, zinc supplementation decreased the ratio of apoptosis to proliferation in the liver of mice after long-term ethanol exposure, thereby providing beneficial effects on alcoholic liver injury.
Liver regeneration in response to the loss of hepatic tissue involves large number of factors, mainly growth factors and cytokines.46,47 Mechanistic studies on ethanol-impaired liver regeneration also demonstrated that chronic ethanol exposure affects multiple pathways involved in the process of liver regeneration. Early investigations have shown that chronic ethanol intake impairs partial hepatectomy-induced liver regeneration in association with an inhibition of the induction of ornithine decarboxylase (the rate-limiting enzyme for polyamine biosynthesis) and polyamines.48,49,50 The role of cytokines such as TNF-α and IL-6 in liver regeneration has also been studied under the condition of ethanol exposure. Although ethanol feeding inhibited recovery of liver mass after hepatectomy, the expressions of TNF-α and IL-6 in liver and adipose tissue were increased by ethanol.43,51 However, the induction by hepatectomy of cell cycle-related proteins including cyclin D1, cyclin D3, cdk-1, and p53 were uniformly inhibited by ethanol consumption.43 Chronic ethanol feeding inhibited activation of p42/44 MAPK, p38 MAPK and JNK induced either by partial hepatectomy or by cell growth factors such as epidermal growth factor, insulin, HGF and TNF.39,40,41 These data indicate that chronic ethanol intake impairs liver regeneration through multiple pathways involved in a variety of factors. The present study determined the effect of zinc on liver regeneration without second stimulus. The regulator for the liver regeneration is likely HNF-4α.
HNF-4α is one of the most abundant transcription factors in the liver, and it is involved in regulation of more than one thousand of genes.32 Previous studies have shown that deficiency in hepatic HNF-4α correlates with the cirrhotic liver damage and hepatoma progression.35,36 Zinc deficiency in the liver is one of the most consistent biochemical/nutritional complication of alcoholic liver disease, as well as other chronic liver diseases.1,2,10 However, the relation between hepatic zinc homeostasis and HNF-4α function has not been defined. We have shown that HNF-4α, at least partially, mediates the protective effect of zinc on D-galactosamine/TNF-α hepatotoxicity.12 The present study demonstrated that chronic ethanol intake caused hepatic zinc deficiency and decreased the protein level of HNF-4α. Zinc supplementation normalized hepatic zinc levels and partially preserved HNF-4α protein level. However, the mRNA levels of HNF-4α were not affected by ethanol or ethanol plus zinc. The results suggest that chronic ethanol exposure suppresses the protein level of HNF-4α at a posttranscriptional level. Our previous report has shown that chronic ethanol exposure generates reactive oxygen species in the liver.10 It is known that oxidation of zinc finger motif by reactive oxygen species can cause zinc release, leading to dysfunction and degradation of zinc finger transcription factors.29,30 Clinical data from alcoholic patients has shown that the imbalance between cell death and proliferation in the liver correlates inversely with glutathione levels and directly with lipid peroxidation.19 Our previous report also showed that zinc supplementation normalizes zinc levels and inhibits oxidative stress in the liver of mice chronically fed ethanol.10 Therefore, the preservation of HNF-4α protein level by zinc could result from both the normalization of zinc level in the liver and the protective effect on zinc finger motifs from oxidative stress.
The in vitro data from the present study demonstrated a direct link of zinc status to HNF-4α in regulating hepatocyte proliferation and cell proliferation-related proteins. First, zinc deficiency by TPEN decreased the protein levels of HGF, IGF-I, IGFBP1, MT, and cyclin D1 in association with inhibition of hepatocyte proliferation. These results indicate that zinc status critically regulates cell proliferation by modulating cell proliferation-related proteins. Second, zinc deficiency by TPEN decreased the DNA binding ability of HNF-4α without affecting its protein levels. Reversal of TPEN effect by zinc supplementation fully restored HNF-4α function. This finding indicates that removal of zinc from the zinc finger motif will lead to loss of the DNA binding function of HNF-4α. Third, HNF-4α siRNA transfection inhibited hepatocyte proliferation in association with down-regulation of IGF-I, IGFBP1, MT, and cyclin D1. These results indicate that HNF-4α is a critical factor in regulation of hepatocyte proliferation. Interestingly, HNF-4α siRNA transfection not only caused MT depletion, but also abrogated the zinc induction of MT. MTs are low-molecular weight proteins involved in many physiological processes, including cell proliferation.52 Induction and nuclear translocation of MT have been observed in rat liver regeneration after hepatic injury.53 Impaired hepatic regeneration was found in MT-null mice after partial hepatectomy.54 Our studies have shown that MT has a protective role in ethanol-induced liver injury and chronic ethanol exposure reduced MT levels in the liver.10,55 These data suggest that MT deficiency in the liver could be an important factor in ethanol-impaired liver regeneration.
