Abstract
The sesquiterpene (E)-β-caryophyllene is emitted by maize (Zea mays) leaves in response to attack by lepidopteran larvae like Spodoptera littoralis and released from roots after damage by larvae of the coleopteran Diabrotica virgifera virgifera. We identified a maize terpene synthase, Terpene Synthase 23 (TPS23), that produces (E)-β-caryophyllene from farnesyl diphosphate. The expression of TPS23 is controlled at the transcript level and induced independently by D. v. virgifera damage in roots and S. littoralis damage in leaves. We demonstrate that (E)-β-caryophyllene can attract natural enemies of both herbivores: entomopathogenic nematodes below ground and parasitic wasps, after an initial learning experience, above ground. The biochemical properties of TPS23 are similar to those of (E)-β-caryophyllene synthases from dicotyledons but are the result of repeated evolution. The sequence of TPS23 is maintained by positive selection in maize and its closest wild relatives, teosinte (Zea sp) species. The gene encoding TPS23 is active in teosinte species and European maize lines, but decreased transcription in most North American lines resulted in the loss of (E)-β-caryophyllene production. We argue that the (E)-β-caryophyllene defense signal was lost during breeding of the North American lines and that its restoration might help to increase the resistance of these lines against agronomically important pests.
INTRODUCTION
In natural ecosystems, plants are usually part of a complex web of interactions with other organisms that may influence their growth and survival. To be successful in such an environment, plants have to respond correctly to a multitude of different herbivores, pathogens, competitors, and mutualists. Much plant biology research today is devoted to unraveling the molecular and biochemical processes that provide plants with flexible and appropriate responses to these various enemies and friends. Plant responses to herbivory often include the formation of secondary metabolites, especially phenolic and terpene compounds that act as toxins and feeding deterrents when ingested by the herbivore (Karban and Baldwin, 1999). Another defense tactic involves the recruitment of natural enemies of herbivores with induced volatiles. This so-called indirect defense has already been identified in >10 plant species (Dicke, 1999; Dicke and van Loon, 2000; Meiners and Hilker, 2000; Kessler and Baldwin, 2002).
A well-studied example of indirect defense is found in maize (Zea mays), in which foliar damage by lepidopteran larvae results in the release of a complex volatile mixture containing indole, lipoxygenase pathway products, and a variety of monoterpene and sesquiterpene olefins. These volatiles attract parasitic wasps like Cotesia marginiventris to the site of damage, where they oviposit in the lepidopteran larvae (Turlings et al., 1990). Parasitized lepidopteran larvae feed less than unparasitized larvae and die upon emergence of the adult wasp, which can result in a considerable reduction in damage to the plant (Hoballah et al., 2002, 2004). The identification of the precise volatile compound(s) that attracts the wasp to the plant for oviposition is a complex and difficult task (Turlings et al., 1991; D'Alessandro and Turlings, 2005). We previously isolated the gene responsible for the biosynthesis of (E)-β-farnesene and (E)-α-bergamotene (Schnee et al., 2006), the predominant sesquiterpenes released upon caterpillar attack by maize (Köllner et al., 2004a). Overexpression of this gene in Arabidopsis thaliana demonstrated that the parasitic wasp C. marginiventris can use these sesquiterpenes for host finding after an initial learning experience (Schnee et al., 2006).
An additional sesquiterpene often emitted after herbivore damage is (E)-β-caryophyllene. This volatile compound has been found in response to herbivore damage in several wild relatives of maize (Gouinguené et al., 2001) and in cultivated maize lines from European breeding programs, but it is absent from maize lines originating from North American breeding programs (Degen et al., 2004). Below ground, (E)-β-caryophyllene has been found to serve as an important signal in the attraction of enemies to another maize herbivore, the root-feeding pest western maize rootworm (Diabrotica virgifera virgifera) (Rasmann et al., 2005). In contrast with the complex volatile blend emitted by caterpillar-damaged leaves, maize roots only release (E)-β-caryophyllene upon damage by D. v. virgifera, which attracts entomopathogenic nematodes (Rasmann et al., 2005).
In the biosynthesis of terpenes, the large class of terpene synthase enzymes converts linear prenyl diphosphate precursors into the large diversity of terpene skeletons encountered in plants. For example, the sesquiterpene synthases convert the C15 farnesyl diphosphate to sesquiterpene olefin products. A characteristic feature of terpene synthases is the formation of multiple terpenes from a single substrate (Gershenzon and Kreis, 1999). In the course of our work on maize terpene biosynthesis, we have characterized the multiproduct sesquiterpene synthases TPS1, TPS4, TPS5, and TPS10, which contribute to the overall terpene composition of the aboveground plant parts (Schnee et al., 2002, 2006; Köllner et al., 2004b). However, no gene has been described yet for the biosynthesis of (E)-β-caryophyllene in maize.
The bicyclic sesquiterpene (E)-β-caryophyllene is of particular interest in maize interactions with other organisms since it is released both above and below ground (Turlings et al., 1998; Rasmann et al., 2005). A gene encoding its biosynthesis would provide a useful tool to study its regulation and function. Here, we describe the properties of maize Terpene Synthase 23 (TPS23), which catalyzes the cyclization of farnesyl diphosphate to (E)-β-caryophyllene and the complex regulation of the tps23 gene in leaves and roots in response to damage by different herbivores. We also show that the TPS23 product, (E)-β-caryophyllene, can function as a signal both above and below ground, thereby contributing to the plant's defense against herbivores with completely different sites and modes of attack. Finally, we demonstrate that the ability to produce (E)-β-caryophyllene is under positive selection pressure among the wild relatives of maize but was lost during the breeding of most North American maize varieties, not because of direct mutation of the tps23 gene itself but due to alteration of the regulatory network that results in its transcription.
RESULTS
Caryophyllene Can Attract Two Types of Herbivore Enemies, Entomopathogenic Nematodes and Parasitic Wasps
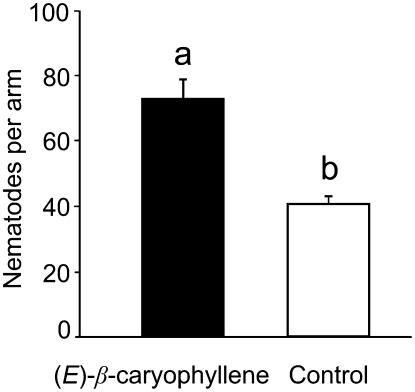
In our attempt to identify the maize volatiles that are responsible for interactions with other organisms, we previously identified (E)-β-farnesene and (E)-α-bergamotene as major constituents of a blend used by parasitic wasps to find their lepidopteran hosts (Schnee et al., 2006). Many maize lines, especially those originating from European breeding programs, also emit the sesquiterpene (E)-β-caryophyllene after damage by lepidopterans (Degen et al., 2004). The hybrid line Delprim, which is of European origin, also emits high concentrations of this compound from leaves and roots after herbivore induction (Figure 1). In roots, (E)-β-caryophyllene is the sole compound released in significant amounts after damage by the herbivore D. v. virgifera (Rasmann et al., 2005). To test whether this amount of (E)-β-caryophyllene attracts the entomopathogenic nematode Heterorhabditis megidis Poinar, we applied (E)-β-caryophyllene from a volatile source in one arm of the six-arm olfactometer (Figure 2). Approximately twice as many nematodes were recovered on average from the arm of the olfactometer spiked with biologically relevant amounts of authentic caryophyllene compared with the average for the five remaining arms that were not spiked (F1,34 = 13.13; P < 0.0001).
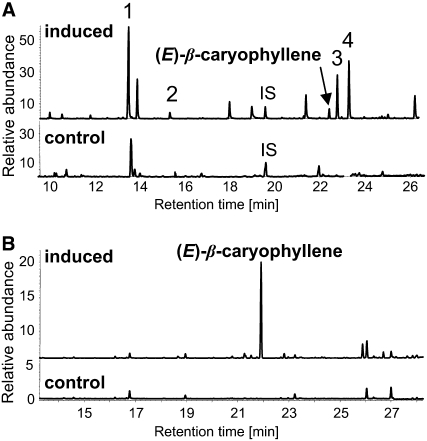
Figure 1.
(E)-β-Caryophyllene Is Emitted in Response to Both Damage of the Leaves by S. littoralis and Attack of the Roots by D. v. virgifera.
(A) Volatiles from control leaves and leaves damaged by S. littoralis were collected and separated by gas chromatography. The major terpene compounds were identified as linalool (peak 1), 4,8-dimethylnona-1,3,7-triene (peak 2), (E)-α-bergamotene (peak 3), and (E)-β-farnesene (peak 4). Depicted are traces of the total ion current detector. IS, internal standard (nonylacetate).
(B) Volatiles from control roots and roots damaged by D. v. virgifera.
Figure 2.
(E)-β-Caryophyllene Attracts Nematodes.
