Abstract
Dendritic cells (DCs) have been proposed to play a pivotal role in the initiation and perpetuation of rheumatoid arthritis (RA) by presentation of arthritogenic antigens to T cells. We investigated the in vivo characteristics of two major DC subsets, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), in RA synovial tissue (ST) by measuring their frequency, phenotype, distribution, and cytokine expression. ST was obtained by arthroscopy from 20 RA, 8 psoriatic arthritis, and 10 inflammatory osteoarthritis patients. Levels of CD1c+ mDCs and CD304+ pDCs present in ST were quantified by digital image analysis, and their distribution was assessed by double immunolabeling with antibodies against CD3 and CD8. The maturation status and cytokine profile of mDCs and pDCs were quantified by double-immunofluorescence microscopy. In RA patients, the number of CD304+ pDCs exceeded that of CD1c+ mDCs, with the majority of infiltrating DCs being CD83− or DC-LAMP−. Synovial pDC numbers were especially increased in RA patients who were positive for rheumatoid factor and anti-citrullinated peptide antibody. mDCs and pDCs were localized adjacent to lymphocyte aggregates. In ST from RA patients, both mDCs and pDCs expressed interleukin (IL)-15. IL-18 and interferon (IFN)-α/β were mainly expressed by pDCs whereas IL-12p70 and IL-23p19 expression was predominant in mDCs. These data characterize the phenotypes of mDCs and pDCs in inflammatory synovitis and define for the first time the cytokine expression profile of these DC subsets.
Dendritic cells (DCs) comprise a complex network of heterogeneous antigen-presenting cells, critical not only to the initiation and regulation of adaptive immunity, but also the maintenance of both central and peripheral tolerance. As such, DCs have been implicated in the initiation and perpetuation of chronic autoimmune disease through the abolition of self-tolerance and subsequent emergence of self-reactive lymphocytes. Significantly, it has recently been shown that the aberrant accumulation of DCs in tissue, but not of T cells or B cells, is sufficient in itself to induce symptoms of autoimmunity including the production of antinuclear antibodies.1 There is considerable intra- and intertissue variation in the phenotype, morphology, function, and tissue localization of different DC populations.2 Human blood DCs have recently been divided into five distinct subsets: CD1b/c+, CD16+, BDCA3+, CD123+ [interleukin (IL)-3R α-chain], and CD34+ DCs.3 In particular, the so-called myeloid DCs (mDCs), which are CD1c (BDCA1)+/CD11c+/CD45RO+/CD123lo, have the ability to produce IL-12 in response to bacterial compounds or CD40L, and require GM-CSF for survival.4 Conversely, plasmacytoid DCs (pDCs) are CD303 (BDCA2)+/CD304(BDCA4)+/CD11c−/CD45RA+/CD123high and require the presence of IL-3 for survival.5 On viral or bacterial infection or exposure to immune complexes consisting of anti-double-stranded DNA, pDCs produce high amounts of type I interferons (IFN-α and IFN-β).6,7
In rheumatoid arthritis (RA) DCs, along with T cells, macrophages, B cells, and plasma cells, comprise part of the massive infiltration of leukocytes to the primary target tissue of disease, the synovial tissue (ST).8 Furthermore, DC infiltration to the inflamed synovial compartment occurs early in disease pathology, and DCs are enriched in both the synovial fluid (SF) and ST of affected joints.9,10 It has been suggested that DCs may play a role in the initiation and perpetuation of RA by presentation of arthritogenic antigen(s) to autoreactive T cells.9,11 Moreover, these DCs may activate infiltrating T cells and this might be sufficient to drive organ inflammation and disease. In view of these observations, we propose that DCs in the inflamed synovial compartment are not only crucial for (auto)antigen capture leading to autoimmunity and disease initiation, but also have a crucial role in established inflammation. Thus, DCs represent a promising target of investigation. However, remarkably little is known regarding the distribution, phenotype, maturation status, and functional profile of DCs within the inflamed synovial compartment.
Recently, we reported a significant reduction of circulating peripheral blood DC subsets in RA and psoriatic arthritis (PsA) patients and concomitant accumulation of these subsets in SF of these patients.12 The analysis of specific subsets and the nature of their in situ functional profile in ST have been hindered by complex methodologies and a lack of specific surface markers. However, novel markers useful to human DC studies have been defined that resolve these issues.13 In the present study we have therefore used CD1c and CD304, rather than the less specific CD11c and CD123, to more accurately identify mDC and pDC subsets, respectively. For the first time we describe a quantitative and comparative analysis of the distribution and phenotype of mDCs and pDCs within, and between, RA, PsA, and inflammatory osteoarthritis (OA) ST. Furthermore, we characterize in detail the cytokine profile of DCs in situ and show that mDCs and pDCs within RA ST possess distinct and unique cytokine profiles.
Materials and Methods
Patients and Tissue Samples
Twenty RA patients,14 ten inflammatory OA patients, and eight PsA patients15 were included in this study (Table 1). OA patients fulfilled established criteria16 and had a joint effusion in the absence of rheumatological disease other than OA. All patients gave informed consent, and the study protocol was approved by the Medical Ethics Committee of the Academic Medical Center in Amsterdam. Patients were allowed to use certain disease-modifying anti-rheumatic drugs such as methotrexate, hydroxychloroquine, or sulfasalazine, provided that the dose had been stable for at least 2 months. Nonsteroidal anti-inflammatory drugs were allowed, provided that the dose and frequency had been stable for 30 days.
