Abstract
Research suggests that monocytes differentiate into unique lineage-determined macrophage subpopulations in response to the local cytokine environment. The present study evaluated the atherogenic potential of two divergent lineage-determined human monocyte-derived macrophage subpopulations. Monocytes were differentiated for 7 days in the presence of alternative macrophage development cytokines: granulocyte-macrophage colony-stimulating factor to produce granulocyte-macrophage-CSF macrophages (GM-Mac), or macrophage colony-stimulating factor (M-CSF) to produce M-Mac. Gene chip analyses of three monocyte donors demonstrated differential expression of inflammatory and cholesterol homeostasis genes in the macrophage subpopulations. Quantitative PCR confirmed a fivefold elevation in the expression of genes that promote reverse cholesterol transport (PPAR-γ, LXR-α, and ABCG1) and macrophage emigration from lesions (CCR7) in GM-Mac compared to that in M-Mac. Immunocytochemistry confirmed enhanced expression of the proinflammatory marker CD14 in M-Mac relative to GM-Mac. M-Mac spontaneously accumulated cholesterol when incubated with unmodified low-density lipoprotein whereas GM-Mac only accumulated similar levels of cholesterol after protein kinase C activation. Immunostained human coronary arteries showed that macrophages with similar antigen expression to that of M-Mac (CD68+/CD14+) were predominant within atherosclerotic lesions whereas macrophages with antigen expression similar to GM-Mac (CD68+/CD14−) were predominant in areas devoid of disease. The identification of macrophage subpopulations with different gene expression patterns and, thus, different potentials for promoting atherosclerosis has important experimental and clinical implications and could prove to be a valuable finding in developing therapeutic interventions in diseases dependent on macrophage function.
The monocyte-derived macrophage plays a pivotal role in vascular lipid deposition and the subsequent progression of atherosclerosis. Research has demonstrated that monocytes enter the vascular wall where they differentiate into macrophages and accumulate lipid to form an atherosclerotic plaque.1 Lipid-laden macrophages will then produce cytokines that perpetuate the inflammatory process and secrete enzymes that degrade the extracellular matrix.2 Monocyte-derived macrophages complete the evolution of an atherosclerotic lesion through the production of coagulation factors that contribute to thrombus formation producing the clinical manifestations of atherosclerotic disease.3
Macrophages have been identified as heterogeneous cells with a variety of physiological and pathophysiological functions.4 Investigators have hypothesized that monocytes will differentiate into specific macrophage subpopulations in response to alternative cytokine environments.5,6 Akagawa and colleagues7 has described two distinct monocyte-derived macrophage lineages in culture. Human monocytes differentiated in human serum or in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) added to fetal bovine serum produce cells that resemble the tissue macrophages in lung alveoli. In contrast, monocytes differentiated with macrophage colony-stimulating factor (M-CSF) added to fetal bovine serum produce cells that resemble peritoneal macrophages. The distinct macrophage subpopulations differ in their morphology and protein expression resulting in divergent functional lineages. However, the potential function of these divergent macrophage subpopulations has not been thoroughly explored in the context of atherosclerosis.
Human atherosclerotic lesions are heterogeneous structures with a variety of cell types creating a complex cytokine milieu. M-CSF is expressed by vascular endothelium, smooth muscle cells, and macrophages and intimal smooth muscle cells also secrete GM-CSF within atherosclerotic lesions.8,9 Because of this, human monocytes entering the vessel wall may be capable of differentiating into alternative lineage-determined macrophage subpopulations in response to the local cytokine environment. In concordance with this hypothesis, previous studies have demonstrated heterogeneity among the macrophages infiltrating human atherosclerotic lesions.10 However, the functional role of these heterogeneous macrophage subpopulations in the pathogenesis of atherosclerotic disease has not yet been elucidated.
The present study characterizes divergent atherogenic potential of human monocyte-derived macrophages differentiated with M-CSF or GM-CSF in vitro. Gene expression analysis suggests significant variations in gene expression related to inflammation and cholesterol homeostasis among the macrophage subpopulations. Furthermore, immunostaining of human coronary arteries demonstrates a greater proportion of macrophages resembling M-CSF monocyte-derived macrophages in atherosclerotic lesions and a greater proportion of macrophages resembling GM-CSF monocyte-derived macrophages in regions devoid of disease.
Materials and Methods
Cell Culture
Human monocytes were obtained through monocytopheresis of normal human donors and subsequently purified using counterflow centrifugal elutriation. This procedure typically yielded a purity of greater than 90% monocytes with the remaining cells consisting of lymphocytes as assessed by electronic volume analysis using a coulter counter. The monocytes were diluted in RPMI 1640 medium (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and seeded in treated culture plates (Plastek C; MatTek, Ashland, MA) at a density of 2 × 105 cells/cm2. The monocytes were incubated for 2 hours before the nonadherent cells were removed with three rinses of RPMI 1640. Adherent monocytes could then be harvested for further experimentation or differentiated in RPMI 1640 with 10% fetal bovine serum and alternative cytokine environments for 7 days. Monocytes differentiated in the presence of 50 ng/ml of recombinant human M-CSF and 25 ng/ml of recombinant human interleukin (IL)-10 (Peprotech, Rocky Hill, NJ) produced M-CSF macrophages (M-Mac). IL-10 has been shown to contribute to a homogeneous macrophage phenotype when monocytes are differentiated with M-CSF.11 Alternatively, monocytes differentiated in the presence of 50 ng/ml of recombinant human GM-CSF (Peprotech) produced GM-CSF macrophages (GM-Mac). The media and cytokines were replaced after 4 days of culture.
Gene Expression
Gene expression analysis was performed in three different monocyte donors. Adherent monocytes and differentiated macrophages were detached using a commercially available subculture kit containing trypsin and ethylenediaminetetraacetic acid (Cambrex, East Rutherford, NJ). The viability of the detached cells in each subpopulation exceeded 85% as determined by trypan blue exclusion (Sigma-Aldrich, St. Louis, MO). RNA was extracted from aliquots of 5 × 105 cells according to the protocols provided with the RNeasy micro kit (Qiagen, Valencia, CA). Isolated RNA was amplified for gene expression microarray analysis as previously described.12,13 Approximately 25 ng of total RNA from each monocyte or macrophage subpopulation was used to synthesize amplified labeled cDNA using the Ovation Biotin kit (Nugen, San Carlos, CA). The labeled cDNA was hybridized, washed, and stained on U133A 2.0 microarray chips according to protocols supplied by the manufacturer (Affymetrix, Santa Clara, CA). These microarray chips contained 22,277 probe sets to analyze the expression profiles of 18,400 transcripts including 14,500 well characterized human genes.
