Abstract
The orphan nuclear receptor pregnane X receptor (PXR) plays an important role in the detoxification of foreign and endogenous chemicals, including bile acids. PXR promotes bile acid elimination by activating bile acid-detoxifying enzymes and transporters. Certain bile acids are known to promote colonic carcinogenesis by inducing colon cancer cell apoptosis. However, whether and how PXR plays a role in colon cancer apoptosis has not been reported. In this study, we showed that activation of PXR by genetic (using a constitutively activated PXR) or pharmacological (using PXR agonist rifampicin) means protected the PXR-overexpressing colon cancer HCT116 cells from deoxycholic acid-induced apoptosis. Interestingly, activation of PXR also protected HCT116 cells from adriamycin-induced cell death, suggesting that the antiapoptotic effect of PXR was not bile acid specific. Moreover, the antiapoptotic effect of PXR in HCT116 cells appeared to be independent of xenobiotic enzyme regulation, because these cells had little basal and inducible expression of bile acid-detoxifying enzymes. Instead, SuperArray analysis showed that PXR-mediated deoxycholic acid resistance was associated with up-regulation of multiple antiapoptotic genes, including BAG3, BIRC2, and MCL-1, and down-regulation of proapoptotic genes, such as BAK1 and TP53/p53. Treatment with rifampicin in colon cancer LS180 cells, a cell line known to express endogenous PXR, also inhibited apoptosis. Activation of PXR in transgenic mice inhibited bile acid-induced colonic epithelial apoptosis and sensitized mice to dimethylhydrazine-induced colonic carcinogenesis, suggesting that the antiapoptotic effect of PXR is conserved in normal colon epithelium. In summary, our results have established the antiapoptotic role of PXR in both human colon cancer cells and normal mouse colon epithelium.
COLON CANCER IS the second most common fatal malignancy in the Western world. The United States is one of the countries with the highest incidence of colon cancer (1). Numerous factors have been implicated in the etiology of colon cancer. These include, but are not limited to, genetic abnormalities, such as the tumor suppressor gene adenomatous polyposis coli (APC) (2) and cyclooxygenase 2 (COX-2) (3,4); and environmental factors, such as high-fat Western-style diet (5,6) and toxic bile acids (7,8).
Bile acids are metabolic derivatives of cholesterol that are synthesized in the liver and excreted into the small intestine, where they aid in the absorption of dietary fats and lipophilic nutrients (9,10). Most bile acids are reabsorbed in the intestine. However, a small quantity remains unabsorbed and passes into the colon where they are converted to secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA), by enteric bacteria. Both epidemiological and animal studies have suggested that bile acids can function as endogenous colon tumor promoters (7,8). In addition to the direct effect of bile acids as colon cancer promoters, increased secretion of bile acids and exposure of the colorectal epithelium to fecal bile acids have been thought to be important contributing factors for the colon cancer-causing effect of high-fat Western diet (11,12).
Bile acid-induced colon epithelial apoptosis is believed to be an important mechanism for its participation in colon cancer development (13,14). It was believed that the ability of bile acids to induce apoptosis in colon epithelium can render selective survival and repopulation of apoptosis-resistant cells over a long-term exposure to bile acids, which ultimately lead to colonic carcinogenesis. Consistent with this notion, patients with a history of colon cancer or familial adenomatous polyposis were found to have a reduced ability to undergo induced apoptosis compared with individuals with no history of colon neoplasia (13). The hydrophobic DCA is one of the best-described apoptotic bile acids, but the mechanism of DCA-induced apoptosis has been a subject of debate. In human colon cancer cells, DCA has been shown to promote carcinogenesis by stimulating proteosome-mediated p53 protein degradation (15). The effects of DCA on apoptosis were subjected to modulation by the mitogen-activated kinases, bcl-2, and protein kinase C (16,17,18). In certain colon cancer cells, such as the HCT116 cells, DCA can induce cytochrome c release and caspase activation, leading to apoptosis (19).
Pregnane X receptor (PXR) was isolated as a xenobiotic receptor that regulates the expression of drug metabolizing/detoxifying enzymes and transporters (for reviews, see Refs. 20 and 21). PXR has been shown to be important in xeno- and endobiotic responses, including bile acid detoxification. PXR promotes bile acid detoxification by activating bile acid- metabolizing enzymes and transporters. These include the phase I enzyme cytochrome P450 3A (CYP3A) (22,23), phase II enzyme sulfotransferase 2A9 (SULT2A9) (24), and drug transporter multidrug resistance-associated protein 2 (25). Having known the role of PXR in bile acid detoxification, it is unclear whether activation of PXR can prevent colon cancer cells from bile acid-induced apoptosis, and if so, whether the activation of bile acid-detoxifying enzymes is necessary for this antiapoptotic effect.
In this report, we showed that genetic or pharmacological activation of PXR was sufficient to inhibit DCA-, adriamycin-, and staurosporine-induced apoptosis in human colon cancer cells. Gene expression analysis showed that the antiapoptotic effect of PXR was associated with the regulation of multiple apoptosis-related genes. The antiapoptotic effect of PXR was also observed in normal mouse colon epithelium.
RESULTS
Creation of Colon Cancer Stable Cell Lines that Express the Wild-Type or Activated PXR
A retroviral transfection method (26) was used to create colon cancer HCT116 cells that express the wild-type human PXR or the constitutively activated PXR (VP-PXR). VP-PXR was created by fusing the viral protein 16 (VP16) activation domain of the herpes simplex virus to the amino terminus of human PXR (21). The expression of transduced PXR and VP-PXR mRNA was confirmed by real-time RT-PCR analysis using PXR-specific probes. As shown in Fig. 1, the vector-transfected HCT116 cells had a low level expression of endogenous PXR. In contrast, PXR mRNA expression was readily detectable in PXR and VP-PXR-transfected HCT116 cells (Fig. 1A). We have previously created colon cancer LS180 cells stably transfected with vector or VP-PXR using the same retroviral transfection method (26). The expression of transduced VP-PXR mRNA in LS180-VP-PXR cells was also confirmed by real-time PCR analysis (Fig. 1B). Human liver RNA isolated from three cases of primary human hepatocytes was included as a positive control for PXR expression.