Table 1.
Clinical Features of RA, PsA, and OA Patients Included in the Study
| Diagnosis | Characteristic | Median (range) |
|---|---|---|
| RA (n = 20) | Median age (years) | 53 (26 to 77) |
| Male:female | 9:11 | |
| Disease duration (months) | 46 (4 to 412) | |
| ESR (mm/hour) | 50.5 (4 to 112) | |
| CRP (mg/L) | 22.5 (<3 to 122) | |
| RF (+/−) | 16 (+), 4 (−) | |
| ACPA (+/−) | 9 (+), 4 (−) (ND = 4) | |
| PsA (n = 8) | Median age (years) | 49 (34 to 71) |
| Male:female | 5:3 | |
| Disease duration (months) | 198 (12 to 456) | |
| ESR (mm/hour) | 44.5 (7 to 50) | |
| CRP (mg/L) | 27.5 (1 to 51) | |
| RF (+/−) | 8 (−) | |
| ACPA (+/−) | 8 (−) | |
| OA (n = 10) | Median age (years) | 69 (54 to 81) |
| Male:female | 1:7 (ND = 2) | |
| Disease duration (months) | 39 (6 to 240) (ND = 2) | |
| ESR (mm/hour) | 18.5 (14 to 63) (ND = 2) | |
| CRP (mg/L) | 7 (2 to 60) (ND = 2) | |
| RF (+/−) | 8 (−) (ND = 2) | |
| ACPA (+/−) | 8 (−) (ND = 2) |
RA, rheumatoid arthritis; PsA, psoriatic arthritis; OA, inflammatory osteoarthritis; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; ACPA, anti-citrullinated peptide antibodies; ND, not determined.
Median (50th percentile) and range is given for each characteristic.
Small-bore arthroscopy was performed under local anesthesia and ST samples were obtained from multiple sites of an actively inflamed joint using 2-mm grasping forceps (Storz, Tuttlingen, Germany) as previously described.17 Synovial biopsy samples were collected and snap-frozen in TissueTek OCT (Miles, Elkhart, IN). Frozen blocks were stored in liquid nitrogen until sectioned for staining. Sections (5 μm) were cut in a cryostat and mounted on Star Frost adhesive glass slides (Knittelgläser, Braunschweig, Germany) that were stored at −80°C until immunohistochemical analysis.
Antibodies
The following primary mouse monoclonal antibodies (mAbs) were used: biotin-conjugated anti-CD1c (BDCA1, IgG2a; Miltenyi Biotec, Bergisch, Gladbach, Germany), biotin-conjugated anti-CD304 (BDCA4, IgG1; Miltenyi Biotec), anti-CD1c (BDCA1, IgG2a; Miltenyi Biotec), anti-CD304 (BDCA4, IgG1; Miltenyi Biotec), fluorescein isothiocyanate (FITC)-conjugated anti-CD1c (BDCA1, IgG2a; Miltenyi Biotec), FITC-conjugated anti-CD303 (BDCA2, IgG1; Miltenyi Biotec), anti-CD11c (IgG1; BD Pharmingen, San Diego, CA), phycoerythrin-conjugated anti-CD123 (IgG1, BD Biosciences, San Jose, CA), anti-CD83 (IgG1, BD Pharmingen), DC-LAMP (CD208, IgG1; Immunotech, Marseille, France), anti-CD3 (IgG1, BD Biosciences), anti-CD8 (IgG1, BD Biosciences), anti-IL-12p70 (IgG1; R&D Systems, Minneapolis, MN), anti-IL-15 (IgG1; Diaclone SAS, Besançon, France), anti-IL-18 (IgG1; MD Biosciences, St. Paul, MN), and anti-IFN-α and IFN-β (both IgG1; PBL Biomedical Laboratories, Florence, Italy). Polyclonal rabbit anti-human IL-23p19 subunit was kindly provided by Dr. J. Pirhonen (Department of Microbiology, National Public Health Institute, Helsinki, Finland). BAFF/BLyS was purchased from Alexis Biochemicals Corporation (mouse IgG1, clone 4D3; Zandhoven, Belgium) and CD1a-FITC by BD Biosciences.
Immunohistochemical Staining
Acetone-fixed cryosections were incubated with mAbs against CD1c (BDCA1) or CD304 (BDCA4) for 1 hour at room temperature after blocking endogenous peroxidase activity with H2O2 and sodium azide (NaN3). As negative controls, the primary antibodies were omitted or irrelevant isotype-matched antibodies were applied. After incubation with goat anti-mouse horseradish peroxidase (HRP)-conjugated (DakoCytomation, Glostrup, Denmark) for 30 minutes at room temperature, sections were incubated for 15 minutes with biotinylated tyramine (Perkin Elmer, Boston, MA), followed by incubation with streptavidin-HRP (strep-HRP, DakoCytomation). Peroxidase activity was revealed using amino-ethylcarbazole substrate kit (SK-4200; Vector Laboratories, Burlingame, CA). With this procedure CD1c (BDCA1)+ mDCs and CD304 (BDCA4)+ pDCs stained red. Sections were counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO), dried, and mounted with glycerol/gelatin.
