Abstract
Xanthine dehydrogenase/oxidase (XDH/XO) is associated with various pathological conditions related to the endothelial injury. However, the molecular mechanism underlying the activation of XDH/XO by hypoxia remains largely unknown. In this report, we determined whether the Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) signaling pathway is involved in hypoxia-induced activation of XDH/XO in primary cultures of lung microvascular endothelial cells (LMVEC). We found that hypoxia significantly increased interleukin 6 (IL6) production in a time-dependent manner in LMVEC. Hypoxia also markedly augmented phosphorylation/activation of JAKs (JAK1, JAK2 and JAK3) and the JAK downstream effectors STATs (STAT3 and STAT5). Hypoxia-induced activation of STAT3 was blocked by IL6 antibodies, the JAK inhibitor AG490 and the suppressor of cytokine signaling 3 (SOCS3), implying that hypoxia-promoted IL6 secretion activates the JAK/STAT pathway in LMVEC. Phosphorylation and DNA-binding activity of STAT3 was also inhibited by the p38 MAPK inhibitor SB203580 and the phosphatidylinositol 3-kinase inhibitor LY294002, suggesting that multiple signaling pathways involved in STAT activation by hypoxia. Importantly, hypoxia promoted XDH/XO activation in LMVEC, which was markedly reversed by inhibiting the JAK/STAT pathway using IL6 antibodies, AG490 and SOCS3. These data demonstrated that JAKs, STATs and XDH/XO were sequentially activated by hypoxia. These data also provide the first evidence indicating that the JAK-STAT pathway is involved in hypoxia-mediated XDH/XO activation in LMVEC.
Keywords: anoxia, interleukin 6, Janus kinases, signal transducers and activators of transcription, xanthine dehydrogenase/oxidase, lung microvascular endothelial cells
1. Introduction
Xanthine oxidase (XO), a major generator of the reactive O2 species (ROS), has been implicated in various pathological conditions related to the endothelial injury, such as ischemia-reperfusion injury and hypoxic-reoxygenation-induced lung injury (Berry & Hare, 2004; Cai, 2005; Beetsch et al., 1998). XO is derived from xanthine dehydrogenase (XDH) through posttranslational modifications. XDH and XO catalyze the oxidation of hypoxanthine to xanthine, and xanthine to uric acid, respectively, resulting in the formation of ROS. It has been well demonstrated that hypoxia induces XDH/XO activation and that XDH/XO activation is directly linked to their phosphorylation (Terada et al., 1992; Dupont et al., 1992; Kayyali et al., 2001; Mervaala et al., 2001; Terada et al., 1997; Poss et al., 1996; Sohn et al., 2003; Kang et al., 2006). However, the molecular mechanism underlying the activation of XDH/XO by hypoxia remains largely undefined.
A number of studies have demonstrated that secretion of interleukins is facilitated by hypoxia (Yan et al., 1995, 1997; Tamm et al., 1998; Ziesche et al., 1996; Kotake-Nara et al., 2005; Sawa et al., 1998; Hartmann et al., 2000). Several studies have also indicated that Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) are activated by interleukin 6 (IL6) in cardiomyocytes, macrophages, carcinoma cells and neurons and by hypoxia in pulmonary arterial smooth muscle cells, rat cardiomyocytes, retinal microvascular endothelial cells (Negoro et al., 2001; Mascareno et al., 2005; Wang et al., 2005; Dawn et al., 2004; Lee et al., 2006; Yamauchi et al., 2006; Terrell et al., 2006). STATs comprise a family of seven transcription factors that are activated by the JAK family of protein tyrosine kinases in response to stimulation by cytokines, hormones and growth factors. Once phosphorylated by JAKs, STATs undergo dimerization and translocation to the nucleus. Phosphorylated STATs in nucleus function as transcriptional factors activating transcriptional expression of a number of genes (Levy & Darnell, 2002; Vinkemeier, 2004). STATs are critical mediators of signal transduction and transcription in many cell types, including human umbilical vein endothelial cells, murine aortic endothelial cells and smooth muscle cells (Levy & Darnell, 2002; Vinkemeier, 2004; Deo et al., 2004; Ni et al., 2004). However, whether hypoxia could induce IL release and subsequently activate JAK-STAT pathway and the role of the JAK-STAT signaling pathway in the activation of XDH/XO in lung microvascular endothelial cells (LMVEC), which are critical targets in both hypoxia- and regeneration-mediated lung injury, have not been defined.
In this report, our data demonstrated that hypoxia induces the production of IL6 and the activation of JAKs/STATs and XDH/XO, and that the XDH/XO activation can be blocked by attenuating the JAK/STAT signaling pathway, providing the first evidence indicating a link between XDH/XO activation and the JAK/STAT pathway in hypoxic LMVEC.
2. Experimental procedures
2.1. Materials
Rats were obtained from Chongqing City Laboratory Animal Center, Chongqing, China. The suppressor of cytokine signaling 3 (SOCS3) in the pEF-FLAG-1 vector was kindly provided by Dr. Tracy Willson (The Walter and Eliza Hall Institute of Medical Research, Victoria, Australia). Dulbecco's modified Eagle's medium (DMEM), trypsin and fetal bovine serum (FBS) were purchased from GIBCO (Invitrogen, Carlsbad, CA). AG490 (a JAK2 inhibitor), SB202190 (a p38 inhibitor), PD98059 (an MEK inhibitor) and LY294002 [a phosphatidylinositol 3-kinase (PI3K) inhibitor) were obtained from Calbiochem. Monoclonal antibodies against IL6 was purchased from R&D Systems Inc. (Minneapolis, MN). Antibodies against hypoxanthine phosphoribosyl transferase (HPRT) and phospho-JAK3 were from Santa Cruz Biotechnology. Antibodies against JAK1, JAK3, phospho-JAK1, STAT3, STAT5, phosphor-STAT3 and phosphos-STAT5 were from Cell Signaling technology (Beverly, MA). Antibodies against JAK2 and phosphor-JAK2, horseradish peroxidase-conjugated goat anti-rabbit IgG were from Upstate Cell Signaling Solutions (Lake Placid, NY). All other materials were described elsewhere (Wang et al., 2005; Wu et al., 2003).
2.2. Cell culture and hypoxia
LMVEC were isolated from the peripheral lung tissue of rats (Chen et al., 1995). Briefly, the lung tissue was cut into small pieces and cultured with DMEM containing 20% heat-inactivated low endotoxin FBS, 90 μg/ml heparin, 4 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 95% air - 5% CO2. The cells were passaged by harvesting with 0.25% trypsin containing 0.02% EDTA and cultured in 25-cm2 flask in DMEM with 10% FBS as described (Wu et al., 2003). LMVEC between passages 4 and 12 were grown on 35-mm plates to 95% confluence and then cultured in DMEM containing 0.1% FBS for 24 h. LMVEC were transferred into an air-tight hypoxic chamber (Thermo electron, USA)) containing 3% O2 - 5% CO2 - N2 for different periods of time.