Analysis of Microarray Data
Normalization of the microarray data were performed using Affymetrix MAS 5.1. Further analysis of the gene expression profiles was performed with GeneSpring (Silicon Genetics, Redwood City, CA). A filter was created to identify genes that were expressed in at least two of three donor samples for each macrophage subpopulation. The expression of these genes was then normalized to the respective monocyte precursor to account for donor variability. Differential regulation of gene expression was set as a more than or equal to fivefold up-regulation or down-regulation among the average of three donors. Gene ontology data mining (level 5) and Expression Analysis Systemic Explore (EASE) biological theme analysis were conducted as previously described.14,15,16
Real-Time PCR
Real-time PCR was performed in three donors as previously described.17 Ten ng of total RNA from each macrophage phenotype (GM-CSF and M-CSF) was reverse-transcribed using the SuperScript II RS kit (Invitrogen, Carlsbad, CA). The resulting cDNA was probed for expressed genes using the TaqMan gene expression assays measured on an ABI Prism 7900 HT (Applied Biosystems, Foster City, CA). Relative gene expression was assessed after normalization to a β-actin control using the method described by Livak and Schmittgen.18
Immunostaining of Cultured Monocyte-Derived Macrophages
Adherent cultured monocytes and differentiated macrophage subpopulations were rinsed three times with phosphate-buffered saline (PBS) (Invitrogen). The adherent cells were fixed in culture plates with methanol at −20°C (VWR International, West Chester, PA) for 5 minutes and blocked with PBS plus 10% normal human serum (Pel-Freez, Milwaukee, WI) for 20 minutes. The cells were then incubated for 60 minutes with 2 μg/ml of primary antibody (goat anti-human CD68 IgG and mouse anti-human CD14 IgG from Santa Cruz Biotechnology, Santa Cruz, CA, or mouse anti-human 25F9 IgG from BMA Biomedicals, Augst, Switzerland). Control staining was performed with nonspecific goat IgG and mouse IgG (Santa Cruz Biotechnology) as the primary antibody. This was followed by a 45-minute incubation with 5 μg/ml of biotinylated secondary antibody (biotinylated horse anti-goat IgG or biotinylated goat anti-mouse IgG; Vector Laboratories, Burlingame, CA) and a 15-minute incubation with 10 μg/ml avidin-Alexa 488 or avidin-Alexa 594 (Molecular Probes, Eugene, OR) to visualize bound antibody. Three rinses with PBS followed all incubations and all steps were performed at room temperature.
Cytokine Analysis
Differentiated macrophages from three different monocyte donors were rinsed three times with RPMI 1640 medium. GM-Mac and M-Mac were then incubated for 48 hours with 1 ml of RPMI 1640 medium containing appropriate differentiation cytokines without serum. The macrophage-conditioned media was then screened for 120 different cytokines using the Human Cytokine Antibody Array C Series 1000 (Raybiotech, Norcross, GA) as previously described.19 Please see Supplemental Table S1 at http://ajp.amjpathol.org for a complete list of cytokines assayed.
Macrophage Cholesterol Content
Cholesterol accumulation assays were performed as previously described.20 GM-CSF and M-CSF differentiated macrophages were incubated in triplicate with 0 or 2 mg/ml of low-density lipoprotein (LDL) and the appropriate differentiation cytokines for 24 hours. The rinsed macrophages were harvested and cholesterol content determined after normalization to protein content as previously described.21,22,23 Data are presented as the mean ± SD for three monocyte donors. Statistical comparisons of means were made using a paired two-tailed t-test. A P value ≤0.05 was considered statistically significant.
Coronary Artery Immunofluorescence
Left coronary arteries were obtained from human autopsy specimens (National Disease Research Interchange, Philadelphia, PA) and immediately snap-frozen in OCT embedding media (Electron Microscopy Sciences, Hatfield, PA). Fifteen frozen tissue sections (10 μm) obtained at 0.6-mm intervals were prepared from each donor and applied to Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −70°C until further experimentation. The sections were double-labeled with biotinylated sheep anti-human CD14 (catalogue number BAF383; R&D, Minneapolis, MN) and mouse anti-human CD68 (clone KP1; DAKO, Carpinteria, CA) or mouse anti-human 25F9 as follows. The frozen sections were fixed 6 minutes with cold acetone, blocked 30 minutes with Dulbecco’s phosphate-buffered saline plus Mg2+, Ca2+ (DPBS) containing 10% human serum, and 15 minutes each with avidin and biotin blocking solutions (Vector Laboratories). Sections were subsequently incubated 30 minutes at room temperature with 5 μg/ml of anti-CD14 antibody diluted in DPBS containing 2% human serum. After three rinses with DPBS, sections were stained with 5 μg/ml Alexa 488-conjugated streptavidin (green fluorescence) to visualize the anti-CD14 primary antibody. After a single rinse, sections were incubated with anti-CD68 primary antibody (as prepared by the manufacturer) or 5 μg/ml anti-human 25F9 (diluted in DPBS containing 2% human serum) for 30 minutes and then rinsed with three 5-minute rinses. Subsequently, 2.5 μg/ml Alexa 594-conjugated chicken anti-mouse secondary antibody (red fluorescence) (Molecular Probes) diluted in DPBS containing 2% human serum was used to visualize the anti-CD68 or anti-25F9 antibody label during a 30-minute incubation. Control incubations were performed by substituting the specific primary anti-CD14 sheep and anti-CD68 or 25F9 mouse antibodies with biotinylated sheep IgG (BI-1004, Vector Laboratories) and the mouse N-Universal-negative control (N1698, DAKO), respectively. Slides were mounted with Vectashield (Vector Laboratories) containing 4,6-diamino-2-phenylindole (DAPI) nuclear stain (blue fluorescence). The immunostained sections were digitally imaged using an Olympus IX81 fluorescence microscope.
Quantification of immunohistochemical staining was done by counting CD14 and CD68 or CD14 and 25F9 immunolabeled cells with DAPI-stained nuclei in the intimal region of the 15 sections prepared from each coronary artery. There were between 1000 and 3000 cells per artery for lesions and between 100 and 1200 cells for normal regions. CD14+/CD68−, CD14−/CD68+, and CD14+/CD68+ doubly positive cells were counted in one experiment and CD14+/25F9−, CD14−/25F9+, and CD14+/25F9+ doubly positive cells were counted in another experiment. Results are expressed as a percentage of total positive immunolabeled cells for each sample. Statistical analysis was performed comparing atherosclerotic lesion versus normal intima by the unpaired t-test. A P < 0.05 was considered significant.
Coronary Artery Lipid Staining
A section immediately after the immunostained section was stained for lipid using oil-red-O as previously described.24 Sections with only some scattered macrophages but no macrophage foam cells were considered normal regions. These normal regions of vessel sometimes showed perifibrous lipid. Sections with dense infiltration of macrophages and macrophage foam cells were considered to be lesions. Lesions always showed areas with pools of extracellular lipid. Other sections were stained with oil-red-O and then mounted temporarily in DAPI-containing mounting medium. Then, oil-red-O red fluorescence and DAPI blue fluorescence were imaged. Next, slides were briefly immersed in water to remove the mounting medium and coverslip. The sections were then fixed in three 5-minute rinses of cold acetone to fix the sections and to remove the oil-red-O dye. This was followed by immunostaining for CD68 and CD14 as described elsewhere. The same regions imaged for oil-red-O were then imaged for immunostaining and co-localization of signals was assessed.
Results
Macrophage Morphology
The monocyte-derived macrophages formed different morphologies in response to alternative cytokine microenvironments (Figure 1). Human monocytes (Figure 1A) differentiated in the presence of GM-CSF for 7 days maintained the rounded shape of the monocyte precursors resembling the fried egg phenotype described in previous studies (Figure 1B).25 The same human monocytes differentiated in the presence of M-CSF for 7 days produced macrophages with an elongated shape and numerous vacuoles (Figure 1C).
Figure 1.
Morphology of differentiated macrophages. A: Human monocytes cultured for 2 hours were differentiated for 7 days in the presence of M-CSF or GM-CSF cytokines. B: Macrophages differentiated with GM-CSF maintained a spherical shape during differentiation. C: Macrophages differentiated with M-CSF were elongated with numerous vacuoles. Similar results were obtained with more than 10 monocyte donors. Scale bar = 10 μm (applies to all).
Gene Expression Analysis
The gene expression profiles in the two macrophage subpopulations were compared with one another. This comparison identified 731 transcripts representing 588 unique genes that showed a fivefold difference in expression levels between M-Mac and GM-Mac. Gene ontology data mining of this gene list demonstrated large differences in genes related to the defense response (7%) and the response to pest/pathogen/parasite (5%). EASE demonstrated an overrepresentation of genes related to the immune response, defense response, and the response to biotic stimuli among the macrophage subpopulations (EASE score, <0.05). A complete analysis is available in Supplemental Tables S2 and S3 at http://ajp.amjpathol.org.