Figure 1.
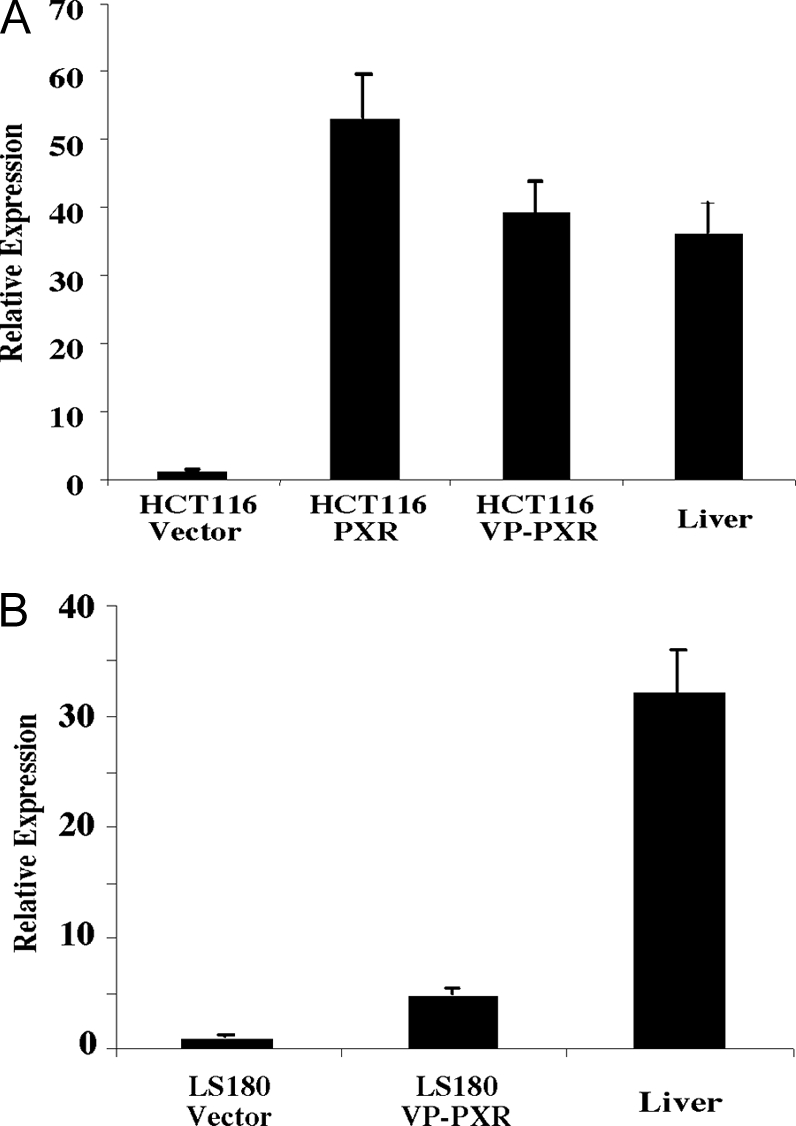
Creation of Colon Cancer Stable Cell Lines that Express the Wild-Type or Activated PXR
A, Real-time PCR analysis on HCT116 cells stably transfected with vector, wild-type human PXR, or activated VP-PXR. B, Real-time PCR analysis on LS180 cells stably transfected with vector, or activated VP-PXR. Results are presented as averages and sd from three independent clones for each cell line. Human hepatocytes from three patients (all Caucasians, including a 45-yr-old male, a 67-yr-old female, and an 84-yr-old male) were included as a control.
Genetic or Pharmacological Activation of PXR Inhibited DCA-Induced HCT116 Cell Apoptosis
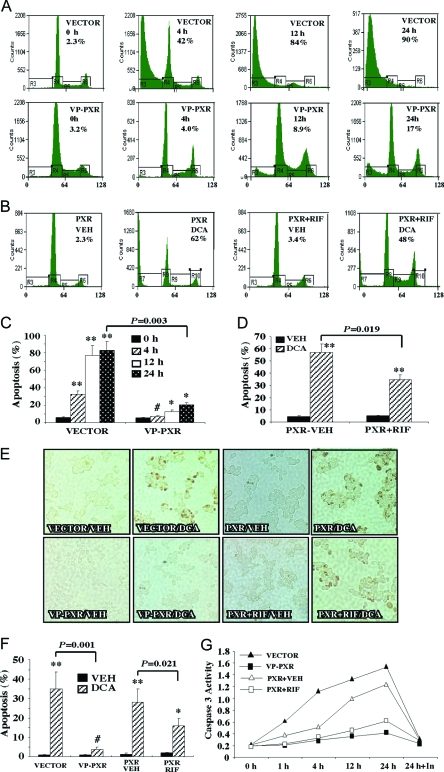
DCA is known to induce apoptosis in HCT116 cells (15,16,17,18). Flow cytometry was first used to investigate the effect of PXR activation on DCA-induced HCT116 cell apoptosis. In this experiment, HCT116-Vector and HCT116-VP-PXR cells were treated with DCA over a time course before harvesting for flow cytometry analysis. Our preliminary study showed that HCT116 apoptosis was DCA dose dependent, and nearly 50% apoptosis was achieved after treatment with 250 μm DCA for 4 h (data not shown). We therefore chose the 250 μm dose in subsequent experiments. Results in Fig. 2A showed that, after a 4-h treatment, 42% Vector cells became apoptotic, which was evidenced by the enrichment of the sub-G1 population in cell cycle profile. In sharp contrast, the percentage of apoptotic VP-PXR cells (4%) was near the basal level. At 12–24 h after treatment, 84–90% of the Vector cells were apoptotic, compared with 8.9–17% seen in the VP-PXR cells (Fig. 2A). The antiapoptotic effect was also seen in HCT116-PXR cells treated with the human PXR agonist rifampicin (RIF). In this experiment, RIF treatment (10 mm) started 24 h before the DCA exposure and continued until the completion of the experiment. As shown in Fig. 2B, treatment with RIF caused a significant inhibition of DCA-induced apoptosis at 12 h, although the inhibitory effect of PXR ligand was not as dramatic as that observed in the VP-PXR cells. The quantification of DCA-induced apoptosis and the effect of PXR activation were summarized in Fig. 2, C and D. A similar pattern of PXR effect was observed when additional individual clones or pooled clones were used (see Fig. 2 legend for details).