2.3. Drug treatment and cell transfection
For drug treatment, cultured LMVEC were preincubated with 30 μM AG490, 10μM SB202190, 15 μM LY294002 or 25 μM PD98059 for 45 min before hypoxia. To determine the effect of the IL6 antibodies on the activation of STATs and XDH/XO, the antibodies diluted in DMEM were added to the culture medium at a final concentration of 3 μg/ml and incubated for 24 h.
To determine the effect of SOCS3 on the activation of STAT3 and XDH/XO, LMVEC were transiently transfected with SOCS3 or empty pEF-FLAG-1 vector using Lipofectamine 2000 (Invitrogen) as described previously (Duvernay et al., 2004). Briefly, LMVEC were cultured on 24-well plates at 90% confluence and transfected with 1 μg of SOCS3 or empty pEF-FLAG-1 vector using 2 μl of Lipofectamine 2000. After incubation for 48 h, the cells were transferred to the hypoxic incubator for additional 8 h. Transient transfection of the empty pEF-FLAG-1 did not produce any effect on the activation of both STAT3 and XDH/XO as compared with cells without transfection.
2.4. Quantification of IL6 concentrations
The IL-6 concentrations in cultured LMVEC medium were measured by ELISA from R & D systems Inc. (Minneapolis, MN) following the manufacturer's instructions. Culture medium was collected at 2, 6, 8, 12 h intervals after hypoxia and each sample was measured in duplicate.
2.5. Electrophoretic mobility shift assays (EMSA)
Cells were washed in cold PBS, lysed in buffer (15 mM KCl, 10 mM HEPES, pH 7.6, 2 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.1% Nonidet P-40, 0.5 mM PMSF, 2.5 μg/ml leupeptin, 5 μg/ml antipain and 5 μg/ml aprotinin) for 10 min on ice, and centrifuged at 14,000 × g for 20 sec at 4°C. Proteins in the nuclei were extracted by incubation at 4°C with vigorous vortex in buffer A (420 mM NaCl, 20 mM HEPES, pH 7.9, 0.2 mM EDTA, 25% glycerol, 1 mM DTT, 0.5 mM PMSF, 2.5 μg/ml leupeptin, 5 μg/ml antipain, and 5 μg/ml aprotinin) followed by centrifugation at 13,000 × g for 30 min at 4°C. The supernatant extract was collected and stored at −80°C. The probes were double-stranded oligonucleotides containing a STAT3 consensus oligonucleotide (5'-GATCCTTCTGGGAATTCCTAGATC-3'; Santa Cruz Biotechnology, Santa Cruz, CA) and end-labeled with [γ-32P]-ATP (Yahui Biological and Medical Engineering, Beijing). DNA binding reactions were performed in a 25-μl reaction mixture containing 6 μl of nuclear extract (1 mg/ml) and 5 μl of 5 × binding buffer (20% Ficoll, 50 mM HEPES, pH 7.9, 5 mM EDTA and 5 mM DTT). The remainder of the reaction mixture contained 50 mM KCl, 0.1% Nonidet P-40, 1 μg of poly(dI-dC) and 200 pg of the probes. Samples were separated through 5.5% polyacrylamide gels and then exposed to x-ray film.
2.6. Measurement of XDH/XO activity
The XDH/XO activity was measured using a fluorimetric assay as described (Kayyali et al., 2001, 2003; Beckman et al., 1989). Cells were collected and sonicated in 50 mM sodium phosphate (pH 7.4), 1.5 mg/ml DTT and 1 × protease inhibitor mixture 3 (Calbiochem). After centrifugation at 10,000 × g for 5 min, the supernatant was collected and XDH/XO activity assayed immediately or stored at −80°C.
2.7. Immunoblotting
Western blotting was carried out as described previously (Duvernay et al., 2004). Twenty μg of protein samples was separated by 4−12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The signal was detected by enhanced chemiluminescence detection system (ECL Plus, Amersham, Buckinghamshire, England). Densitometric analysis was performed using Quantity One software (Bio-Rad). The phosporylation of JAKs and STATs are normalized to their total protein expression.
2.8. Statistical analysis
Statistical comparisons were performed using the paired, two-tailed Student's t-test for experiments consisting of two groups only and with one-way ANOVA with a multiple comparison method for experiments consisting of more than two groups. Results were considered statistically significant when P < 0.05. Data are presented as mean ± S.E.
3. Results
3.1. Effect of hypoxia on the production of IL6 in rat LMVEC
Rat LMVEC were cultured and exposed to hypoxia for 2 to 12 h and IL6 production in culture medium was measured by ELISA. IL6 production was time-dependently increased in LMVEC upon exposure to hypoxia (Fig. 1). IL6 expression was increased by 2.2 folds (59.8 pg/ml) at 2 h hypoxia compared with normoxia control (27.2 pg/ml). At 12 h, the level of IL6 in the medium reached 104.6 pg/ml. Pretreatment with the IL6 monoclonal antibodies at for 24 h profoundly blocked IL6 production by 94%. These results indicate that hypoxia induces IL-6 secretion to the medium from LMVEC.
Fig. 1.
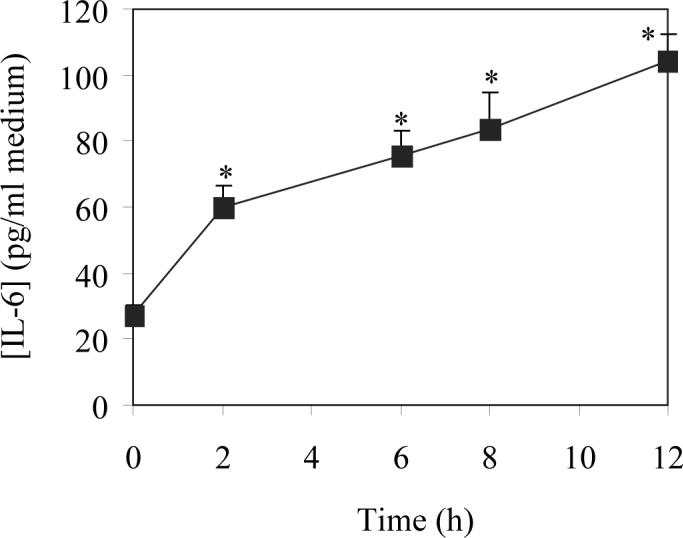
Effect of hypoxia on the secretion of IL6 in LMVEC. Isolated LMVEC were cultured and subjected to hypoxia for 2, 6, 8 and 12 h as described under “Experimental procedures”. The concentrations of IL6 in cultured medium were measured by ELISA. The data are presented as the means ± SE of three separated experiments. * P<0.05 versus normoxic control (time 0).