The attractiveness of (E)-β-caryophyllene to the entomopathogenic nematode H. megidis was demonstrated in six-arm olfactometers filled with moist sand. Of the nematodes that were released in the centers of the olfactometers, a significantly larger number was recovered from the arm connected to a pot spiked with 0.2 μL of (E)-β-caryophyllene than from each of the five control arms (P < 0.0001; n = 12). Different letters above the bars indicate a significant difference. Means and se are shown.
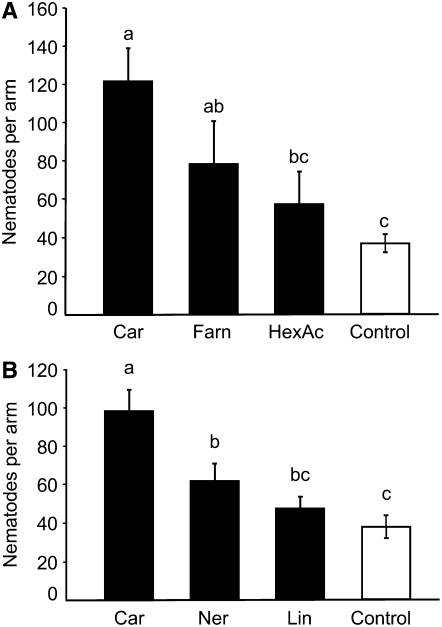
To test whether the attraction of nematodes is specific to (E)-β-caryophyllene, we compared it with another sesquiterpene olefin, (E)-β-farnesene, the sesquiterpene alcohol (E)-nerolidol, the monoterpene alcohol linalool, and a common volatile originating from the lipoxygenase pathway, (Z)-3-hexenyl acetate (Figures 3A and 3B). With the exception of (E)-β-farnesene, the remaining plant volatile compounds were less attractive [F3,44 = 9.39; P < 0.001 for (E)-β-farnesene and (Z)-3-hexenyl acetate, F3,68 = 8.26; P < 0.001 for (E)-nerolidol and linalool] than (E)-β-caryophyllene, indicating that (E)-β-caryophyllene is particularly effective at promoting plant–nematode interaction.
Figure 3.
Nematode Attraction Is Specific to (E)-β-Caryophyllene.
The attractiveness of plant volatile compounds to the entomopathogenic nematode H. megidis was demonstrated in six-arm olfactometers filled with moist sand. The nematodes were released in the center of the olfactometer and chose between arms in which 0.2 μL of (E)-β-caryophyllene (Car), (E)-β-farnesene (Farn), and (Z)-3-hexenyl acetate (HexAc) (A) or (E)-β-caryophyllene, (E)-nerolidol (Ner), and linalool (Lin) (B) were added and a sand-only control. Different letters above the bars indicate significant differences at P < 0.05. Means and se of n = 8 (A) or n = 12 (B) repetitions are shown.
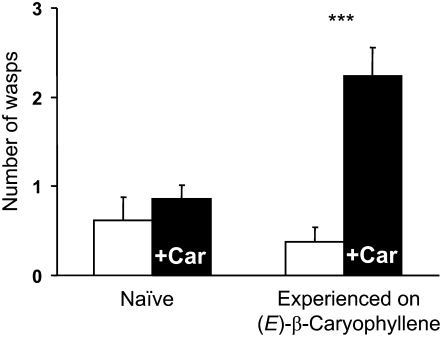
To test for a possible aboveground role of (E)-β-caryophyllene in attracting the parasitic wasp C. marginiventris, we used the pure compound for bioassays in a four-arm olfactometer. Naive wasps without any oviposition experience were not attracted (F1.82 = 0.98; P = 0.32). However, wasps preferred air containing (E)-β-caryophyllene to pure air (F1.82 = 52.06; P < 0.0001) after they had experienced laying eggs in host larvae while perceiving (E)-β-caryophyllene (Figure 4). This effect of associative learning was reflected in a significant treatment effect (F1.166 = 34.04; P < 0.0001) and a significant treatment–experience interaction (F1.164 = 13.49; P < 0.001). These results confirm that (E)-β-caryophyllene by itself is a key attractant for the nematodes, whereas above ground this bicyclic sesquiterpene olefin is a component of a blend of leaf volatiles produced after herbivore damage that can be perceived and learned as a host location cue by herbivore parasitoids, as has been found for several other volatiles within this blend (D'Alessandro et al., 2006; Schnee et al., 2006).
Figure 4.
The Parasitic Wasp C. marginiventris Is Attracted by (E)-β-Caryophyllene after Previous Oviposition Experience.
Responses of the parasitic wasp C. marginiventris to (E)-β-caryophyllene. The attraction of parasitoid females to a pure standard of (E)-β-caryophyllene was tested in a four-arm olfactometer. Two groups of parasitoids were tested: naive wasps and wasps with a previous oviposition experience on host larvae in the presence of (E)-β-caryophyllene. The parasitoids were tested in groups of six (n = 14). The asterisks indicate a significant preference (P < 0.0001) of experienced wasps for the odor of (E)-β-caryophyllene (black bars) versus pure air (white bars show average preference for one arm). Naive wasps did not show this preference. Means and se are shown.
Cloning of the Maize Terpene Synthase Gene tps23
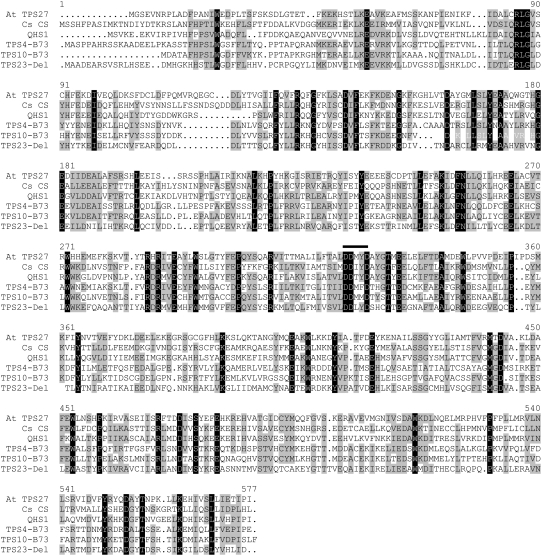
To find the terpene synthase gene(s) producing (E)-β-caryophyllene from farnesyl diphosphate in maize, we screened a public maize genome database (http://maize.tigr.org/) for sequences with similarity to known terpene synthases. One of the resulting fragments, AZM4_53695, contained the two exons flanking the last intron of a putative sesquiterpene synthase gene. The 5′ end of this fragment was extended using a cDNA library from herbivore-induced leaves of the maize cultivars Graf and Delprim to obtain the complete open reading frame (ORF). Both cDNAs contained an identical ORF of 1644 bp designated as tps23 that encodes a protein with a predicted molecular mass of 63.6 kD. Numerous amino acid motifs throughout its sequence are highly conserved among plant terpene synthases (Figure 5). The most characteristic element is an Asp-rich DDxxD motif in the C-terminal part that was implicated in the binding of the divalent metal cofactor in a sesquiterpene synthase from tobacco (Nicotiana tabacum) (Starks et al., 1997). The deduced amino acid sequence of TPS23 shows similarities to sequences of other terpene synthases from maize, for example, 40.5% amino acid identity with TPS10 (Schnee et al., 2006) and 37.8% amino acid identity with TPS4 (Köllner et al., 2004b). Among maize terpene synthases, the sequences are most highly conserved in the regions encoding the active site, which are situated toward the C terminus (Figure 5). Considerable effort was expended to search for maize terpene synthase sequences with higher similarity by repeated PCR with maize cDNA and rapid amplification of cDNA ends (RACE) libraries as well as searching of all available maize genomic databases. However, no genes encoding proteins with a sequence identity of >50% were found, which was further supported by DNA hybridization analysis with tps23 as a probe under low-stringency conditions (data not shown).
Figure 5.
Comparison of the Deduced Amino Acid Sequence of TPS23-Del with Other Terpene Synthases.