Figure 2.
Genetic or Pharmacological Activation of PXR Inhibited DCA-Induced HCT116 Cell Apoptosis
A and B, Flow cytometry analysis on DCA-treated VP-PXR cells (A), or PXR cells in the presence or absence of RIF treatment (B). Cells were mock-treated or treated with 250 μm DCA for indicated amount of time (A) or 12 h (B) before flow cytometry analysis. The percentages of apoptotic sub-G1 cell populations are labeled. Results shown are representative flow cytometry profiles from a single clone of each cell line. C and D, Quantification of apoptotic cells in panels A and B, respectively. Results shown represent averages and sd derived from two pooled clones and each clone was repeated twice. Each pooled clone represents clones from a 10-cm tissue culture plate in the original retroviral infection and drug selection. *, P < 0.05; **, P < 0.01; #, P > 0.05. P values are compared with 0 h in panel C or the Vehicle (VEH) control in panel D, or the comparisons are labeled. E, TUNEL assay on Vector, VP-PXR, and PXR cells mock treated or treated with 250 mm DCA for 3 h. F, Quantification of TUNEL-positive cells in panel E. Results shown represent averages and sd from two to three independent experiments. *, P < 0.05; **, P < 0.01; #, P > 0.05. P values are compared with the Vehicle (VEH) control, or the comparisons are labeled. G, Caspase 3 activity measured on Vector, VP-PXR, and PXR cells treated with 250 mm DCA for increasing amounts of time. In the “24 h+In” lane, cells were cotreated with the caspase inhibitor Z-VAD-FMK. Results shown represent averages derived from two pooled clones. In panels B, D, and E–G, when applicable, PXR cells were treated with RIF (10 mm) 24 h before DCA exposure, and the treatment continued until the completion of the experiment.
The antiapoptotic effect of PXR was confirmed by TUNEL (terminal deoxynucleotide transferase-mediated dUTP nick end labeling) and caspase 3 activity assays. In the TUNEL assay, cells were treated with 250 μm DCA for 3 h before fixation and TUNEL staining. As shown in Fig. 2E and results summarized in Fig. 2F, approximately 35% of Vector cells were positive for TUNEL staining, whereas only 3% of VP-PXR cells were stained positive after the same DCA treatment. A modest but significant inhibition of DCA-induced TUNEL staining was also seen in HCT116-PXR cells treated with RIF for 24 h before DCA exposure (Fig. 2, E and F).
Caspase 3 is a downstream effector caspase the activation of which indicates the occurrence of apoptosis. A previous report showed that treatment with DCA induced caspase 3 activation in HCT116 cells, and the general caspase inhibitor Z-Val-Ala-Asp-fmk (Z-VAD-FMK) completely inhibited DCA-induced apoptosis (19). Results in Fig. 2G showed that treatment with DCA induced caspase 3 activation in Vector cells in a time-dependent manner, consistent with the previous report (19). Caspase 3 activation can be seen as early as 1 h of DCA treatment. At 24 h, caspase 3 activation was 8-fold that of the untreated cells, but this activation was completely inhibited by Z-VAD-FMK. In contrast, little caspase 3 activation was seen in DCA-treated VP-PXR cells. A similar inhibition of DCA-induced caspase 3 activation was also seen in PXR cells treated with RIF for 24 h before DCA exposure (Fig. 2G). Interestingly, DCA was not able to induce apoptosis in either vector- or VP-PXR-transfected LS180 cells (data not shown), suggesting that the DCA effect was cell type specific.
The Antiapoptotic Effect of PXR in HCT116 Cells Was Not Bile Acid Specific and May Not Rely on the Activation of Bile Acid-Detoxifying Enzymes
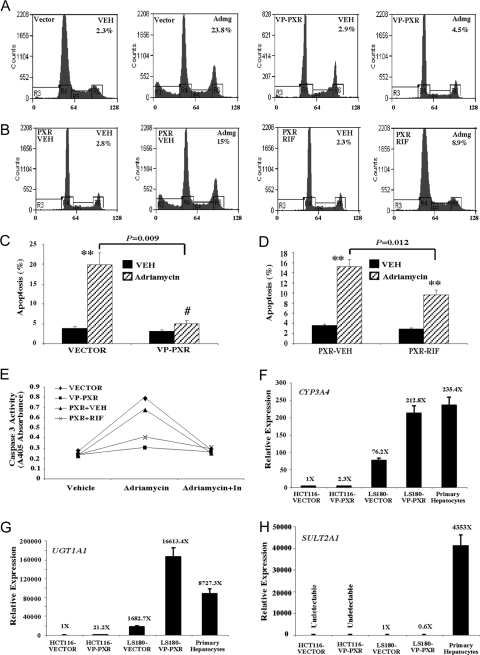
To determine whether the antiapoptotic effect of PXR was bile acid specific, we exposed cells to 0.5 mg/ml adriamycin, another agent known to induce apoptosis in HCT116 cells at this dose range (27), for 24 h. Both flow cytometry (Fig. 3, A–D) and caspase 3 activation (Fig. 3E) assays revealed that VP-PXR or RIF-treated PXR cells showed protection from adriamycin-induced apoptosis. Of note, adriamycin (0.5 mg/ml) appeared to be less effective than DCA (250 μm) in inducing apoptosis in HCT116 cells. Adriamycin treatment for 24 h resulted in an apoptotic rate of 24% in the Vector cells (Fig. 3A), compared with the rate of 90% in DCA-treated counterparts (Fig. 2A). Nevertheless, the resistance to adriamycin-induced apoptosis suggested that the antiapoptotic effect of PXR was not bile acid specific.