3.2. Effect of hypoxia on the activation of JAKs in rat LMVEC
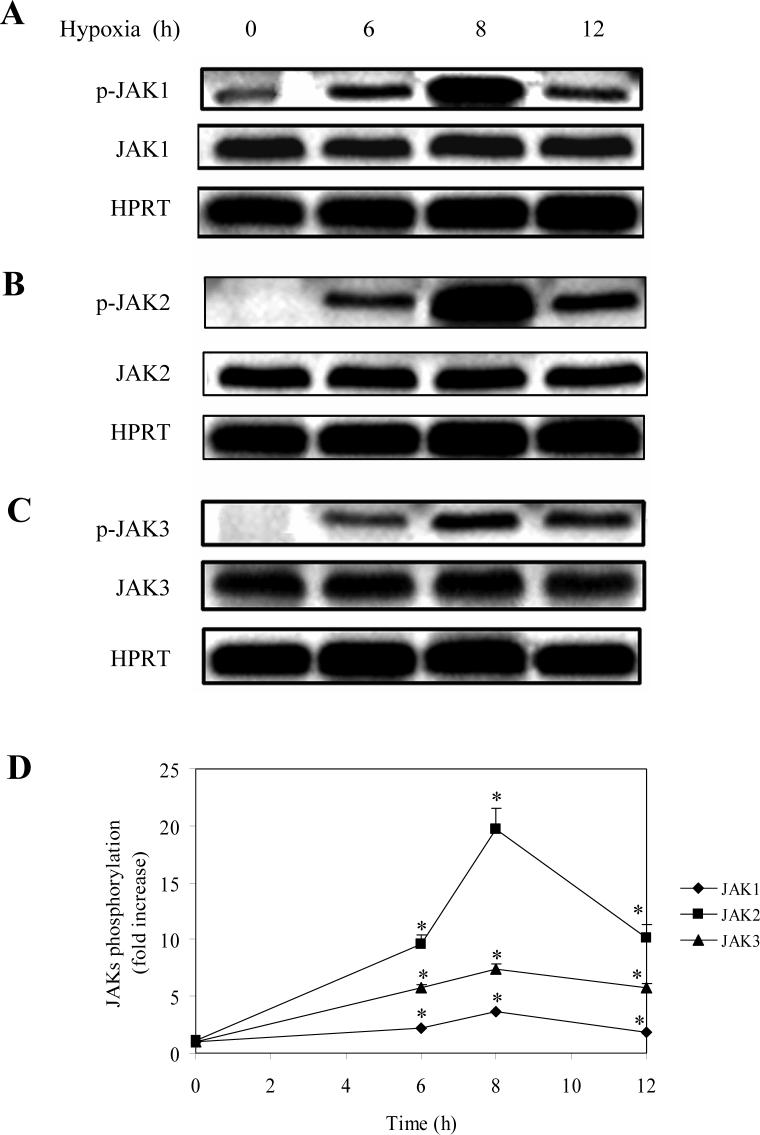
We then determined the effect of hypoxia on the phosphorylation of JAKs. LMVEC was exposed to hypoxia for 6, 8 and 12 h and the phosphorylation of JAK1, JAK2 and JAK3 measured by immunoblotting using specific antibodies against phosphorylated JAKs. Phosphorylation of JAK1, JAK2 and JAK3 was slightly increased at 2 after hypoxia (data not shown) and significantly augmented at 6 h as compared with the normoxia controls (Fig. 2). The maximal increase in JAK1, JAK2 and JAK3 phosphorylation was achieved at 8 h after hypoxia (Fig. 2). Furthermore, hypoxia-induced phosphorylation of JAK2 in LMVEC was blocked by 39% by pretreatment with the JAK2-specific inhibitor AG490 for 30 min (Fig. 3). These data demonstrate that hypoxia strongly induce phosphorylation/activation of JAKs.
Fig. 2.
Effect of hypoxia on the phosphorylation of JAK1, JAK2 and JAK3. LMVEC were subjected to hypoxia for 6, 8 and 10 h. A, B and C. Representative gels showing phosphorylation/activation of JAK1 (A), JAK2 (B) and JAK3 (C). Upper panel – phsophorylated JAKs; middle panel – total JAKs; lower panel – expression of HPRT as loading controls. B. Quantitative data of JAK phosphorylation normalized to the total JAK expression. The data shown are fold increase and are presented as the means ± SE of three individual experiments. * P<0.05 versus their respective normoxic controls (time 0).
Fig. 3.
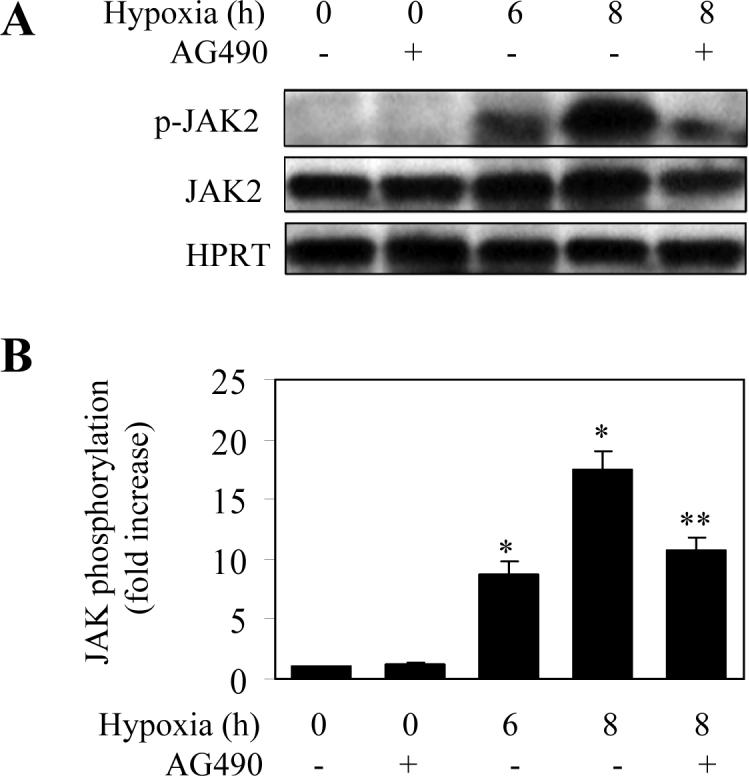
Effect of AG490 on hypoxia-induced phosphorylation of JAK2. LMVEC were subjected to hypoxia for 6 and 8 h and the phosphorylation of JAK2 was determined by Western blotting using the phosphor-JAK2 antibodies. To determine the effect of the JAK inhibitor AG490 on hypoxia-induced JAK2 phosphorylation, LMVEC were preincubated with AG490 at a final concentration of 30 μM for 30 min before hypoxia. A. Representative blots showing phosphorylated JAK2 (upper panel), total JAK2 expression (middle panel) and HPRT expression (lower panel). B. Quantitative data of JAK2 phosphorylation after hypoxia. The data are presented as the means ± SE of three separate experiments. * P<0.05 versus the normoxic control (time 0) and ** P<0.05 versus hypoxia for 8 h.
