Abstract
We show that a previously uncharacterized Arabidopsis thaliana basic helix-loop-helix (bHLH) phytochrome interacting factor (PIF), designated PIF7, interacts specifically with the far-red light–absorbing Pfr form of phyB through a conserved domain called the active phyB binding motif. Similar to PIF3, upon light exposure, PIF7 rapidly migrates to intranuclear speckles, where it colocalizes with phyB. However, in striking contrast to PIF3, this process is not accompanied by detectable light-induced phosphorylation or degradation of PIF7, suggesting that the consequences of interaction with photoactivated phyB may differ among PIFs. Nevertheless, PIF7 acts similarly to PIF3 in prolonged red light as a weak negative regulator of phyB-mediated seedling deetiolation. Examination of pif3, pif4, and pif7 double mutant combinations shows that their moderate hypersensitivity to extended red light is additive. We provide evidence that the mechanism by which these PIFs operate on the phyB signaling pathway under prolonged red light is through maintaining low phyB protein levels, in an additive or synergistic manner, via a process likely involving the proteasome pathway. These data suggest that the role of these phyB-interacting bHLH factors in modulating seedling deetiolation in prolonged red light may not be as phy-activated signaling intermediates, as proposed previously, but as direct modulators of the abundance of the photoreceptor.
INTRODUCTION
Plants have evolved a series of sensory systems to constantly monitor their changing environment and respond appropriately. Light is their most precious energy and informational resource, and plants utilize photoreceptors to perceive changes in light quality, intensity, direction, and periodicity (Chen et al., 2004; Schäfer and Nagy, 2006; Whitelam and Halliday, 2007). All higher plants contain UV-A/blue light–absorbing cryptochromes (Cashmore et al., 1999), UV-A/blue light–absorbing phototropins (Briggs and Olney, 2001), as well as red light (R)- and far-red light (FR)–absorbing phytochromes (phys) (Smith, 2000; Quail, 2002; Wang and Deng, 2004). Together, these different informational photoreceptors perceive and integrate the environmental light signals to regulate photomorphogenic responses throughout the life cycle of plants.
The phytochromes are soluble dimeric chromoproteins, with each monomer consisting of an ∼125-kD polypeptide with a covalently attached chromophore. Phytochromes exist in two interconvertible conformers: a R-absorbing inactive Pr form and a FR-absorbing biologically active Pfr form (Rockwell et al., 2006). The reversible transformation between the two forms is fundamental for the biological function of the phys, acting as a switch to induce or regulate the extent of phy-mediated responses (Kendrick and Kronenberg, 1994). In Arabidopsis thaliana, the phytochromes are encoded by a small gene family of five members, PHYA to PHYE (Sharrock and Quail, 1989). The phyA protein is light-labile, whereas phyB to phyE are more light-stable (Hirschfeld et al., 1998; Hennig et al., 1999). Studies with mutants deficient in individual or multiple phy species have established that phytochromes mediate physiological responses such as seed germination, seedling deetiolation, shade avoidance, and flowering, with individual functions that can be distinct but also overlapping and partly redundant (Quail, 1998; Schäfer and Nagy, 2006; Whitelam and Halliday, 2007). Among the members of the phy family, phyA and phyB have the most prominent functions: phyA is exclusively responsible for the deetiolation responses to continuous FR (FRc) (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993), and phyB is the predominant phy mediating hypocotyl growth regulation in continuous R (Rc) (Somers et al., 1991; Reed et al., 1993). phyC is a weak Rc sensor with a role in deetiolation under Rc that is complementary to phyB (Franklin et al., 2003; Monte et al., 2003), and phyD and phyE are redundant to phyB in the control of several responses (Aukerman et al., 1997; Devlin et al., 1998).
Phytochromes are synthesized in the cytosol and translocate to the nucleus as the active Pfr form in response to light (Sakamoto and Nagatani, 1996; Kircher et al., 1999; Kircher et al., 2002). Nuclear localization of the phytochromes triggers signaling events that alter the expression of target genes within minutes, initiating a cascade that ultimately leads to the modulation of the biological responses (Quail, 2002; Jiao et al., 2007). Members of the basic helix-loop-helix family (bHLHs) of constitutive nuclear transcription factors play a central role in the molecular mechanisms of phytochrome signal transduction (Duek and Fankhauser, 2005). PIF3 (for phytochrome-interacting factor3) was the first member of the bHLH family identified as a specific interactor of light-activated phyA and phyB (Ni et al., 1999). PIF3 colocalizes in the nucleus with active phy in speckles (Bauer et al., 2004). Speckles contain localized concentrations of specific proteins that are visible by immunofluorescence and have been postulated to be important for phy signaling (Kircher et al., 2002; Al-Sady et. al, 2006). In contrast with the initial report based on antisense PIF3 lines (Ni et al., 1998), studies with pif3 mutants have revealed that PIF3 functions in phy-mediated deetiolation in prolonged Rc as a negative regulator of hypocotyl growth and cotyledon expansion and as a positive regulator of anthocyanin accumulation (Kim et al., 2003; Bauer et al., 2004; Monte et al., 2004). We have shown that PIF3 also acts as a positive regulator of chloroplast development in early deetiolation during the dark-to-light transition in 4-d-old etiolated seedlings, regulating the expression of a subset of light-induced genes encoding chloroplast components (Monte et al., 2004).
A number of PIF3-related bHLHs involved in phy signaling have been reported in recent years. They are known as PIFs (for phy-interacting factor) or PILs (for phy-interacting factor-like) and include PIF1/PIL5, PIF4, PIF5/PIL6, PIL1, and the atypical HFR1 (for long hypocotyl in far red), which does not bind phy directly. Studies with mutants of these PIF/PIL proteins have established that they regulate different aspects of phy-regulated development. HFR1 is a positive regulator of phyA signaling (Fairchild et al., 2000; Fankhauser and Chory, 2000; Soh et al., 2000) and a negative regulator of shade avoidance (Sessa et al., 2005), PIF4 and PIF5/PIL6 are negative regulators of phyB signaling and integrate clock and light signaling to regulate diurnal growth rhythms (Huq and Quail, 2002; Fujimori et al., 2004; Nozue et al., 2007), PIL1 is involved in the regulation of shade avoidance (Salter et al., 2003), and PIF1/PIL5 is a negative regulator of seed germination (Oh et al., 2004) and chlorophyll biosynthesis (Huq et al., 2004). They all belong to the PIF3 subfamily (subfamily 15) of evolutionarily related bHLH proteins (Heim et al., 2003; Toledo-Ortiz et al., 2003). Several of them preferentially interact in vitro with the Pfr form of phyA and/or phyB through two different small N-terminal motifs called the active phyA binding (APA) and active phyB binding (APB) domains, respectively (Khanna et al., 2004; Al-Sady et al., 2006). The interaction between PIF4 and phyB is required for PIF4 activity in vivo (Khanna et al., 2004), and recent findings demonstrate that the interaction of PIF3 with phyA and phyB is necessary for the phosphorylation and degradation of PIF3 in the light (Al-Sady et al., 2006). Light-induced degradation has been demonstrated for PIF3 (Bauer et al., 2004; Monte et al., 2004; Park et al., 2004), PIF5 (Shen et al., 2007), and the phyA- and phyB-interacting PIF1 bHLH factor (Shen et al., 2005). Interestingly, an APB-related domain in the atypical bHLH protein HFR1 has been shown to be a determinant of HFR1 stability in the dark (Duek et al., 2004). These results indicate that regulation of protein stability plays an important role in fine-tuning the function of the PIFs in photomorphogenesis. However, despite all of these recent advances, the mechanism by which these phyB-interacting factors regulate photomorphogenesis is still unknown. Evidence of in vitro interaction between active phy and DNA-bound PIF3 suggested the existence of a direct phy transcriptional mechanism (Martinez-Garcia et al., 2000), but despite considerable effort, we have obtained no evidence to support this model.
As part of our efforts to investigate the role of the PIF3-related members of the Arabidopsis bHLH subfamily in phy signaling, we examined the genetic interactions between several members of the PIF family: PIF3, PIF4, and a previously uncharacterized member, PIF7. Our results show that PIF7 is a very low-abundance protein that interacts selectively with the Pfr form of phyB and, unlike previously described PIFs, is light-stable. PIF7 is rapidly induced to migrate to intranuclear speckles upon light exposure. However, this process is not accompanied by either detectable light-induced phosphorylation or degradation. Despite this unique molecular behavior, the role of PIF7 in seedling deetiolation under prolonged irradiation is similar to that of PIF3 and PIF4, acting as a negative regulator of phyB signaling under prolonged Rc. Double mutant analyses show that the role of these PIF factors under prolonged Rc irradiation is additive or synergistic and that this effect could be explained by a mechanism by which the PIFs regulate the levels of phyB posttranscriptionally. These findings require a conceptual shift in how the PIFs function to modulate the signaling output from phyB in prolonged Rc: rather than the previously proposed role as phy-activated signaling intermediates, these data suggest that instead they function to directly regulate photoreceptor abundance in modulating hypocotyl inhibition under these irradiation conditions.
RESULTS
Identification of PIF7, a bHLH Factor That Binds Specifically to the Biologically Active Pfr Form of phyB through the APB Motif
As part of our systematic effort to investigate members of the Arabidopsis bHLH subfamily 15 for possible involvement in phy signaling, we characterized the previously uncharacterized bHLH072 (Arabidopsis Genome Initiative locus number At5g61270) (Bailey et al., 2003; Toledo-Ortiz et al., 2003). An alignment of the predicted full-length bHLH072 protein with PIF3 (Ni et al., 1998), PIF4 (Huq and Quail, 2002), and its closest homolog, bHLH016 (Bailey et al., 2003; Toledo-Ortiz et al., 2003), is shown in Figure 1A. bHLH072 contains the APB motif important for interaction with phyB Pfr (Khanna et al., 2004) (Figure 1A, top), a nuclear localization signal, the bHLH motif involved in DNA binding and dimerization (Shirakata et al., 1993; Massari and Murre, 2000), and a Q-rich motif absent in PIF3 and PIF4 but predicted to be involved in protein–protein interactions and/or transcriptional activity (Freiman and Tjian, 2002) (Figure 1A, bottom).