For double-immunohistochemical staining CD1c (BDCA1) or CD304 (BDCA4) in combination with CD3 or CD8, and CD11c or CD123, respectively, the sections were incubated overnight at 4°C with either biotin-conjugated anti-BDCA1 or with biotin-conjugated anti-BDCA4 as primary mAbs. After incubation with strep-HRP for 30 minutes at room temperature sections were incubated for 15 minutes with biotinylated tyramine, followed by incubation with strep-HRP for 30 minutes. After a 15-minute incubation with 10% normal mouse serum (DakoCytomation), anti-CD3, anti-CD8, anti-CD11c, or anti-CD123 mAbs were applied to the sections and incubated for 1 hour at room temperature followed by incubation with alkaline phosphatase (AP)-conjugated goat anti-mouse (DakoCytomation) for 30 minutes. Peroxidase activity was revealed as stated above and AP activity was detected using the Alkaline Phosphatase Substrate III kit (SK-5300, Vector Laboratories).
Immunofluorescence Staining
For double-immunofluorescence staining, sections were first incubated with FITC-conjugated primary mAbs against anti-CD1c (BDCA1) or CD303 (BDCA2) or CD1a followed by incubation with rabbit anti-FITC (DakoCytomation) and with Alexa-488-conjugated goat anti-rabbit (Molecular Probes Europe, Leiden, The Netherlands). After blocking with normal mouse serum, the sections were incubated with the mouse monoclonal antibodies against CD83, DC-LAMP, IL-12p70, IL-15, IL-18, IFN-α, IFN-β, or BAFF/BLyS, or with the rabbit polyclonal against IL-23p19. After incubation with Alexa-594-conjugated goat anti-mouse or with Alexa-594-conjugated goat anti-rabbit (Molecular Probes Europe), the slides were analyzed using a fluorescence microscope (Leica DMRA, Wetzlar, Germany) coupled to a charge-coupled device camera and Image-Pro Plus software (Media Cybernetics, Dutch Vision Components, Breda, The Netherlands). For co-expression of CD83 or DC-LAMP and mDC or pDC 12 patients with RA, 5 patients with OA, and 7 patients with PsA were analyzed. For the expression of cytokines by mDCs and pDCs five patients with RA were analyzed. The selection of these patients was based on the presence of mDCs and/or pDCs in the synovium. To quantify the data, the numbers of double-positive staining cells (n = 6 RA patients) were counted in a minimum of six microscopic fields and the percentage of double-positive cells was calculated as follows: total number of double-positive cells divided by total number of mDCs (or pDCs) multiplied by 100. In addition, we correlated the number of labeled cells in biopsies to the total cellular infiltration to the synovium, thus accounting for variations in disease severity (percentage of double-positive cells divided by the total nuclei).
Quantification of CD1c (BDCA1)+ mDC and CD304 (BDCA4)+ pDC Numbers by Digital Image Analysis
All sections were coded and randomly analyzed by computer-assisted image analysis. For all markers, 18 high-power fields were analyzed. The images of the high-power fields were analyzed using the Qwin analysis system (Leica, Cambridge, UK), as described previously.18 Of importance, because CD304 (pDC marker) is also expressed by endothelial cells, blood vessels were excluded in the analysis. Briefly, regions identified as endothelium and staining positively for CD304 were excluded by selecting that region. This procedure is identical as the distinction of CD68+ cells present in the intimal lining layer versus the sublining, as previously described.19,20
Statistical Analysis
Data are expressed as mean ± SEM. Differences between three groups were analyzed for statistical significance with the nonparametric Kruskal-Wallis test, and differences between two groups were analyzed for statistical significance with the Mann-Whitney U-test. Correlations between rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA) levels on the one hand and mDC and pDC numbers in RA ST on the other were analyzed by Spearman rank correlation, using the GraphPad InStat software (version 3.00; GraphPad InStat, Inc., San Diego, CA). A P value <0.05 was considered as the level of significance.
Results
Patients
Clinical data for the patients included in this study are presented in Table 1. The median (range) duration of clinical manifestations was 46 (4 to 412) months in RA patients, 198 (12 to 456) months in PsA patients, and 39 (6 to 240) months in inflammatory OA patients. Fourteen RA patients (70%), eight PsA patients (100%), but no inflammatory OA patients (0%) were using disease-modifying anti-rheumatic drugs at the time of synovial biopsy.
Infiltration of Inflamed Synovium by Plasmacytoid and Myeloid DCs
We analyzed the in vivo expression of CD1c+ mDCs and CD304 (BDCA4)+ pDCs in ST by immunohistochemistry. Of importance, because CD304 is also expressed by endothelial cells, these cells were excluded from the analysis as stated in the Material and Methods. In normal healthy controls (our unpublished observations) and in noninflammatory OA patients (see Supplemental Figure S1 at http://ajp.amjpathol.org), CD1c+ and CD304 (BDCA4)+ DCs are (virtually) absent in ST. In contrast, CD1c+ mDCs and CD304 (BDCA4)+ pDCs were dispersed throughout the synovial sublining, but not the intimal lining layer, in all forms of synovitis (RA, PsA, and inflammatory OA) (Figure 1A). The mean number of mDCs in synovium did not differ significantly between the different diagnostic groups (Figure 1B). The results were similar when the values were corrected for the total nuclei/mm2 (data not shown). Interestingly, in both RA (P < 0.001) and PsA (N.S.), but not in OA, the number of synovial pDCs was higher compared to this particular mDC subset (Figure 1C). Although the increase in pDCs compared to this particular mDC subset appears similar for RA and PsA, the difference did not reach statistical significance in the PsA group, presumably because of the lower number of PsA patients included.