A number of differentially expressed genes were related to the inflammatory response and lipid metabolism. An arbitrary selection of genes within these categories has been extracted according to their known role in atherosclerotic disease and presented in Table 1. Each of the genes listed in this table were differentially expressed by at least fivefold in the macrophage subpopulations. A complete list of the differentially expressed genes is available in Supplemental Table S4 (see http://ajp.amjpathol.org).
Table 1.
Gene Expression Profiles of GM-Mac and M-Mac
| Immune response | Δ | Coagulation and proteolysis | Δ | Lipid metabolism | Δ | Cytokine system | Δ |
|---|---|---|---|---|---|---|---|
| GM-Mac | |||||||
| CD1a | 38 | TIMP3 | 20 | LPL | 23 | CCL1 | 124 |
| CD1c | 37 | MMP12 | 19 | FATP3 (SLC27A) | 13 | CCL17 | 124 |
| CD22 | 12 | ProC | 9 | ApoC1 | 12 | CCL22 | 16 |
| ABCB9 | 10 | ApoC2 | 11 | CCL13 | 14 | ||
| PPAR-γ | 10 | TGF-β2 | 9 | ||||
| ApoD | 7 | CCR7 | 4 | ||||
| ABCG1 | 6 | ||||||
| CYP27A1 | 6 | ||||||
| ApoE | 5 | ||||||
| LXR-α | 5 | ||||||
| M-Mac | |||||||
| CD28 | 155 | PAI-2 (SERPINB2) | 48 | PLTP | 41 | IGF-1 | 129 |
| CD16 | 22 | TSP-1 (THBS1) | 43 | EDG1 | 103 | ||
| CD14 | 21 | TFPI-β | 12 | BMP2 | 44 | ||
| CD163 | 20 | DF | 9 | HGF | 22 | ||
| C2 | 8 | FGF-13 | 20 | ||||
| MCP-1 (CCL2) | 10 | ||||||
| GRO-α (CXCL1) | 10 |
The differential expression of arbitrarily selected genes between the macrophage phenotypes is shown according to biological process categories. The fold difference (Δ) is indicated for genes predominant in GM-Mac (top frame) or M-Mac (bottom frame). The results are the means of three monocyte donors.
Cytokine Secretion
A cytokine array was used to evaluate the transcriptional differences in cytokine production identified with the gene chip microarray. Table 2 summarizes the cytokines found to be differentially secreted in the macrophage subpopulations cultured from three monocyte donors (P < 0.05). GM-Mac secreted significantly more macrophage-derived chemokine (MDC), also known as CCL22, that plays a role in monocyte recruitment.26 M-Mac secreted cytokines related to monocyte recruitment (GRO-α, HGF) and smooth muscle proliferation (HGF, IGF-1).27 These findings were consistent with the expression profiles determined by the gene chip analysis. IL-13 was also found to be differentially secreted in the cytokine array with enhanced secretion by M-Mac. However, differential expression of this cytokine was not detected with the gene microarray.
Table 2.
Cytokine Secretion by GM-Mac and M-Mac
| Sample | GM-Mac Mean ± SD | M-Mac Mean ± SD | P value | Fold difference |
|---|---|---|---|---|
| IL-13 | 234 ± 298 | 4420 ± 310 | 0.006 | 18.9 |
| IGF-I | 592 ± 372 | 3500 ± 616 | 0.019 | 5.9 |
| PlGF | 356 ± 310 | 1121 ± 350 | 0.037 | 3.2 |
| HGF | 340 ± 316 | 1063 ± 428 | 0.033 | 3.1 |
| GRO-α | 284 ± 493 | 873 ± 464 | 0.014 | 3.1 |
| LIGHT | 1051 ± 547 | 2796 ± 123 | 0.045 | 2.7 |
| IL-6 R | 1644 ± 1190 | 2686 ± 1278 | 0.010 | 1.6 |
| VEGF-D | 2925 ± 296 | 2493 ± 294 | 0.035 | −1.2 |
| TNF-β | 3518 ± 483 | 2425 ± 629 | 0.006 | −1.5 |
| TGF-β3 | 1685 ± 106 | 860 ± 290 | 0.043 | −2.0 |
| CCL22 | 3638 ± 1078 | 983 ± 228 | 0.045 | −3.7 |
Each of the cytokines listed was differentially secreted by the macrophage subpopulations (P < 0.05). The mean secretion from three donors is expressed in arbitrary units. A positive fold difference indicates predominance in M-Mac whereas a negative fold difference indicates predominance in GM-Mac.
Quantitative PCR
Quantitative PCR was used to confirm differential gene expression related to cholesterol homeostasis identified with the microarray. Table 3 summarizes the relative expression of selected genes implicated in macrophage cholesterol homeostasis. The nuclear transcription factors PPAR-γ and LXR-α enhance the transcription of ABCA1 and ABCG1 inducing cholesterol efflux and facilitating reverse cholesterol transport.28,29 As suggested by the microarray, quantitative PCR confirmed the enhanced expression of these transcription factors by GM-Mac relative to M-Mac. Furthermore, ABCG1, a downstream effecter of cholesterol efflux, was also enhanced in the GM-CSF-derived macrophage subpopulation. Other nuclear transcription factors (PPAR-α and LXR-β) that do not play as significant a role in the regulation of cholesterol metabolism were similar in the macrophage subpopulations.28 GM-Mac demonstrated a significant increase in CCR7 expression, a cytokine associated with dendritic cell migration and maturation.30 The relative expression profiles determined with quantitative PCR correlated with the gene expression microarray for all of the genes assayed by both methods (R2 = 0.57).
Table 3.
Quantitative PCR for Selected Lipid-Related Genes in M-Mac and GM-Mac
| Gene | Fold difference
|
|
|---|---|---|
| qPCR | Microarray | |
| ABCG1 | 18.7 | 5.6 |
| CCR7 | 17.3 | 4.0 |
| PPAR-γ | 7.4 | 9.8 |
| LXR-α | 5.1 | 5.4 |
| ApoE | 3.0 | 5.1 |
| ABCA1 | 1.4 | −1.7 |
| PPAR-α | 1.1 | 2.0 |
| ACAT1 | −1.2 | −1.1 |
| LXR-β | −1.4 | −2.0 |
The genes listed above were investigated with quantitative PCR. A positive fold difference indicates an up-regulation in GM-Mac relative to M-Mac while a negative fold difference indicates a down-regulation. The quantitative PCR measurements correlated with the gene expression microarray (R2 = 0.57). The results are the means of three monocyte donors.
Inflammatory Markers
Immunocytochemistry was used to evaluate the transcriptional differences in inflammatory markers identified with the gene expression microarray. The cells were initially stained for CD68, a glycoprotein located on the lysosomal membrane of monocytes and macrophages. Previous research has used CD68 as a universal monocyte and macrophage marker in the study of atherosclerotic disease. The monocyte precursors (data not shown) and macrophage subpopulations all stained for CD68 (Figure 2). Further staining identified the proinflammatory marker CD14 on M-Mac without concomitant expression in GM-Mac. CD14 is a glycosylphosphatidylinositol-anchored protein that binds lipopolysaccharide found on the surface of select subpopulations of the monocytic lineage.31,32 25F9 detects an 86-kDa differentiation antigen present in some macrophage subpopulations.33 GM-Mac but not M-Mac showed strong staining for the 25F9 antigen (Figure 3). The adherent monocytes stained for CD14 but did not stain for 25F9 (data not shown).
Figure 2.