Figure 1.
bHLH072 Is a phy-Interacting Protein, Now Designated PIF7.
(A) Alignment of PIF7, PIF3, PIF4, and bHLH016 full-length proteins. Two different aligned regions are shown: amino acids 1 to 26 of PIF7, including the APB domain (top); and amino acids 152 to 270 of PIF7, including the nuclear localization signal (NLS), the bHLH region, and the Q-rich motif present in PIF7 (bottom). Schematic diagrams of the PIF7 protein domains are also shown.
(B) PIF7 binds conformer-specifically to phyB Pfr. The GAL4 transcriptional activation domain (GAD) alone or fusions of GAD to full-length PIF7 (PIF7:GAD) or PIF3 (GAD:PIF3) were used as bait in in vitro coimmunoprecipitation assays. The Pfr and Pr forms of phyB were used as prey. All proteins were synthesized as [35S]Met-labeled products in TnT reactions. Schematic diagrams at top show the design of the experiment, and the SDS-PAGE separations of the pellet fractions and the phyB input (5%) are shown in the center. A quantification of the binding, expressed as percentage of the total phyB input recovered as phyB bound to the bait, is shown at bottom. Data from a representative experiment are shown.
(C) PIF7 binds selectively to phyB. A GAD:full-length PIF7 fusion protein was used as bait in in vitro coimmunoprecipitation assays. The Pfr and Pr forms of phy (A to E) were used as prey. All proteins were synthesized as [35S]Met-labeled products in TnT reactions. SDS-PAGE separations of the pellet fractions and the inputs (5%) are shown.
(D) The APB motif mediates the binding of PIF7 to phyB Pfr. GAD fusions with full-length wild-type or mutated PIF7 were used as bait in in vitro coimmunoprecipitation assays. Mutated PIF7 carries E8A and G14A substitution mutations in the APB motif. Pfr and Pr forms of phyB were used as prey. All proteins were synthesized as [35S]Met-labeled products in TnT reactions. Schematic diagrams at top show the design of the experiment, and the SDS-PAGE separations of the pellet fractions and the phyB input (5%) are shown at bottom.
To determine whether At5g61270 was capable of direct interaction with the photoreceptor molecule, we tested for binding to the five phy family members using an in vitro coimmunoprecipitation assay. At5g61270 does bind specifically to the active conformer Pfr of phyB (Figure 1B) but not to phyA, phyC, phyD, or phyE (Figure 1C). Therefore, we designated At5g61270 PHYTOCHROME-INTERACTING FACTOR7 (PIF7). The binding affinity of PIF7 with phyB in this experiment, however, was ∼85% lower than that of PIF3 (Figure 1B). We also used the light-switchable yeast in vivo expression system (Shimizu-Sato et al., 2002) and showed that PIF7 interacts with active phyB but not with active phyA (see Supplemental Figure 1 online), confirming the in vitro results. These data also verify that PIF7 has an apparently lower affinity for photoactivated phyB than does PIF3, although the magnitude of the difference appears to be smaller than for the pull-down experiments (see Supplemental Figure 1 online). It remains to be determined whether the GAD fusion protein configuration (the order in which the proteins are fused together) influences this difference.
Unlike other members of bHLH subfamily 15, PIF7 contains the APB motif at its extreme N terminus (Figure 1A, top). The APB domain has been shown to be crucial for mediating the interaction with phyB Pfr (Khanna et al., 2004) and is present in most of the bHLH class proteins involved in phy signaling. Although necessary, it is not sufficient, given that at least one APB-containing bHLH (bHLH124, corresponding to PIL1) does not interact with phyB Pfr (Khanna et al., 2004). To determine whether the APB of PIF7 mediates the binding to phyB Pfr, we mutated two residues in the APB motif of PIF7 that were shown to abolish binding to phyB (Khanna et al., 2004). The doubly mutated PIF7 (E8A and G14A) lost the ability to bind to phyB Pfr (Figure 1D), suggesting that the APB domain does mediate the binding of PIF7 to phyB Pfr.
PIF7 Is a Light-Stable Protein That Colocalizes with phyB in Nuclear Speckles
Because of the known APB-dependent light-induced phosphorylation and degradation of PIF3 (Al-Sady et al., 2006), we wanted to test whether PIF7 behaves similarly. We generated polyclonal antibodies that were able to recognize as little as 30 pg of in vitro TnT-produced PIF7 (see Supplemental Figure 2 online). However, despite extensive experiments, this antibody was unable to detect PIF7 in protein extracts prepared from wild-type seedlings grown in the dark and transferred to Rc (see Supplemental Figure 2 online), suggesting that PIF7 is an extremely low-abundance protein. This is supported by RNA gel blot analysis. Although Rc induced an increase in PIF7 transcript levels that was detectable after 2 h of light exposure, the mRNA was barely detectable in dark-grown seedlings (see Supplemental Figure 3 online).
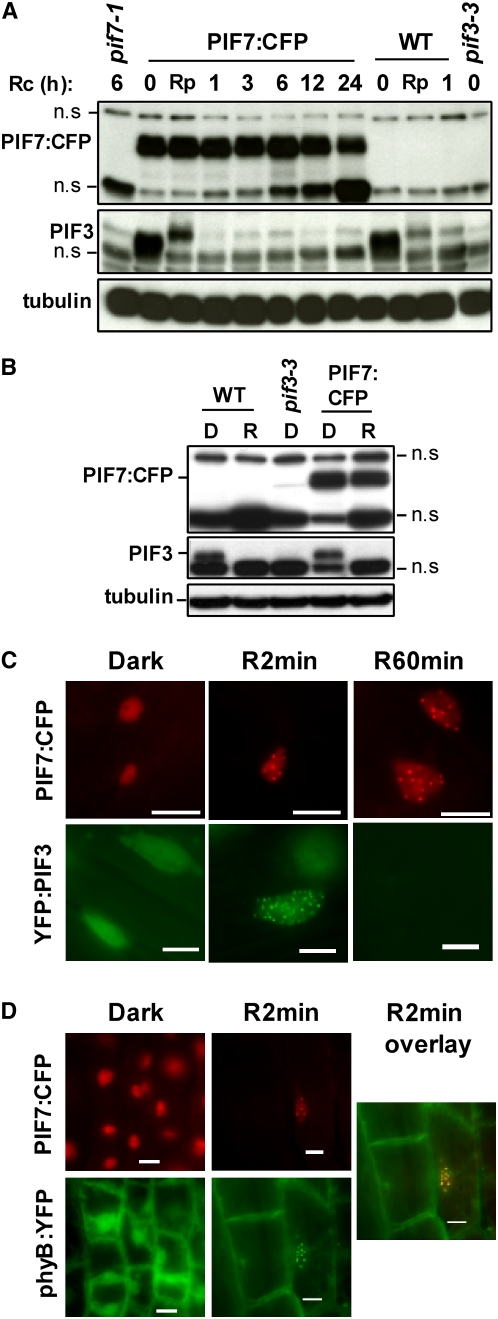
Therefore, we generated transgenic lines overexpressing PIF7 fused to the cyan fluorescent protein (CFP) driven by the cauliflower mosaic virus 35S promoter (35S:PIF7:CFP). The promoter and fusion are equivalent to those described by Bauer et al. (2004) for PIF3 protein stability studies. Despite PIF7:CFP overexpression appearing to cause a somewhat short hypocotyl phenotype in the dark, the seedlings showed reduced sensitivity to the red light compared with the wild type (see Supplemental Figure 4 online). This result indicates that the fusion protein is active in the cell in a manner similar to other previously reported PIFs, including PIF1, PIF3, PIF4, and PIF5 (Huq and Quail, 2002; Kim et al., 2003; Bauer et al., 2004; Fujimori et al., 2004; Oh et al., 2004), and therefore likely reflects the behavior of the endogenous PIF7 protein. In the 35S:PIF7:CFP lines, the anti-PIF7 antibody recognized a protein of the expected size of the fusion protein that was not detectable in wild-type, pif7, and pif3 seedlings (Figure 2A). A time course experiment of etiolated seedlings either after a saturating pulse of red light (Rp) or after transferring to Rc for different times (1, 3, 6, 12, and 24 h) showed that the PIF7 fusion protein did not exhibit the mobility shift indicative of phosphorylation and was stable upon light exposure (Figure 2A).
Figure 2.
PIF7 Is a Light-Stable Nuclear Protein That Colocalizes with phyB in Rc-Induced Early Speckles.
(A) Immunoblot of protein extracts from T2 segregating 35S:PIF7:CFP seedlings. Seedlings were grown in the dark for 2 d (0), transferred to Rc for a 30-s pulse, followed by 10 min of dark (Rp), or transferred to Rc for 1, 3, 6, 12, or 24 h. PIF7-specific polyclonal antibody (top) and a PIF3-specific polyclonal antibody (middle) were used as probes. As controls, protein extracts from the wild type, pif7-1, and pif3-3 are included. Tubulin was used as a loading control (bottom). n.s., nonspecific, cross-reacting bands.
(B) Immunoblot of protein extracts from 35S:PIF7:CFP seedlings. Seedlings were grown for 4 d in the dark (D) or in Rc at 6.2 μmol·m−2·s−1 (R). PIF7-specific polyclonal antibody (top) and a PIF3-specific polyclonal antibody (middle) were used as probes. As controls, protein extracts from the wild type and pif3-3 are included. Tubulin was used as a loading control (bottom).
(C) Epifluorescence imaging of YFP (displayed in green) and CFP (displayed in red) fluorescence in nuclei of Arabidopsis transgenic seedlings expressing PIF7:CFP (top panels) or YFP:PIF3 (bottom panels). Seedlings were grown in the dark for 4 d and then either maintained in darkness (Dark), exposed to Rc for 2 min (R2min) provided by passing the microscope light through a red filter (140 μmol·m−2·s−1), or exposed to Rc for 60 min of 7 μmol·m−2·s−1 (R60min). Images were recorded within 2 min of termination of the Rc treatments. Bars = 10 μm.