3.3. Effect of hypoxia on the activation of STAT3 and STAT5 in rat LMVEC
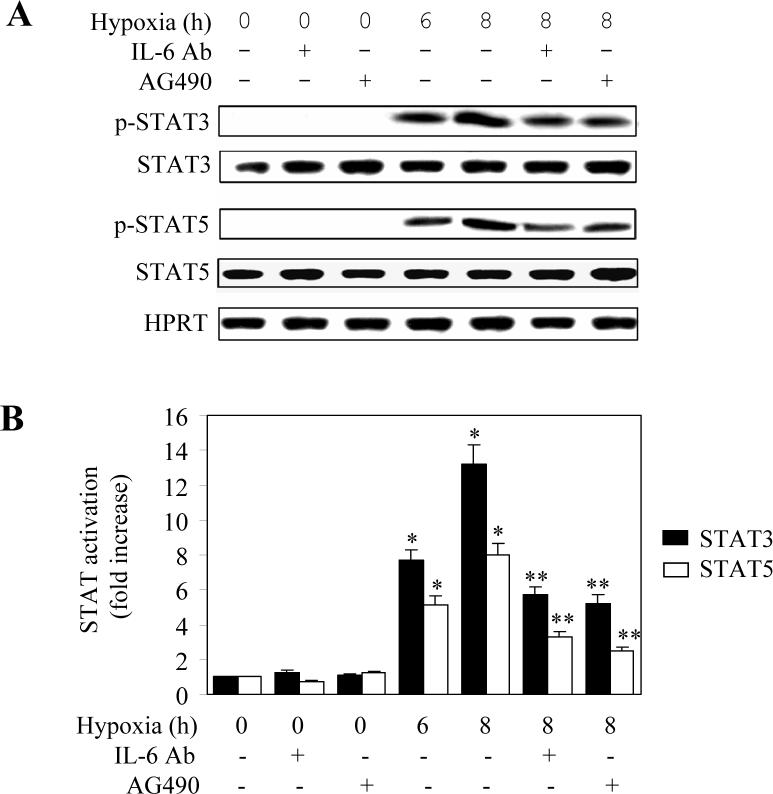
As JAK family members are the upstream activators of STATs, we sought to investigate whether hypoxia could induce STAT activation through activating the IL6-JAK pathway in LMVEC. Activation of STAT3 and STAT5 was measured by Western blot using antibodies against their phosphorylated forms. The phosphorylation levels of STAT3 or STAT5 were dramatically increased at 6 and 8 h after hypoxia (Fig. 4A and 4B). Hypoxia-induced phosphorylation of STAT3 or STAT5 was attenuated by greater than 60% by pretreatment with IL6 antibodies and the JAK2-specific inhibitor AG490 (Fig. 4A and 4B). Furthermore, hypoxia-induced phosphorylation of STAT3 was markedly blocked by transient expression of SOCS3 (Fig. 4C). These data indicate that activation of STAT3 and STAT5 in hypoxic LMVEC is mediated, at least in part, through the IL6-JAK pathway.
Fig. 4.
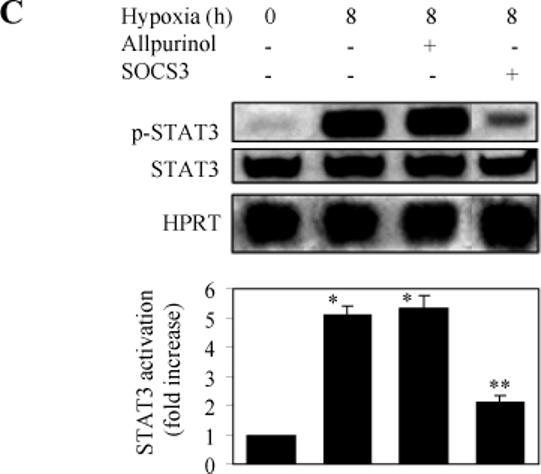
Effect of hypoxia on the phosphorylation of STAT3 and STAT5. LMVEC were subjected to hypoxia for 6 and 8 h and the phosphorylation of STAT3 and STAT5 was measured by Western blotting using the phosphor-STAT-specific antibodies. Cultured LMVEC were preincubated with AG490, anti-IL6 antibodies, allopurinol, or transient transfection of SOCS3 as described under “Experimental procedures”. A. Representative Western blots showing phosphorylated JAK2, total JAK2 expression and HPRT expression. B. Quantitative data of STAT3 and SAT5 phosphorylation after hypoxia. The data are presented as the means ± SE of three separate experiments. * P<0.05 versus their respective normoxic controls (time 0) and ** P<0.05 versus their respective cells subjected to hypoxia for 8 h. C. Effect of allopurinol and SOCS3 on the hypoxia-induced STAT3 phosphorylation. Upper panel - representative Western blots showing phosphorylated STAT3, total STAT3 and HPRT expression; Lower panel - quantitative data of STAT3 phosphorylation. The data are presented as the means ± SE of three separate experiments. * P<0.05 versus the normoxic control (time 0) and ** P<0.05 versus hypoxia for 8 h.