Figure 1.
Expression of CD1c (BDCA1)+ mDCs and CD304 (BDCA4)+ pDCs in ST from patients with RA, PsA, and inflammatory OA. A: Representative sections of ST are shown. Insets show high magnification of the CD1c+ mDCs or CD304+ pDCs. Because CD304 is also expressed by endothelial cells, these cells were excluded from the analysis. B: No significant differences were observed between the numbers of both mDCs and pDCs present in RA, PsA, and inflammatory OA synovia. C: In RA synovium the numbers of pDCs are significantly higher compared to mDCs. Results are shown as mean numbers of positive cells/mm2 ± SEM of 20 patients with RA, 8 patients with PsA, and 10 patients with inflammatory OA (B and C). *P < 0.05, **P < 0.01, ***P < 0.001. Original magnifications, ×400.
Numbers of Myeloid and Plasmacytoid DCs Are Increased in the Synovium of Autoantibody-Positive Patients with RA
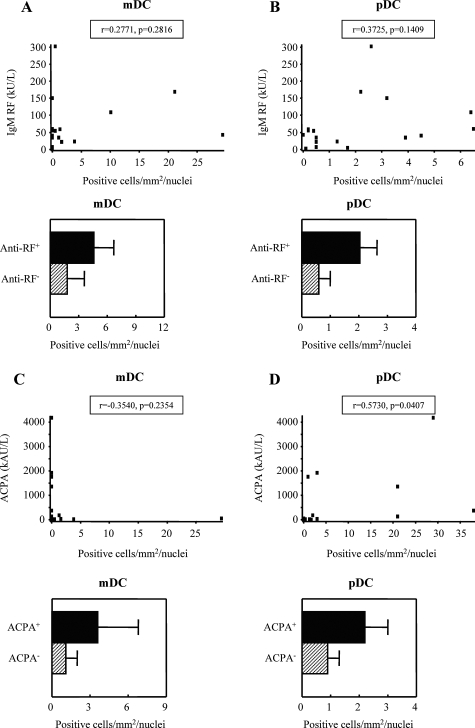
Because DCs are able to regulate B-cell responses and antibody production,21,22 we assessed a possible relationship between ST DC numbers on the one hand and RF and ACPA serum levels on the other. Although no consistent correlation between the numbers of ST mDCs and autoantibody levels was found (Figure 2, A and C), ST pDC numbers showed a positive correlation with ACPA levels in RA (Figure 2, B and D). Consistent with the notion that pDCs might regulate the humoral response in RA, especially patients who were either RF-positive or ACPA-positive showed elevated numbers of pDCs in the synovium (Figure 2, B and D). Although the number of mDCs was slightly elevated in RF+ and ACPA+ patients, this effect was less evident compared to pDCs. However, at this sample size the data did not reach statistical significance. Consistent with the role of DCs in B-cell regulation, analysis of B-cell-activating factor (BAFF/BLyS) expression by synovial DC subsets namely CD1a, CD1c, and CD303/BDCA2, was performed. Interestingly, part of the CD1a, CD1c, and CD303/BDCA2 DC subsets co-express BAFF/BLyS (see Supplemental Figure S2 at http://ajp.amjpathol.org), suggesting a possible role of these subsets in B-cell regulation in RA ST.
Figure 2.
Correlation between mDC and pDC numbers in RA ST to RF (A and B, n = 17) and ACPA serum levels (C and D, n = 13). ST mDC and pDC numbers in RF+/− (positive, n = 16; negative, n = 4) and ACPA+/− (positive, n = 9; negative, n = 4) patients. Data expressed as positive cells/mm2/total nuclei (values are corrected for the global cell infiltration of the synovium, eg, number of nuclei). Spearman rank correlation was used where a P value <0.05 was considered as the level of significance.
Phenotype of Myeloid and Plasmacytoid DCs in Rheumatoid Synovium
To determine the myeloid and plasmacytoid DC phenotype in rheumatoid ST, we examined the co-expression of CD11c and CD123 (IL-3Rα) by CD1c+ mDCs and CD304 (BDCA4)+ pDCs, respectively. We confirmed that all CD1c+ cells were also CD11c+ and therefore mDCs, and that all CD304 (BDCA4)+ cells were also CD123 (IL-3Rα)+ and therefore pDCs (Figure 3A, insets). Moreover, this analysis revealed the presence of CD1c−/CD11c+ cells and CD304 (BDCA4)−/CD123+ cells, not belonging to either DC subset, thus highlighting the importance of using combinations of specific antibodies to accurately identify the respective DC subsets and exclude non-DC leukocytes from analysis.
Figure 3.