(D) Epifluorescence imaging of YFP (displayed in green) and CFP (displayed in red) fluorescence in nuclei of Arabidopsis transgenic seedlings coexpressing PIF7:CFP (top panels) and phyB:YFP (bottom panels) in the phyB-9 background. Seedlings were grown in the dark for 4 d and then either maintained in darkness (Dark) or exposed to Rc for 2 min (R2min) provided by passing the microscope light through a red filter (140 μmol·m−2·s−1). Images were recorded within 2 min of termination of the Rc treatments. The overlay of the PIF7:YFP and the phyB:CFP signals after a Rc irradiation is indicated in yellow in the merged image (right panel). Bars = 10 μm.
While it is certainly possible that PIF7 phosphorylation events not detected by the mobility shift assay may occur, such events would not appear to be involved in the light-induced instability of the protein. On the other hand, in extracts from the same experiment, PIF3 showed the expected light-induced phosphorylation and degradation described previously (Bauer et al., 2004; Monte et al., 2004; Al-Sady et al., 2006), suggesting that the lack of degradation observed for PIF7:CFP is not due to the possible saturation of the proteolytic machinery in response to the overexpression. Because an apparent small decrease in the PIF7:CFP protein level was observed after 24 h of exposure to Rc (Figure 2A), we checked the level under more prolonged Rc irradiation. As shown in Figure 2B, seedlings grown for 4 d in Rc appear to have slightly less PIF7:CFP protein than dark-grown seedlings. This is in contrast with PIF3, in which the basal levels in Rc appear to be much more reduced, below the detection levels (Figure 2B). Together with the light-induced PIF7 mRNA accumulation pattern, these results suggest that PIF7 is a light-stable bHLH factor, compared with PIF3, that accumulates upon Rc light exposure, albeit to very low levels.
Previous data showed that PIF3 migrates to nuclear speckles following light treatment, where it colocalizes with phyA and phyB (Bauer et al., 2004). PIF3 nuclear speckle formation depends on the interaction with phyA and phyB (Al-Sady et al., 2006) and PIF3 is necessary for phyB speckle formation (Bauer et al., 2004); therefore, these speckles were proposed to be where phosphorylation and degradation of PIF3 may occur (Al-Sady et al., 2006). To determine whether PIF7 also forms nuclear speckles, we examined our PIF7:CFP lines for speckle formation upon illumination. Figure 2C (top panels) shows that PIF7:CFP is a constitutively nuclear protein with a diffuse distribution in the dark and that it rapidly (within 2 min) forms speckles when first exposed to a brief, saturating red light treatment (early speckles). This behavior is similar to that observed for PIF3 (Figure 2C) (Bauer et al., 2004). We still observed the PIF7 speckles after 60 min of Rc illumination, in agreement with the protein being light-stable (Figure 2C). This PIF7 nuclear speckle stability was in contrast with PIF3, which, although speckling rapidly after initial exposure to 2 min of Rc, was undetectable after 1 h of Rc due to protein degradation (Figure 2C, bottom panels), consistent with Bauer et al. (2004).
We next examined whether PIF7 and phyB colocalize in the early light-induced speckles using plants carrying PIF7:CFP and phyB:yellow fluorescent protein (YFP) constructs (Figure 2D). A 2-min Rc treatment induced speckle formation of PIF7 (top right panel), as expected, and also of phyB (bottom right panel), consistent with Bauer et al. (2004). The merged image (right panel) shows the colocalization of the PIF7 and phyB speckles. Together, these data suggest that light induces the migration of PIF7 to nuclear speckles, where it presumably interacts with the phyB Pfr form but remains stable, in contrast with what was shown for PIF3 (Al-Sady et al., 2006).
To determine whether PIF7 could function as a transcription factor potentially regulating phy-mediated gene expression, we investigated the DNA binding and the gene transactivation properties of PIF7. Gel-shift assays showed that PIF7 is able to bind the G-box DNA motif (CACGTG) (see Supplemental Figure 5A online), as predicted from molecular phylogenetic analysis (Toledo-Ortiz et al., 2003). The binding was lost when using a mutated version of the G-box CACGGG, indicating that this interaction is sequence-specific (see Supplemental Figure 5A online). We were unable to detect simultaneous interaction of the G-box–bound PIF7 with photoactivated phyB (data not shown), a behavior reported previously for PIF1 (Huq et al., 2004) and PIF4 (Huq and Quail, 2002) that is in contrast with PIF3 (Martinez-Garcia et al., 2000). We next examined whether PIF7 could activate transcription using an in vivo transient assay described previously for PIF1 (Huq et al., 2004). For this purpose, PIF7 and PIF1 were expressed as fusion proteins with the GAL4 DNA binding domain under the control of the 35S promoter (see Supplemental Figure 5B online). Each of these constructs (or a DNA binding domain–only control) was expressed transiently by bombardment in etiolated Arabidopsis seedlings together with a Luciferase reporter gene driven by a minimal 35S promoter with the GAL4 DNA binding site (see Supplemental Figure 5B online). We found that PIF7 was able to induce Luciferase activity 2.8 times more than the GAL4 DNA binding domain alone in etiolated Arabidopsis seedlings, indicating that PIF7 has the capacity to function as a transcriptional activator in vivo (see Supplemental Figure 5B online). We also observed that this response was lost in etiolated seedlings treated with 18 h of Rc (see Supplemental Figure 5B), suggesting that phyB can suppress the transcriptional activity of PIF7. The R-induced suppression of PIF7 activity is similar to that of PIF1 (Huq et al., 2004). In the case of PIF1, that effect might be explained simply by degradation of the PIF1 protein. However, since our data suggest that PIF7 is light-stable, the relevance of this light-dependent suppression of PIF7 transcriptional activity is unclear.
Given the presence of a Gln-rich domain in the C-terminal region of PIF7 (Figure 1A), with a possible role in transcriptional activity (Freiman and Tjian, 2002), we tested the activity of a truncated PIF7 that removed that domain. Supplemental Figure 5C online shows that this construct had higher transcriptional activity in a transient assay done in onion (Allium cepa) epidermal cells, suggesting that the Q-rich domain functions to modulate the transcriptional activity of PIF7 in vivo.
PIF7 Participates in the Control of Seedling Deetiolation under Rc
To investigate the biological function of PIF7, we identified two T-DNA insertional alleles, designated pif7-1 and pif7-2 (Figure 3A). pif7-1 was obtained in a screen of the Salk collection (Alonso et al., 2003; http://signal.salk.edu) and carries a T-DNA insertion in the second exon. pif7-2 was obtained from the public Syngenta collection of Arabidopsis insertional mutants (Sessions et al., 2002) and carries a T-DNA insertion in the fifth exon. The pif7-1 allele produced no detectable PIF7 transcript and is likely null, whereas pif7-2 produced an altered size transcript that, if translated, would produce a truncated protein lacking the C-terminal end of the bHLH domain, known to be necessary for dimerization and DNA binding (Figure 3B).
Figure 3.
T-DNA Insertional pif7 Mutants Are Hypersensitive to Rc.
(A) Mutations identified in the Arabidopsis PIF7 gene (At5g61270). T-DNA insertions in pif7-1 and pif7-2 are indicated at positions 24,657,427 and 24,656,638, respectively, on chromosome V. The location of the bHLH domain is indicated. The coding region is represented by shaded boxes, and the untranslated regions are represented by open boxes (exons), with introns shown as short lines connecting the boxes.
(B) RNA gel blots of 4-d-old Rc-grown (10 μmol·m−2·s−1) wild-type Columbia (Col-0) and pif7 mutant seedlings probed with a PIF7-specific probe. As a loading control, the blot was reprobed with an ACTIN-specific probe.
(C) Visual phenotype of 4-d-old Rc-grown (10 μmol·m−2·s−1) wild-type and pif7-1 and pif7-2 mutant seedlings.
(D) Fluence response curves for hypocotyl length in 4-d-old wild-type and pif7-1 (left) and pif7-2 (right) seedlings grown in Rc. Mean and se values are representative of at least 25 seedlings for each light treatment.
(E) Fluence response curves for hypocotyl length in 4-d-old wild-type (Col-0), phyB-9, pif7-1, and pif7 phyB seedlings grown in Rc. Mean and se values are representative of at least 25 seedlings for each light treatment.
PIF7 mRNA accumulates to low levels in 4-d-old Rc-grown seedlings but is almost undetectable in dark- or FRc-grown seedlings (see Supplemental Figure 6 online). To test whether PIF7 might play a role in deetiolation under prolonged irradiation, we examined the photoresponsiveness of the pif7 mutants in Rc and FRc. Figure 3C shows that pif7 mutant seedlings displayed shorter hypocotyls than do wild-type seedlings when grown under Rc for 4 d. This phenotype was observed for both pif7-1 and pif7-2 mutant alleles over a range of Rc fluence rates (Figure 3D), indicating that pif7 mutants are hypersensitive to all Rc fluence rates tested. However, pif7 mutant cotyledons were equally or only marginally more expanded than the corresponding wild-type cotyledons in Rc (see Supplemental Figure 7 online). The pif7 phyB double mutant was indistinguishable from the phyB single mutant in its lack of responsiveness to a broad range of Rc fluence rates (Figure 3E), indicating that PIF7 is involved in this phyB-mediated response. Supplemental Figure 8 online shows that pif7 mutants were indistinguishable from wild-type seedlings when grown under FRc, indicating that PIF7 does not participate in seedling deetiolation under these conditions.