The sequence of TPS23 was compared with sequences of (E)-β-caryophyllene synthases from other plants (Arabidopsis At TPS27, C. sativus Cs CS, and A. annua QHS1) and two sesquiterpene synthases from maize (TPS10-B73 and TPS4-B73). Amino acids identical in all six proteins are indicated by black boxes. Amino acids identical in at least four proteins or representing conservative changes are highlighted with gray boxes. The highly conserved DDxxD region is marked with a bar.
tps23 Encodes an (E)-β-Caryophyllene Synthase
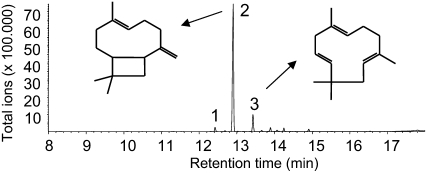
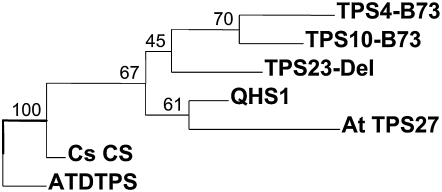
Since the product specificity of a putative terpene synthase cannot be predicted from its amino acid sequence, we cloned tps23 into a bacterial expression system and incubated the recombinant protein with the potential substrates geranyl diphosphate (GPP; C10), farnesyl diphosphate (FPP; C15), and geranylgeranyl diphosphate (GGPP; C20). The enzyme did not accept GPP or GGPP as a substrate (data not shown), converting only FPP to terpene products (Figure 6). The major product formed from FPP was identified as (E)-β-caryophyllene by mass spectrometry and cochromatography with an authentic standard, and the two minor products were α-humulene and δ-elemene. A similar spectrum of terpene products was observed from Os TPS3, an (E)-β-caryophyllene synthase from rice (Oryza sativa) (Cheng et al., 2007). Although this enzyme has 50% amino acid identity to TPS23, the present genomic information on rice and maize is not sufficient to establish homology between these two species. Caryophyllene synthases identified from dicotyledonous plants, including those from Arabidopsis (At TPS27), Cucumis sativus (Cs CS), and Artemisia annua (Aa QHS1), show only low amino acid identity to TPS23: 33.9, 30.3, and 35.7%, respectively. A dendrogram analysis demonstrates that TPS23 is much more closely related to the functionally unrelated terpene synthases of maize than to terpene synthases of similar function in other plant species, suggesting a repeated evolution of the ability to make (E)-β-caryophyllene in monocotyledonous grasses and dicotyledonous plants (Figure 7; see Supplemental Data Set 1 online).
Figure 6.
Sesquiterpene Products of TPS23.
The enzyme was expressed in Escherichia coli, extracted, partially purified, and incubated with the substrate (E,E)-FPP. The resulting terpene products were collected with a solid-phase microextraction (SPME) fiber and analyzed by gas chromatography–mass spectrometry. The products were identified as δ-elemene (peak 1), (E)-β-caryophyllene (peak 2), and α-humulene (peak 3) by comparison of their retention times and mass spectra with those of authentic standards.
Figure 7.
TPS23 Shows Low Sequence Identity to Other (E)-β-Caryophyllene Synthases.
Dendrogram analysis of TPS23 with two closely related maize sesquiterpene synthases (TPS10-B73 and TPS4-B73) and several (E)-β-caryophyllene synthases from other plants (Arabidopsis At TPS27, C. sativus Cs CS, and A. annua QHS1). The analysis was conducted using a neighbor-joining algorithm. Bootstrap values are shown in percentage and were generated with a sample of n = 1000. ATDTPS indicates ent-kaurene synthase from Arabidopsis, a functionally different diterpene synthase that was used as an outgroup.
Biochemical Characterization of TPS23
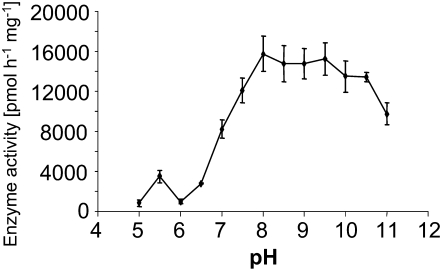
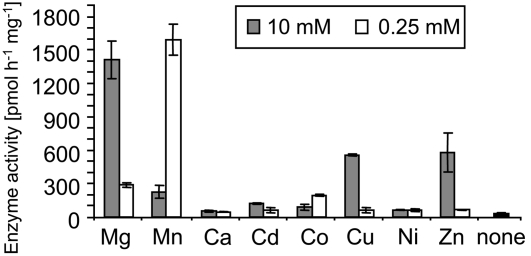
The biochemical properties of TPS23 were determined with purified enzyme and tritium-labeled FPP substrate. The enzyme exhibited a broad catalytic optimum from pH 8.0 to 9.5 but still showed substantial activity at typical cytoplasmic pH conditions (Figure 8). A divalent metal ion cofactor was required for enzyme activity (Figure 9). A range of potential cofactor species were tested at different concentrations, with Mg2+ ions at a concentration of 10 mM and Mn2+ ions at a concentration of 0.25 mM giving maximal activities. The Km values were 183 ± 34 and 28 ± 6 μM for Mg2+ and Mn2+, respectively (Table 1). Both the Km and the kcat values for the FPP substrate were similar to those of most characterized plant sesquiterpene synthases identified to date (Chen et al., 1996; Crock et al., 1997; Picaud et al., 2005, 2006).
Figure 8.
pH Dependence of the Enzymatic Activity of TPS23.
The catalytic activity of the purified enzyme was measured in the presence of 10 mM Mg2+. The pH values were adjusted with the following buffers: pH 5.0 and 5.5, acetate buffer (100 mM); pH 6.0, MES buffer (100 mM); pH 6.5 to 9.5, bis-Tris-propane buffer (100 mM); pH 10.0 to 11, CAPS buffer (10 mM). Means ± se of triplicate assays are shown.
Figure 9.
Metal Cofactors Affect the Enzymatic Activity of TPS23.
The catalytic activity of the purified enzyme was measured in the presence of various divalent metal ions at concentrations of 10 and 0.25 mM. Means ± se of triplicate assays are shown.
Table 1.
Kinetic Constants for TPS23-Del Heterologously Expressed in E. coli
| Constant | FPP (10 mM MgCl2) | FPP (0.25 mM MnCl2) | Mg2+ | Mn2+ |
|---|---|---|---|---|
| Km (μM) | 3.7 ± 0.5 | 1.1 ± 0.3 | 183 ± 34 | 28 ± 5 |
| kcat (s−1) | (1.91 ± 0.09) × 10−3 | (1.13 ± 0.07) × 10−3 |
Herbivory above and below Ground Induces Transcript Levels of tps23 Independently
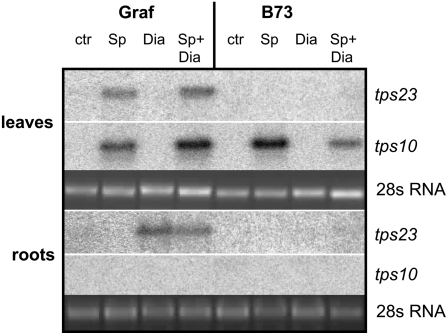
To determine whether TPS23 is involved in the herbivore-induced synthesis of (E)-β-caryophyllene, we measured the transcript levels of tps23 in response to feeding damage by S. littoralis and D. v. virgifera and compared them with the expression pattern of tps10, a gene known to be involved in aboveground, herbivore-induced sesquiterpene synthesis in maize (Schnee et al., 2006). The (E)-β-caryophyllene–producing line Graf accumulated transcripts of tps23 in the leaves only after leaf damage by S. littoralis, but no transcripts were detectable in roots after S. littoralis leaf damage (Figure 10). Conversely, root damage by D. v. virgifera resulted in the accumulation of tps23 transcripts in the roots but not in the shoots. The high transcript levels in the roots of Graf correlated with the production of more (E)-β-caryophyllene in this line (Rasmann et al., 2005). Simultaneous feeding by both herbivores resulted in the accumulation of tps23 transcripts in both leaves and roots. The inbred line B73, which does not emit (E)-β-caryophyllene, had no detectable levels of tps23 transcript throughout the plant, indicating the lack of tps23 transcription or low transcript stability as the cause of the lack of (E)-β-caryophyllene production. As expected, the transcript of the leaf-specific terpene synthase tps10 accumulated only in response to the aboveground damage by S. littoralis but was not induced by belowground attack by D. v. virgifera. Transcripts of tps10 are also present in caryophyllene-producing and non-caryophyllene-producing plants alike, consistent with earlier reports on the emission of TPS10 volatiles by line B73 (Köllner et al., 2004a).
Figure 10.
tps23 Is Selectively Induced by Herbivory of S. littoralis and D. v. virgifera.
The transcript levels of tps23 and tps10 were determined in leaves and roots of the maize cultivars Graf and B73 after feeding of S. littoralis (Sp), D. v. virgifera (Dia), and S. littorals plus D. v. virgifera (Sp+Dia) or in undamaged controls (ctr). RNA isolated from 2-week-old plants was hybridized with probes specific for tps23 or tps10. The bottom panels show the 28S RNA band of the ethidium bromide–stained RNA gels.