Differential expression of macrophage markers. Human monocytes were differentiated for 7 days in the presence of M-CSF or GM-CSF. Then, macrophage cultures were immunostained for the macrophage marker CD68 (green) and then stained for CD14 (red). Dual labeling demonstrated the presence of CD14 (B) on M-Mac without similar staining on GM-Mac (A). Similar results were obtained with two other monocyte donors. Scale bar = 10 μm (applies also to B).
Figure 3.
Differential expression of macrophage marker 25F9. Human monocytes were differentiated for 7 days in the presence of GM-CSF (A and B) or M-CSF (B and D). Macrophage cultures were then immunostained for the macrophage marker 25F9 (green). A and C show fluorescence whereas B and D show phase microscopic images of the macrophages. Similar results were obtained with another monocyte donor. Scale bar = 50 μm (applies to all).
Cholesterol Accumulation
The differential expression of genes related to cholesterol homeostasis prompted further investigation of the cholesterol accumulation potential of the two macrophage phenotypes. Macrophage subpopulations from three monocyte donors were incubated with 2 mg/ml of unmodified LDL. Figure 4 summarizes the spontaneous cholesterol accumulation in both macrophage subpopulations. GM-Mac accumulated 107 ± 47 nmol cholesterol/mg cellular protein whereas M-Mac accumulated twice as much cholesterol at 217 ± 41 nmol/mg (P < 0.05). After activating the macrophage subpopulations with phorbol 12-myristate 13-acetate (PMA), GM-Mac accumulated the same amount of cholesterol (250 ± 48 nmol/mg) as M-Mac (284 ± 84 nmol/mg).25,34 PMA did not significantly increase the cholesterol accumulation of M-Mac.
Figure 4.
Cholesterol accumulation of differentiated macrophages. Human monocytes were differentiated for 7 days in the presence of M-CSF or GM-CSF. Macrophage cultures were subsequently incubated in triplicate with 0 or 2 mg/ml LDL for 24 hours and their cholesterol and protein contents were determined. Shown is the net cholesterol accumulation of macrophages incubated with LDL compared with macrophages incubated without LDL for the indicated conditions. M-Mac spontaneously accumulated significant quantities of LDL-derived cholesterol relative to GM-Mac (P < 0.05). Activation of the subpopulations with PMA (1 μg/ml) increased cholesterol accumulation in GM-Mac without a change in M-Mac. Data are presented as the mean ± SD for three monocyte donors.
Coronary Artery Immunofluorescence
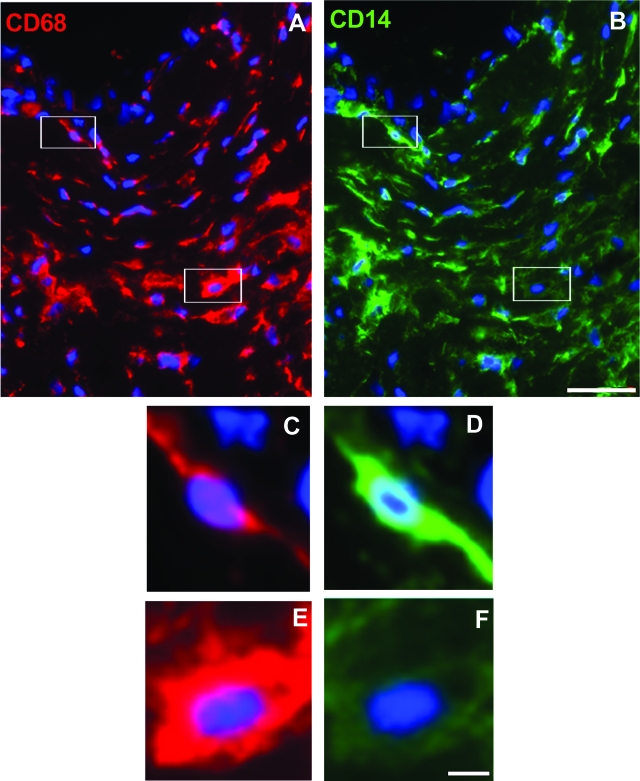
Dual-immunofluorescence staining for CD14 and the pan-macrophage marker CD68 was used to determine the predominant macrophage phenotype in normal and atherosclerotic human coronary arteries. In concordance with the in vitro macrophage immunostaining, CD68+/CD14+ double-labeled cells were considered to resemble the M-Mac phenotype whereas CD68+/CD14− single-labeled cells were considered to resemble the GM-Mac phenotype. Consistent with this was the observation that CD68+/CD14+ double-labeled cells showed an elongated phenotype, whereas CD68+/CD14− single-labeled cells showed a rounded phenotype (Figure 5). Thus, the morphology of the in vivo macrophage resembled the morphology of the cultured macrophage having the same marker phenotype. Both GM-Mac and M-Mac phenotypes were present in normal intimal regions as well as atherosclerotic lesions of coronary arteries (Figure 5). In addition, rare cells labeled with CD14 alone (CD68−/CD14+) were also present within the intima. Quantification of intimal immunostained cells demonstrated that macrophages resembling M-Mac (CD68+/CD14+) were more prevalent in atherosclerotic lesions (75 ± 4%) compared with normal intimal regions of coronary arteries (39 ± 3%). In contrast, macrophages resembling GM-Mac (CD68+/ CD14−) were more prevalent in normal intimal regions (56 ± 2%) compared with atherosclerotic lesions of coronary arteries (21 ± 5%) (Table 4).
Figure 5.
Analysis of macrophage phenotype in coronary artery atherosclerotic lesions. Fluorescence immunostaining of CD68 and CD14 in an atherosclerotic lesion is shown. Cells were visualized by DAPI nuclear staining (blue fluorescence) and immunolabeled for CD 68 (red fluorescence; A, C, and E) and CD14 (green fluorescence; B, D, and F). C and D: Higher magnification (top left-hand boxed region in A and B) demonstrates CD68+/CD14+ macrophages. E and F: Higher magnification (bottom right-hand boxed region in A and B) demonstrates CD68+/CD14− macrophages. Scale bars: 10 μm (B, and also applies to A); 2.5 μm (F, and also applies to C–E).
Table 4.
Quantification of Macrophage Staining in Human Coronary Artery Sections
| Sample number | Intimal region type | Sex | Age | Macrophage phenotype (% of total immunolabeled cells)
|
||
|---|---|---|---|---|---|---|
| CD68+ CD14+ | CD68+ CD14− | CD68− CD14+ | ||||
| 1 | Normal | Female | 70 | 33 | 53 | 14 |
| 2 | Normal* | Female | 57 | 45 | 55 | 0 |
| 3 | Normal | Male | 72 | 39 | 61 | 15 |
| mean ± SEM | 39 ± 3† | 56 ± 2‡ | 10 ± 5 | |||
| 4 | Lesion | Male | 73 | 73 | 27 | 0 |
| 5 | Lesion | Female | 57 | 82 | 12 | 6 |
| 6 | Lesion | Male | 49 | 69 | 24 | 7 |
| mean ± SEM | 75 ± 4† | 21 ± 5‡ | 4 ± 2 | |||
Normal intimal region in vessel wall opposite of sample 5 lesion.
Comparison between normal and atherosclerotic lesions, P < 0.05.
Comparison between normal and atherosclerotic lesions, P < 0.05.