Figure 3.
The Antiapoptotic Effect of PXR in HCT116 Cells Was Not Bile Acid Specific and May Not Rely on the Activation of Bile Acid-Detoxifying Enzymes
A and B, Flow cytometry analysis on adriamycin (Admg)-induced apoptosis in HCT116-VP-PXR cells (A) and HCT116-PXR cells in the presence or absence of RIF (B). Cells were treated with adriamycin (0.5 mg/ml) for 24 h before flow cytometry analysis. The percentages of apoptotic sub-G1 cell populations are labeled. Results shown are representative flow cytometry profiles from the same clones used in Fig. 2, A and B. C and D, Quantification of apoptotic cells in panels A and B, respectively. Results shown represent averages and sd derived from the same pooled clones used in Fig. 2, C and D. **, P < 0.01; #, P > 0.05. P values are compared with the Vehicle (VEH) control, or the comparisons are labeled. E, Adriamycin-induced apoptosis was measured by caspase 3 activation. Adriamycin treatment was the same as in panels A and B. In the “Adriamycin+In” lane, cells were cotreated with the caspase inhibitor Z-VAD-FMK. Results shown represent averages derived from two pooled clones. F–H, Real-time PCR analysis on the expression of bile acid-metabolizing enzymes CYP3A4 (F), UGT1A1 (G), and SULT2A1 (H) in Vector- or VP-PXR-transfected HCT116 and LS180 cells. RNA prepared from untreated primary human hepatocytes from three patients (all Caucasians, including a 45-yr-old male, a 67-yr-old female, and an 84-yr-old male) was included as positive controls.
Although PXR is known to promote bile acid detoxification, the apoptotic resistance to the non-bile acid adriamycin suggested that the antiapoptotic effect of PXR may not rely on the activation of bile acid-detoxifying enzymes. To test this hypothesis, the expression of major bile acid-metabolizing enzymes, including CYP3A4, glucuronosyltransferase 1A1 (UGT1A1), and SULT2A1, was evaluated by real-time RT-PCR. Compared with LS180-Vector, LS180-VP-PXR, and primary human hepatocytes, the expression of both CYP3A4 and UGT1A1 was much lower and the expression of SULT2A1 was undetectable in either vector or VP-PXR-transfected HCT116 cells (Fig. 3, F–H). The low basal and PXR-inducible expression of major bile acid-metabolizing enzymes suggested that the antiapoptotic effect of PXR in HCT116 cells may not have resulted from the activation of bile acid metabolism.
Pharmacological Activation of Endogenous PXR in LS180 Cells Inhibited Staurosporine-Induced Apoptosis
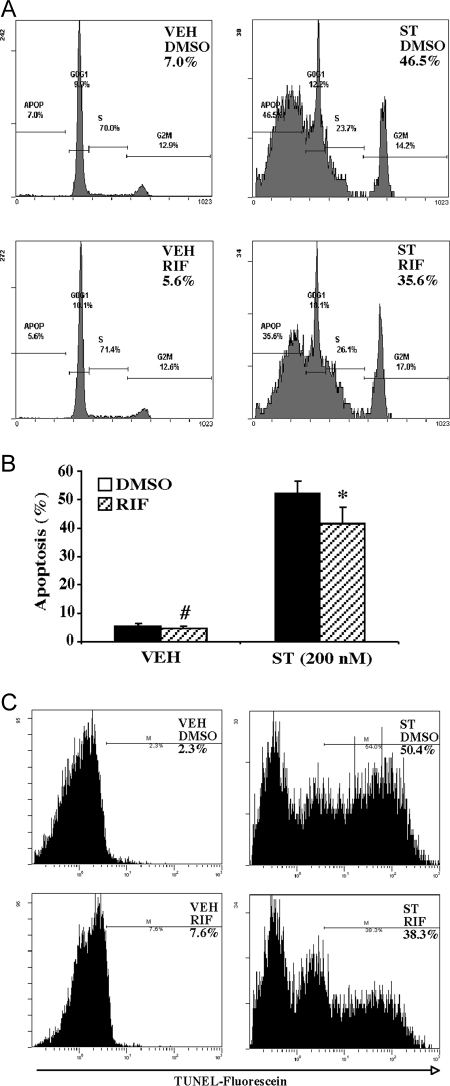
Having demonstrated the antiapoptotic effect of PXR in PXR-overexpressing HCT116 cells, we went on to determine whether activation of the endogenous PXR in colon cancer cells is sufficient to inhibit apoptosis. For this purpose, we used the parent LS180 cells, which are known to express PXR, and treatment with RIF activated PXR target gene expression in these cells (28,29). Staurosporine was chosen as the apoptotic agent because it has been used to show the antiapoptotic effect of PXR in hepatocytes (30), and our preliminary results showed that LS180 cells were sensitive to this drug. As shown in Fig. 4, treatment with RIF (10 μm) was sufficient to inhibit staurosporine (200 nm)-induced apoptosis as measured by flow cytometry (Fig. 4, A and B) or TUNEL assay (Fig. 4C). In this experiment, RIF treatment started 24 h before staurosporine exposure (48 h) and continued until the completion of the experiment. RIF was also able to inhibit adriamycin-induced apoptosis in LS180 cells (data not shown), again suggesting that the antiapoptotic effect of PXR was not drug specific.
Figure 4.
Pharmacological Activation of Endogenous PXR in LS180 Cells Inhibited Staurosporine-Induced Apoptosis
A and B, Flow cytometry analysis with the percentages of apoptotic sub-G1 cell populations labeled (A) and quantification of sub-G1 cells (B) in staurosporine (200 nm)-treated parent LS180 cells in the presence or absence of RIF (10 μm). Results shown in panel B represent averages and sd from three independent experiments. C, Apoptosis measured by TUNEL assay followed by flow cytometry. The percentages of TUNEL-positive cells are labeled. ST, Staurosporine; VEH, vehicle. *, P < 0.05; #, P > 0.05, compared with their respective dimethylsulfoxide (DMSO) control groups.