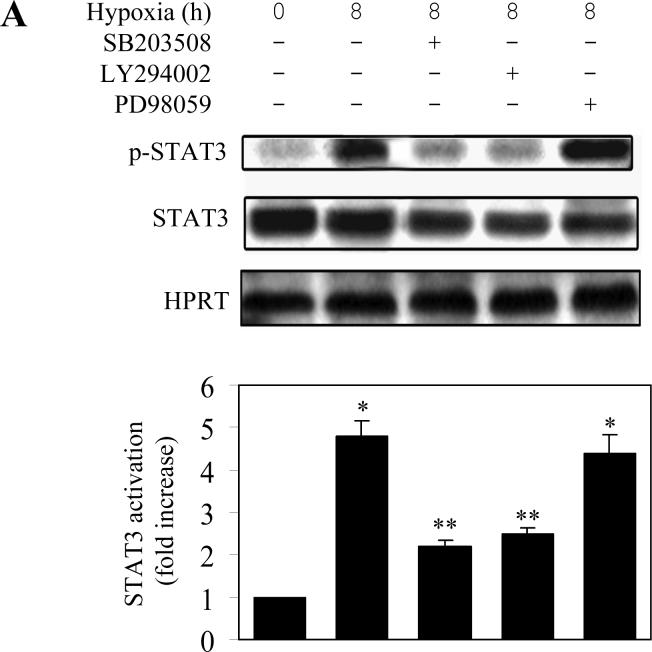
It has been demonstrated that, in addition to the JAK-STAT pathway, IL6 also activates the MAPK cascade and the PI3K pathway (Lee et al., 2007; Lehmann et al., 2003; Zauberman et al., 1999; Zhao et al., 1999). It has also been demonstrated that hypoxia activates the MAPK and PI3K pathways (Eguchi et al., 2007; Jin et al., 2007; Kayyali et al., 2001). To further dissect the pathway mediating STAT activation by hypoxia, we determined whether MAPK and PI3K were involved. The hypoxia-induced STAT3 phosphorylation was significantly blocked by pretreatment with the p38 MAPK inhibitor SB203580 and the PI3K inhibitor LY294002 (Fig. 5A). In contrast, pretreatment with the ERK1/2 inhibitor PD98059 did not produce any clear effect on the STAT3 activation induced by hypoxia (Fig. 5A).
Fig. 5.
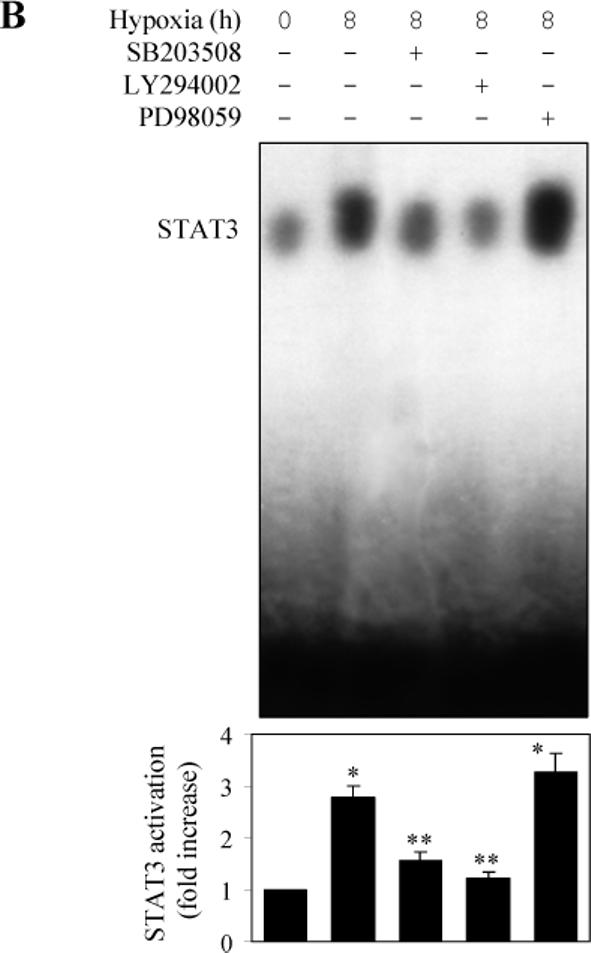
Effect of protein kinase inhibitors on hypoxia-induced STAT3 activation in LMVEC. LMVEC were incubated with the p38 MAPK inhibitor SB203508 (10 μM), the PI3K inhibitor LY29002 (15 μM) or the MEK inhibitor PD98059 (25 μM) for 45 min and then subjected to hypoxia for 8 h. A. effect on hypoxia-induced STAT3 phosphorylation of the protein kinase inhibitors as determined by Western blotting. Upper panel - representative Western blots; lower panel – quantitative data. B. Effect on STAT3 DNA-binding activity of the protein kinase inhibitors as measured by electrophoretic mobility shift assays using a [γ-32P]-labeled double-stranded oligonucleotides containing a STAT3 consensus oligonucleotide as described under “Experimental Procedures”. Upper panel - representative image; lower panel – quantitative data. In A and B, quantitative data are presented as the means ± SE of three separate experiments. * P<0.05 versus the normoxic control (time 0) and ** P<0.05 versus hypoxia for 8 h.
To confirm whether p38 and PI3K were involved in the STAT3 activation, we determined whether the DNA-binding activity of STAT3 could be altered by these kinase inhibitors in the electrophoretic mobility-shift assay. The intensity of the STAT3 DNA-binding band was much stronger at 8 h after hypoxia as compared with control. Pretreatment with SB203580 and LY294002, but not PD98059, profoundly attenuated the DNA-binding ability of STAT3 induced by hypoxia (Fig. 5B). These results suggest that the p38 MAPK and PI3K may be involved in hypoxia-induced STAT3 activation in LMVEC.
3.4. Effect of hypoxia on the activation of XDH/XO in rat LMVEC
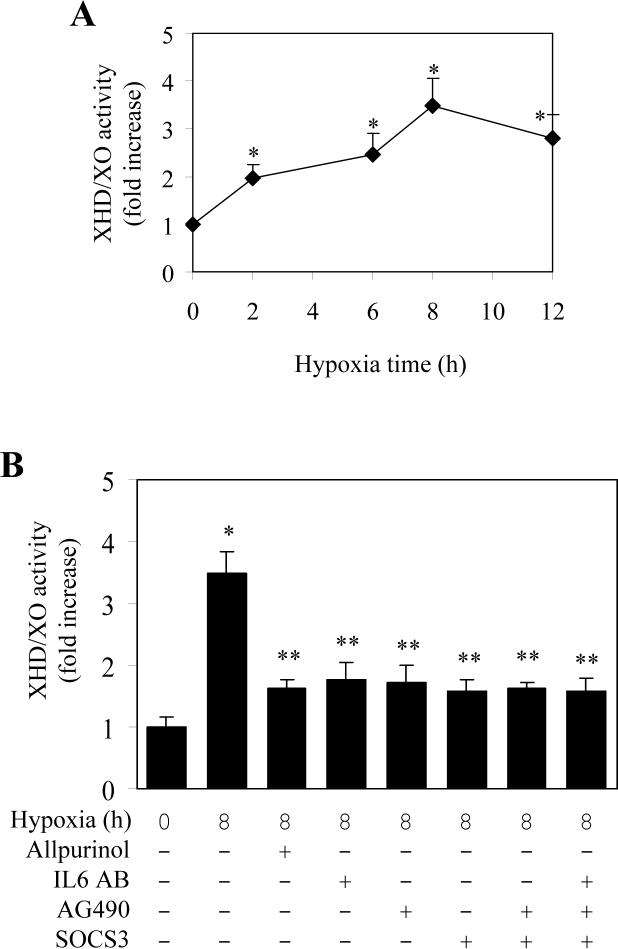
The preceding results have demonstrated that hypoxia induced activation of IL6-JAK-STAT pathway. We next determined if this pathway is involved in the activation of XDH/XO. LMVEC were exposed to hypoxia for different lengths of time and effect of hypoxia on the total XDH/XO enzymatic activity was measured. Hypoxia induced activation of XDH/XO in a time-dependent manner. A significant increase in XDH/XO activity was observed after exposing the LMVEC to hypoxia for 2 h and the total XDH/XO activity was increased by 3.5 folds at 8 h after hypoxia (Fig. 6A). Pretreatment with allopurinol, an inhibitor and scavenger of XDH/XO, attenuated the XDH/XO activation, but had no influence on the activation of STAT3 (Fig. 4C).