PIF3, PIF4, and PIF7 Act Additively in the Regulation of Long-Term Seedling Morphological Responses under Rc
The slight hypersensitivity of PIF7-deficient mutants to prolonged Rc is similar to that described previously for mutants deficient in PIF3 (Kim et al., 2003; Monte et al., 2004), PIF4 (Huq and Quail, 2002), and PIF5/PIL6 (Fujimori et al., 2004). This was somewhat surprising given the differences in phyB affinity and/or protein accumulation and degradation kinetics observed between PIF3, PIF4, and PIF7. This prompted us to explore the genetic interactions among these PIF factors. We constructed pif3 pif7, pif4 pif7, and pif3 pif4 double mutants and examined the photoresponsiveness of the single and double mutants to Rc. The degree of hypocotyl hypersensitivity to Rc was different among the monogenic mutants: pif7 showed the mildest phenotype, especially at higher Rc fluences, whereas pif4 had slightly greater sensitivity to Rc over the fluence rate curve (Figure 4; see Supplemental Figure 9A online). The degree of hypocotyl inhibition in the three double mutant combinations was greater than either parental single mutant, indicating that the hypersensitive phenotypes of pif3, pif4, and pif7 to Rc are additive (Figure 4; see Supplemental Figure 9 online). As expected, double mutants deficient in PIF4 and either PIF3 or PIF7 were more hypersensitive than pif3 pif7 (Figure 4; see Supplemental Figure 9A online), since pif4 has the strongest phenotype of the single mutants. A marginal additive effect was observed in the cotyledons at various Rc intensities (see Supplemental Figure 9B online).
Figure 4.
Hypersensitivity of pif3, pif4, and pif7 to Prolonged Rc Is Additive.
(A) Visual phenotypes of 4-d-old Rc-grown (10 μmol·m−2·s−1) wild-type and pif seedlings.
(B) Hypocotyl length in 4-d-old Rc-grown wild-type and pif seedlings. Rc fluence rate was 0.8 μmol·m−2·s−1 for seedlings in all panels except for the middle right panel, in which it was 0.3 μmol·m−2·s−1. Mean and se values are representative of at least 25 seedlings.
PIF3, PIF4, and PIF7 Regulate phyB Levels Posttranscriptionally
The above results indicate that PIF3, PIF4, and PIF7 have similar roles and act negatively and additively on phyB signaling under long-term Rc. Despite their different protein accumulations and degradation dynamics after initial Rc exposure, it is possible that these PIFs regulate hypocotyl length through a common mechanism involving direct association with the Pfr form of phyB. The observation that their action on phyB signaling is additive led us to postulate that these PIFs might act to regulate the amount of phyB photoreceptor available. Small changes in the amount of phyB photoreceptor are known to greatly affect hypocotyl growth, as shown by studies with phyB heterozygous mutants (Koornneef et al., 1980; Wester et al., 1994) and phyB overexpressor lines (Wagner et al., 1996), including transgenic lines having increasing copy numbers of the PHYB gene (Wester et al., 1994). Using both the single pif mutants with moderate hypocotyl hypersensitivity to Rc and the double pif mutants displaying a more severe hypocotyl phenotype, we tested whether the regulation of phyB abundance was a mechanism by which the PIFs regulate hypocotyl inhibition in long-term Rc.
We performed immunoblot analysis using anti-phyB monoclonal antibodies to test whether phyB levels are elevated during long-term Rc in single and double pif mutants compared with phyB levels in wild-type seedlings irradiated similarly (Figure 5). Visual analysis indicates that the levels of phyB in 4-d-old Rc-grown seedlings are somewhat higher in the pif3, pif4, and pif7 single mutants compared with the levels in their respective wild-type Col-0 seedlings (Figure 5A), and double pif mutants have even higher levels (Figure 5A). We quantified the effect of the absence of each PIF on phyB levels in at least three independent experiments by measuring the amount of phyB signal in each of the mutant lines, normalized to the levels of tubulin (Figure 5B; see Supplemental Figure 10A online). Consistent with the qualitative results (Figure 5A; see Supplemental Figure 10A online), the data show that phyB levels are increased by 1.6-fold in pif3, 1.6- to 1.8-fold in pif4, and 1.11-fold in pif7, relative to the wild-type levels, and in the double pif mutants, they are increased by 2.1-fold in pif3 pif7, 2.3-fold in pif4 pif7, and 3.0-fold in pif3 pif4. Although these changes appear to be only marginal to modest, each is statistically significant (see Supplemental Figure 10B online). In particular for PIF7, the analysis of six independent biological replicates (Figure 5B; see Supplemental Figure 10A online) indicates that the differences between the pif7 monogenic mutant and the wild type are the most marginal but statistically significant (see Supplemental Figure 10B online).
Figure 5.
phyB Levels Are Elevated in pif3, pif4, and pif7 Single and Double Mutant Seedlings Grown under Prolonged Rc.
phyA and phyB levels in 4-d-old wild-type Col-0 and pif mutant seedlings. Set 1 and set 2 correspond to two sets of seeds that were harvested at different times.
(A) Representative immunoblots of protein extracts of wild-type and pif seedlings grown in Rc (0.9 μmol·m−2·s−1) and probed with phyB-specific monoclonal antibodies (top) and phyA-specific monoclonal antibodies (bottom). Tubulin was used as a loading control (middle).
(B) phyA and phyB protein levels in protein extracts of 4-d-old wild-type and pif seedlings grown in Rc (0.9 μmol·m−2·s−1). phyA and phyB signal normalized to the tubulin was quantified using NIH Image software in at least three independent biological replicates. To ensure the linearity of phyA, phyB, and tubulin chemiluminescence signals, a protein extract dilution curve was always run and exposed in parallel. Fold increase for each set was calculated considering the corresponding wild-type value as 1. Mean fold increase value were calculated from three (set 1), four (set 2 except for pif7), or six (wild type and pif7) independently grown replicates. Two additional technical replicates quantified in the Odyssey infrared imaging system were included in the analysis of phyB levels of set 1 seeds. Immunoblots corresponding to all of the replicates analyzed are shown in (A) and Supplemental Figure 10A online. Error bars indicate se, based on biological replicates.
(C) Representative immunoblots of protein extracts of 4-d-old wild-type and pif seedlings. Seedlings were grown in the dark, and extracts were probed with phyB-specific monoclonal antibodies (top). Tubulin was used as a loading control (bottom).
(D) Correlation of the level of phyB protein with the hypocotyl length displayed by wild-type and pif seedlings grown in Rc (0.9 μmol·m−2·s−1). Plotted hypocotyl and phyB values are shown as mean fold increase over the wild type. The best-fit curve by eye is shown. Error bars indicate se, based on biological replicates.
A quantitatively more compelling contribution of PIF7 is observed in the absence of PIF3, as shown in the pif3 pif7 double mutant (Figure 5B), in which PIF7 displays a statistically significant synergistic effect with PIF3 (see Supplemental Figure 10B online). A similar apparent additive/synergistic effect is seen between PIF7 and PIF4 in the pif4 pif7 double mutant (Figure 5B), which falls just above the statistically significant P value threshold of 0.050 (P = 0.055). Importantly, the levels of phyA were also measured in the same extracts as an internal control, and no apparent increases were observed in any of the genotypes (Figures 5A and 5B; see Supplemental Figure 10A online). When the pif mutant lines were grown at higher Rc fluence rates, similar effects on phyB levels were observed (see Supplemental Figure 11 online). These data indicate that the PIFs act to downregulate phyB levels in prolonged Rc, with a relatively robust and additive effect in the case of PIF3 and PIF4 and with a marginal and additive/synergistic effect in the case of PIF7 and PIF3 or PIF4 (Figure 5B; see Supplemental Figure 10B online).
These differences do not appear to be determined at the transcriptional level, because we did not detect any comparable increases in PHYB transcript levels in RNA extracts from the same mutant lines (see Supplemental Figure 12 online). The data show that the PHYB mRNA levels in the majority of the mutant lines are, if anything, slightly lower than in the wild type (see Supplemental Figure 12 online), the direct opposite of the increased phyB protein levels observed for these lines (Figure 5; see Supplemental Figure 10 online). Control experiments indicate that our mRNA quantitation procedures can detect statistically significant differences in PHYB transcript levels as little as 1.5-fold (see Supplemental Figure 13 online). Because the phyB protein level increases detected are mostly 1.6-fold or greater (Figure 5B), the data are consistent with the conclusion that the effect is likely posttranscriptional. In contrast with what we observed in Rc, phyB levels in the dark were similar in all genotypes (Figure 5C; see Supplemental Figure 14 online), indicating that the differences are only induced upon photoactivation of the photoreceptor.
In good agreement with the lines showing the mildest and the strongest short hypocotyl phenotype (Figure 4), pif7 showed a marginal increase in phyB levels, whereas the increase was greatest in pif3 pif4 (Figure 5B). When we plotted both parameters in each single and higher order PIF-deficient mutant line (Figure 5D), we observed a good correlation between the level of phyB overaccumulation and the severity of the hypocotyl phenotype. The correlation curve has a first steep phase in which relatively small changes in phyB correlate with strong effects on hypocotyl growth inhibition, followed by a second phase in which the effect of increased phyB levels on the hypocotyl growth tends to saturate (Figure 5D). Whereas the pif double mutants cluster in the second phase, the pif monogenic mutants fall into the first steep phase, further supporting the notion that the marginal effects observed for the pif monogenic mutants, especially for pif7, can have a strong impact on hypocotyl growth. Together, these data suggest that the pif mutant lines tested regulate hypocotyl growth responses in prolonged Rc by regulating phyB photoreceptor abundance at the posttranscriptional level.
We wanted to examine the alternative possibility that pif mutant–induced changes in hypocotyl elongation rates are the cause, rather than the consequence, of the increases in phyB levels. To do this, we performed a time-course experiment measuring hypocotyl elongation and phyB levels, in parallel, in wild-type and pif3 mutant seedlings in response to Rc. Differences in phyB levels precede the differences in hypocotyl growth inhibition observed between the pif3 mutant and the wild type (Figure 6). Whereas statistically significant increases in phyB levels are already detectable at 36 h in pif3 compared with the wild type, statistically significant differences in hypocotyl elongation are not apparent until 48 to 60 h. These data are more consistent with the idea that the elevated phyB levels are the cause of the short hypocotyl phenotype in the pif mutant rather than the consequence of this phenotype.
Figure 6.
Changes of phyB Levels in pif3 Mutant Seedlings Precede the Changes in Hypocotyl Elongation.