Maize tps23 and Its Teosinte Orthologs Are Maintained by Positive Selection
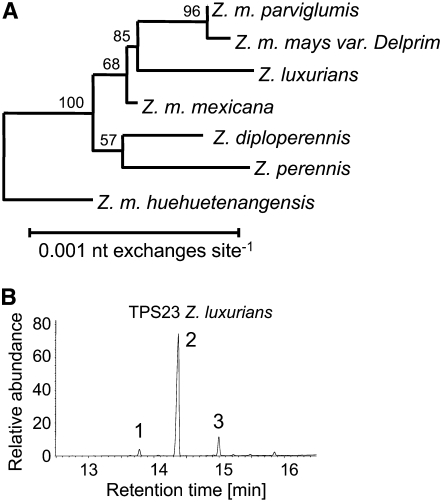
The appearance of (E)-β-caryophyllene in herbivore-induced volatiles of many grasses related to maize suggests that this compound has a widespread role in indirect defense (Gouinguené et al., 2001; Degen et al., 2004). To learn more about the evolution of (E)-β-caryophyllene formation, we isolated the apparent orthologs of tps23 from six teosinte (Zea sp) taxa utilizing PCR (Figure 11A; see Supplemental Figure 1 and Supplemental Data Set 2 online). After expression in a bacterial system, all tps23 apparent orthologs produced the (E)-β-caryophyllene main product as well as the characteristic by-products α-humulene and δ-elemene (Figure 11B; see Supplemental Figure 2 online), demonstrating complete functional conservation of tps23 among maize and its close relatives. A dendrogram analysis of tps23 apparent orthologs followed the phylogeny generally observed among the teosinte species (Buckler et al., 2006) and showed high levels of amino acid identity (Figure 11A). A positive selection pressure for the maintenance of (E)-β-caryophyllene synthase function is evident from the high average number of synonymous nucleotide changes relative to nonsynonymous changes among tps23 from maize and its teosinte apparent orthologs (dS/dN = 6.88).
Figure 11.
TPS23 Is Functionally Conserved among Relatives of Maize.
(A) Dendrogram analysis of TPS23 from maize (Z. m. mays) and its teosinte orthologs from Z. m. parviglumis, Z. luxurians, Z. m. mexicana, Z. diploperennis, Z. perennis, and Z. m. huehuetenangensis. The analysis was conducted as described in Methods. The teosinte Z. m. huehuetenangensis was the designated outgroup due to its overall distance from the other teosinte varieties used in the analysis.
(B) Sesquiterpene products of a putative TPS23 ortholog from Z. luxurians. The enzyme was expressed in E. coli, extracted, partially purified, and incubated with the substrate (E,E)-FPP. The resulting terpene products were collected with a SPME fiber and analyzed by gas chromatography–mass spectrometry. The products were identified as δ-elemene (peak 1), (E)-β-caryophyllene (peak 2), and α-humulene (peak 3) by comparison of their retention times and mass spectra with those of authentic standards.
Reduced Transcription of tps23 Prevents (E)-β-Caryophyllene Formation in Most North American Maize Lines
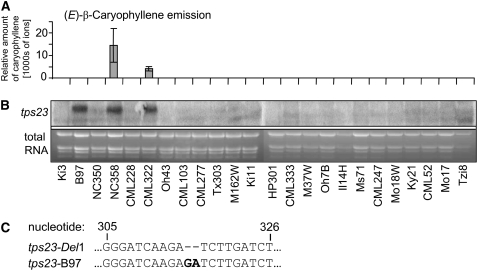
Initial studies by Degen et al. (2004) and Rasmann et al. (2005) suggested that maize lines originating from North American breeding programs have largely lost the ability to produce the (E)-β-caryophyllene signal. To determine the extent to which this defense trait was lost during domestication, we studied (E)-β-caryophyllene production in a set of 24 inbred founder lines assembled to reflect ∼85% of the polymorphisms in North American maize (Liu et al., 2003). Of these 24 lines, only 2, NC358 and CML322, were found to produce (E)-β-caryophyllene in response to herbivore damage, suggesting that this trait is indeed largely absent from North American breeding lines (Figure 12A). The two (E)-β-caryophyllene–producing lines displayed high concentrations of transcripts of tps23, while very low levels or no tps23 transcripts were observed in all other lines except one (Figure 12B). This exceptional line, B97, accumulated high concentrations of tps23 transcripts despite no (E)-β-caryophyllene production. Sequencing of the tps23-B97 allele, however, showed a 2-bp insertion at position 315, which results in a frameshift that prevents the correct translation of the protein and thereby blocks (E)-β-caryophyllene production (Figure 12C).
Figure 12.
Most Maize Lines of North American Origin Do Not Produce (E)-β-Caryophyllene.
(A) A set of 24 inbred lines was tested for (E)-β-caryophyllene production in herbivore-damaged leaves. The averages ± se of triplicate measurements of the (E)-β-caryophyllene amounts are shown.
(B) Accumulation of tps23 transcript in herbivore-damaged leaves. The bottom panel shows the total RNA on the ethidium bromide–stained gel as a loading control.
(C) The tps23 allele of the inbred line B97 contains a 2-bp insertion at nucleotide 315 compared with tps23-Del, which results in an inactive enzyme.
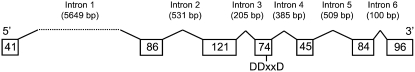
To understand how domestication and breeding may have caused the loss of this defense signal, we compared the tps23 alleles of six (E)-β-caryophyllene–producing lines (the hybrids Graf and Delprim and the inbred lines F2, F476, Du101, and W401) with four nonproducing lines (the hybrid Pactol and the inbred lines B73, F7001, and F670). All lines tested contained an active tps23 allele, indicating that the lack of transcript in some of the lines is not due to differences in the ORF (Table 2). Next, the genomic structure of tps23 was determined by analysis of the alleles tps23-B73 and tps23-F2. The structure of both alleles consists of seven exons and is generally similar to that of other terpene synthases from maize (Shen et al., 2001; Köllner et al., 2004b) and class III terpene synthases from other plants (Trapp and Croteau, 2001). Unlike other terpene synthases, however, the first intron is very large and contains transposon sequences, indicating that this intron was enlarged by transposon insertion from a size of ∼121 bp, usually observed in terpene synthases, to 5.6 kb (Figure 13). Since this insertion is observed in the tps23 alleles of all lines, regardless of (E)-β-caryophyllene production, it too is not likely to be responsible for the inactivation of the gene in the nonproducing lines. Furthermore, we tested a 1.8-kb promoter fragment for specific differences that might regulate the transcriptional activity of tps23 in the different maize lines. Two types of promoter sequence were found, which are distinguished by 18 single base pair changes throughout the fragment. A single base pair change that created an EcoRI restriction site was located 425 bp upstream of the transcription start. Among the hybrid lines, Pactol has only promoter type 1, Graf has only type 2, and Delprim has both. However, since all inbred lines had type 1 promoters regardless of their ability to produce (E)-β-caryophyllene, the changes in the 1.8-kb fragment did not account for the differences in the transcriptional activity of tps23. Similarly, the 880-bp 3′ untranslated region of all alleles was shown to be identical and therefore cannot cause differences in transcriptional activity or changes in mRNA stability.
Table 2.
Properties of the tps23 Alleles and Their Promoters in the Hybrid Lines Delprim, Graf, and Pactol and the Inbred Lines B73, F2, F467, Du101, W401, F670, and F7001
| Line | Caryophyllene | 5.6-kb Intron | Promoter Allele 1 | Promoter Allele 2 | ORF Allele 1 | 3′ Untranslated Region Allele 1 |
|---|---|---|---|---|---|---|
| Graf | + | + | − | + | + | + |
| Delprim | + | + | + | + | + | + |
| F2 | + | + | + | − | + | + |
| F476 | + | + | + | − | Not tested | + |
| Du101 | + | + | + | − | Not tested | + |
| W401 | + | + | + | − | Not tested | + |
| F670 | − | + | + | − | Not tested | + |
| F7001 | − | + | + | − | Not tested | + |
| B73 | − | + | + | − | + | + |
| Pactol | − | + | + | − | + | + |
Figure 13.
Exon–Intron Structure of tps23.
The seven exons are represented by boxes showing the number of amino acids they contain. The first intron is enlarged by the insertion of a transposon-like sequence element of ∼5.4 kb. DDxxD marks the position of the Asp-rich region in the active center of the protein. The dotted line marks the transposon-like sequence included in the first intron.
DISCUSSION
TPS23 Provides a Signal for the Attraction of Herbivore Enemies Both above and below Ground
Plants produce a large arsenal of terpenes, phenolics, and other presumed defensive metabolites whose exact functions are largely unknown. Here, we confirm that the sesquiterpene olefin (E)-β-caryophyllene can play a role in two spatially separate modes of induced defenses against herbivores: the attraction of parasitic wasps above ground that oviposit on lepidopteran larvae, such as S. littoralis, and the attraction of nematodes below ground that can attack larvae of the beetle D. v. virgifera (Rasmann et al., 2005). Both volatile signals are produced by a single enzyme, the terpene synthase TPS23. The attraction of the nematode to (E)-β-caryophyllene is innate (Rasmann et al., 2005) (Figure 2), whereas females of the parasitic wasp are attracted only after associative learning (Figure 4). (E)-β-Caryophyllene is one of many volatiles released by maize leaves that can serve as cues for parasitoids to find their herbivore hosts, but this specific compound is essential for attracting nematodes. A comparison with authentic versions of volatiles that are typically released from maize leaves has revealed that none diffuses as readily in sand and soil as (E)-β-caryophyllene (Hiltpold and Turlings, 2008), providing a possible reason why this substance is so much more attractive to the nematode than other typical maize volatiles (Rasmann et al., 2005).