Phenotype and maturation status of mDCs and pDCs in RA synovium. A: Double-immunohistochemistry stainings were performed to investigate the co-expression of CD11c (myeloid marker) or CD123 with CD1c (BDCA1) and CD304 (BDCA4), respectively. CD1c (BDCA1)+ mDCs (red) co-express CD11c (blue) and CD304 (BDCA4)+ pDCs (red) co-express CD123 (blue). A representative double immunostaining of RA synovium from one patient is shown. Insets show high magnification of double-positive CD1c+/CD11c+ mDCs and CD304+/CD123+ pDCs. Arrows show CD11c and CD123 single-positive cells. The percentage of both mDCs and pDCs co-expressing the DC maturation marker CD83 (B) or DC-LAMP (C) in RA synovium did not differ significantly compared to PsA and inflammatory OA synovia. Representative double-immunofluorescence stainings of RA synovium from one patient is shown. CD1c (BDCA1)+ and CD303 (BDCA2)+ DCs are shown in green, CD83+ cells and DC-LAMP+ cells in red, and double-positive cells in yellow. Insets show high magnification of double-positive CD1c+/CD83+ or DC-LAMP+ mDCs and CD303+/CD83+ or DC-LAMP+ pDCs. Original magnifications, ×400.
Inflamed Synovium Contains More CD83−/DC-LAMP− than CD83+/DC-LAMP+ Myeloid and Plasmacytoid DCs
We determined the maturation status of mDCs and pDCs in RA, PsA, and inflammatory OA ST using the DC maturation markers CD83 and DC-LAMP. Mature CD83+ (Figure 3B) and DC-LAMP+ (Figure 3C) mDCs and pDCs were identified in all patient groups. The mean percentage of mature CD83+ DCs, as a proportion of all DCs, was low in all patient groups (mDCs, 9.9 ± 3.6; and pDCs, 21.0 ± 3.6; mean ± SEM), indicating that 89.1% of mDCs and 79% of pDCs in ST retain an immature phenotype. Low numbers of mature mDCs were especially observed in RA and PsA synovium (Figure 3B). The data were confirmed by using DC-LAMP as a maturation marker (Figure 3C).
Myeloid and Plasmacytoid DCs Are Localized Near T-Cell Aggregates in Rheumatoid ST
We next sought the precise tissue location of DC subsets, particularly relating to T-cell subsets. We analyzed the distribution of mDCs and pDCs in RA ST in relation to T-cell infiltration. To this end, double immunohistochemistry was performed using antibodies against CD3 and CD8, but not CD4 because both CD1c+ and CD304 (BDCA4)+ DCs also express the CD4 antigen13 Importantly, both mDCs and pDCs were identified in close proximity to clusters of CD3- and CD8-positive cells in RA ST (Figure 4).
Figure 4.
mDCs and pDCs are localized in lymphocyte aggregates in RA ST. Double-immunohistochemistry stainings were performed to investigate the distribution of CD1c (BDCA1)+ mDCs and CD304 (BDCA4)+ pDCs in relation with CD3- and CD8-positive T cells. Both mDCs and pDCs in RA synovium can be identified in close proximity to clusters of CD3- and CD8-positive cells. A representative double immunostaining of RA synovium from one patient is shown. Original magnifications, ×400.
Differential Expression of Cytokines by Myeloid and Plasmacytoid DCs in Rheumatoid ST
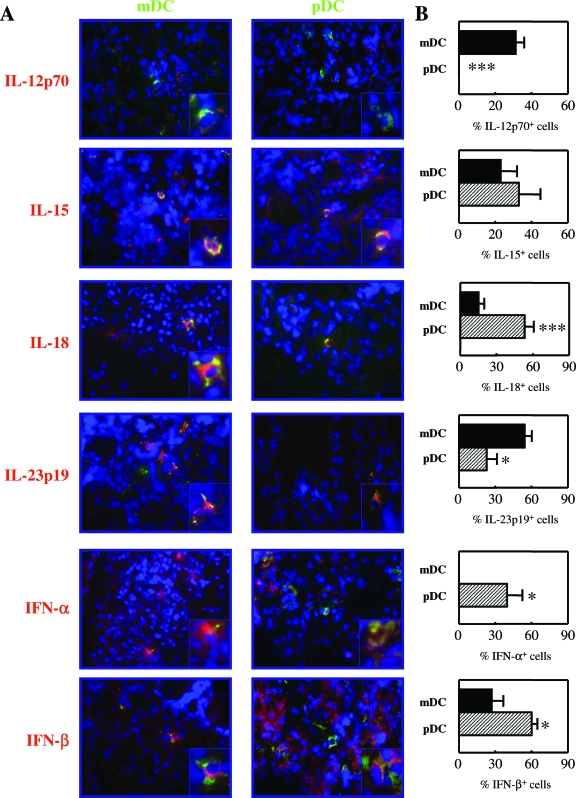
The propensity of DC subsets to direct the immune system toward either tolerance or immunity can primarily be assessed by their specific cytokine profile. Because RA is considered a predominantly Th1/Th17 skewed disorder, we analyzed the expression of IL-12p70, IL-15, IL-18, IL-23-p19, IFN-α, and IFN-β by each DC subset in RA ST. In Figure 5A, representative sections of RA ST staining are shown. Quantification of mDCs and pDCs expressing the aforementioned cytokines is depicted in Figure 5B and described in the Material and Methods [note that we have correlated the number of labeled cells (mDCs or pDCs double-positive for the depicted cytokines) in biopsies to the total cellular infiltration to the synovium, thus accounting for variations in disease severity]. Of particular interest, IL-23p19 expression by mDCs was significantly higher compared to pDCs (P = 0.0145). In addition, and as expected, IL-12p70 was expressed by mDCs only and not by pDCs. The expression of IL-18 was however significantly higher by pDCs as compared to mDCs (P = 0.0020). IL-15 was expressed in relatively equal proportions by both subsets (Figure 5) and also by CD1a+ mDCs (see Supplemental Figure S3 at http://ajp.amjpathol.org). As anticipated, IFN-α was significantly expressed by pDCs only and not by mDCs (P = 0.0320). The expression of IFN-β was also significantly expressed by this subset compared to mDCs (P = 0.0426).