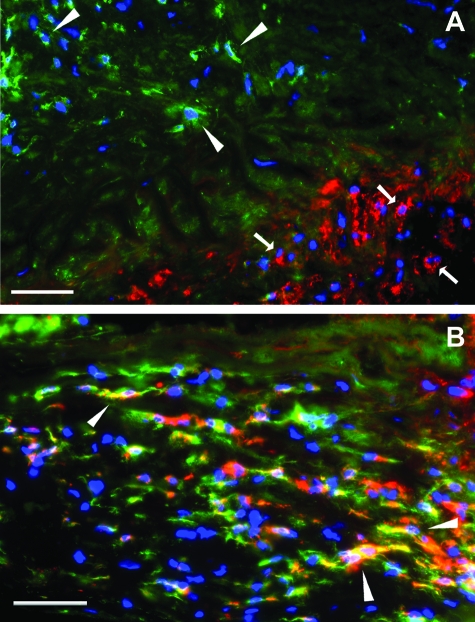
The presence of 25F9, the macrophage differentiation antigen present on GM-Mac in vitro, was also assessed in coronary atherosclerotic lesions. Clusters of macrophages that labeled with either 25F9 or CD14 were observed in atherosclerotic lesion areas (Figure 6A) consistent with these markers identifying distinct macrophage phenotypes similar to cultured GM-Mac and M-Mac. However, a third macrophage population showing labeling with both markers was also observed in lesions (Figure 6B). Quantification of the macrophage subpopulations demonstrated that 24% of the total CD14+ macrophages co-stained with 25F9 (Table 5). This staining pattern distinguishes this macrophage subpopulation from the cultured macrophage phenotypes that stained exclusively for 25F9 in the case of cultured GM-Mac or exclusively for CD14 in the case of cultured M-Mac. Nevertheless, the M-Mac phenotype (25F9−/CD14+) was also predominant in this immunostaining experiment showing that 62 ± 1% of intimal macrophages resembled M-Mac and only 15 ± 4% of macrophages resembled the GM-Mac phenotype (25F9+/CD14−). In normal regions of intima, the percentage of GM-Mac phenotype indicated by 25F9+/CD14− immunolabeling (2 ± 1%) was substantially less than the percentage of GM-Mac suggested by CD68+/CD14+ immunolabeling (56 ± 2%). 25F9 antigen is absent on monocytes and is acquired during differentiation of GM-Mac in culture.35 Thus, these results suggest that the normal intima may contain predominately immature CD68+/CD14− GM-Mac without expression of 25F9.
Figure 6.
Analysis of 25F9 macrophage immunolabeling in coronary artery atherosclerotic lesions. Fluorescence immunostaining of 25F9 and CD14 in an atherosclerotic lesion is shown. Cells were visualized by DAPI nuclear staining (blue fluorescence) and immunolabeled for 25F9 (red fluorescence) and CD14 (green fluorescence). Separate clusters of 25F9+/CD14− (bottom right, arrows) and 25F9−/CD14+ macrophages (top left, arrowheads) are shown in A. Arrowheads in B indicate minor cell subpopulation showing both 25F9 (red fluorescence) and CD14 (green fluorescence) immunolabeling. Scale bars = 50 μm.
Table 5.
Quantification of 25F9 Macrophage Staining in Human Coronary Artery Sections
| Sample number* | Intimal region type | Macrophage phenotype (% of total immunolabeled cells)
|
|||
|---|---|---|---|---|---|
| 25F9− CD14+ | 25F9+ CD14− | 25F9+ CD14+ | |||
| 1 | Normal | 100 | 0 | 0 | |
| 2 | Normal† | 95 | 4 | 1 | |
| 3 | Normal | 87 | 3 | 10 | |
| Mean ± SEM | 94 ± 4 | 2 ± 1 | 4 ± 3 | ||
| 4 | Lesion‡ | ||||
| 5 | Lesion | 62 | 11 | 27 | |
| 6 | Lesion | 61 | 18 | 21 | |
| Mean ± SEM§ | 62 ± 1 | 15 ± 4 | 24 ± 3 | ||
Same samples as in Table 4.
Normal intimal region in vessel wall opposite sample 5 lesion.
Sample lost because of laboratory accident.
All means comparing macrophage phenotypes for normal and lesions were significant, P < 0.05.
The immunostaining also revealed the presence of CD68+/CD14+ double-labeled cells throughout the adventitia in both normal and diseased regions of the coronary arteries. Cells with CD68 and/or CD14 immunolabeling were not present within the media of normal coronary arteries. However, the media of human coronary arteries with advanced atherosclerotic lesions showed CD68+/CD14+ double-labeled cells extending from the adventitia through the media into the intima (see Supplemental Figure S1 at http://ajp.amjpathol.org). Some CD14 single-labeled cells were also present within the media at these sites. The CD68+/CD14+ double-labeled cells in the intima were observed close to gaps in the internal elastic membrane and these macrophages were uniquely oriented with their long axis perpendicular to the internal elastic membrane consistent with migration toward or away from the internal elastic membrane.
Immunostaining of markers that localized to areas without nuclei could be because of shedding of these proteins markers from the plasma membrane,36 or could represent remnants of dead macrophages. Some immunostaining could also be macrophages whose nuclei were not included within the plane of the tissue section. For all markers, immunostaining that was not associated with nuclei was not included in the cell counts.
Coronary Artery Lipid Staining
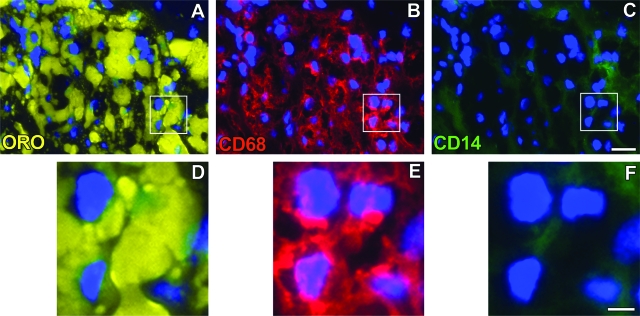
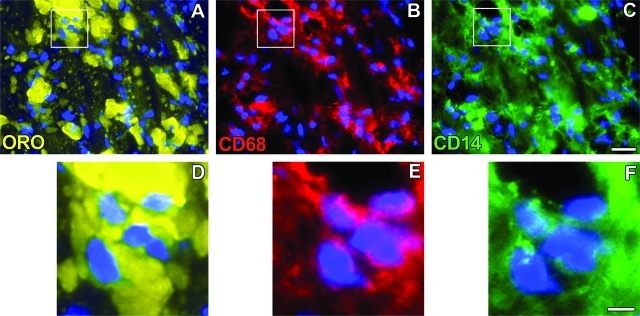
Subsequent staining sought to determine whether both CD68+/CD14+ and CD68+/CD14− macrophage phenotypes accumulated lipid in vivo as assessed by co-localization of macrophage phenotype markers with oil-red-O-stained lipid. As shown in Figures 7 and 8, both macrophage phenotypes showed accumulation of oil-red-O-stained lipid demonstrating that both types of macrophages can become foam cells within atherosclerotic lesions.
Figure 7.
Co-localization of GM-Mac with lipid. Co-localization of fluorescence oil-red-O staining of lipid (A and D) and fluorescence immunostaining of CD68 (red fluorescence, B and E) and CD14 (green fluorescence, C and F) in an advanced atherosclerotic lesion is shown. Cells were visualized by DAPI nuclear staining (blue fluorescence). The boxed region is shown at higher magnification at the bottom and demonstrates lipid-containing CD68+/CD14− macrophages (ie, GM-CSF phenotype). Scale bars: 20 μm (C; also applies to A and B); 5 μm (F; also applies to D and E).
Figure 8.
Co-localization of M-Mac with lipid. Co-localization of fluorescence oil-red-O staining of lipid (A and D) and fluorescence immunostaining of CD68 (red fluorescence, B and E) and CD14 (green fluorescence, C and F) in an advanced atherosclerotic lesion is shown. Cells were visualized by DAPI nuclear staining (blue fluorescence). The boxed region is shown at higher magnification at the bottom and demonstrates lipid-containing CD68+/CD14+ macrophages (ie, M-CSF phenotype). Scale bars: 20 μm (C; also applies to A and B); 5 μm (F; also applies to D and E).