Molecular Mechanism by Which PXR Inhibited DCA-Induced Apoptosis in HCT116 Cells
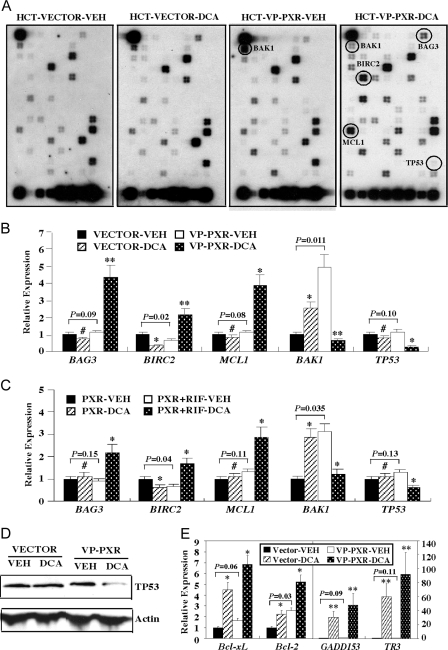
To understand the molecular mechanism by which PXR inhibited apoptosis, we profiled the expression of apoptosis-related genes by using apoptosis pathway-focused oligonucleotide SuperArray from SuperArray Bioscience (Frederick, MD). In this experiment, total RNA was extracted from the HCT116-Vector and HCT116-VP-PXR cells treated with DCA (250 μm) or mock treated for 2 h and subjected to SuperArray analysis. The means of nine housekeeping genes were used to normalize the hybridization signals. Results in Fig. 5A highlight several genes the expression of which was dramatically altered in VP-PXR cells, and the relative fold expressions are summarized in Table 1. Most notable changes were seen in the DCA-treated VP-PXR cells. These included the up-regulation of several antiapoptotic genes, including Bcl-2-associated athanogene 3 (BAG3, also known as CAIR-1) (31), baculoviral inhibitor of apoptosis repeat-containing protein 2 (BIRC2) (32), and myeloid cell leukemia sequence 1 (MCL-1) (33), whereas the expression of proapoptotic genes, including Bcl2-antagonist/killer 1 (BAK1) (34) and tumor protein 53 (TP53, or p53) (35) was down-regulated in the same cells. The SuperArray results were confirmed by real-time RT-PCR analysis (Fig. 5B). Interestingly, activation of PXR alone, in the absence of DCA treatment, significantly suppressed the expression of BIRC2 and induced the expression of BAK1, but VP-PXR had little effect on the basal expression of BAG3, MCL1, and TP53. A similar pattern of apoptotic and antiapoptotic gene regulation was observed in DCA- and RIF-treated HCT116-PXR cells (Fig. 5C). The inhibition of TP53 protein expression in DCA-treated HCT116-VP-PXR cells was also confirmed by Western blot analysis (Fig. 5D). The activation of antiapoptotic genes and inhibition of proapoptotic genes provided a plausible mechanism for the antiapoptotic effect of PXR.
Figure 5.
Molecular Mechanism by Which PXR Inhibited DCA-Induced Apoptosis in HCT116 Cells
A, SuperArray analysis on RNA samples derived from Vector and VP-PXR cells mock treated or treated with 250 μm DCA for 2 h. Genes the expression of which notably altered are circled and labeled. B, Real-time PCR analysis of BAG3, BIRC2, MCL-1, BAK1, and TP53 mRNA expression on RNA samples described in panel A. C, Real-time PCR analysis on RNA samples derived from PXR cells mock treated or treated with 250 μm DCA for 2 h in the presence or absence of RIF treatment. The RIF (10 mm) treatment started 24 h before DCA exposure and continued until the completion of the experiment. D, Decreased expression of TP53 protein in DCA-treated VP-PXR cells as revealed by Western blot analysis. E, Real-time PCR analysis of Bcl-xL, Bcl-2 (left panel), GADD153 and TR3 (right panel) mRNA expression on RNA samples described in panel A. Results in panels B, C, and E represent averages and sd from triplicate assays. *, P < 0.05; **, P < 0.01; #, P > 0.05. P values are compared with the Vehicle (VEH) control of the same cells, or the comparisons are labeled.
Table 1.
SuperArray Analysis on the Expression of Apoptosis-Related Genes in HCT-Vector and HCT-VP-PXR Cells Treated with DCA (250 μm) or Vehicle for 2 h
| Gene Name | GenBank Accession No. | Normalized Relative Expression
|
|||
|---|---|---|---|---|---|
| Vector-VEH | Vector-DCA | VP-PXR-VEH | VP-PXR-DCA | ||
| BAG3 | NM-004281 | 1 | 1.12 | 0.91 (1) | 2.84 (3.12) |
| BIRC2 | NM-001166 | 1 | 0.53 | 0.86 (1) | 2.89 (3.36) |
| MCL-1 | NM-021960 | 1 | 1.12 | 1.37 (1) | 4.39 (3.2) |
| BAK1 | NM-001188 | 1 | 2.15 | 4.83 (1) | 0.82 (0.19) |
| TP53/p53 | NM-000546 | 1 | 1.12 | 1.23 (1) | 0.21 (0.17) |
VEH, Vehicle.