Fig. 6.
Hypoxia-mediated XDH/XO activation and its inhibition by blocking the JAK/STAT pathway in LMVEC. A. Effect of hypoxia on the activation of XDH/XO. LMVEC were cultured and subjected to hypoxia for 2, 6, 8 and 12 h and the activation of XDH/XO was measured as described under “Experimental Procedures”. The data are presented as the means ± SE of three separate experiments. * P<0.05 versus normoxic control (time 0). B. Effect of protein inhibitors on hypoxia-induced XDH/XO activation. LMVEC were transiently transfected with SOCS3 or without transfection and then incubated with allopurinol, IL6 antibodies or AG490 individually or in combination. The LMVEC were then subjected to hypoxia for 8 h. The data are presented as the means ± SE of three separate experiments. * P<0.05 versus the normoxic control (time 0) and ** P<0.05 versus hypoxia for 8 h.
To define if the IL6-JAK-STAT pathway was involved in the activation of XDH/XO, we determined the effect of IL-6 antibodies and the JAK2 inhibitor AG490 on the XDH/XO activation. Pretreatment with IL-6 antibodies and AG490 inhibited hypoxia-mediated XDH/XO activation by approximately 50% (Fig. 6B). Transient expression of SOCS3 also blocked XDH/XO activation induced by hypoxia (Fig. 6B). These data strongly indicate that XDH/XO activation induced by hypoxia in LMVEC is mediated through IL6-JAK-STAT pathway.
As the treatment with IL-6 antibodies and AG490 and transient expression of SOCS3 only partially inhibited XDH/XO activation induced by hypoxia, we then determined the effect of combination of these inhibitors on the activation of XDH/XO by hypoxia. Similar to individual inhibitor, combination of AG490 and SOCS3 as well as IL-6 antibodies, AG490 and SOCS3 did not increase their inhibitory effect on hypoxia-induced XDH/XO activation (Fig. 6B). These data suggest that, in addition to the IL-6-JAKs-STATs pathway, other signaling pathways may also be involved in the activation of XDH/XO by hypoxia.
4. Discussion
It has been demonstrated that hypoxia activates XDH/XO, an important source of reactive oxygen species (ROS) (Terada et al., 1992; Dupont et al., 1992; Kayyali et al., 2001; Mervaala et al., 2001; Terada et al., 1997; Poss et al., 1996; Sohn et al., 2003; Kang et al., 2006). However, the molecular mechanism underlying the hypoxia-mediated activation of XDH/XO remains largely unknown. In this report, we demonstrated that hypoxia promotes the secretion of IL6, augments the JAK-STAT signaling and activates XDH/XO in LMVEC. Most interestingly, our data indicate that the potentiation of IL6-JAK-STAT pathway is involved in the activation of XDH/XO induced by hypoxia in LMVEC.
IL6 secretion has been demonstrated to be facilitated by hypoxia in human umbilical vein and pulmonary endothelial cells, primary human pulmonary fibroblasts and vascular smooth muscle cells, and Kupffer cells as well as whole pulmonary artery organoid cultures (Yan et al., 1995, 1997; Tamm et al., 1998; Ziesche et al., 1996; Kotake-Nara et al., 2005; Sawa et al., 1998; Hartmann et al., 2000). Our previous studies found that the level of IL-6 was increased in pulmonary tissues of rats with hypoxia-induced pulmonary hypertension (Wang et al., 2005). Consistent with these reports, our present data indicate that IL6 production was markedly increased by hypoxia in LMVEC and increase in IL6 production is dependent on the hypoxic time.
It has been well documented that the JAK/SATA signal pathway is activated by interleukins, including IL-6 (Levy & Darnell, 2002; Vinkemeier, 2004; Heinrich et al., 2003; Saura et al., 2006; Severgnini et al., 2004). Our data showed that phosphorylation, reflecting activation, of JAK1, JAK2 and JAK3 were dramatically activated in hypoxic rat LMVEC. Furthermore, hypoxia-stimulated JAK2 phosphorylation was markedly inhibited by pretreatment with the JAK2-specific inhibitor AG490. These data indicate that hypoxia activates JAKs in LMVEC.
JAK family members are the most commonly involved in the activation of STATs and the JAK-STAT signaling pathway participates in many signal transduction systems, providing an almost universal paradigm for signaling from cytokine receptors and a commonly used system for growth factor receptors and influencing growth, survival, apoptosis, host defense, stress and differentiation functions (Levy & Darnell, 2002). We demonstrated that hypoxia augmented the phosphorylation of STAT3 and STAT5, which was significantly blocked by AG490 and IL-6 antibodies. These data indicate that the IL6 and JAKs are involved in the signaling pathway mediating STAT activation by hypoxia in LMVEC.
In addition to JAKs, the p38 MAPK and PI3K may also play an important role in hypoxia-induced STAT activation in LMVEC, as we demonstrated that the p38 MAPK inhibitor SB 203580 and the PI3K inhibitor LY294002 markedly inhibited the STAT3 phosphorylation. It has been reported that hypoxia strongly activates the p38 MAPK and Rho kinase in rat pulmonary microvascular endothelial cells (An et al., 2005). Other studies have also demonstrated that peroxide treatment results in STAT3 tyrosine phosphorylation and nuclear translocation (Carballo et al., 1999]. These data suggest that hypoxia-induced tyrosine phosphorylation or activation of STATs in LMVEC may be mediated through multiple signaling pathways involving JAKs, p38 and PI3K.