Figure 5.
Expression of cytokines by mDCs and pDCs in RA synovium. Double-immunofluorescence stainings were performed to investigate the expression of IL-12p70, IL-15, IL-18, IL-23p19, IFN-α, or IFN-β by CD1c (BDCA1)+ mDCs and CD303 (BDCA2)+ pDCs. A: A representative double-immunofluorescence staining of RA synovium from one patient is shown. CD1c (BDCA1)+ and CD303 (BDCA2)+ DCs are shown in green, cytokines in red, and double-positive cells in yellow. Insets show high magnification of double-positive CD1c+ mDCs or CD303+ pDCs expressing the indicated cytokines. B: Quantification of cytokine expression by mDCs and pDCs. (n = 6, *P < 0.05, **P < 0.01, ***P < 0.001). Data are expressed as the percentage of double-positive cells (corrected for the global cell infiltration of the synovium, eg, number of nuclei). Original magnifications, 400.
Discussion
Identifying the phenotype, distribution, and activation potential of DC subsets in human autoimmune conditions is an important prerequisite in the development of novel tolerance-inducing therapies. We here provide a detailed investigation of the distribution and phenotype of CD1c+ mDCs and CD303/4+ pDCs within, and between, RA, PsA, and inflammatory OA. Furthermore, the results presented here show that mDCs and pDCs within RA synovium possess distinct and unique cytokine profiles.
We identified infiltration of two particular mDC and pDC subsets in the synovial sublining of inflamed ST. Because mDCs and pDCs are absent from healthy ST (our unpublished observations) it is most likely that mDCs and pDCs are recruited from the circulation to the synovial compartment, which is supported by previous observations showing that mDCs and pDCs are significantly decreased in the peripheral blood of RA patients.12 In addition, peripheral blood monocytes cultured in the presence of factors that mimic the RA microenvironment (synovial fibroblast-conditioned medium and co-cultures) did not account for the expression of CD1c or CD303/4 (M.C.L., unpublished observations).
Of particular interest, we show for the first time the remarkable observation that pDC numbers are specifically and significantly higher in inflamed RA ST compared to this particular mDC subset (CD1c+). In PsA a similar trend was observed, although the difference did not reach statistical significance at this sample size. Because pDCs are the dominant type I IFN producers in the body and maintain this function in the ST, the enrichment of pDCs in ST may be associated with both pro- and anti-inflammatory mechanisms. Consistent with their main function,4 we did indeed observe that RA synovial pDCs are major producers of IFN-α/β. Type I interferons have pleiotropic effects and may enhance isotype switching and humoral autoimmunity as well as potently stimulate the development of human Th1 cells and activation of autoreactive T cells, but IFN-β may also inhibit arthritis activity.23,24,25 Consistent with a proinflammatory effect of pDCs in the synovium, we observed a specific increase in pDCs in RF-positive and in ACPA-positive patients, in line with the observation that these cells may regulate the humoral response.21,22,26 Although the numbers of mDCs appear to be slightly increased in RF-positive and in ACPA-positive patients, this phenomenon is not as clear as for pDCs. Importantly, the numbers of pDCs in RA ST are positively correlated with the levels of serum ACPA. These data, together with the localization of synovial pDCs in the vicinity of CD19-expressing B cells and CD38-expressing plasma cells (M.C.L., unpublished observations) and the expression of BAFF/BLyS by synovial pDCs, suggest that pDCs might regulate the production of autoantibodies (ACPA) in the synovium. On the other hand, a regulatory role for pDCs in inflamed synovia cannot be excluded. Supporting this notion, it has been demonstrated that pDC prime allogeneic CD4+CD25− T cells to differentiate into CD4+CD25+ regulatory T cells (Tregs)27 and that Tregs accumulate around lymphocyte aggregates in ST of patients with juvenile idiopathic arthritis.28 Paradoxically, type I interferons produced by pDCs could also directly inhibit the production of IL-12 by mature DCs and reduce Th1 development.29 Thus, the ultimate, probably pleiotropic, role of pDCs in the inflamed synovium remains to be elucidated.
Although the coexistence of both immature and mature DCs in RA synovium has been reported earlier,30,31,32 there is poor understanding of how DC phenotype differs between inflammatory arthropathies. We found that the majority of the mDCs and pDCs present in different forms of arthritis (especially RA and PsA) do not express the maturation markers CD83 and DC-LAMP. These findings are consistent with the observations that DC maturation is incomplete in the inflamed SF12 and ST.33 The large pool of CD83− and DC-LAMP− DCs present in RA ST might be recruited wholly from the blood.
To our knowledge, cytokine expression by mDCs and pDCs at the site of synovial inflammation has not been shown previously. During the acute and chronic inflammatory response, cytokines convey pro- and anti-inflammatory signals between and within cells and as such are crucial in the pathogenesis of RA. In this respect, RA ST is associated with increased production of an array of cytokines by several cell types.34,35 As expected, IL-12p70 was confined to mDCs. IL-12 production may induce or enhance IFN-γ production by NK cells and effector CD4+ T cells,36 a function enhanced synergistically by IL-18,37 thus magnifying the Th1 phenotype of disease and subsequent inflammatory tissue damage.