Discussion
The present study demonstrates that human monocytes can be differentiated into divergent macrophage phenotypes (ie, M-Mac and GM-Mac) with potentially alternative functions in human atherosclerotic disease. Gene expression analysis suggests that the resulting macrophage lineage-determined subpopulations exhibit significant variation in gene expression related to the immune response, cytokine secretion, cholesterol homeostasis, and coagulation. Research suggests that the regulation of macrophage cholesterol metabolism may be an extension of the inflammatory process because similar transcription factors affect both systems.37,38 The differential expression of inflammatory and cholesterol homeostasis genes may contribute to divergent atherosclerotic potentials for these macrophage phenotypes. In concordance with this hypothesis, immunofluorescence of human coronary arteries confirmed that the CD68+/CD14+ (similar to M-Mac) macrophage subpopulation predominates in atherosclerotic lesions whereas the CD68+/CD14− (similar to GM-Mac) macrophage subpopulation predominates in areas devoid of disease. 25F9, a marker acquired by GM-Mac as they differentiate in culture,35 was not prominent in normal intima suggesting that the GM-Mac in normal intima were not well differentiated.
The gene expression analysis demonstrated differential expression of inflammatory response genes that have been associated with atherosclerosis and cardiovascular disease. M-Mac express the proinflammatory marker CD14. CD14 participates in the innate immune response through the recognition of bacterial-derived lipopolysaccharide and the subsequent activation of macrophages through toll-like receptor 4.39,40 Research has identified polymorphisms in CD14 as an independent risk factor for cardiovascular disease indicating a potential role for this receptor in atherosclerosis.41,42 Thus, the presence of CD14 on M-Mac could facilitate the activation of the innate immune response involved in plaque formation. M-Mac also showed increased gene expression of CD16 relative to GM-Mac. Human monocyte subpopulations that vary in expression of CD14, CD16, CD64, and CD33 have been described.43,44 An increased proportion of monocytes expressing both CD14 and CD16 has been associated with hypercholesterolemia and an increased incidence of coronary artery disease.43,45 Ly-6Clo monocytes, a subpopulation of mouse monocytes, actively contribute to plaque macrophages in this species.46,47 It remains to be determined what human monocyte subsets give rise to M-Mac and GM-Mac phenotypes.
Further analysis of the gene expression data revealed differential expression of genes associated with antigen presentation. Although antigen presentation has been attributed to dendritic cells, monocyte-derived macrophages can also serve as antigen-presenting cells.48 GM-Mac demonstrated enhanced expression of genes related to antigen processing of lipids [CD1a, CD1c, and apolipoprotein E (apoE)]. CD1 glycoproteins bind lipids for subsequent presentation to T lymphocytes. Previous studies demonstrated that apoE functions in macrophage cholesterol efflux and reverse cholesterol transport.49 Also, apoE has recently been shown to bind and deliver lipid antigens to CD1 glycoproteins thereby facilitating antigen processing.50 Atherosclerotic plaques contain numerous lipid particles with glycosphingolipids and phospholipids that would require CD1-mediated antigen presentation.51 Furthermore, CD1 glycoproteins have been localized within human atherosclerotic plaque macrophages.52 The enhanced expression of lipid antigen presentation genes in GM-Mac suggests an underlying similarity to dendritic cells in mediating immune responses within atherosclerotic lesions.
Cytokine secretion among the macrophage subpopulations also demonstrated alternative inflammatory potentials. M-Mac secreted significantly more LIGHT, GRO-α, HGF, IGF-1, and IL-13 relative to GM-Mac (Table 2). GRO-α and HGF are chemotactic cytokines thought to be involved in monocyte recruitment and smooth muscle proliferation in murine atherosclerotic lesions.27,53 IL-13 has been associated with increased oxidation of LDL by cultured macrophages whereas IGF-1 has been suggested to promote atherosclerosis in some studies and to be atheroprotective in other studies.54,55,56 The gene expression microarray also identified enhanced expression in M-Mac relative to GM-Mac of the NF-κB responsive monocyte chemotactic protein-1 (MCP-1), also known as CCL2. MCP-1 stimulates monocyte infiltration into the vessel wall and vessel accumulation of oxidized lipid.57 Furthermore, research has demonstrated that removal of the primary receptor for MCP-1 (CCR2) reduces atherosclerotic burden in murine models.58 GM-Mac secreted significantly more than twofold CCL22 relative to M-Mac. CCL22 has been shown to attenuate mast cell chemotaxis in response to other chemokines.59 This might be a significant atheroprotective function of GM-Mac because research has suggested that mast cells may contribute to atherosclerotic plaque erosion and rupture.60
The alternative inflammatory characteristics of the lineage-determined macrophage subpopulations also encompass divergent regulation of cholesterol homeostasis. Recent work has identified PPAR-γ and LXR-α as important regulators of cholesterol homeostasis in macrophages within atherosclerotic lesions.28 These transcription factors induce increased expression of ABCA1 and ABCG1 facilitating cholesterol efflux and reverse cholesterol transport. PPAR-γ and LXR-α also regulate innate immunity and inhibit the inflammatory response induced by NF-κB.37 Agonists of PPAR-γ and LXR-α attenuate the inflammatory response and atherosclerosis progression in murine models.28,61 In the present study, GM-Mac expressed five times more PPAR-γ and LXR-α when compared with M-Mac (Table 3). The up-regulation of these genes in GM-Mac was accompanied by an increase in ABCG1 and apoE, genes that are regulated by these transcription factors and directly mediate cholesterol efflux. Although ABCA1 expression is also usually up-regulated by PPAR-γ and LXR-α, there was no difference in ABCA1 expression between the two macrophage phenotypes. This suggests that other factors may also affect regulation of ABCA1 in GM-Mac. This is consistent with recent reports that ABCA1 expression can be selectively down-regulated compared with ABCG1 by oxysterol-binding protein-related protein 8 and interleukin-1β.62,63 However, neither of these genes regulating ABCA1 was differentially expressed in the two macrophage phenotypes.
Both M-Mac and GM-Mac phenotypes are capable of accumulating lipid and forming foam cells in vitro. Both macrophage phenotypes take up LDL by a receptor-independent mechanism and accumulate cholesterol when incubated with native LDL. M-Mac show spontaneous fluid-phase pinocytosis of LDL, whereas GM-Mac show fluid-phase pinocytosis of LDL only when these macrophages are activated through protein kinase C.20,25,64 Our results show that both macrophage phenotypes accumulate lipid in vivo. Fluid-phase pinocytosis of LDL in M-Mac can be down-regulated by the LXR agonist T0901317.65 Thus, the high gene expression of LXR-α in GM-Macs relative to M-Macs may result in the down-regulation of fluid-phase pinocytosis of LDL in vitro as well as up-regulation of downstream effectors of cholesterol efflux. In vivo, this up-regulation of LXR-α might also decrease the inflammatory response of the GM-Macs within the vessel wall.
Human coronary arteries contain a different proportion of macrophage subpopulations in atherosclerotic lesions compared with disease-free regions of vessel. We have shown that atherosclerotic lesions contain a large percentage of CD68+/CD14+ cells resembling M-Mac, whereas normal coronary intimal vasculature predominantly contains CD68+/CD14− cells resembling GM-Mac. In atherosclerotic lesions, smooth muscle cells express GM-CSF and endothelial, smooth muscle, and macrophage cells express M-CSF.8,9,66 Our observation that GM-CSF-independent (CD68+/CD14−) and M-CSF-dependent (CD68+/CD14+) macrophage phenotypes occur within human atherosclerotic plaques suggests that these locally produced macrophage-differentiating factors promote differentiation of monocytes that enter the vessel wall.