In conclusion, the present study provides evidence that alcohol-induced liver damage initiates hepatocyte proliferation, and zinc supplementation accelerates liver regeneration through up-regulating cell proliferation-related proteins. Preservation of HNF-4α is likely an important mechanism involved in the zinc effect on hepatocyte proliferation. These results suggest that dietary zinc supplementation may have beneficial effects in alcoholic liver disease through enhancing liver regeneration.
Acknowledgments
We thank Xinguo Sun for technical assistance. YJK and CJM are Distinguished University Scholars of the University of Louisville.
Footnotes
Address reprint requests to Zhanxiang Zhou, Department of Medicine, University of Louisville School of Medicine, 511 South Floyd St., MDR 529, Louisville, KY 40292, E-mail: z0zhou01@louisville.edu.
Supported in part by the National Institutes of Health and Office of Dietary Supplements grants, and the Veterans Administration, Louisville.
References
- McClain CJ, Antonow DR, Cohen DA, Shedlofsky S. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–589. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605–1609. doi: 10.1002/hep.1840080622. [DOI] [PubMed] [Google Scholar]
- Dinsmore WW, Callender ME, McMaster D, Love AH. The absorption of zinc from a standardized meal in alcoholics and in normal volunteers. Am J Clin Nutr. 1985;42:688–693. doi: 10.1093/ajcn/42.4.688. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno F, González-Reimers E, Santolaria-Fernández F, Galindo-Martín L, Hernandez-Torres O, Batista-López N, Molina-Perez M. Zinc, copper, manganese, and iron in chronic alcoholic liver disease. Alcohol. 1997;14:39–44. doi: 10.1016/s0741-8329(96)00103-6. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Adams L, Shedlofsky S. Zinc and the gastrointestinal system. Prasad AS, editor. New York: Alan R. Liss Inc,; Essential and Toxic Trace Elements in Human Health and Disease. 1988:pp 55–73. [Google Scholar]
- McNall AD, Etherton TD, Fosmire GJ. The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin-like growth factor I and growth hormone receptor genes. J Nutr. 1995;125:874–879. doi: 10.1093/jn/125.4.874. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Gerstmayer B, Bosio A, Windisch W. Effect of zinc deficiency on the mRNA expression pattern in liver and jejunum of adult rats: monitoring gene expression using cDNA microarrays combined with real-time RT-PCR. J Nutr Biochem. 2003;14:691–702. doi: 10.1016/j.jnutbio.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Dieck HT, Döring F, Roth HP, Daniel H. Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J Nutr. 2003;133:1004–1010. doi: 10.1093/jn/133.4.1004. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Lambert JC, Saari JT, Kang YJ. Metallothionein-independent zinc protection from alcoholic liver injury. Am J Pathol. 2002;160:2267–2274. doi: 10.1016/S0002-9440(10)61174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–1690. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis f actor-alpha production and liver injury. Am J Pathol. 2004;164:1547–1556. doi: 10.1016/s0002-9440(10)63713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Kang X, Jiang Y, Song Z, Feng W, McClain CJ, Kang YJ. Preservation of hepatocyte nuclear factor-4alpha is associated with zinc protection against TNF-alpha hepatotoxicity in mice. Exp Biol Med. 2007;232:622–628. [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nature Rev Mol Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Inhibition of hepatic regeneration in rats by acute and chronic ethanol intoxication. Gastroenterology. 1979;77:528–531. [PubMed] [Google Scholar]
- Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Effect of acute and chronic ethanol intoxication on hepatic regeneration. Adv Exp Med Biol. 1980;132:663–670. doi: 10.1007/978-1-4757-1419-7_69. [DOI] [PubMed] [Google Scholar]