We also evaluated the expression of several other apoptosis-related genes, including Bcl-2, Bcl-xL, growth arrest- and DNA damage-inducible gene 153 (GADD153), TR3, and MDM-2. Activation of Bcl-2 and Bcl-xL has been implicated in PXR-mediated protection from staurosporine-induced apoptosis in hepatocytes (30). GADD153 is known to be induced in DCA-treated HCT116 cells (36). TR3 (also known as Nur77, NGFI-B, TIS1, or NAK-1) is an orphan receptor the expression of which can be rapidly induced in various apoptotic cancer cells (37,38). Activation of MDM-2 by the constitutive androstane receptor (CAR) has been associated with the effect of CAR in promoting cell proliferation and inhibiting p53-dependent apoptosis in the liver (39). We found that, compared with vector-transfected cells, the expression of Bcl-2 and Bcl-xL was similarly induced in DCA-treated VP-PXR cells, although the basal expression of these two genes was modestly elevated in the VP-PXR cells (Fig. 5E, left panel). These results suggested that Bcl-2 and Bcl-xL induction per se may not account for the protective effect of PXR. The basal expression of GADD153 and TR3 was not significantly altered in the VP-PXR cells, and the expression of both genes was dramatically and similarly induced in DCA-treated Vector or VP-PXR cells (Fig. 5E, right panel). The expression of MDM-2 was unchanged between the Vector and VP-PXR cells regardless of DCA treatment (data not shown), suggesting that the antiapoptotic mechanism of PXR may differ from that of CAR.
Activation of PXR Inhibited Bile Acid-Induced Apoptosis and Sensitized Mice to Dimethylhydrazine (DMH)-Induced Colonic Carcinogenesis in Vivo
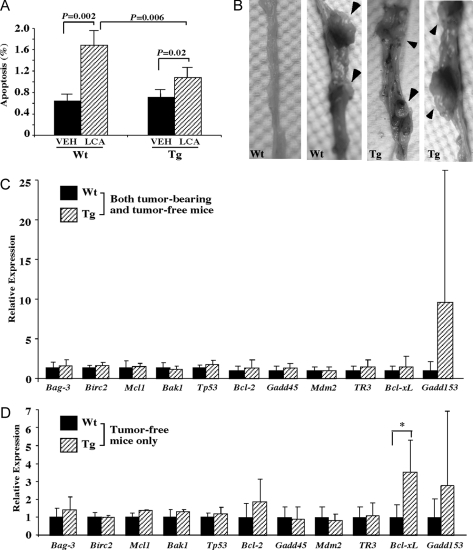
We next evaluate whether PXR also has an anti-apoptotic effect in vivo. For this purpose, we used the fatty acid-binding protein (FABP)-VP-PXR transgenic mice that express the activated VP-PXR in the intestinal tracts, including the colon, under the control of FABP gene promoter (26). In this experiment, wild-type and VP-PXR transgenic mice were challenged with the toxic secondary bile acid LCA by enema for 5 wk. Mice were then killed and colon tissues were harvested for TUNEL assay for detection of apoptotic cells. LCA administration in the colon is known to promote apoptosis (40). As expected, TUNEL assay showed that the percentage of apoptotic cells increased from 0.6% to 1.7% (∼2.5-fold increase) in wild-type mice after LCA treatment (Fig. 5A). In contrast, the apoptotic rate in LCA-treated transgenic mice (1.1%) was significantly lower than their wild-type counterparts (Fig. 6A), suggesting that PXR also has an antiapoptotic effect in vivo.
Figure 6.
Activation of PXR Inhibited Bile Acid-Induced Apoptosis and Sensitized Mice to DMH-Induced Colonic Carcinogenesis in Vivo
A, Wild-type (Wt, n = 3) and FABP-VP-PXR transgenic (Tg, n = 4) mice treated with vehicle or LCA enema for 5 wk. Colon tissues were then harvested and subjected to TUNEL assay for the detection of apoptotic cells. Results shown are percentages of apoptotic cells and represent averages and sd from three mice in each group. P values and comparisons are labeled. B, Representative tumor-free and tumor-bearing colon tissues derived from DMH-treated Wt and Tg mice. Mice received DMH treatment for 18 wk and were killed 12 wk after the last dose. Arrowheads indicate tumor nodules. C and D, Expression of 11 apoptosis-related genes in DMH-treated Wt and Tg mice when both tumor-bearing and tumor-free mice (C) or only the tumor-free mice (D) were included in the analysis. *, P < 0.05, compared with the Wt mice. VEH, Vehicle.
To determine whether activation of PXR affects colonic carcinogenesis, wild-type and VP-PXR transgenic mice were subjected to DMH treatment (10 mg/kg) for 18 wk, an established model of chemical colonic carcinogenesis (41). DMH-treated mice were killed, and all of the colon tissues were dissected and inspected for tumor formation 12 wk after the last dose of DMH. Carcinogenesis was scored by the appearance of tumor nodules. We found that 44% (four of nine mice) of the wild-type mice developed tumors, an incidence close to what has been reported (41). In contrast, 81% (13 of 16 mice) of the VP-PXR transgenic mice developed tumors. Figure 6B shows representative tumor-free and tumor-bearing colon tissues from the wild-type and transgenic mice. The tumor identify of the nodules was confirmed by paraffin section and hematoxylin and eosin staining (data not shown).
When the expression of apoptosis-related genes was measured in DMH-treated mice, we found that the expression of none of the 11 genes analyzed in human colon cancer cells (Fig. 5) was significantly altered in the transgenic group when both tumor-bearing and tumor-free mice (Fig. 6C) or only the tumor-bearing mice (data not shown) were included in the analysis. Interestingly, when only the tumor-free mice were included in the analysis, the expression of Bcl-xL, an antiapoptotic survival protein, was found to be significantly higher in the transgenic group (Fig. 6D), consistent with an earlier report in primary hepatocytes (30).
DISCUSSION
In this study, we found that PXR can protect human colon cancer HCT116 cells from DCA- and adriamycin-induced apoptosis. The antiapoptotic effect was also observed in PXR transgenic mice treated with LCA, a known apoptotic bile acid in vivo (40). Subsequent gene expression analysis showed that the antiapoptotic effect of PXR in HCT116 cells was associated with the activation of multiple antiapoptotic genes and inhibition of several proapoptotic genes.