RA ST macrophages, another important antigen-presenting cell of myeloid lineage, have previously been shown to produce IL-18.38 In the present study we found that in RA ST pDCs, rather than mDCs, are the dominant IL-18 producers within the DC group. An accumulating body of evidence supports the role of IL-18 in the pathogenesis of RA. RA synovial expression of IL-18 is accompanied by the co-expression of IL-1β and tumor necrosis factor-α and is associated with local inflammation.39 Interestingly, IL-18 has been shown to recruit mDCs40 and pDCs41 to areas of inflammation, in particular under Th1 cytokine conditions as observed in RA. Thus, an intriguing model emerges whereby DCs provide a paracrine expansion mechanism by which additional DCs are recruited to the inflamed synovial compartment. This mechanism might also explain the preferential accumulation of pDCs in RA ST.
In RA ST IL-15, which we have previously demonstrated in association with macrophages, endothelial cells, and fibroblast-like synoviocytes in RA ST,42,43 was expressed by both CD1c+ mDCs, CD1a+ mDCs, and pDCs. As such, IL-15 may be integral to the perpetuation of synovial inflammation, particularly when in association with DC/T-cell clustering, as our results indicate. IL-15 induces proliferation and survival of activated T cells, chemoattraction of T cells, and induces proliferation and immunoglobulin synthesis by human B cells.44 In addition, IL-15 is sufficient to drive monocyte conversion to mDCs45 and may therefore represent a local cytokine-mediated feedback loop whereby DC IL-15 release promotes local CD1a+ mDC differentiation. Significantly, targeting IL-15 has proven therapeutically beneficial in RA.46
Of particular current interest, we observed that in RA ST mDCs are the main producers of IL-23 compared to pDCs. IL-23 promotes the expansion of IL-17-producing cells (Th17 cells),47 that are involved in RA pathology. In this respect, elevated levels of IL-17 have been detected in the SF from patients with RA, but not with OA48 and explants of the rheumatoid ST were found to express and release IL-17.49 The important role of IL-23 in arthritis is supported by the observation that p19-deficient mice do not develop any clinical signs of joint after immunization with collagen type II.50 Thus, in view of the above mentioned observations, IL-23 derived from mDCs and to a lesser extent pDCs may contribute to the expansion of Th17 cells in RA synovium and contribute to RA pathology.51
At inflammatory sites, multiple cellular cross-regulatory interactions may occur. Synovial DCs might be activated by various stimuli, including immune complexes, DNA or RNA molecules, or by apoptotic cell-derived CpG-DNA molecules. This activation results in cytokine production by mainly DCs that have not yet up-regulated CD83 and/or DC-LAMP maturation markers. Moreover, the pDC marker used in our double-immunohistochemistry cytokine stainings, CD303 is only expressed by immature pDCs, being down-modulated after maturation.13,52 Although this concept might be controversial, there are several studies that support this notion. In the colonic mucosa of patients with Crohn’s disease for instance, IL-12 and IL-18 could not be detected in CD83-expressing DCs.53
Based on our investigations presented here and published previously12 we propose a model whereby mDCs and pDCs migrate from the blood to, and potentially traffic between, the SF and ST (Figure 6). In this model, CD83− and/or DC-LAMP− pDCs and mDCs release of proinflammatory cytokines may not only contribute to synovial pathology, but also promote the further recruitment to, and differentiation within the inflamed synovial compartment.
Figure 6.
Schematic representation of the proposed model. mDC and pDC migration from the blood circulation into inflamed RA synovium results in their reduced frequency in blood circulation. Because of as yet unknown factors, pDCs are preferentially accumulated in RA synovium compared to mDCs. Within RA synovium, pDC-derived type I IFNs might reach the SF and account for the differentiation of monocytes (that are shed from the synovial lining layer) into mDCs. This event might explain the preferential accumulation of mDCs in the synovial fluid compared to pDCs.
The results presented here indicate that synovial DCs may play an important role in synovial inflammation, conceivably via stimulation of memory T cells. Moreover, synovial DCs might contribute to the balance toward Th1 responses observed in RA54 via the release of proinflammatory and Th1-inducing cytokines. In this respect, IL-12 together with IL-18 may enhance IFN-γ production by effector T cells. Type I IFNs may play a role in (auto-) antibody production by B cells and IL-23 may pivotally induce expansion of the newly described Th17 cell subset. These results suggest that immunomodulation by interfering with these mechanisms and specifically targeting synovial DCs could provide a novel anti-rheumatic strategy.
Acknowledgments
We thank P. Reinders-Blankert and S. Aarrass for performing immunohistochemical analysis, M. Vinkenoog for helping with computer-assisted image analysis, and Drs. M.A. van Maanen and C.E. Vergunst for providing the patient characteristics.
Footnotes
Address reprint requests to Maria Cristina Lebre, Ph.D., Academic Medical Center/University of Amsterdam, Division of Clinical Immunology and Rheumatology, K0-134, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands. E-mail: c.lebre@amc.uva.nl.
Supported by the European League Against Rheumatism (young investigator award to M.C.L.) and the Dutch Arthritis Association (“Reumafonds”) (to T.J.S.).