Generation of ROS is one of the characteristics for hypoxia and especially for reoxygenation. Of the ROS, hydrogen peroxide (H2O2) and superoxide (O2-) are both produced in a number of cellular reactions, including the iron-catalysed Fenton reaction, and by various enzymes including XO. We found that hypoxia-mediated XDH/XO activation is dependent on the JAK/STAT pathway in primary cultures of LMVEC. We demonstrated that the XDH/XO activity significantly increased by hypoxia, consistent with the increased phosphorylation or activation of XDH/XO in response to hypoxia in other cells (Terada et al., 1992; Dupont et al., 1992; Kayyali et al., 2001; Mervaala et al., 2001; Terada et al., 1997; Poss et al., 1996; Sohn et al., 2003; Kang et al., 2006).
Interestingly, our data demonstrated that XDH/XO activation induced by could be inhibited by either depletion of IL6 using IL6 antibodies, pretreatment with the JAK2 inhibitor AG490 or transient expression of the JAK-STAT pathway blocker SOCS3 and their inhibitory effect on the XDH/XO activation was at a similar magnitude. Our data also showed that IL-6 antibodies, AG490 and SOCS3 attenuated the activation of STATs. These data strongly suggest that the IL6-JAK-STAT pathway is involved in the activation of XDH/XO by hypoxia. However, combinational use of these inhibitors did not produce more inhibitory effect on the activation of XDH/XO as compared with individual inhibitors. These data suggest that, in addition to the IL6-JAK-STAT pathway, other signaling pathways may also be involved in the activation of XDH/XO by hypoxia in LMVEC. Consistent with this possibility, it has been demonstrated that XDH/XO phosphorylation is partially mediated by p38 kinase and casein kinase II (Kayyali et al., 2001). Therefore, similar to STAT activation, XDH/XO activation by hypoxia in LMVEC is activated by multiple signal transduction pathways.
In conclusion, we have demonstrated that hypoxia activates the IL6-JAK-STAT pathway. This pathway is involved in the activation of XDH/XO by hypoxia. As the JAK-STAT-XDH/XO pathway, together with p38 MAPK, PI3K, or ROS signaling pathways, are very important for the function of cytokines and immune regulation in LMVEC, further studies of these signaling pathways may help designing therapeutic strategies for the hypoxic LMVEC-related disease.
ACKNOWLEDGEMENTS
This study was funded by the National Research Program of China (No. 2006CB504100) and University Grants of TMMU 2006HG04 (to G. Wang) and by National Institutes of Health Grant GM076167 (to G. Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. The American Journal of Physiology - Cell Physiology. 2005;289:C521–530. doi: 10.1152/ajpcell.00429.2004. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Parks DA, Pearson JD, Marshall PA, Freeman BA. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radical Biology and Medicine. 1989;6:607–615. doi: 10.1016/0891-5849(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Beetsch JW, Park TS, Dugan LL, Shah AR, Gidday JM. Xanthine oxidase-derived superoxide causes reoxygenation injury of ischemic cerebral endothelial cells. Brain Research. 1998;786:89–95. doi: 10.1016/s0006-8993(97)01407-8. [DOI] [PubMed] [Google Scholar]
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. The Journal of Physiology. 2004;16:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovascular Research. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Carballo M, Conde M, Bekay R, El., Martin-Nieto J, Camacho MJ, Monteseirin J, Conde J, Bedoya FJ, Sobrino F. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. The Journal of Biological Chemistry. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- Chen SF, Fei X, Li SH. A new simple method for isolation of microvascular endothelial cells avoiding both chemical and mechanical injuries. Microvascular Research. 1995;50:119–128. doi: 10.1006/mvre.1995.1044. [DOI] [PubMed] [Google Scholar]
- Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, Wu WJ, Tan W, Bolli R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovascular Research. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Torre P, Diaz-Sanjuan T, Garcia-Ruiz I, Esteban E, Canga F, Munoz-Yague T, Solis-Herruzo JA. Interleukin-6 increases rat metalloproteinase-13 gene expression through Janus kinase-2-mediated inhibition of serine/threonine phosphatase-2A. Cellular Signalling. 2005;17:427–435. doi: 10.1016/j.cellsig.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Deo DD, Bazan NG, Hunt JD. Activation of platelet-activating factor receptor-coupled G alpha q leads to stimulation of Src and focal adhesion kinase via two separate pathways in human umbilical vein endothelial cells. The Journal of Biological Chemistry. 2004;279:3497–3508. doi: 10.1074/jbc.M304497200. [DOI] [PubMed] [Google Scholar]
- Dudley AC, Thomas DM, Best J, Jenkins A. A VEGF/JAK2/STAT5 axis may partially mediate endothelial cell tolerance to hypoxia. Biochemical Journal. 2005;390:427–436. doi: 10.1042/BJ20050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont GP, Huecksteadt TP, Marshall BC, Ryan US, Michael JR, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity and gene expression in cultured rat pulmonary endothelial cells. The Journal of Clinical Investigation. 1992;89:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. The Journal of Biological Chemistry. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Eguchi R, Suzuki A, Miyakaze S, Kaji K, Ohta T. Hypoxia induces apoptosis of HUVECs in an in vitro capillary model by activating proapoptotic signal p38 through suppression of ERK1/2. Cellular Signalling. 2007;19:1121–1131. doi: 10.1016/j.cellsig.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, Tschop K, Hautmann H, Endres S, Toepfer M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemical Journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HO, An S, Lee HC, Woo SH, Seo SK, Choe TB, Yoo DH, Lee SB, Um HD, Lee SJ, Park MJ, Kim JI, Hong SI, Rhee CH, Park IC. Hypoxic condition- and high cell density-induced expression of Redd1 is regulated by activation of hypoxia-inducible factor-1alpha and Sp1 through the phosphatidylinositol 3-kinase/Akt signaling pathway. Cellular Signalling. 2007;19:1393–1403. doi: 10.1016/j.cellsig.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kang SM, Lim S, Song H, Chang W, Lee S, Bae SM, Chung JH, Lee H, Kim HG, Yoon DH, Kim TW, Jang Y, Sung JM, Chung NS, Hwang KC. Allopurinol modulates reactive oxygen species generation and Ca2+ overload in ischemia-reperfused heart and hypoxia-reoxygenated cardiomyocytes. European Journal of Pharmacology. 2006;535:212–219. doi: 10.1016/j.ejphar.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Budhiraja R, Pennella CM, Cooray S, Lanzillo JJ, Chalkley R, Hassoun PM. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicology and Applied Pharmacology. 2003;188:59–68. doi: 10.1016/s0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. The Journal of Biological Chemistry. 2001;276:14359–14365. doi: 10.1074/jbc.M010100200. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Heo JS, Suh HN, Lee MY, Han HJ. Interleukin-6 stimulates 14C-{alpha}-methyl-D-glucopyranoside uptake in renal proximal tubule cells: Involvement of STAT3, PI3K/Akt, MAPKs, and NF-kB. American Journal of Physiology: Renal Physiology. 2007 doi: 10.1152/ajprenal.00034.2007. (in press) [DOI] [PubMed] [Google Scholar]