- Duguay L, Coutu D, Hetu C, Joly JG. Inhibition of liver regeneration by chronic alcohol administration. Gut. 1982;23:8–13. doi: 10.1136/gut.23.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinati F, Cardin R, D’Errico A, De Maria N, Naccarato R, Cecchetto A, Grigioni W. Hepatocyte proliferative activity in chronic liver damage as assessed by the monoclonal antibody MIB1 Ki67 in archival material: the role of etiology, disease activity, iron, and lipid peroxidation. Hepatology. 1996;23:1468–1475. doi: 10.1053/jhep.1996.v23.pm0008675166. [DOI] [PubMed] [Google Scholar]
- Farinati F, Cardin R, Fiorentino M, D’errico A, Grigioni W, Cecchetto A, Naccarato R. Imbalance between cytoproliferation and apoptosis in hepatitis C virus related chronic liver disease. J Viral Hepat. 2001;8:34–40. doi: 10.1046/j.1365-2893.2001.00267.x. [DOI] [PubMed] [Google Scholar]
- Cardin R, D’Errico A, Fiorentino M, Cecchetto A, Naccarato R, Farinati F. Hepatocyte proliferation and apoptosis in relation to oxidative damage in alcohol-related liver disease. Alcohol Alcohol. 2002;37:43–48. doi: 10.1093/alcalc/37.1.43. [DOI] [PubMed] [Google Scholar]
- Koteish A, Yang S, Lin H, Huang J, Diehl AM. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2002;26:1710–1718. doi: 10.1097/01.ALC.0000036923.77613.59. [DOI] [PubMed] [Google Scholar]
- Hsu MK, Qiao L, Ho V, Zhang BH, Zhang H, Teoh N, Dent P, Farrell GC. Ethanol reduces p38 kinase activation and cyclin D1 protein expression after partial hepatectomy in rats. J Hepatol. 2006;44:375–382. doi: 10.1016/j.jhep.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Cytokines and the molecular mechanisms of alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:1419–1424. [PubMed] [Google Scholar]
- Fang JW, Bird GL, Nakamura T, Davis GL, Lau JY. Hepatocyte proliferation as an indicator of outcome in acute alcoholic hepatitis. Lancet. 1994;343:820–823. doi: 10.1016/s0140-6736(94)92025-7. [DOI] [PubMed] [Google Scholar]
- Connell P, Young VM, Toborek M, Cohen DA, Barve S, McClain CJ, Hennig B. Zinc attenuates tumor necrosis factor-mediated activation of transcription factors in endothelial cells. J Am Coll Nutr. 1997;16:411–417. doi: 10.1080/07315724.1997.10718706. [DOI] [PubMed] [Google Scholar]
- Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med. 2001;226:103–111. doi: 10.1177/153537020122600207. [DOI] [PubMed] [Google Scholar]
- Cui L, Schoene NW, Zhu L, Fanzo JC, Alshatwi A, Lei KY. Zinc depletion reduced Egr-1 and HNF-3beta expression and apolipoprotein A-I promoter activity in Hep G2 cells. Am J Physiol Cell Physiol. 2002;283:C623–C630. doi: 10.1152/ajpcell.00308.2001. [DOI] [PubMed] [Google Scholar]
- Meerarani P, Reiterer G, Toborek M, Hennig B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J Nutr. 2003;133:3058–3064. doi: 10.1093/jn/133.10.3058. [DOI] [PubMed] [Google Scholar]
- Schott-Ohly P, Lgssiar A, Partke HJ, Hassan M, Friesen N, Gleichmann H. Prevention of spontaneous and experimentally induced diabetes in mice with zinc sulfate-enriched drinking water is associated with activation and reduction of NF-kappa B and AP-1 in islets, respectively. Exp Biol Med. 2004;229:1177–1185. doi: 10.1177/153537020422901113. [DOI] [PubMed] [Google Scholar]
- Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- Wilcox DE, Schenk AD, Feldman BM, Xu Y. Oxidation of zinc-binding cysteine residues in transcription factor proteins. Antioxid Redox Signal. 2001;3:549–564. doi: 10.1089/15230860152542925. [DOI] [PubMed] [Google Scholar]
- Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol Rev. 2002;54:129–158. doi: 10.1124/pr.54.1.129. [DOI] [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:266–277. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI, Engelhardt NV, Duncan SA. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology. 2004;39:1038–1047. doi: 10.1002/hep.20155. [DOI] [PubMed] [Google Scholar]