(A) Time course of hypocotyl length and phyB level in wild-type Col-0 and pif3-3 mutant seedlings. Seedlings were germinated and grown as described in Methods (Monte et al., 2004). Germination was induced for 3 h in white light followed by 21 h of darkness, and the seedlings were then moved to Rc (7.7 μmol·m−2·s−1) for up to 36, 48, 60, or 96 h after inducing germination before measuring hypocotyl length (top) and phyB levels (bottom). Mean values of hypocotyl length are representative of at least 25 seedlings. phyB normalized to tubulin (phyB/tub) was quantified as in Figure 5B, and mean values from three biological replicates are shown (bottom). Bars represent se. P values from a t test analysis for differences between wild-type and pif3-3 mutant seedlings are shown.
(B) Immunoblots corresponding to all of the replicates (rep.) analyzed in (A), bottom panel, are shown.
To begin to explore the mechanism by which phyB levels are regulated, we examined the effect of the proteasome inhibitor MG132 on phyB protein abundance. The data show that the slow Rc-induced decline in phyB levels appears to involve the 26S proteasome pathway (see Supplemental Figure 15 online). Together with the apparent absence of an effect of the pif mutations on PHYB mRNA levels, this result suggests that the PIF proteins may modulate phyB levels by regulating the rate of turnover of the photoreceptor molecule via the ubiquitin-proteasome pathway.
DISCUSSION
Despite considerable progress in recent years, the role of the PIF proteins in light signaling is still incomplete (Duek and Fankhauser, 2005). Different PIFs modulate different facets of phy-mediated photomorphogenesis, often acting as apparent negative regulators at the phenotypic level, at least under prolonged irradiation. However, the mode of action is still unknown. Studies with PIF1 (Shen et al., 2005), PIF3 (Bauer et al., 2004; Monte et al., 2004; Park et al., 2004), and PIF5 (Shen et al., 2007) show that these PIFs are downregulated by phytochrome-induced degradation of the protein during initial deetiolation. This occurs as a result of rapid Pfr-dependent light-induced phosphorylation and probable ubiquitination in the case of PIF3 (Al-Sady et al., 2006) and PIF5 (Shen et al., 2007). Studies with PIF4 indicate that interaction with phyB is necessary for its activity in regulating hypocotyl inhibition (Khanna et al., 2004). In addition, some PIFs, like PIF1/PIL5 (Huq et al., 2004; Oh et al., 2004), PIF3 (Kim et al., 2003; Monte et al., 2004), and PIF4 and PIF5 (Fujimori et al., 2004; Khanna et al., 2007; Nozue et al., 2007), are implicated in more than one facet of photomorphogenesis and, in the case of PIF3, with seemingly contradictory roles. PIF3 acts as a positive regulator of anthocyanin and chlorophyll accumulation but as a negative regulator of phy-imposed inhibition of hypocotyl elongation (Kim et al., 2003; Monte et al., 2004; Shin et al., 2007). In this study, by analyzing the molecular properties of PIF7 and its genetic interactions with PIF3 and PIF4 in phyB-mediated inhibition of hypocotyl length, we show that PIF7 has features different from those of other PIFs described to date and provide evidence of a possible novel mode of action of the PIFs on phenotypic responses in prolonged Rc signaling.
Several of our findings indicate that PIF7 is unique among the PIF3-related proteins. First, while PIF7 specifically binds to the Pfr form of phyB through the APB motif, as do other PIFs, the binding affinity is lower (Figures 1B to 1D). Second, studies with PIF7:CFP-overexpressing plants show that PIF7 exhibits no evidence of phy-induced phosphorylation and is a light-stable protein (Figure 2A; see Supplemental Figure 2 online), in contrast with the light-induced phosphorylation and degradation of PIF3 (Bauer et al., 2004; Monte et al., 2004; Park et al., 2004), PIF1 (Shen et al., 2005), and PIF5 (Shen et al., 2007). Third, although PIF7 is a constitutively nuclear protein that colocalizes with phyB in early nuclear speckles within 2 min of exposure to R (Figure 2C), speckle formation did not lead to protein degradation (Figure 2C). For PIF7, direct interaction with phyB in nuclear speckles might initiate light signaling through a mechanism that does not involve phosphorylation and degradation. Fourth, our data indicate that the Q-rich domain in PIF7 might act to repress transcription (see Supplemental Figure 5C online).
Despite these distinct molecular properties, loss-of-function mutations in PIF7 resulted in Rc-specific hypersensitivity under prolonged illumination (Figures 3 and 4). In this respect, PIF7 is similar to PIF3 (Kim et al., 2003; Monte et al., 2004), PIF4 (Huq and Quail, 2002), and PIF5/PIL6 (Fujimori et al., 2004). PIF3, PIF4, and PIF5 have been proposed previously to have roles as negative-acting phyB-regulated signaling intermediates based on (1) the Rc-specific enhanced inhibition of hypocotyl growth displayed by the mutants under prolonged irradiation (Huq and Quail, 2002; Kim et al., 2003; Fujimori et al., 2004; Monte et al., 2004), (2) their preferential interaction with the Pfr form of phyB (Ni et al., 1999; Huq and Quail, 2002; Khanna et al., 2004), and (3) the requirement of this interaction for PIF4 activity (Khanna et al., 2004). However, in this study, by analyzing single and double mutants deficient in PIF3, PIF4, and PIF7, we provide evidence that challenges the hypothesis that these proteins act as signaling intermediates under these prolonged irradiation conditions.
Genetic analyses of pif3 pif4, pif3 pif7, and pif4 pif7 double mutants show that the Rc-hypersensitive phenotypes of the pif3, pif4, and pif7 single mutants are additive (Figure 4; see Supplemental Figure 9 online). This indicates that the PIFs act collectively and additively in the modulation of phyB signaling in prolonged Rc, with antagonistic effects on the phyB-mediated inhibition of hypocotyl elongation. These results support the notion that the mechanism by which different PIFs operate in prolonged Rc to regulate hypocotyl length might be similar, each PIF having a qualitatively similar but quantitatively different contribution to the output from phyB (Figure 4; see Supplemental Figure 9 online).
Analysis of phyB levels in the pif single and double mutants provided evidence that the mechanism by which these PIFs act in Rc might be through the direct regulation of the amount of photoreceptor (Figure 5A). Consistent with the observed hypocotyl responses, quantification of the increased phyB levels (Figure 5B) indicates that the PIFs act additively or synergistically to suppress phyB levels in prolonged Rc. The strong correlation observed between the progressively increasing phyB levels in the pif single and double mutant lines and the severity of the hypocotyl response (Figure 5D) further reinforces the idea that the PIFs act by regulating phyB protein abundance in Rc. The correlation curve appears to be double-phased (Figure 5D), with an initial steepness indicating high hypocotyl growth sensitivity to relatively small changes in phyB levels, followed by a second phase of lower sensitivity to changes in phyB levels, where the effect tends to saturate. The initial phase of the curve is represented by the pif7 monogenic mutant, which has a significant difference in hypocotyl photosensitivity to prolonged Rc in spite of showing only marginal differences in phyB levels (Figures 5B and 5D).
This observation is in agreement with previous reports using a series of transgenic lines engineered to contain increasing copy numbers of the PHYB gene, driven by its native promoter, in which a 1.2-fold increase in phyB protein levels already produced a 0.8-mm (10%) reduction in hypocotyl elongation (Wester et al., 1994). At the other extreme, pif3 pif4 double mutants show a threefold increase in phyB levels with respect to the wild type (Figure 5B) and already show an almost saturated hypocotyl response (Figure 5D). Similar double-phased correlation curves have been described for phyB-overexpressing lines showing progressively increasing levels of phyB (Wagner et al., 1996), in which only a threefold to fourfold increase of phyB was enough to saturate the hypocotyl response. Although other possible mechanistic actions of PIFs in negatively regulating phyB signaling cannot be ruled out, the recurrent reports of the high sensitivity of hypocotyl growth to small changes in phyB levels (Koornneef et al., 1980; Wester et al., 1994; Wagner et al., 1996) suggests that increases in phyB levels alone could account for the hypersensitivity of the single and double pif mutants to Rc (Figure 5D).
The alternative possibility, that autonomous, pif mutant–induced reductions in hypocotyl elongation rate may be the cause rather than the consequence of the observed increases in phyB levels, does not appear to be supported by the data. First, where examined (pif1, pif3, pif4, pif5, and pif7), no difference in hypocotyl length is apparent between wild-type and pif mutant seedlings grown in darkness (Figure 3D) (Huq and Quail, 2002; Kim et al., 2003; Fujimori et al., 2004; Monte et al., 2004; Oh et al., 2004; Ito et al., 2007; Khanna et al., 2007). This indicates that these PIFs have no role in regulating intrinsic hypocotyl elongation rates in the absence of light signals. Similarly, where examined (pif1, pif3, pif4, pif5, and pif7), no significant difference in hypocotyl photoresponsiveness is observed between the phyB single mutant and the five corresponding phyB pif double mutant combinations across all fluence rates of Rc tested. All such mutants completely lack any detectable Rc-imposed hypocotyl inhibition (Figure 3E) (Huq and Quail, 2002; Oh et al., 2004; Ito et al., 2007; Khanna et al., 2007; Al-Sady et al., 2008). These data establish both that these PIFs have no detectable autonomous role in regulating hypocotyl elongation in the light and that they are not capable of regulating hypocotyl elongation in response to Rc light signals perceived by other phy family members (phyA, -C, -D, or -E) in the absence of phyB.
Second, time-course experiments indicate that, where tested (pif3), the increase in phyB level in the pif3 mutant compared with wild-type seedlings observed in prolonged Rc clearly precedes the decrease in pif3 hypocotyl elongation rate compared with the wild type (Figure 6). This result is consistent with the increase in phyB levels in pif3 being the cause rather than the consequence of the enhanced repression of hypocotyl elongation in the mutant in response to Rc. Third, as alluded to above, genetic and molecular genetic experiments with phyB mutants and phyB overexpressors show that changes in phyB levels generate phenotypes opposite to those observed in the pif mutants and overexpressors, respectively. This indicates that such changes in phyB levels, generated by an independent means, are sufficient to cause the observed hypocotyl Rc responsivity changes, in contrast with PIF abundance changes, which, as detailed above, are not autonomously sufficient to cause such responsivity changes (Figures 3D and 3E) (Koornneef et al., 1980; Wester et al., 1994; Wagner et al., 1996; Huq and Quail, 2002; Oh et al., 2004; Ito et al., 2007; Khanna et al., 2007; Al-Sady et al., 2008).