- Lee C, Lim HK, Sakong J, Lee YS, Kim JR, Baek SH. Janus kinase-signal transducer and activator of transcription mediates phosphatidic acid-induced interleukin (IL)-1beta and IL-6 production. Molecular Pharmacology. 2006;69:1041–1047. doi: 10.1124/mol.105.018481. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Schmitz J, Weissenbach M, Sobota RM,, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki A, Schneider-Mergener J, Yoshimura A, Neel BG, Heinrich PC, Schaper F. SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. The Journal Biological Chemistry. 2203;278:661–671. doi: 10.1074/jbc.M210552200. [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr. STATs: transcription control and biological impact. Nature Reviews Molecular Cell Biology. 2002;3:656–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Mascareno E, Beckles DL, Siddiqui MA. Janus kinase-2 signaling mediates apoptosis in rat cardiomyocytes. Vascular Pharmacology. 2005;43:327–335. doi: 10.1016/j.vph.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Mervaala EM, Cheng ZJ, Tikkanen I, Lapatto R, Nurminen K, Vapaatalo H, Muller DN, Fiebeler A, Ganten U, Ganten D, Luft FC. Endothelial dysfunction and xanthine oxidoreductase activity in rats with human renin and angiotensinogen genes. Hypertension. 2001;37:414–418. doi: 10.1161/01.hyp.37.2.414. [DOI] [PubMed] [Google Scholar]
- Nara Y, Kotake E, Takizawa S, Quan J, Wang H, Saida K. Cobalt chloride induces neurite outgrowth in rat pheochromocytoma PC-12 cells through regulation of endothelin-2/vasoactive intestinal contractor. Journal of Neuroscience Research. 2005;81:563–571. doi: 10.1002/jnr.20568. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Ni CW, Hsieh HJ, Chao YJ, Wang DL. Interleukin-6-induced JAK2/STAT3 signaling pathway in endothelial cells is suppressed by hemodynamic flow. The American Journal of Physiology - Cell Physiology. 2004;287:C771–780. doi: 10.1152/ajpcell.00532.2003. [DOI] [PubMed] [Google Scholar]
- Ottino P, Taheri F, Bazan HE. Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: imbalance between the gene expression of MMP-9 and TIMP-1 and −2. Experimental Eye Research. 2002;74:393–402. doi: 10.1006/exer.2001.1135. [DOI] [PubMed] [Google Scholar]
- Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. The American journal of physiology - Lung cellular and molecular physiology. 1996;270:L941–946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M, Lowenstein CJ. Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression. The Journal of Biological Chemistry. 2006;281:30057–30062. doi: 10.1074/jbc.M606279200. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Ichikawa H, Kagisaki K, Ohata T, Matsuda H. Interleukin-6 derived from hypoxic myocytes promotes neutrophil-mediated reperfusion injury in myocardium. Journal of Thoracic & Cardiovascular Surgery. 1998;116:511–517. doi: 10.1016/S0022-5223(98)70018-2. [DOI] [PubMed] [Google Scholar]
- Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, Cochran BH, Simon AR. Activation of the STAT pathway in acute lung injury. The American journal of physiology - Lung cellular and molecular physiology. 2004;286:L1282–1292. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- Sohn HY, Krotz F, Gloe T, Keller M, Theisen K, Klauss V, Pohl U. Differential regulation of xanthine and NAD(P)H oxidase by hypoxia in human umbilical vein endothelial cells. Role of nitric oxide and adenosine. Cardiovascular Research. 2003;58:638–646. doi: 10.1016/s0008-6363(03)00262-1. [DOI] [PubMed] [Google Scholar]
- Tamm M, Bihl M, Eickelberg O, Stulz P, Perruchoud AP, Roth M. Hypoxia-induced interleukin-6 and interleukin-8 production is mediated by platelet-activating factor and platelet-derived growth factor in primary human lung cells. American Journal of Respiratory Cell and Molecular Biology. 1998;19:653–661. doi: 10.1165/ajrcmb.19.4.3058. [DOI] [PubMed] [Google Scholar]
- Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS, Piermattei D, Shibao GN, McManaman JL, Wright RM. Hypoxia regulates xanthine dehydrogenase activity at pre- and posttranslational levels. Archives of Biochemistry and Biophysics. 1997;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226–234. doi: 10.1097/01.shk.0000226341.32786.b9. [DOI] [PubMed] [Google Scholar]
- Vinkemeier U. Getting the message across, STAT! Design principles of a molecular signaling circuit. The Journal of Cell Biology. 2004;167:197–201. doi: 10.1083/jcb.200407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GS, Qian GS, Zhou DS, Zhao JQ. JAK-STAT signaling pathway in pulmonary arterial smooth muscle cells is activated by hypoxia. Cell Biology International. 2005;29:598–603. doi: 10.1016/j.cellbi.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1 independent transport of a G protein-coupled receptor. The Journal of Biological Chemistry. 2003;278:47062–47069. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Osuka K, Takayasu M, Usuda N, Nakazawa A, Nakahara N, Yoshida M, Aoshima C, Hara M, Yoshida J. Activation of JAK/STAT signalling in neurons following spinal cord injury in mice. Journal of Neurochemistry. 2006;96:1060–1070. doi: 10.1111/j.1471-4159.2005.03559.x. [DOI] [PubMed] [Google Scholar]
- Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. The Journal of Biological Chemistry. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- Yan SF, Zou YS, Mendelsohn M, Gao Y, Naka Y, Du Yan S., Pinsky D, Stern D. Nuclear factor interleukin 6 motifs mediate tissue-specific gene transcription in hypoxia. The Journal of Biological Chemistry. 1997;272:4287–4294. doi: 10.1074/jbc.272.7.4287. [DOI] [PubMed] [Google Scholar]
- Zhao L, Hart S, Cheng J, Melenhorst JJ,, Bierie B, Ernst M, Stewart C, Schaper F, Heinrich PC, Ullrich A, Robinson GW, Hennighausen L. Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. The Journal of Biological Chemistry. 1999;279:44093–44100. doi: 10.1074/jbc.M313131200. [DOI] [PubMed] [Google Scholar]
- Zauberman A, Zipori D, Krupsky M, Ben-Levy R. Stress activated protein kinase p38 is involved in IL-6 induced transcriptional activation of STAT3. Oncogene. 1999;18:3886–3893. doi: 10.1038/sj.onc.1202738. [DOI] [PubMed] [Google Scholar]