(E)-β-Caryophyllene is also suitable for defense signaling above ground, as it diffuses rapidly in the air. However, compared with most other volatile monoterpenes and sesquiterpenes, (E)-β-caryophyllene is unstable in the atmosphere, reacting readily with ozone and other reactive oxygen species (Grosjean et al., 1993). Thus, (E)-β-caryophyllene may be diagnostic as a short-range cue for host or prey location. (E)-β-Caryophyllene also has antimicrobial activity (Sabulal et al., 2006) and might have been initially selected as a direct defense against pathogen attack. Its signaling function in indirect defense could have evolved secondarily.
Maize (E)-β-Caryophyllene Synthase Is a Product of Convergent Evolution
The properties of maize TPS23 are similar to those of other plant caryophyllene synthases from A. annua (Cai et al., 2002), C. sativus (Mercke et al., 2004), and Arabidopsis (Tholl et al., 2005) in kinetic parameters and cofactor requirement. These enzymes also have a common reaction mechanism, as indicated by the formation of the same minor products, α-humulene and δ-elemene.
Despite these similarities, sequence comparisons indicate that the (E)-β-caryophyllene synthases are products of convergent or repeated evolution (Pichersky and Gang, 2000). TPS23 is more closely related to other maize terpene synthases than to (E)-β-caryophyllene synthases isolated from dicotyledons. One such closely related maize terpene synthase is TPS10, which might share with TPS23 a common ancestor involved in indirect defense. The mechanism to produce (E)-β-caryophyllene may subsequently have been acquired by TPS23. Such convergent evolution is probably facilitated by the ability of terpene synthases to alter product specificity in response to only a few amino acid changes (Köllner et al., 2004b, 2006; Yoshikuni et al., 2006).
Regulation of tps23 Transcript Allows Independent Expression in Different Organs
Enzyme activities in plants are often controlled by differential regulation of the members of a gene family. In terpene biosynthesis, for example, enzymes catalyzing important regulatory steps, such as 3-hydroxy-3-methylglutaryl-CoA synthase (Enjuto et al., 1995; Daraselia et al., 1996; Korth et al., 1997), 1-deoxy-xylulose phosphate synthase (Walter et al., 2002), and isoprenyl diphosphate synthases (Cunillera et al., 1997; Okada et al., 2000), are encoded by small gene families with differential expression. However, tps23 provides an example of a single gene with two distinct expression patterns in different organs. The aboveground induction of tps23 is similar to that of tps10, a maize terpene synthase gene that is activated after herbivory by lepidopteran larvae on leaves and produces most of the herbivore-induced sesquiterpene hydrocarbon volatiles of maize (Schnee et al., 2006). By contrast, only tps23, and not tps10, is activated below ground. It is conceivable that the tps23 and tps10 genes share the same regulatory mechanism for aboveground induction but that tps23 contains an additional promoter element that activates the gene in the root after herbivory. We will test this hypothesis of a modular regulatory system by comparing the promoters of tps23 and tps10.
(E)-β-Caryophyllene Emission Was Lost Due to a Decrease in tps23 Transcription during the Breeding of North American Maize Lines
(E)-β-Caryophyllene is emitted from all tested maize lines from European breeding programs and from species of teosinte, the closest wild relative of maize. On the other hand, a range of inbred lines that represent ∼85% of the genetic diversity of North American maize lines showed (E)-β-caryophyllene production in <10% of the lines. Therefore, we assume that this defensive trait was largely lost during the breeding of North American maize lines. All lines that did not produce (E)-β-caryophyllene show very low or no tps23 transcript (with one easily rationalized exception), indicating that the (E)-β-caryophyllene polymorphism results from differences in transcription. Differences in transcript stability can be ruled out, since the hypothetical tps23 transcript is identical in all maize lines analyzed regardless of (E)-β-caryophyllene production. The strongly reduced transcription in most North American lines might be due to the inactivation of a transcription factor or the corruption of an enhancer element outside of the assayed promoter region. Whatever the identity of this factor or enhancer element, it is clearly not necessary for the activation of tps10, which has a very similar expression profile to that of tps23 in leaves after S. littoralis attack.
The loss of defensive traits during crop domestication has frequently been postulated, but the genetic basis of this process, such as the inactivation of tps23 expression described here, has rarely been elucidated (Sotelo, 1997). The loss of tps23 expression might be ascribed to several causes. First, a null allele of a required transcription factor may be closely linked to a trait that is known to differ between North American and European maize lines, like flowering time. Breeding efforts to alter this trait could then have resulted in the accumulation of the null allele for (E)-β-caryophyllene production. Alternatively, the release of (E)-β-caryophyllene might be disadvantageous under conditions specific to North American agriculture and therefore may have been selected against. A possible disadvantage of (E)-β-caryophyllene release in this scenario could be its reported attractiveness to adult females of D. v. virgifera (Hammack, 2001).
The use of natural enemies, such as entomopathogenic nematodes, is an important component of many integrated pest control programs and could reduce damage by D. v. virgifera, an economically important maize pest that causes extensive yield losses. The failure of past efforts to control this pest with nematodes in North America (Ellsbury et al., 1996; Jackson, 1996) may be due to the lack of (E)-β-caryophyllene release from maize lines under cultivation. (E)-β-Caryophyllene release is correlated with increased nematode attraction to maize in the field (Rasmann et al., 2005). The identification of tps23 provides a molecular tool to devise alternative strategies for D. v. virgifera control. The restoration of (E)-β-caryophyllene production in nonproducing maize lines should enhance their attractiveness to nematodes and thus increase D. v. virgifera mortality. We are currently transforming a non-(E)-β-caryophyllene–producing maize line with an (E)-β-caryophyllene synthase and will evaluate its performance in an agronomical setting.
Another strategy is the use of the tps23 promoter to control the expression of toxins, such as the Bacillus thuringensis Cry3 Bb 1 protein, that are effective against D. v. virgifera. This could provide the plant with an efficient, timely, and well-localized defense against this pest.
METHODS
Plant and Insect Material
Plants of the maize (Zea mays) varieties B73 (KWS Seeds), Graf (Landi), Delprim (Delley Samen und Pflanzen), and Pactol (Syngenta) were kindly provided by their respective breeders. The inbred lines F2, F476, Du101, W401, F7001, and F670 and seeds of the teosinte (Zea sp) species were a gift from the Station de Génétique Végétale, Institut National de la Recherche Agronomique, and the 24 North American inbred lines (small diversity panel) was supplied by the National Germplasm System of the USDA Agricultural Research Service. The plants were grown in commercially available potting soil in a climate-controlled chamber with a 16-h photoperiod, 1 mmol·m−2·s−1 photosynthetically active radiation, a temperature cycle of 22/18°C (day/night), and 65% RH. Twelve- to 15-d-old plants (20 to 30 cm high, four to five expanded leaves) were used in all experiments. Eggs of Spodoptera littoralis (Lepidoptera: Noctuidae) were obtained from Aventis and were reared on an artificial wheat germ diet (Heliothis mix; Stonefly Industries) for ∼10 to 15 d at 22°C under an illumination of 750 μmol·m−2·s−1. For the S. littoralis treatments, three third instar larvae were enclosed on the middle portion of each plant for 20 h in a cage made out of two halves of a Petri dish (9 cm diameter) with a circle cut out of each side and covered with gauze to allow for ventilation (Röse et al., 1996). Larvae of Diabrotica virgifera virgifera were obtained from CABI BioSience, and nematodes of the species Heterorhabditis megidis were supplied by Andermatt Biocontrol. For the D. v. virgifera treatment, each maize plant was subjected to four second instar or third instar larvae for 2 d. The solitary endoparasitoid Cotesia marginiventris that was used in the experiments originated from the USDA Agricultural Research Service, Biological Control and Mass Rearing Research Unit. For the rearing of parasitoids, 25 young caterpillars (3 to 4 d old) were offered to a single mated female (4 to 7 d old) for 3 h in a plastic box (9.5 cm diameter, 5 cm high). The caterpillars were further reared on artificial diet in an incubator (25°C, 16 h of light/8 h of dark) until cocoon formation. Cocoons were kept in Petri dishes until adult emergence. Emerging adults were sexed and kept in cages (30 × 30 × 30 cm) at a male:female ratio of 1:2, with distilled water on cotton and honey as a food source. The cages were kept in the laboratory under ambient light and temperature conditions.
Bioassays
Attraction of the nematode H. megidis toward (E)-β-caryophyllene was tested with belowground six-arm olfactometer assays (Rasmann et al., 2005). The apparatus consisted of a central glass chamber with six evenly distributed side arms that connect it to six glass pots. The entire system was filled with moist sand (10% water). To test the attractiveness of (E)-β-caryophyllene, amber glass vials (1.5 mL; Supelco and Sigma-Aldrich) were half filled with glass wool and 200 mL of authentic (E)-β-caryophyllene was added. Vials were closed with an open screw cap containing a septum, through which a 100-μL capillary (Hirschmann Laborgeräte) was inserted into the vial's saturated head space. The vial was then placed upside down with the capillary projecting into the sand in one of the outer pots of the six-arm olfactometer, ensuring a constant release of (E)-β-caryophyllene into the sand.