Given (1) the existing evidence that PIF3 acts early in regulating the rapid (within 1 h), phy-induced changes in gene expression triggered by the initial exposure of dark-grown seedlings to light (Monte et al., 2004) and (2) the evidence presented here that the PIFs examined apparently act on phyB protein levels to modulate long-term hypocotyl growth inhibition under prolonged irradiation, two questions arise. First, does this long-term response also require ongoing, PIF-dependent mediation of the phy-regulated gene expression, which is presumably necessary to implement the observed light-induced changes in hypocotyl cell expansion rates? Conversely, is there evidence that the PIF proteins modulate phyB levels rapidly enough following the initial irradiation of previously dark-grown seedlings to influence early-response gene expression? As outlined below, the answer to both questions appears to be no.
First, it follows from the absence of hypocotyl length differences between the wild type and pif mutants in the dark referred to above (Figure 3D) (Huq and Quail, 2002; Kim et al., 2003; Fujimori et al., 2004; Monte et al., 2004; Oh et al., 2004; Ito et al., 2007; Khanna et al., 2007) that any potential changes in gene expression that might have been caused by the mutation have no detectable autonomous role in regulating hypocotyl cell expansion rates. Second, it follows similarly from the absence of differences between the phyB and phyB pif mutants in hypocotyl photoresponsiveness to Rc mentioned above (Figure 3E) (Huq and Quail, 2002; Oh et al., 2004; Ito et al., 2007; Khanna et al., 2007; Al-Sady et al., 2008) that any potential gene expression changes possibly caused by the pif mutations also have no detectable autonomous role in regulating hypocotyl cell expansion rates in the light. Third, recent work in a separate study shows that mutated PIF3, which is blocked in DNA binding, loses its capacity to regulate rapid-response (1 h) target gene expression but fully retains its capacity to regulate phyB levels and hypocotyl growth in response to prolonged Rc (Al-Sady et al., 2008). Conversely, mutated PIF3, blocked in phyB binding, loses its capacity to regulate phyB levels and hypocotyl growth but fully retains its capacity to regulate early target gene expression. These results indicate that PIF3 lacking intrinsic transcriptional regulatory activity is still capable of regulating hypocotyl elongation rates in the absence of its capacity to regulate gene expression. It may be concluded, therefore, that the phyB-regulated changes in gene expression presumed to drive Rc-imposed hypocotyl inhibition in prolonged irradiation occur via pathways not involving PIF3-controlled transcription. Finally, by contrast, dark-grown pif3 mutant seedlings exhibit no detectable difference in phyB levels compared with the wild type over the first several hours of Rc exposure (Monte et al., 2004), indicating that the observed differences in early-response gene expression are due to the absence of PIF3 and not to altered photoreceptor abundance.
Our data thus suggest a previously undescribed mode of action by which the PIF class of bHLH transcription factors may modulate seedling responsivity to prolonged Rc irradiation during deetiolation. The data indicate that PIF3, PIF4, and PIF7 act directly on the photoreceptor to regulate the photosensitivity of the system by modulating the amount of phyB available. Similar data have also been obtained for PIF5 (Khanna et al., 2007), indicating that the function described here and elsewhere (Al-Sady et al., 2008) for PIF3, PIF4, and PIF7 can be extended to other members of the phyB-interacting bHLH group of proteins. The emerging picture, therefore, suggests that these PIFs, rather than acting as phyB signaling intermediates under long-term irradiation, may act as homeostatic regulators of phy output by controlling photoreceptor abundance. The presence of a battery of PIFs displaying different sensitivity to Rc and/or affinity to photoactivated phyB potentially provides a very sensitive system to tightly regulate phyB protein levels. This fine-tuning of phyB output is proposed to eventually determine the optimal growth response of the seedling to a particular light environment.
Because the PIFs do not appear to regulate either phyB protein levels in the dark (see Supplemental Figure 14 online) or PHYB transcript levels in prolonged Rc (see Supplemental Figure 12 online), PIF function may require direct interaction between the photoactivated photoreceptor and the PIF proteins through the APB motif. In retrospect, this notion agrees with previous findings for PIF4, in which an APB-mutated variant of PIF4 failed to rescue the pif4 hypersensitive phenotype (Khanna et al., 2004). The specific molecular mechanism triggered by the presumptive interaction between the PIFs and activated phyB Pfr in prolonged Rc is currently unknown. However, the consequence of this interaction appears to be increased degradation of the photoreceptor protein at least partially via the proteasome system (see Supplemental Figure 15 online). A simple model would be that these PIF–phyB interactions target phyB for degradation. This would be the opposite of what has been shown for PIF3 and PIF5 upon the initial exposure of dark-grown seedlings to light (as opposed to prolonged Rc), in which the interaction with phyB (as well as with phyA) regulates the levels of PIF3 and PIF5 by inducing rapid phosphorylation and degradation (Al-Sady et al., 2006; Shen et al., 2007). This implies a homeostatic feedback loop in which each of the two interacting partners negatively regulates the other.
Collectively, then, our data suggest a novel dichotomy in the mechanism by which PIF3, and possibly other PIFs, act both early (at the onset of the initial illumination of dark-grown seedlings, when PIF3 acts as positive regulator of Rc-induced transcription and greening) and late (under prolonged Rc, when PIF3 acts as a negative regulator of hypocotyl elongation) during seedling development. On the one hand, PIF3 appears to exert conventional transcriptional regulatory control when participating in early (within 1 h of initial Rc exposure) phy-induced changes in gene expression (Monte et al., 2004; Tepperman et al., 2006) without inducing changes in phyB levels. By contrast, PIF3 and the other PIFs examined display an unexpected capacity to posttranscriptionally modulate phyB photoreceptor abundance later, under prolonged irradiation, thereby altering the sensitivity of the phy system to incoming light signals. Thus, whereas PIF3 (and possibly other PIFs) appear to function initially as signaling intermediates in mediating transcriptional responses to early light signals, they apparently function subsequently, at steady state, as more global homeostatic modulators of photosensory sensitivity. We are currently investigating whether other PIFs, like PIF4 and PIF7, are also involved in the regulation of different facets of early phy-mediated seedling deetiolation. In contrast with their common late mechanism of action by the modulation of phyB levels described here, it is possible that the early function of each PIF is specialized and will reflect its distinct molecular properties.
METHODS
Cloning of Full-Length PIF7 cDNA
PIF7 full-length cDNA was obtained by PCR amplification from a cDNA library prepared from total RNA extracted from 4-d-old Rc-grown Arabidopsis thaliana Col-0 seedlings. Primers used were EMO218 (5′-GGAGAAGAATATGAGCTCGATGTCGAATTATGGAGTTAAAGAACTCACATGG-3′ (which introduces a silent G-to-A mutation to avoid a SacI site) and EMO219 (5′-CCTCTTCTTATACTCGAGCTACTAATCTCTTTTCTCATGATTCGAAGAAC-3′). The cDNA was cloned in the TA vector (Invitrogen) and confirmed by sequencing. The sequence of the open reading frame agrees with that predicted from the Arabidopsis Col-0 sequenced DNA (www.Arabidopsis.org).
RNA Analysis
Total RNA extraction, loading, and hybridization conditions were as described (Monte et al., 2003). The PIF7-specific probe was amplified from the PIF7 cDNA by PCR using primers EMO96 (5′-CCGGGTGTGAGACTTTGGA-3′) and EMO111 (5′-CCCGTCGTCCATTAGATCTACCTGCTTCTC-3′) and corresponds to the region between the APB motif and the bHLH region (nucleotides Ala-119 to Glu-493, downstream of the predicted ATG). The probe to detect 18S RNA was described by Cantón and Quail (1999). The probe to detect actin corresponds to ACTIN2.
Seedling and Plant Growth and Measurements
Seeds were sterilized and induced for germination as described (Monte et al., 2003), then placed in darkness at 21°C. For prolonged Rc and FRc treatments, seedlings were moved after 21 h in darkness to Rc or FRc for 3 d at 21°C. Dark control seedlings were kept in darkness. Measurements of light, fluence rates, hypocotyl length, and cotyledon area were as described (Monte et al., 2003).
Isolation of PIF7 Mutant Alleles
pif7-1 was obtained from the Ecker/Alonso collection of Arabidopsis T-DNA insertional mutants (http://signal.salk.edu; Alonso et al., 2003) and screened by PCR for disruption of the PIF7 gene. pif7-2 was obtained from the Syngenta collection of sequenced T-DNA insertional mutants (line 622) (Sessions et al., 2002).
Seeds were grown and DNA was extracted from leaves (Edwards et al., 1991) to identify genotype and to map the precise T-DNA insertion site as described (Monte et al., 2003). For pif7-1, primers EMO88 (5′-CATCCTCTGGTTTATCCTATCACGCCG-3′) and EMO89 (5′-CCGTTCATGGTCTAGGCG-3′) were used to detect the PIF7 wild-type copy, and primers EMO88 and EMO48 (5′-TGATAGTGACCTTAGGCGACTTTTGAACGC-3′) were used to detect the presence of the T-DNA in the PIF7 gene. PCR conditions were 94°C for 2 min and 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. The wild-type band was ∼0.65 kb, and the T-DNA band was ∼0.85 kb. For pif7-2, primers EMO103 (5′-GGAGAGCCATAGAGTTGG-3′) and EMO108 (5′-CGACATCTGAAACTGTTGC-3′) were used to detect the wild-type PIF7 gene, and primers EMO103 and EMO138 (5′-TAGCATCTGAATTTCATAACCAATCTCGATACAC-3′) were used to detect the presence of the T-DNA insertion in the PIF7 gene. PCR conditions were as for pif7-1. The wild-type band was ∼0.7 kb, and the T-DNA band was ∼0.6 kb. Homozygous pif7-1 and pif7-2 mutant plants were outcrossed once to their wild-type Col-0, and the resulting F2 segregating population was genotyped to select individual homozygous and wild-type sibling lines for phenotypic analyses.