The predominance of macrophages in atherosclerotic lesions resembling M-Mac differentiated with M-CSF suggests this macrophage phenotype promotes development or maintenance of these lesions. M-CSF is required for the development of M-Mac in vivo and M-CSF is necessary for the development of atherosclerotic lesions in mouse models of atherosclerosis. ApoE/M-CSF-deficient mice have decreased atherosclerosis in the aorta despite elevations in plasma cholesterol when compared with apoE-deficient mice.67,68,69 Furthermore, intraperitoneal injection of antibodies against the M-CSF receptor (c-fms) has been shown to reduce the initial events of atherogenesis in apoE-deficient mice.70 In contrast to the pathogenic role of M-CSF in atherosclerosis, recent research is less clear concerning the role for GM-CSF in atherogenesis. One study has demonstrated that GM-CSF deficiency increases atherosclerosis in apoE-deficient mice.71 Furthermore, the protective role of GM-CSF was mediated primarily by PPAR-γ and ABCG1, genes shown to be enhanced in our GM-Mac. In contrast, another study showed decreased atherosclerosis in GM-CSF-deficient mice that genetically lacked the LDL receptor.72 Further research should clarify the role of GM-CSF in the pathogenesis of human atherosclerotic disease.
The predominance of macrophages similar to GM-Mac in vascular regions devoid of disease suggests that this subpopulation may contribute to vessel wall homeostasis. Recent data has demonstrated that induction of LXR-α accompanied by an increase in CCR7 activation can lead to regression of atherosclerotic lesions from apoE-deficient hypercholesterolemic mice transplanted into normolipemic wild-type mice.30 Previous work had suggested that this regression may be related to monocyte-derived dendritic cells/macrophages migrating from the atherosclerotic lesions into the blood and draining lymph nodes in response to CCR7 activation.73 GM-Mac demonstrate dendritic cell characteristics in the present study and express 17-fold more CCR7 than M-Mac indicating a possible explanation for a predominance of this macrophage phenotype in regions devoid of disease. It is possible that GM-Mac-derived foam cells facilitate cellular transport of cholesterol out of the vessel by CCR7-dependent emigration from the vessel wall. Further investigation could clarify the role of this macrophage subpopulation in vivo.
As with all in vitro microarray experiments, the present study is hindered by various limitations. The in vitro environment that we have created is a simplistic model containing few cytokines in isolation and may not adequately represent the complex cytokine milieu that is present in human disease. Because of this, the macrophage subpopulations in vitro may represent extremes on a spectrum of different in vivo phenotypes. The macrophage markers used to identify similar phenotypes in human coronary arteries may not be specific enough to fully characterize the phenotype in vivo. Experiments are underway to further identify the gene expression patterns of particular macrophage subpopulations in human coronary arteries and correlate them with our in vitro models. Gene expression microarray studies are plagued by the inability to adequately analyze all gene data. Pathway analysis and literature searches of interesting genes are always biased and limited by the current scientific knowledge. It is possible that our analysis has overlooked vitally important genes that have not yet been investigated in the context of human disease. Despite these limitations, though, the present study does provide a contribution to our understanding of macrophage biology and atherosclerosis.
In conclusion, our findings demonstrate that human monocytes will differentiate into unique lineage-determined macrophage subpopulations with different inflammatory potentials in response to the cytokine environment. The GM-Mac phenotype that predominates in normal intima expresses genes known to mediate reverse cholesterol transport and macrophage emigration from the vessel wall. The M-Mac phenotype that predominates in diseased intima is relatively deficient in these same atheroprotective genes but instead expresses many proinflammatory genes. The identification of macrophage subpopulations with different gene expression patterns and thus different potentials for promoting atherosclerosis has important experimental and clinical implications. Experimental models of atherosclerosis may be influenced by a particular predisposition toward differentiating certain macrophage phenotypes in different species. Patients with a predisposition toward developing more inflammatory macrophage subpopulations could be at an increased risk for developing atherosclerotic disease. Divergent macrophage phenotypes that make alternative contributions to disease pathogenesis are not limited to cardiovascular disease. Research has demonstrated that most macrophages in the normal liver are CD68+/CD14− (GM-Mac), whereas macrophages in patients with inflammatory liver disease are CD68+/CD14+ (M-Mac).74,75 The modulation of macrophage differentiation could prove to be a valuable therapeutic intervention in diseases dependent on macrophage function.
Acknowledgments
We thank the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for providing elutriated monocytes; Rani Rao for technical assistance; and Kiyoko Akagawa for scientific discussion.
Footnotes
Address reprint requests to Howard S. Kruth, M.D., Section of Experimental Atherosclerosis, National Heart, Lung, and Blood Institute, National Institutes of Health, Building 10, Room 5N-113, 10 Center Dr. MSC 1422, Bethesda, MD 20892-1422. E-mail: kruthh@nhlbi.nih.gov.
Supported by the Intramural Research Program of the National Institutes of Heath, National Heart, Lung, and Blood Institute; and The Howard Hughes Medical Institute (the National Institutes of Heath Medical Research Scholar Program to S.W.W).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- Crawley J, Lupu F, Westmuckett AD, Severs NJ, Kakkar VV, Lupu C. Expression, localization, and activity of tissue factor pathway inhibitor in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2000;20:1362–1373. doi: 10.1161/01.atv.20.5.1362. [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst ES, Jelk J, Asmis R. Differential expression of CD14. CD36 and the LDL receptor on human monocyte-derived macrophages A novel cell culture system to study macrophage differentiation and heterogeneity. Histochem Cell Biol. 1998;110:231–241. doi: 10.1007/s004180050285. [DOI] [PubMed] [Google Scholar]
- Williams L, Jarai G, Smith A, Finan P. IL-10 expression profiling in human monocytes. J Leukoc Biol. 2002;72:800–809. [PubMed] [Google Scholar]
- Akagawa KS, Kamoshita K, Tokunaga T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J Immunol. 1988;141:3383–3390. [PubMed] [Google Scholar]
- Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- Plenz G, Koenig C, Severs NJ, Robenek H. Smooth muscle cells express granulocyte-macrophage colony-stimulating factor in the undiseased and atherosclerotic human coronary artery. Arterioscler Thromb Vasc Biol. 1997;17:2489–2499. doi: 10.1161/01.atv.17.11.2489. [DOI] [PubMed] [Google Scholar]
- Poston RN, Hussain IF. The immunohistochemical heterogeneity of atheroma macrophages: comparison with lymphoid tissues suggests that recently blood-derived macrophages can be distinguished from longer-resident cells. J Histochem Cytochem. 1993;41:1503–1512. doi: 10.1177/41.10.7504008. [DOI] [PubMed] [Google Scholar]
- Hashimoto SI, Komuro I, Yamada M, Akagawa KS. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J Immunol. 2001;167:3619–3625. doi: 10.4049/jimmunol.167.7.3619. [DOI] [PubMed] [Google Scholar]