To compare the attractiveness of (E)-β-caryophyllene with that of other substances, an aliquot of 0.2 μL of authentic (E)-β-caryophyllene (98% pure; Sigma-Aldrich), (E)-β-farnesene (Bedoukian), linalool (95% pure; Sigma-Aldrich), nerolidol (98% pure; Sigma-Aldrich), or (Z)-3-hexenyl acetate (98% pure; Sigma-Aldrich) was injected into one of the glass pots, and the five untreated pots were used as controls. About 2000 H. megidis were released in a drop of water in the center of the central arena. Ultrafine screens at the end of each olfactometer arm prevented the nematodes from entering the pots. These arms consist of detachable parts from which nematodes can be recovered (for details, see Rasmann et al., 2005). Our study used six belowground olfactometers simultaneously. Twenty-four hours after H. megidis release, the belowground olfactometers were disassembled and the sand from each arm was placed on separate cotton filter discs (Hoeschele) in Bearmann extractors (Curran, 1992; Hass et al., 1999). The next day, recovered nematodes were counted.
To test the aboveground role of (E)-β-caryophyllene in attracting herbivore enemies, S. littoralis caterpillars and the solitary endoparasitoid wasp, C. marginiventris, were reared as described above. We tested mated, 2- to 5-d-old females, both naive and experienced individuals. Experienced females were obtained by placing them in a tube containing 20 S. littoralis larvae on top of a vessel that was connected via a glass capillary to a 2-mL glass vial filled with 300 μL of synthetic (E)-β-caryophyllene. The release rate was calibrated to the range of (E)-β-caryophyllene concentrations that are released by maize plants. The wasps were released in the tube one at a time and removed after three to five ovipositions. For each replicate, six wasps were provided with this oviposition experience.
The attractiveness of the (E)-β-caryophyllene to C. marginiventris females was tested in a four-arm olfactometer as described by D'Alessandro and Turlings (2005). In all experiments, the (E)-β-caryophyllene–releasing device was installed in the airflow of one of the four olfactometer arms. Cleaned and humidified air entered each vessel at 1.2 L/min carrying the volatiles via the arms to a central cylinder. Simultaneously, 0.6 L/min air was pulled out through volatile collection traps containing the adsorbent Super Q (80/100 mesh; Alltech), which were connected to a port at the top of each vessel. Wasps of the same experience type were released in groups of six into the central glass cylinder and could choose to enter one of the four arms. After entering an arm, their passage was blocked by a stainless steel screen and eventually they oriented toward a light source into glass bulbs, where they were counted and removed. Wasps that did not enter an arm within 30 min were considered as having made no choice, while wasps that chose an arm were considered responsive. A total of four groups of six wasps were tested during a 3-h period, alternating between the two experience types [naive or experienced with (E)-β-caryophyllene]. The experiment was repeated seven times.
The behavioral responses of parasitoids and entomopathogenic nematodes to (E)-β-caryophyllene were analyzed with a log-linear model. The data did not conform to the simple variance assumptions implied in using the multinomial distribution. Therefore, we used quasi-likelihood functions to compensate for the overdispersion of wasps within the olfactometer and for the fact that not all of the wasps made a choice during the 30 min of the trial. The adequacy of the model was assessed through likelihood ratio statistics and examination of residuals in the software package R (R Foundation of Statistical Software, version 2.4.0; www.r-project.org) (Turlings et al., 2004; Ricard and Davison, 2007). We tested treatment effects (i.e., odor sources) for naive and experienced wasps individually. In addition, we tested for a significant effect of the experience and an interaction between treatment and experience.
Volatile Collection
For the analysis of volatile terpenes, leaf material was frozen in liquid nitrogen and pulverized in a mortar. An aliquot of 0.2 g of plant powder was placed in a glass vial with a septum in the lid. A 100-μm PDMS SPME fiber (Supelco) was inserted through the septum and exposed for 60 min at 40°C. The compounds adsorbed onto the fiber were analyzed by gas chromatography–mass spectrometry.
cDNA Library Construction
Ten-day-old maize plants of cv Delprim were subjected to herbivory by S. littoralis for 4 h. One gram of leaf material was ground in a mortar to a fine powder in liquid nitrogen and added to 10 mL of Trizol reagent (Gibco BRL). The mixture was treated with a Polytron (Kinematika) for 1 min and incubated for 3 min on ice. Total RNA was isolated according to the manufacturer's instructions. From ∼80 μg of total RNA, the mRNA was isolated using poly(T)-coated ferromagnetic beads (Dynal). The mRNA was transcribed into cDNA, and a Marathon RACE library was constructed according to the manufacturer's instructions (Clontech).
Isolation of tps23 cDNA from Maize and Teosinte
Sequences with high similarity to plant terpene synthases were identified in BLAST searches of The Institute for Genomic Research Maize Database (http://maize.tigr.org/). One of these fragments (AZM4_53695) was cloned, sequenced, and extended toward the 5′ end by the Marathon RACE procedure (Clontech) from a cDNA library of herbivore-induced leaves of the maize cultivars Graf and Delprim. The complete sequence, amplified with the primers BH3fwd (5′-ATGGCAGCTGATAGGCAAGATCCG-3′) and BH2rev (5′-TTAGTCTATTAGATGCACATACAATG-3′) from a cDNA and introduced into the sequencing vector pCR4-TOPO (Invitrogen), contains an ORF of 1644 bp. The apparent orthologs of tps23 from the teosinte species were cloned using cDNA from herbivore-induced leaves of each of the teosinte species and the primers mentioned above. The genomic clones from tps23 were cloned from genomic DNA of the respective maize varieties using the same set of primers. To avoid sequence errors, all putative orthologs were cloned from two independent amplification reactions. No evidence for additional genes with high sequence identity was found in any of the teosinte species or maize varieties.
Phylogenetic Analysis
Sequence analysis was performed with the DNAstar suite of programs (Lasergene), and nucleotide substitution rates were determined with the program Syn-SCAN (http://hivdb.standford.edu/pages/synscan.html) according to the method of Nei and Gojobori (1986). For dendrogram analysis, the ORFs of (E)-β-caryophyllene synthases (Figure 5) and ORFs of putative tps23 orthologs (see Supplemental Figure 1 online) were aligned with DNAstar utilizing a ClustalW algorithm (matrix, PAM250; gap penalty,10; gap length, 0.2; delay divergent sequence, 20; DNA transition weight, 0.5) with no additional adjustment. The dendrograms were created using the TREECON 1.3b software package (Van de Peer and De Wachter, 1994) using a neighbor-joining algorithm with bootstrap values from 1000 trials.
Isolation of the 5′ and 3′ Flanking Regions and Intron 1 of tps23
For each of the maize lines assayed, genomic DNA was prepared with the DNeasy plant mini kit (Qiagen) according to the manufacturer's instructions. The Universal GenomeWalker kit (Clontech) was used to isolate a 1.8-kb DNA fragment upstream of the tps23 open reading frame. To compare promoter alleles from different maize cultivars, the promoter fragments were amplified from genomic DNA by nested PCR using the primers BH13fwd (5′-GTTAGTCCAATATTTGTGTTGGGC-3′), BH12rev (5′-GACGGATCTTGCCTCATCAGCTGCC-3′) and BH14fwd (5′-TTCAACCACCAAAATTAATACTGGG-3′), BH11rev (5′-GTATACTAGCTAGCTACTCTCCTGC-3′), respectively, cloned into the sequencing vector pCR4-TOPO (Invitrogen), and fully sequenced. To analyze the downstream region of tps23, a 1.5-kb fragment containing 880 bp of the untranslated 3′ region was amplified with the primers BH31fwd (5′-GTGCTATAATGCCGAGACAGAATGGCGTGACAAG-3′) and BH32rev (5′-CAATTCATGTGGATTGGGTAGGATTGAGTGGGTTTC-3′), cloned, and sequenced as described above. To test for the presence of the unusually large intron 1, PCR was performed with the gene-specific primer BH3fwd (5′-ATGGCAGCTGATAGGCAAGATCCG-3′) located on exon 1 and the intron-specific primer BH26rev (5′-GATCTAAGGCCGTGTTTTATTCGC-3′). The resulting 600-bp fragment was cloned and sequenced. The complete intron 1 was isolated from the inbred lines B73 and F2 using nested PCR with the primers BH3fwd, BH22rev (5′-AGTAACATTTTCTTCACCTCCTCC-3′) and BH27fwd (5′-CACAGTGAGGAGGACATGCATGGG-3′), BH21rev (5′-ATTTCGACGTTATCCTTCATAATC-3′), respectively.