Construction of Double pif Mutants and Transgenic PIF7 Overexpressor Lines
pif7 phyB double mutant lines were obtained by crossing pif7-1 to phyB-9 (Reed et al., 1993). pif3 pif7 double mutant lines were obtained by crossing pif7-1 to pif3-3 (Monte et al., 2004). pif3 pif4 and pif4 pif7 double mutant lines were obtained by crossing pif4-2 to pif3-3 (Monte et al., 2004) or pif7-1 (pif4 is a mutant allele in the Col-0 background that was obtained from the Syngenta collection [line 1288] and has a T-DNA insertion in the sixth intron [E. Huq and P.H. Quail, unpublished data]). Selection of the double mutants in the F2 segregants was performed by PCR analysis, following PCR conditions detailed above for PIF7 and as described elsewhere for PIF3 (Monte et al., 2004). For pif4-2, primers EMO206 (5′-ACCTCCTCAAGTCATGGTTAAGCCTAAGCC-3′) and EMO207 (5′-TCCAAACGAGAACCGTCGGT-3′) were used to detect the wild-type PIF4 gene and primers EMO138 (5′-TAGCATCTGAATTTCATAACCAATCTCGATACAC-3′) and EMO207 were used to detect the presence of the T-DNA insertion. PCR conditions were as for pif7-1, with an extension time of 3 min to detect the PIF4 wild-type band and of 1 min to detect the T-DNA. The wild-type band was ∼1.4 kb, and the T-DNA band was ∼0.3 kb.
For the construction of the PIF7 overexpressor lines, the PIF7 open reading frame was cloned into the EcoRI and BamHI sites in pEZS-NL (D. Ehrhardt, Carnegie Institution of Washington, Stanford, CA) with the eGFP replaced with the eCFP (Clontech). Using the NotI site, the 35S:PIF7:CFP construct was then moved into the binary vector pART27 (Gleave, 1992) and used to transform Arabidopsis Col-0 plants (Clough and Bent, 1998).
For the generation of transgenic plants overexpressing both phyB:YFP and PIF7:CFP, the above-mentioned 35S:PIF7:CFP construct was used to transform phyB-9 mutant plants carrying a 35S:phyB:YFP expression cassette (Bauer et al., 2004).
Protein Extraction, Immunoblots, and Quantification
A nonconserved DNA fragment encoding amino acids 53 to 162 of PIF7 was amplified and cloned into the pDEST 17 vector (Invitrogen) in-frame with DNA encoding a 6xHis tag. The fusion protein was produced in BL21AI Escherichia coli cells (Invitrogen) and purified using the nickel-nitrilotriacetic acid agarose purification system (Invitrogen). Polyclonal antisera were produced in rabbits (Covance), and the crude serum was purified using an affinity column containing a glutathione S-transferase–PIF7 (amino acids 53 to 162) fusion protein as described (Al-Sady et al., 2006).
Protein extracts of Arabidopsis seedlings were prepared as described (Al-Sady et al., 2006) for immunodetection of PIF3 and PIF7. For phyA and phyB immunoblots, the following modifications were made to the extraction buffer: 2% SDS and 50 mM metabisulfite instead of 5% SDS and 40 mM β-mercaptoethanol. Total protein was quantified using a Protein DC kit (Bio-Rad), and β-mercaptoethanol was added just before loading. Aliquots from each sample containing equal amounts of protein were subjected to PAGE as described (Al-Sady et al., 2006). As specified, some seedlings were treated with 30 μM MG132 (Calbiochem) for 2 h in darkness followed by 12 h in Rc.
Immunodetections of PIF3 and PIF7 were performed using the purified anti-PIF3 (Al-Sady et al., 2006) and anti-PIF7 antibodies. Mouse monoclonal anti-phyB (B1 and B7) and anti-phyA (073d) antibodies (Somers et al., 1991) were used to immunodetect phyB and phyA, respectively. A mouse monoclonal antibody against α-tubulin (Sigma-Aldrich) was used as a control for loading. Anti-rabbit horseradish peroxidase and anti-mouse horseradish peroxidase were used as secondary antibodies (Promega), and ECL or ECL-plus chemiluminescence kits (Amersham) were used for detection.
For the quantification of phyA, phyB, and tubulin levels, a protein extract dilution curve was run in parallel with the wild-type and mutant extracts and exposed at the same time to check the linearity of the ECL signal. Multiple exposures were recorded on x-ray films, and only those giving a good linear fit for the standard curve were quantified using NIH Image software. Relative amounts of phyA and phyB normalized to tubulin were calculated and represented as fold increase over the wild-type levels. Mean fold increase of phyA and phyB levels over the wild-type was calculated from at least three biological replicates. As an alternative, where indicated, phyB was quantified with the Odyssey infrared imaging system (LI-COR). An anti-mouse IRDye 800CW was then used as a secondary antibody.
In Vitro Coimmunoprecipitation Assay
In vitro coimmunoprecipitation experiments were essentially as described (Khanna et al., 2004). Briefly, each protein was expressed from T7 promoters using the TnT in vitro transcription/translation system (Promega). The PIF7:GAD construct was the PIF7 full-length open reading frame fused to the N terminus of the GAL4 activation domain, cloned into the pET17b vector (Invitrogen). The other constructs used were described previously: GAD:PIF3 (Ni et al., 1998), phyB (Ni et al., 1999), phyA (Fairchild et al., 2000), and phyC-E (Khanna et al., 2004). Bait and prey were prepared as specified (Khanna et al., 2004). Light treatments were done for 4 min of R or for 4 min of R + 4 min of FR. Binding and final washes were as described (Khanna et al., 2004). Separation of the pellets and the inputs was performed on either 8% (Figures 1C and 1D) or 10% (Figure 1B) SDS-PAGE gels. Signals were quantified with a Storm 860 PhosphorImager (Molecular Dynamics).
Yeast Two-Hybrid Assay
GBD:PIF7 (GAL4 DNA binding domain fused to full-length PIF7 in the pGBKT7 vector from Clontech) and GAD:PIF3 (full-length PIF3 fused to the GAL4 activation domain in the pGAD424 vector) (Ni et al.,1998) were used. Yeast transformation and liquid β-galactosidase assays were according to the Yeast Protocol Handbook from Clontech.
Electromobility Shift Assay
Electromobility shift assays were according to Martinez-Garcia et al. (2000), but with pLUC control plasmid added to the binding reactions, as described by Toledo-Ortiz et al. (2003). All proteins were expressed from T7 promoters using the TnT in vitro transcription/translation system (Promega). Naked PIF3 construct was described by Fairchild et al. (2000), GAD:PIF3 was described by Ni et al. (1999), and naked PIF7 was as described above.
Epifluorescence Microscopy
Epifluorescence microscopy analysis was according to Bauer et al. (2004) using an Axiovert 200 microscope (Zeiss). Excitation and detection of YFP and CFP fluorophores were performed with YFP and CFP filter sets (Zeiss). Images were recorded with a digital Axiocam camera (Zeiss) and processed for optimal presentation using Adobe Photoshop (Adobe Systems).
Accession Numbers
Sequence data can be found in the Arabidopsis Genome Initiative database under accession numbers At1g09530 (PIF3), At2g43010 (PIF4), At5g61270 (PIF7), and At4g00050 (bHLH016).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. PIF7 Binds to the Pfr Form of phyB but Not phyA in the Yeast Light-Switch Two-Hybrid Assay.
Supplemental Figure 2. Endogenous PIF7 Protein Is Not Detectable by Immunoblot in Young Seedlings.
Supplemental Figure 3. PIF7 Transcript Is Induced by Light and Accumulates to Very Low Levels.
Supplemental Figure 4. PIF7:CFP Overexpression Causes Reduced Sensitivity to Rc.
Supplemental Figure 5. PIF7 Is a Transcriptional Activator That Binds to the G-Box DNA Motif.
Supplemental Figure 6. PIF7 Transcript Levels Are at or below the Limits of Detection in Dark-Grown and FRc-Grown Seedlings but Increase in Rc-Grown Seedlings.
Supplemental Figure 7. Rc-Induced Cotyledon Expansion Is Marginally Enhanced at High Fluence Rate in the pif7 Mutant under Prolonged (4 d) Irradiation Conditions.
Supplemental Figure 8. pif7 Mutants Are Unaffected in Responsiveness to Prolonged FRc.
Supplemental Figure 9. Rc-Induced Hypocotyl Inhibition and Cotyledon Expansion in pif3 pif7, pif3 pif4, and pif4 pif7 Double Mutants under Prolonged (4 d) Irradiation Conditions.
Supplemental Figure 10. Supportive Material for the Quantification of phyA and phyB Levels in pif3, pif4, and pif7 Single and Double Mutant Seedlings Shown in Figure 5B.
Supplemental Figure 11. phyB Levels Are Elevated in pif3, pif4, and pif7 Single and Double Mutant Seedlings Grown under a Higher Rc Fluence.
Supplemental Figure 12. PHYB RNA Levels Are Not Affected in pif3, pif4, and pif7 Single Mutant or in pif3 pif4, pif3 pif7, and pif4 pif7 Double Mutant Seedlings Grown under Prolonged Rc.
Supplemental Figure 13. PHYB RNA Dilution Curve.
Supplemental Figure 14. phyB Levels Are Not Affected in pif3, pif4, and pif7 Single and Double Mutant Seedlings Grown in the Dark.
Supplemental Figure 15. Downregulation of phyB Levels in Rc Is Partially Blocked by the Proteasome Inhibitor MG-132.
Supplementary Material
Acknowledgments
We thank Enamul Huq for providing us with the pif4-2 allele and Eberhard Schäfer for the phyB:YFP lines. We thank Giovanni Mele for his assistance in obtaining the PIF7 cDNA and Tiffany Liu for technical assistance in obtaining the data for Supplemental Figure 4 online. We are grateful to Jim Tepperman for his help with the preparation of the figures and to Sheila McCormick for her valuable comments on the manuscript. This work was supported by a postdoctoral fellowship from the Spanish Ministry of Education and Science to P.L. and by National Institutes of Health Grant GM-47475, Department of Energy Grant DEFG03-87ER13742, and USDA Agricultural Research Service Current Research Information System Grant 5335-21000-017-00D to P.H.Q.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Peter H. Quail (quail@nature.berkeley.edu).