- Singh R, Maganti RJ, Jabba SV, Wang M, Deng G, Heath JD, Kurn N, Wangemann P. Microarray based comparison of three amplification methods for nanogram amounts of total RNA. Am J Physiol Cell Physiol. 2005:C1179–C1189. doi: 10.1152/ajpcell.00258.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Roden J, Shapiro BE, Wold BJ, Bhatia S, Forman SJ, Bhatia R. Reproducibility, fidelity, and discriminant validity of mRNA amplification for microarray analysis from primary hematopoietic cells. J Mol Diagn. 2005;7:48–56. doi: 10.1016/S1525-1578(10)60008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for Annotation. Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Technol. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- Kruth HS, Skarlatos SI, Lilly K, Chang J, Ifrim I. Sequestration of acetylated LDL and cholesterol crystals by human monocyte-derived macrophages. J Cell Biol. 1995;129:133–145. doi: 10.1083/jcb.129.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Kruth HS. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am J Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- Kruth HS, Huang W, Ishii I, Zhang WY. Macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2002;277:34573–34580. doi: 10.1074/jbc.M205059200. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Hakkinen T, Lucas AD, Liddiard K, Jones E, Quinn CM, Senaratne J, Green FR, Tyson K, Boyle J, Shanahan C, Weissberg PL, Gordon S, Yla-Hertualla S. Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus- and activation-regulated chemokine, are expressed in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:923–929. doi: 10.1161/01.atv.21.6.923. [DOI] [PubMed] [Google Scholar]
- Ma H, Calderon TM, Kessel T, Ashton AW, Berman JW. Mechanisms of hepatocyte growth factor-mediated vascular smooth muscle cell migration. Circ Res. 2003;93:1066–1073. doi: 10.1161/01.RES.0000102867.54523.7F. [DOI] [PubMed] [Google Scholar]
- Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers PA, Zhou D, Powell D, Lichenstein H, Kelley M, Pironkova R. Endotoxin receptors (CD14) are found with CD16 (Fc gamma RIII) in an intracellular compartment of neutrophils that contains alkaline phosphatase. J Immunol. 1995;155:2085–2095. [PubMed] [Google Scholar]
- Simmons DL, Tan S, Tenen DG, Nicholson-Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- Zwadlo G, Brocker EB, von Bassewitz DB, Feige U, Sorg C. A monoclonal antibody to a differentiation antigen present on mature human macrophages and absent from monocytes. J Immunol. 1985;134:1487–1492. [PubMed] [Google Scholar]
- Ma HT, Lin WW, Zhao B, Wu WT, Huang W, Li Y, Jones NL, Kruth HS. Protein kinase C beta and delta isoenzymes mediate cholesterol accumulation in PMA-activated macrophages. Biochem Biophys Res Commun. 2006;349:214–220. doi: 10.1016/j.bbrc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Zwadlo G, Schlegel R, Sorg C. A monoclonal antibody to a subset of human monocytes found only in the peripheral blood and inflammatory tissues. J Immunol. 1986;137:512–518. [PubMed] [Google Scholar]
- Pedron T, Girard R, Chaby R. Variation of LPS-binding capacity, epitope expression, and shedding of membrane-bound CD14 during differentiation of human monocytes. J Immunol. 1995;155:1460–1471. [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Hubacek JA, Rothe G, Pit’ha J, Skodova Z, Stanek V, Poledne R, Schmitz G. C(-260)–>T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. doi: 10.1161/01.cir.99.25.3218. [DOI] [PubMed] [Google Scholar]
- Unkelbach K, Gardemann A, Kostrzewa M, Philipp M, Tillmanns H, Haberbosch W. A new promoter polymorphism in the gene of lipopolysaccharide receptor CD14 is associated with expired myocardial infarction in patients with low atherosclerotic risk profile. Arterioscler Thromb Vasc Biol. 1999;19:932–938. doi: 10.1161/01.atv.19.4.932. [DOI] [PubMed] [Google Scholar]
- Rothe G, Gabriel H, Kovacs E, Klucken J, Stohr J, Kindermann W, Schmitz G. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996;16:1437–1447. doi: 10.1161/01.atv.16.12.1437. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Kruth HS, Skarlatos SI, Gaynor PM, Gamble W. Production of cholesterol-enriched nascent high density lipoproteins by human monocyte-derived macrophages is a mechanism that contributes to macrophage cholesterol efflux. J Biol Chem. 1994;269:24511–24518. [PubMed] [Google Scholar]
- van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- Mukhin DN, Chao FF, Kruth HS. Glycosphingolipid accumulation in the aortic wall is another feature of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1607–1615. doi: 10.1161/01.atv.15.10.1607. [DOI] [PubMed] [Google Scholar]
- Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol. 1999;155:775–786. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folcik VA, Aamir R, Cathcart MK. Cytokine modulation of LDL oxidation by activated human monocytes. Arterioscler Thromb Vasc Biol. 1997;17:1954–1961. doi: 10.1161/01.atv.17.10.1954. [DOI] [PubMed] [Google Scholar]
- Kawachi S, Takeda N, Sasaki A, Kokubo Y, Takami K, Sarui H, Hayashi M, Yamakita N, Yasuda K. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:617–621. doi: 10.1161/01.ATV.0000154486.03017.35. [DOI] [PubMed] [Google Scholar]
- Kaplan RC, Strickler HD, Rohan TE, Muzumdar R, Brown DL. Insulin-like growth factors and coronary heart disease. Cardiol Rev. 2005;13:35–39. doi: 10.1097/01.crd.0000134914.10407.40. [DOI] [PubMed] [Google Scholar]
- Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Juremalm M, Olsson N, Nilsson G. CCL17 and CCL22 attenuate CCL5-induced mast cell migration. Clin Exp Allergy. 2005;35:708–712. doi: 10.1111/j.1365-2222.2005.02203.x. [DOI] [PubMed] [Google Scholar]
- Lindstedt KA, Kovanen PT. Mast cells in vulnerable coronary plaques: potential mechanisms linking mast cell activation to plaque erosion and rupture. Curr Opin Lipidol. 2004;15:567–573. doi: 10.1097/00041433-200410000-00011. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci USA. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li W, Wang N, Zhu Y, Wang X. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am J Physiol. 2007;292:C1493–C1501. doi: 10.1152/ajpcell.00016.2006. [DOI] [PubMed] [Google Scholar]
- Yan D, Mayranpaa MI, Wong J, Perttila J, Lehto M, Jauhiainen M, Kovanen PT, Ehnholm C, Brown AJ, Olkkonen VM. OSBP-related protein 8 (ORP8) suppresses ABCA1 expression and cholesterol efflux from macrophages. J Biol Chem. 2008;283:332–340. doi: 10.1074/jbc.M705313200. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li Y, Buono C, Waldo SW, Jones NL, Mori M, Kruth HS. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF). J Biol Chem. 2006;281:15757–15762. doi: 10.1074/jbc.M510714200. [DOI] [PubMed] [Google Scholar]
- Buono C, Li Y, Waldo SW, Kruth HS. Liver X receptors inhibit human monocyte-derived macrophage foam cell formation by inhibiting fluid-phase pinocytosis of LDL. J Lipid Res. 2007;48:2411–2418. doi: 10.1194/jlr.M700170-JLR200. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Yla-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300. [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Yokode M, Kataoka H, Imabayashi T, Yoshida H, Sano H, Nishikawa S, Nishikawa S, Kita T. Intraperitoneal administration of anti-c-fms monoclonal antibody prevents initial events of atherogenesis but does not reduce the size of advanced lesions in apolipoprotein E-deficient mice. Circulation. 1999;99:1740–1746. doi: 10.1161/01.cir.99.13.1740. [DOI] [PubMed] [Google Scholar]
- Ditiatkovski M, Toh BH, Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Yamamoto K, Kobashi H, Ohmoto M, Tsuji T. Immunohistochemical phenotyping of liver macrophages in normal and diseased human liver. Hepatology. 1994;20:317–325. [PubMed] [Google Scholar]
- Leicester KL, Olynyk JK, Brunt EM, Britton RS, Bacon BR. CD14-positive hepatic monocytes/macrophages increase in hereditary hemochromatosis. Liver Int. 2004;24:446–451. doi: 10.1111/j.1478-3231.2004.0943.x. [DOI] [PubMed] [Google Scholar]