Heterologous Expression of Terpene Synthases
For expression with an N-terminal 8× His tag, the ORF of tps23 was amplified with the primers BH8fwd (5′-ATTGCCATGGCGCAGCTGATGAGGCAAGATCC-3′) and BH9rev (5′-ATTAGAATTCTTAGTCTATTAGATGCACATAC-3′) and cloned as a NcoI-EcoRI fragment into the expression vector pHIS8-3. The construct was introduced into the Escherichia coli strain BL21 (DE3) and fully sequenced to avoid errors introduced by DNA amplification. Liquid cultures of the bacteria harboring the expression constructs were grown at 37°C to an OD600 of 0.6. Then, isopropyl-β-thiogalactopyranoside was added to a final concentration of 1 mM, and the cultures were incubated for 20 h at 18°C. The cells were collected by centrifugation and disrupted by a 4 × 30 s treatment with a sonicator (Bandelin UW2070) in chilled extraction buffer (50 mM MOPSO, pH 7.0, with 5 mM MgCl2, 5 mM sodium ascorbate, 0.5 mM phenylmethylsulfonyl fluoride, 5 mM DTT, and 10% [v/v] glycerol). The cell fragments were removed by centrifugation at 14,000g, and the supernatant was desalted into assay buffer (10 mM MOPSO, pH 7.0, 1 mM DTT, and 10% [v/v] glycerol) by passage through an Econopac 10DG column (Bio-Rad). For kinetic studies, the His-tagged enzyme was further purified on a nickel-nitrilotriacetate agarose column (Qiagen) according to the manufacturer's instructions.
Assay for Terpene Synthase Activity
To determine the catalytic activity of the terpene synthase TPS23, enzyme assays containing 50 μL of the bacterial extract and 50 μL of assay buffer with 10 μM (E,E)-FPP, 10 mM MgCl2, 0.05 mM MnCl2, 0.2 mM NaWO4, and 0.1 mM NaF in a Teflon-sealed, screw-capped 1-mL gas chromatograph glass vial were performed. A SPME fiber consisting of 100-μm polydimethylsiloxane (Supelco) was placed into the head space of the vial for a 1-h incubation at 30°C. For analysis of the adsorbed reaction products, the SPME fiber was inserted directly into the injector of the gas chromatograph.
For the determination of metal ion cofactors, Km values, and effects of pH, an assay containing 1 μM purified TPS23 protein, 10 μM [1-3H](E,E)-FPP (37 GBq/mol; American Radiolabeled Chemicals), and 10 mM MgCl2 in 100 μL of assay buffer was used. The assay was overlaid with 1 mL of pentane to trap volatile products and incubated for 20 min at 30°C. The reaction was stopped by mixing, and 0.5 mL of the pentane layer was taken for the measurement of radioactivity by liquid scintillation counting in 2 mL of Lipoluma cocktail (Packard Bioscience) using a Packard Tricarb 2300TR liquid scintillation counter (3H efficiency = 61%).
The pH optimum was determined in buffers from pH 5.0 to 11.0. Assay results are reported as means of three independent replicate assays, and each experiment was repeated two to three times with similar results. The Km values were determined using seven substrate concentrations with four repetitions each. The enzyme activity was stable for at least 1 month when stored at −80°C. The concentration of the purified protein was determined by the method of Bradford (1976) using the Bio-Rad reagent with BSA as a standard.
Gas Chromatography–Mass Spectrometry
A Hewlett-Packard model 6890 gas chromatograph was employed with the carrier gas He at 1 mL/min, splitless injection (injector temperature of 220°C), a Chrompack CP-SIL-5 CB-MS column [5% (phenyl)-methylpolysiloxane, 25 m × 0.25 mm i.d. × 0.25 μm film thickness; Varian], and a temperature program from 40°C (3-min hold) at 5°C/min to 240°C (3-min hold). The coupled mass spectrometer was a Hewlett-Packard model 5973 with a quadrupole mass selective detector, transfer line temperature of 230°C, source temperature of 230°C, quadrupole temperature of 150°C, ionization potential of 70 eV, and a scan range of 40 to 350 atomic mass units. Products were identified by comparison of retention times and mass spectra with authentic reference compounds.
RNA Hybridization
Plant RNA was prepared with the RNeasy plant mini kit (Qiagen) according to the manufacturer's instructions. A 400-bp fragment containing the first two exons of tps23 was used as a probe, generated by linear PCR with the primer 5′-GAACTTCAAAAATACATCAGA-3′ and the complete ORF as a template. The probe was labeled with [32P]ATP using the Strip-EZ PCR procedure (Ambion). Blotting on a Nytran-Plus nylon membrane (Schleicher and Schuell), hybridization, and washing were performed (Sambrook, 1989). The blots were scanned with a Storm 840 PhosphorImager (Molecular Dynamics). All RNA hybridization experiments were performed in two biological replicates.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database. The cDNA sequences for tps23 alleles and its promoters from the different maize lines were deposited in GenBank (http://www.ncbi.nlm.nih.gov) with the accession numbers EU259634 (genomic sequence from inbred line B73), EU259632 (coding sequence tps23-Graf1), EU259633 (coding sequence tps23-Del1), EU259635 (coding sequence tps23-Del2), EU259636 (1.8-kb promoter fragment tps23-Del1), and EU259637 (1.8-kb promoter fragment tps23-Del2). The apparent orthologs of tps23 in teosinte species have the accession numbers EU259638 (Z. diploperennis), EU259639 (Z. m. huehuetenangensis), EU259640 (Z. luxurians), EU259641 (Z. m. mexicana), EU259642 (Z. m. parviglumis), and EU259643 (Z. perennis). The accession numbers for the maize terpene synthases TPS10 and TPS4 are AAS88571 and AAX99146, respectively. The accession numbers of (E)-β-caryophyllene synthases from other plants are AAO85539 (Arabidopsis At TPS27), AAL79181 (Artemisia annua QHS1), AAU05952 (Cucumis sativus), and ABJ16553 (Oryza sativa Os TPS3), and the accession number of the Arabidopsis diterpene synthase At DTPS is NP_178064.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Amino Acid Alignment of the Putative TPS23 Orthologs from Maize and the Teosinte Species Z. parviglumis, Z. luxurians, Z. mays mexicana, Z. diploperennis, Z. perennis, and Z. huehuetenangensis.
Supplemental Figure 2. Sesquiterpene Products of the Putative TPS23 Orthologs.
Supplemental Data Set 1. Amino Acid Alignment Used to Produce the Dendrogram Presented in Figure 7.
Supplemental Data Set 2. Amino Acid Alignment Used to Produce the Dendrogram Presented in Figure 11.
Supplementary Material
Acknowledgments
We thank Jens Wurlitzer for excellent technical assistance. We are also indebted to KWS Seeds for maize B73 seeds and Syngenta for Spodoptera littoralis. The inbred lines were kindly provided by the Station de Génétique Végétale, Institut National de la Recherche Agronomique Ferme du Moulon, and the National Germplasm System of the USDA Agricultural Research Service. This work was supported by the German National Science Foundation (Grant DE-837/2-2) and the Max Planck Society and in part by the Swiss National Center of Competence in Research, Plant Survival.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jörg Degenhardt (degenhardt@ice.mpg.de).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. [DOI] [PubMed] [Google Scholar]
- Buckler, E.S., Goodman, M.M., Holtsford, T.P., Doebley, J.F., and Sanchez, J. (2006). Phylogeography of the wild subspecies of Zea mays. Maydica 51 123–134. [Google Scholar]
- Cai, Y., Jia, J.W., Crock, J., Lin, Z.X., Chen, X.Y., and Croteau, R. (2002). A cDNA clone for β-caryophyllene synthase from Artemisia annua. Phytochemistry 61 523–529. [DOI] [PubMed] [Google Scholar]
- Chen, X.J., Wang, M., Chen, Y., Davisson, V.J., and Heinstein, P. (1996). Cloning and heterologous expression of a second (+)-δ-cadinene synthase from Gossypium arboreum. J. Nat. Prod. 59 944–951. [DOI] [PubMed] [Google Scholar]
- Cheng, A.-X., Xiang, C.-Y., Li, J.-X., Yang, C.-Q., Hu, W.-L., Wang, L.-J., Lou, Y.-G., and Chen, X.-Y. (2007). The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry. 68 1632–1641. [DOI] [PubMed] [Google Scholar]
- Crock, J., Wildung, M., and Croteau, R. (1997). Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha × piperita, L.) that produces the aphid alarm pheromone (E)-β-farnesene. Proc. Natl. Acad. Sci. USA 94 12833–12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera, N., Boronat, A., and Ferrer, A. (1997). The Arabidopsis thaliana fps1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. J. Biol. Chem. 272 15381–15388. [DOI] [PubMed] [Google Scholar]
- Curran, J. (1992). Influence of application method and pest population size on the field efficacy of entomopathogenic nematodes. J. Nematol. 24 631–636. [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, M., Held, M., Triponez, Y., and Turlings, T.C.J. (2006). The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol. 32 2733–2748. [DOI] [PubMed] [Google Scholar]
- D'Alessandro, M., and Turlings, T.C.J. (2005). In situ modification of herbivore-induced plant odors: A novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses 30 739–753. [DOI] [PubMed] [Google Scholar]
- Daraselia, N.D., Tarchevskaya, S., and Narita, J.O. (1996). The promoter for tomato 3-hydroxy-3-methylglutaryl coenzyme A reductase gene 2 has unusual regulatory elements that direct high-level expression. Plant Physiol. 11 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen, T., Dillmann, C., Marion-Poll, F., and Turlings, T.C.J. (2004). High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 135 1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke, M. (1999). Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91 131–142. [Google Scholar]
- Dicke, M., and van Loon, J.J.A. (2000). Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97 237–249. [Google Scholar]
- Ellsbury, M.M., Jackson, J.J., Woodson, W.D., Beck, D.L., and Stange, K.A. (1996). Efficacy, application distribution, and concentration by stemflow of Steinernema carpocapsae (Rhabditida: Steinernematidae) suspensions applied with a later-move irrigation system for corn rootworm (Coleoptera: Chrysomelidae) control in maize. J. Econ. Entomol. 89 74–81. [Google Scholar]
- Enjuto, M., Lumbreras, V., Marín, C., and Boronat, A. (1995). Expression of the Arabidopsis HMG2 gene, encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase, restricted to meristematic and floral tissues. Plant Cell 7 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon, J., and Kreis, W. (1999). Biosynthesis of monoterpenes, sesquiterpenes, diterpenes, sterols, cardiac glycosides and steroid saponins. In Biochemistry of Plant Secondary Metabolism, Annual Plant Reviews, Vol. 2, M. Wink, ed (Sheffield, UK: Sheffield Academic Press), pp. 222–299.