Online version contains Web-only data.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Al-Sady, B., Kikis, E.A., Monte, E., and Quail, P.H. (2008). Mechanistic duality of transcription factor function in phytochrome signaling. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- Al-Sady, B., Ni, W., Kircher, S., Schafer, E., and Quail, P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation as a prelude to proteasome-mediated degradation. Mol. Cell 23 439–446. [DOI] [PubMed] [Google Scholar]
- Aukerman, M.J., Hirschfeld, M., Wester, L., Weaver, M., Clack, T., Amasino, R.M., and Sharrock, R.A. (1997). A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, P.C., Martin, C., Toledo-Ortiz, G., Quail, P.H., Huq, E., Heim, M.A., Jakoby, M., Werber, M., and Weisshaar, B. (2003). Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, D., Viczian, A., Kircher, S., Nobis, T., Nitschke, R., Kunkel, T., Panigrahi, K.C.S., Adam, E., Fejes, E., Schafer, E., and Nagy, F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., and Olney, M.A. (2001). Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 125 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantón, F.R., and Quail, P.H. (1999). Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 121 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D.M. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284 760–765. [DOI] [PubMed] [Google Scholar]
- Chen, M., Chory, J., and Fankhauser, C. (2004). Light signal transduction in higher plants. Annu. Rev. Genet. 38 87–117. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., Patel, S.R., and Whitelam, G.C. (1998). Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek, P.D., Elmer, M.V., van Oosten, V.R., and Fankhauser, C. (2004). The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr. Biol. 14 2296–2301. [DOI] [PubMed] [Google Scholar]
- Duek, P.D., and Fankhauser, C. (2005). bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10 51–54. [DOI] [PubMed] [Google Scholar]
- Edwards, K., Johnstone, C., and Thompson, C. (1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild, C.D., Schumaker, M.A., and Quail, P.H. (2000). HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14 2377–2391. [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C., and Chory, J. (2000). RSF1, an Arabidopsis locus implicated in phytochrome A signaling. Plant Physiol. 124 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K.A., Davis, S.J., Stoddart, W.M., Vierstra, R.D., and Whitelam, G.C. (2003). Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. Plant Cell 15 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman, R.N., and Tjian, R. (2002). Perspectives. Neurodegeneration—A glutamine-rich trail leads to transcription factors. Science 296 2149–2150. [DOI] [PubMed] [Google Scholar]
- Fujimori, T., Yamashino, T., Kato, T., and Mizuno, T. (2004). Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 45 1078–1086. [DOI] [PubMed] [Google Scholar]
- Gleave, A.P. (1992). A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20 1203–1207. [DOI] [PubMed] [Google Scholar]
- Heim, M.A., Jakoby, M., Werber, M., Martin, C., Weisshaar, B., and Bailey, P.C. (2003). The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 20 735–747. [DOI] [PubMed] [Google Scholar]
- Hennig, L., Buche, C., Eichenberg, K., and Schafer, E. (1999). Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings. Plant Physiol. 121 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld, M., Tepperman, J.M., Clack, T., Quail, P.H., and Sharrock, R.A. (1998). Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics 149 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq, E., Al-Sady, B., Hudson, M., Kim, C., Apel, K., and Quail, P.H. (2004). PHYTOCHROME-INTERACTING FACTOR 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305 1937–1941. [DOI] [PubMed] [Google Scholar]
- Huq, E., and Quail, P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, S., Nakamichi, N., Nakamura, Y., Niwa, Y., Kato, T., Murakami, M., Kita, M., Mizoguchi, T., Niinuma, K., Yamashino, T., and Mizuno, T. (2007). Genetic linkages between circadian clock-associated components and phytochrome-dependent red light signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 48 971–983. [DOI] [PubMed] [Google Scholar]
- Jiao, Y., Lau, O.S., and Deng, X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8 217–230. [DOI] [PubMed] [Google Scholar]
- Khanna, R., Huq, E., Kikis, E.A., Al-Sady, B., Lanzatella, C., and Quail, P.H. (2004). A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, R., Shen, Y., Marion, C.M., Tsuchisaka, A., Theologis, A., Schäfer, E., and Quail, P.H. (December 7, 2007). The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell http://dx.doi.org/10.1105/tpc.107.051508. [DOI] [PMC free article] [PubMed]
- Kendrick, R.E., and Kronenberg, G.H.M. (1994). Photomorphogenesis in Plants. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Kim, J.Y., Yi, H.K., Choi, G., Shin, B., Song, P.S., and Choi, G.S. (2003). Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Gil, P., Kozma-Bognar, L., Fejes, E., Speth, V., Husselstein-Muller, T., Bauer, D., Adam, E., Schafer, E., and Nagy, F. (2002). Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bognar, L., Kim, L., Adam, E., Harter, K., Schafer, E., and Nagy, F. (1999). Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Rolff, E., and Spruit, C.J.P. (1980). Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.). Z. Pflanzenphysiol. 100 147–160. [Google Scholar]
- Martinez-Garcia, J.F., Huq, E., and Quail, P.H. (2000). Direct targeting of light signals to a promoter element-bound transcription factor. Science 288 859–863. [DOI] [PubMed] [Google Scholar]
- Massari, M.E., and Murre, C. (2000). Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte, E., Alonso, J.M., Ecker, J.R., Zhang, Y., Li, X., Young, J., Austin-Phillips, S., and Quail, P.H. (2003). Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways. Plant Cell 15 1962–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte, E., Tepperman, J.M., Al-Sady, B., Kaczorowski, K.A., Alonso, J.M., Ecker, J.R., Li, X., Zhang, Y.L., and Quail, P.H. (2004). The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 101 16091–16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani, A., Reed, J.W., and Chory, J. (1993). Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 102 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 657–667. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400 781–784. [DOI] [PubMed] [Google Scholar]
- Nozue, K., Covington, M.F., Duek, P.D., Lorrain, S., Fankhauser, C., Harmer, S.L., and Maloof, J.N. (2007). Rhythmic growth explained by coincidence between internal and external cues. Nature 448 358–361. [DOI] [PubMed] [Google Scholar]
- Oh, E., Kim, J., Park, E., Kim, J.I., Kang, C., and Choi, G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16 3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E., Kim, J., Lee, Y., Shin, J., Oh, E., Chung, W.I., Liu, J.R., and Choi, G. (2004). Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 45 968–975. [DOI] [PubMed] [Google Scholar]
- Parks, B.M., and Quail, P.H. (1993). hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell 5 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H. (1998). The phytochrome family: Dissection of functional roles and signalling pathways among family members. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353 1399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H. (2002). Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 3 85–93. [DOI] [PubMed] [Google Scholar]
- Reed, J.W., Nagpal, P., Poole, D.S., Furuya, M., and Chory, J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell, N.C., Su, Y.S., and Lagarias, J.C. (2006). Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57 837–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, K., and Nagatani, A. (1996). Nuclear localization activity of phytochrome B. Plant J. 10 859–868. [DOI] [PubMed] [Google Scholar]
- Salter, M.G., Franklin, K.A., and Whitelam, G.C. (2003). Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426 680–683. [DOI] [PubMed] [Google Scholar]
- Schäfer, E., and Nagy, F. (2006). Photomorphogenesis in Plants and Bacteria. (Dordrecht, The Netherlands: Springer).
- Sessa, G., Carabelli, M., Sassi, M., Ciolfi, A., Possenti, M., Mittempergher, F., Becker, J., Morelli, G., and Ruberti, I. (2005). A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 19 2811–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions, A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock, R.A., and Quail, P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3 1745–1757. [DOI] [PubMed] [Google Scholar]
- Shen, H., Moon, J., and Huq, E. (2005). PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J. 44 1023–1035. [DOI] [PubMed] [Google Scholar]
- Shen, Y., Khanna, R., Carle, C.M., and Quail, P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato, S., Huq, E., Tepperman, J.M., and Quail, P.H. (2002). A light-switchable gene promoter system. Nat. Biotechnol. 20 1041–1044. [DOI] [PubMed] [Google Scholar]
- Shin, J., Park, E., and Choi, G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49 981–994. [DOI] [PubMed] [Google Scholar]
- Shirakata, M., Friedman, F.K., Wei, Q., and Patterson, B. (1993). Dimerization specificity of myogenic helix-loop-helix DNA binding factors directed by nonconserved hydrophilic residues. Genes Dev. 7 2456–2470. [DOI] [PubMed] [Google Scholar]
- Smith, H. (2000). Phytochromes and light signal perception by plants—An emerging synthesis. Nature 407 585–591. [DOI] [PubMed] [Google Scholar]
- Soh, M.S., Kim, Y.M., Han, S.J., and Song, P.S. (2000). REP1,a basic helix-loop-helix protein, is required for a branch pathway of phytochrome A signaling in Arabidopsis. Plant Cell 12 2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers, D.E., Sharrock, R.A., Tepperman, J.M., and Quail, P.H. (1991). The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3 1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman, J.M., Hwang, Y.S., and Quail, P.H. (2006). phyA dominates in transduction of red-light signals to rapidly-responding genes at the initiation of Arabidopsis seedling deetiolation. Plant J. 48 728–742. [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz, G., Huq, E., and Quail, P.H. (2003). The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, D., Koloszvari, M., and Quail, P.H. (1996). Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell 8 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., and Deng, X.W. (2004). Phytochrome signaling mechanism. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), doi/10.1199/tab.0074.1, http://www.aspb.org/publications/arabidopsis/.
- Wester, L., Somers, D.E., Clack, T., and Sharrock, R.A. (1994). Transgenic complementation of the hy3 phytochrome-B mutation and response to PHYB gene copy number in Arabidopsis. Plant J. 5 261–272. [DOI] [PubMed] [Google Scholar]
- Whitelam, G.C., and Halliday, K.J. (2007). Light and Plant Development. (Oxford, UK: Blackwell Publishing).
- Whitelam, G.C., Johnson, E., Peng, J., Carol, P., Anderson, M.L., Cowl, J.S., and Harberd, N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.