- Berasain C, Herrero JI, Garcia-Trevijano ER, Avila MA, Esteban JI, Mato JM, Prieto J. Expression of Wilms’ tumor suppressor in the liver with cirrhosis: relation to hepatocyte nuclear factor 4 and hepatocellular function. Hepatology. 2003;38:148–157. doi: 10.1053/jhep.2003.50269. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E. Glutamate-oxaloacetate transaminase: colorimetric assay of Reitman and Frankel. Bergmeyer HU, editor. New York: Academic Press,; 1974:pp 760–764. [Google Scholar]
- Greenwell A, Foley JF, Maronpot RR. An enhancement method for immunohistochemical staining of proliferating cell nuclear antigen in archival rodent tissues. Cancer Lett. 1991;59:251–256. doi: 10.1016/0304-3835(91)90149-c. [DOI] [PubMed] [Google Scholar]
- Chen J, Ishac EJ, Dent P, Kunos G, Gao B. Effects of ethanol on mitogen-activated protein kinase and stress-activated protein kinase cascades in normal and regenerating liver. Biochem J. 1998;334:669–676. doi: 10.1042/bj3340669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bao H, Sawyer S, Kunos G, Gao B. Effects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liver. Biochem Biophys Res Commun. 1997;239:666–669. doi: 10.1006/bbrc.1997.7531. [DOI] [PubMed] [Google Scholar]
- Chen J, Kunos G, Gao B. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett. 1999;457:162–168. doi: 10.1016/s0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem. 1999;32:571–578. doi: 10.1016/s0009-9120(99)00057-0. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Yin M, Albrecht JH, Diehl AM. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol. 1998;275:G696–G704. doi: 10.1152/ajpgi.1998.275.4.G696. [DOI] [PubMed] [Google Scholar]
- Apte UM, McRee R, Ramaiah SK. Hepatocyte proliferation is the possible mechanism for the transient decrease in liver injury during steatosis stage of alcoholic liver disease. Toxicol Pathol. 2004;32:567–576. doi: 10.1080/01926230490508812. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ: Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43 (2 Suppl 1):S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Abdo S, Brown N. Supplemental putrescine reverses ethanol-associated inhibition of liver regeneration. Hepatology. 1990;12:633–637. doi: 10.1002/hep.1840120402. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Wells M, Brown ND, Thorgeirsson SS, Steer CJ. Effect of ethanol on polyamine synthesis during liver regeneration in rats. J Clin Invest. 1990;85:385–390. doi: 10.1172/JCI114450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AM, Yang SQ, Brown N, Smith J, Raiford D, Gordon R, Casero R. Ethanol-associated alterations in the kinetics of putrescine uptake and metabolism by the regenerating liver. Alcohol Clin Exp Res. 1992;16:5–10. doi: 10.1111/j.1530-0277.1992.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Akerman PA, Cote PM, Yang SQ, McClain C, Nelson S, Bagby G, Diehl AM. Long-term ethanol consumption alters the hepatic response to the regenerative effects of tumor necrosis factor-alpha. Hepatology. 1993;17:1066–1073. [PubMed] [Google Scholar]
- Cherian MG, Kang YJ. Metallothionein and liver cell regeneration. Exp Biol Med. 2006;231:138–144. doi: 10.1177/153537020623100203. [DOI] [PubMed] [Google Scholar]
- Tohyama C, Suzuki JS, Hemelraad J, Nishimura N, Nishimura H. Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology. 1993;18:1193–1201. [PubMed] [Google Scholar]
- Oliver JR, Mara TW, Cherian MG. Impaired hepatic regeneration in metallothionein-I/II knockout mice after partial hepatectomy. Exp Biol Med. 2005;230:61–67. doi: 10.1177/153537020523000108. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, James Kang YJ. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]