M.C.L. and S.L.J. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Liu Y-J, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu Y-J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- Tak PP. Examination of the synovium and synovial fluid. Wollheim FA, Firestein GS, Panayi G, editors. Oxford: Oxford University Press, Inc.,; Rheumatoid ArthritisFrontiers in Pathogenesis and Treatment. 2006:pp 229–241. [Google Scholar]
- Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis: role of CD86 in the synovium. J Immunol. 1996;156:3074–3086. [PubMed] [Google Scholar]
- Thomas R, Lipsky PE. Presentation of self peptides by dendritic cells: possible implications for the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1996;39:183–190. doi: 10.1002/art.1780390202. [DOI] [PubMed] [Google Scholar]
- Summers KL, O’Donnell JL, Williams LA, Hart DN. Expression and function of CD80 and CD86 costimulator molecules on synovial dendritic cells in chronic arthritis. Arthritis Rheum. 1996;39:1287–1291. doi: 10.1002/art.1780390804. [DOI] [PubMed] [Google Scholar]
- Jongbloed SL, Lebre MC, Fraser AR, Gracie JA, Sturrock RD, Tak PP, McInnes IB. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R15. doi: 10.1186/ar1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2. BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Kraan MC, Reece RJ, Smeets TJ, Veale DJ, Emery P, Tak PP. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002;46:2034–2038. doi: 10.1002/art.10556. [DOI] [PubMed] [Google Scholar]
- Haringman JJ, Vinkenoog M, Gerlag DM, Smeets TJ, Zwinderman AH, Tak PP. Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R862–R867. doi: 10.1186/ar1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlag DM, Haringman JJ, Smeets TJ, Zwinderman AH, Kraan MC, Laud PJ, Morgan S, Nash AF, Tak PP. Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum. 2004;50:3783–3791. doi: 10.1002/art.20664. [DOI] [PubMed] [Google Scholar]
- Vos K, Thurlings RM, Wijbrandts CA, van Schaardenburg D, Gerlag DM, Tak PP. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:772–778. doi: 10.1002/art.22400. [DOI] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol Cell Biol. 2005;83:554–562. doi: 10.1111/j.1440-1711.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- Conrad B. Potential mechanisms of interferon-alpha induced autoimmunity. Autoimmunity. 2003;36:519–523. doi: 10.1080/08916930310001602137. [DOI] [PubMed] [Google Scholar]
- Rogge L, D’Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, Adorini L, Sinigaglia F. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567–6574. [PubMed] [Google Scholar]
- van Holten J, Plater-Zyberk C, Tak PP. Interferon-beta for treatment of rheumatoid arthritis? Arthritis Res. 2002;4:346–352. doi: 10.1186/ar598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae BL, Semnani RT, Hayes MP, van Seventer GA. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J Immunol. 1998;160:4298–4304. [PubMed] [Google Scholar]
- Thomas R, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994;152:2613–2623. [PubMed] [Google Scholar]
- Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Thomas R, Lipsky PE. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996;17:559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- Page G, Chevrel G, Miossec P. Anatomic localization of immature and mature dendritic cell subsets in dermatomyositis and polymyositis: interaction with chemokines and Th1 cytokine-producing cells. Arthritis Rheum. 2004;50:199–208. doi: 10.1002/art.11428. [DOI] [PubMed] [Google Scholar]
- Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4:208–217. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- Smeets TJ, Dolhain RJ, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998;186:75–81. doi: 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Barbulescu K, Becker C, Schlaak JF, Schmitt E, Meyer zum Buschenfelde KH, Neurath MF. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J Immunol. 1998;160:3642–3647. [PubMed] [Google Scholar]
- Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Radstake TR, Lubberts E, van den Bersselaar LA, van Riel PL, van Lent PL, Barrera P, van den Berg WB. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Langer K, Mommert S, Wittmann M, Kapp A, Werfel T. Human dendritic cells express the IL-18R and are chemoattracted to IL-18. J Immunol. 2003;171:6363–6371. doi: 10.4049/jimmunol.171.12.6363. [DOI] [PubMed] [Google Scholar]
- Kaser A, Kaser S, Kaneider NC, Enrich B, Wiedermann CJ, Tilg H. Interleukin-18 attracts plasmacytoid dendritic cells (DC2s) and promotes Th1 induction by DC2s through IL-18 receptor expression. Blood. 2004;103:648–655. doi: 10.1182/blood-2002-07-2322. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- Thurkow EW, van der Heiden IM, Breedveld FC, Smeets TJ, Daha MR, Kluin PM, Meinders AE, Tak PP. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997;181:444–450. doi: 10.1002/(SICI)1096-9896(199704)181:4<444::AID-PATH778>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Saikh KU, Khan AS, Kissner T, Ulrich RG. IL-15-induced conversion of monocytes to mature dendritic cells. Clin Exp Immunol. 2001;126:447–455. doi: 10.1046/j.1365-2249.2001.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ, Schuurman J, van de Winkel JG, Parren PW, Gracie JA, Jongbloed S, Liew FY, McInnes IB. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P. IL-17 in rheumatoid arthritis: a new target for treatment or just another cytokine? Joint Bone Spine. 2004;71:87–90. doi: 10.1016/j.jbspin.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune , Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12−IL-18− dendritic cell populations in the colonic mucosa of patients with Crohn’s disease. Eur J Immunol. 2003;33:143–151. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H, Kalden JR. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2001;15:677–691. doi: 10.1053/berh.2001.0187. [DOI] [PubMed] [Google Scholar]