- Gouinguené, S., Degen, T., and Turlings, T.C.J. (2001). Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11 9–16. [Google Scholar]
- Grosjean, D., Williams, E.L., Grosjean, E., Andino, J.M., and Seinfeld, J.H. (1993). Atmospheric oxidation of biogenic hydrocarbons: Reaction of ozone with β-pinene, d-limonene and trans-caryophyllene. Environ. Sci. Technol. 27 2754–2758. [Google Scholar]
- Hammack, L. (2001). Single and blended maize volatiles as attractants for diabroticite corn rootworm beetles. J. Chem. Ecol. 27 1373–1390. [DOI] [PubMed] [Google Scholar]
- Hass, B., Griffin, C.T., and Downes, M.J. (1999). Persistence of Heterorhabditis infective juveniles in soil: Comparison of extraction and infectivity measurements. J. Nematol. 31 508–516. [PMC free article] [PubMed] [Google Scholar]
- Hiltpold, I., and Turlings, T.C.J. (2008). Belowground chemical signaling in maize: When simplicity rhymes with efficiency. J. Chem. Ecol., in press. [DOI] [PubMed]
- Hoballah, M.E., Köllner, T.G., Degenhardt, J., and Turlings, T.C.J. (2004). Costs of induced volatile production in maize. Oikos 105 168–180. [Google Scholar]
- Hoballah, M.E., Tamò, C., and Turlings, T.C.J. (2002). Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J. Chem. Ecol. 28 951–968. [DOI] [PubMed] [Google Scholar]
- Jackson, J.J. (1996). Field performance of entomopathogenic nematodes for suppression of western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 89 366–372. [DOI] [PubMed] [Google Scholar]
- Karban, R., and Baldwin, I.T. (1999). Induced Responses to Herbivory. (Chicago: University of Chicago Press).
- Kessler, A., and Baldwin, I.T. (2002). Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53 299–328. [DOI] [PubMed] [Google Scholar]
- Köllner, T.G., O'Maille, P.E., Gatto, N., Boland, W., Gershenzon, J., and Degenhardt, J. (2006). Two pockets in the active site of maize sesquiterpene synthase TPS4 carry out sequential parts of the reaction scheme resulting in multiple products. Arch. Biochem. Biophys. 448 83–92. [DOI] [PubMed] [Google Scholar]
- Köllner, T.G., Schnee, C., Gershenzon, J., and Degenhardt, J. (2004. a). The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distributions. Phytochemistry 65 1895–1902. [DOI] [PubMed] [Google Scholar]
- Köllner, T.G., Schnee, C., Gershenzon, J., and Degenhardt, J. (2004. b). The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16 1115–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth, K.L., Stermer, B.A., Bhattacharyya, M.K., and Dixon, R.A. (1997). HMG-CoA reductase gene families that differentially accumulate transcripts in potato tubers are developmentally expressed in floral tissues. Plant Mol. Biol. 33 545–551. [DOI] [PubMed] [Google Scholar]
- Liu, K., Goodman, M., Muse, S., Smith, J.S., Buckler, E., and Doebley, J. (2003). Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 164 2117–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners, T., and Hilker, M. (2000). Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol. 26 221–232. [Google Scholar]
- Mercke, P., Kappers, I.F., Verstappen, F.W.A., Vorst, O., Dicke, M., and Bouwmeester, H.J. (2004). Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol. 135 2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M., and Gojobori, T. (1986). Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3 418–426. [DOI] [PubMed] [Google Scholar]
- Okada, K., Saito, T., Nakagawa, T., Kawamukai, M., and Komiya, Y. (2000). Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol. 122 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud, S., Brodelius, M., and Brodelius, P.E. (2005). Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry 66 961–967. [DOI] [PubMed] [Google Scholar]
- Picaud, S., Olsson, M.E., Brodelius, M., and Brodelius, P.E. (2006). Cloning, expression, purification and characterization of recombinant (+)-germacrene D synthase from Zingiber officinale. Arch. Biochem. Biophys. 452 17–28. [DOI] [PubMed] [Google Scholar]
- Pichersky, E., and Gang, D.R. (2000). Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 5 439–445. [DOI] [PubMed] [Google Scholar]
- Rasmann, S., Köllner, T.G., Degenhardt, J., Hiltpold, I., Toepfer, S., Kuhlmann, U., Gershenzon, J., and Turlings, T.C.J. (2005). Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434 732–737. [DOI] [PubMed] [Google Scholar]
- Ricard, I., and Davison, A.C. (2007). Statistical inference for olfactometer data. Appl. Stat. 56 479–492. [Google Scholar]
- Röse, U.S.R., Manukian, A., Heath, R.R., and Tumlinson, J.H. (1996). Volatile semiochemicals released from undamaged cotton leaves. A systemic response of living plants to caterpillar damage. Plant Physiol. 111 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabulal, B., Dan, M., Anil, J.J., Kurup, R., Pradeep, N.S., Valsamma, R.K., and George, V. (2006). Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 67 2469–2473. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schnee, C., Köllner, T.G., Gershenzon, J., and Degenhardt, J. (2002). The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 130 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee, C., Köllner, T.G., Held, M., Turlings, T.C.J., Gershenzon, J., and Degenhardt, J. (2006). The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 103 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, B., Zheng, Z., and Dooner, H.K. (2001). A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: Characterization of wild-type and mutant alleles. Proc. Natl. Acad. Sci. USA 97 14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo, A. ( 1997) Constituents of wild food plants. In Functionality of Food Phytochemicals, T. Jones and J. Romeo, eds (New York: Plenum Press), pp. 89–111.
- Starks, C.M., Back, K., Chappell, J., and Noel, J.P. (1997). Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 277 1815–1820. [DOI] [PubMed] [Google Scholar]
- Tholl, D., Chen, F., Petri, J., Gershenzon, J., and Pichersky, E. (2005). Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 42 757–771. [DOI] [PubMed] [Google Scholar]
- Trapp, S.C., and Croteau, R. (2001). Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158 811–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings, T.C.J., Bernasconi, M., Bertossa, R., Bigler, F., Caloz, G., and Dorn, S. (1998). The induction of volatile emissions in maize by three herbivore species with different feeding habits: Possible consequences for their natural enemies. Biol. Control 11 122–129. [Google Scholar]
- Turlings, T.C.J., Davison, A., and Tamò, C. (2004). A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 29 45–55. [Google Scholar]
- Turlings, T.C.J., Tumlinson, J.H., Heat, R.R., Proveaux, A.T., and Doolittle, R.R. (1991). Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 17 2235–2251. [DOI] [PubMed] [Google Scholar]
- Turlings, T.C.J., Tumlinson, J.H., and Lewis, W.J. (1990). Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253. [DOI] [PubMed] [Google Scholar]
- Van de Peer, Y., and De Wachter, Y. (1994). TREECON for Windows: A software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10 569–570. [DOI] [PubMed] [Google Scholar]
- Walter, M.H., Hans, J., and Strack, D. (2002). Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: Differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J. 31 243–254. [DOI] [PubMed] [Google Scholar]
- Yoshikuni, Y., Ferrin, T.E., and Keasling, J.D. (2006). Designed divergent evolution of enzyme function. Nature 440 1078–1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.