The antiapoptotic effect of PXR was first observed and SuperArray analyses were performed on the VP-PXR-overexpressing cells. VP-PXR represents a gain of function model of receptor activation. Although the receptor is constitutively activated, the target gene regulation and the implication of this regulation in apoptosis have been shown to be consistent with those of the wild-type receptors in response to ligand treatments. The same strategy has been successfully used by us and others to study nuclear receptor functions both in vitro and in transgenic mice (21,26,42,43,44,45). In the current study, the VP-PXR effect on apoptosis and associated gene regulation was confirmed in ligand-treated wild-type PXR-expressing HCT116 cells, although the ligand effects were overall less dramatic. Moreover, treatment with RIF in LS180 cells, a cell line known to express endogenous PXR, was shown to inhibit staurosporine-induced apoptosis.
Although PXR and CAR were isolated as “xenobiotic receptors” postulated to regulate the expression of drug-metabolizing enzymes and transporters, emerging evidence has pointed to a potential role of these receptors in other cellular functions, including apoptosis. PXR was shown to protect from staurosporine-induced apoptosis in hepatocytes, which involved the activation of antiapoptotic genes Bcl-2 and Bcl-xL (30). In HCT116 cells, Bcl-2 and Bcl-xL were efficiently induced in both vector- and VP-PXR-transfected cells upon DCA treatment, suggesting that activation of Bcl-2 and Bcl-xL alone may not account for the antiapoptotic effect of PXR. CAR has been shown to suppress p53-mediated apoptosis in hepatocytes by directly regulating MDM-2 (39). In our study, activation of PXR had no effect on MDM-2 expression (data not shown), suggesting that the mechanism by which PXR suppresses apoptosis is different from that of CAR.
PXR has been shown to promote bile acid detoxification by activating bile acid-metabolizing enzymes, such as CYP3A, SULT2A, and UGT1A1 (22,23,46,47). However, several lines of evidence suggested that the antiapoptotic effect of PXR may not rely on its activation of bile acid-detoxifying enzymes. First, the effect was not bile acid specific, because activation of PXR also prevented adriamycin- and staurosporine-induced apoptosis. Moreover, compared with other colon cancer cells and primary hepatocytes, HCT116 cells had little basal and PXR-inducible expression of major bile acid-detoxifying enzymes. Interestingly, LS180 cells that have relatively high basal and inducible expression of CYP3A4 and UGT1A1 (Fig. 3, F and G), were resistant to DCA-induced apoptosis even in the absence of PXR activation (data not shown). However, LS180 cells were sensitive to staurosporine- (Fig. 4) and adriamycin (data not shown)-induced apoptosis, and treatment with the PXR agonist RIF was antiapoptotic in LS180 cells. The mechanism for cell type specificity of bile acid-induced apoptosis in colon cancer cells remains to be defined.
Our SuperArray and real-time PCR analysis showed that the antiapoptotic effect of PXR was associated with altered expression of multiple apoptosis-related genes. These include the up-regulation of anti-apoptotic genes BAG3, BIRC2, and MCL-1, and down-regulation of proapoptotic genes BAK1 and TP53. BAG3 was identified as a Bcl-2 interacting protein. It was shown that BAG3 itself had only weak antiapoptotic activity, but was synergistic with Bcl-2 in preventing Bax-induced and Fas-mediated apoptosis (48). Interestingly, Bcl-2 was also induced in DCA-treated VP-PXR cells (Fig. 4D). BIRC2 belongs to the inhibitor of apoptosis family of antiapoptotic proteins. Inhibitors of apoptosis inhibit apoptosis by inhibiting the activation of procaspase, as well as directly inhibiting activated caspases, such as caspases 3 and 7 (49). Some BIRC family members were found to be highly expressed in apoptosis-resistant colon cancer cell lines, indicating that BIRC may play a role in the apoptotic resistance in colon cancer cells (49). MCL-1, a Bcl-2 family member, plays a critical role in the survival of cancer cells. Depletion of MCL-1 via antisense oligodeoxynucleotides triggered apoptosis in cancer cells (50). It remains to be determined whether or not BAG3, BIRC2, and MCL-1 are direct transcriptional targets of PXR. Preliminary bioinformatic analysis on the approximate 2- to 3-kb 5′-regulatory sequences of these genes failed to identify conserved PXR response elements. It is also important to note that activation of PXR did not induce the basal expression of these antiapoptotic genes, but rather increased their bile acid-responsive expression. The mechanism for this apoptotic challenge-specific gene regulation remains to be determined. Nevertheless, the up-regulation of antiapoptotic genes provides a plausible explanation for the antiapoptotic effect of PXR.
Among genes down-regulated in PXR-activated and DCA-treated HCT116 cells, TP53/p53 is an important tumor suppressor gene. In the presence of DNA damages, TP53 can be activated and promote cell cycle arrest and allow cells to repair the damaged DNA. If DNA damages are beyond repair, TP53 will then promote cells to apoptosis (35,51,52). DCA has been shown to stimulate proteasome-mediated p53/TP53 degradation (15). Our results suggested that DCA also suppresses TP53 expression at the transcriptional level. BAK1 is another proapoptotic gene. In healthy cells, BAK1 is kept inactive by association with the antiapoptotic MCL-1. In the presence of apoptotic stimuli, such as UV DNA damage and oncogene activation, MCL-1 will be eliminated by proteasome-mediated degradation, thereby unleashing BAK1 to promote apoptosis (53,54,55). Interestingly, BAK1 is one of few apoptotic genes the expression of which was robustly induced by PXR activation in the absence of DCA treatment, but its expression in the same cells was dramatically suppressed upon DCA exposure (Fig. 5, A–C). The significance of PXR-induced higher BAK1 basal expression in the antiapoptotic phenotype of PXR activation is unknown. Nevertheless, the down-regulation of TP53 and BAK1 may have also contributed to the DCA resistance.
The antiapoptotic role of PXR was also observed in the colon of transgenic mice that express the constitutively activated PXR in this tissue. The transgenic mice showed resistance to LCA-induced apoptosis. The sensitization of transgenic mice to DMH-induced colonic carcinogenesis was also consistent with the antiapoptotic role of PXR. The gene regulation responsible for the antiapoptotic effect of PXR in vivo is not completely understood at present. In the LCA model, the mRNA expression pattern of Bag-3, Birc2, Mcl1, Bak1, and Tp53 was not significantly altered in the colon of transgenic mice, regardless of the LCA treatment (data not shown). In the DMH model, the expression of 11 apoptosis-related genes analyzed in human colon cancer cells (Fig. 5) was not significantly altered in the transgenic group when both tumor-bearing and tumor-free mice were included in the analysis (Fig. 6C). However, when only the tumor-free mice were included in the analysis, the expression of the antiapoptotic gene Bcl-xL was significantly increased in the transgenic group (Fig. 6D), consistent with an earlier report in primary hepatocytes (30). The discrepancies between the human cells and mouse tissues could be due to combined factors of apoptotic agents used, species specificity of gene regulation, as well as cancerous cells vs. normal colon epithelium.
In summary, our results have established an antiapoptotic role of PXR in colon cancer cells and normal colon epithelium. Moreover, we showed that the antiapoptotic effect of PXR in colon cancer cells was not bile acid specific and may not involved the much anticipated regulation of bile acid-metabolizing enzymes. The antiapoptotic effect of PXR does not seem to be cell type specific, because similar effects of PXR on apoptosis were seen in hepatocytes (30) and human endometrial cancer cells (57). Future studies are necessary to determine the in vivo relevance of PXR-mediated antiapoptosis in human colorectal carcinogenesis.
MATERIALS AND METHODS
Creation of PXR-Expressing Cell Lines
HCT116 cells were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum. Stable PXR-expressing HCT116 cells were generated by a retroviral transfection method as we have described previously (26). In brief, cDNAs of the wild-type human PXR or constitutively activated VP-PXR were cloned into the pBabe retroviral vector (26). VP-PXR was created by fusing the VP16 activation domain of the herpes simplex virus to the amino terminus of human PXR (21). For retroviral production, pBabe vectors of PXR, VP-PXR, or the empty vector were transfected into Phoenix-Ampho helper-free retroviral-producing cells using Lipofectamine 2000 from Invitrogen (Carlsbad, CA). Cell culture medium that contained the retrovirus were harvested 48 h after transfection and used to infect HCT116 cells. Cells were replaced with medium supplemented with puromycin (2–4 mg/ml) 24 h after infection and selected for 2–3 wk before clone pick and expansion. The creation of LS180-VP-PXR stable cells was previously described (26).
Induction of Apoptosis
Cells were seeded onto six-well tissue culture plates and let grow to subconfluency. Cells were then replaced with fresh medium containing DCA (250 μm), adriamycin (0.5 mg/ml), or staurosporine (200 nm) for various amounts of time to induce apoptosis.
Flow Cytometry
Both floating cells in the medium and adherent cells were collected after apoptotic induction. Cells were then washed with PBS and trypsinized to achieve single cell suspensions. Cells were then stained with propidium iodide and analyzed using an EPICS XL-MCL flow cytometer at the University of Pittsburgh Cancer Institute. Quantitation of the apoptotic sub-G1 portions was performed with WinMD1 Software (http://facs.scripps.edu/software.html).
TUNEL Assay
For the HCT116 cell TUNEL assay, cells were air dried and fixed in 4% paraformaldehyde for 1 h at room temperature. TUNEL assay was performed with the In Situ Cell Death Kit, peroxidase (catalog no. 11684817910) from Roche (Indianapolis, IN), following the manufacturer's instruction. In brief, fixed cells were rinsed in PBS, blocked, and permeabilized. TUNEL reaction mixture was then added to the cells and incubated at 37 C for 1 h. Apoptotic signals were converted with peroxidase and stained with 3,3′-diaminobenzidine tetrahydrochloride. For TUNEL assay in colon tissues, In Situ Cell Death Kit (catalog no. 11684815910) from Roche was used. Paraffin-embedded tissue sections were dewaxed and rehydrated with a graded series of ethanol. Sections were treated with proteinase K and then incubated with TUNEL reaction mixture. Apoptotic cells were detected and scored under fluorescence microscope with 4′,6-diamidino-2-phenylindole counter staining. For the LS180 cell TUNEL assay, apoptotic cells were detected and scored by flow cytometry.
Measurement of Caspase 3 Activity
Caspase 3 activity was determined by using the Caspase Assay Kit (catalog no. 0509011819) from CHEMICON (Temecula, CA), following the manufacturer's instruction. In brief, cells were collected, washed in PBS, and lysed in lysis buffer. Nuclei were removed by centrifugation, and the cytosolic fractions were added into caspase assay buffer. After reaction, the absorbance at 405 nm was measured with an ELISA plate reader. The caspase inhibitor Z-VAD-FMK was included in the Caspase Assay Kit and was applied to the cell culture at the concentration of 10 mm.
SuperArray Analysis
Total RNAs were isolated using TRIZOL reagent from Invitrogen. RNAs were then treated with DNase I (1 U/ml) to eliminate possible genomic DNA contamination. The absence of DNA contamination was confirmed by PCR for 35 cycles with primers of GAPDH (data not shown). The apoptosis pathway-focused oligonucleotide SuperArray membranes (catalog no. OHS012) and related reagents were purchased from SuperArray Bioscience. cRNA synthesis, labeling, and hybridization were performed following the manufacturer's instruction. Hybridization signals were detected with chemiluminescent detection kit. Image analysis and data acquisition were performed using the web-based integrated GEArray Expression Analysis Suite provided by SuperArray Bioscience. We used the means of nine housekeeping genes to normalize the intensity of the signals.
Real-Time RT-PCR
Total RNAs were isolated using TRIZOL reagent. Real-time PCR using the SYBR-green reagent was performed with the ABI 7300 Real-Time PCR System as we have previously described (56). Sequences of the CYBR-green oligonucleotide probes are available upon request.
LCA Animal Experiments and DMH Model of Colonic Carcinogenesis
Age-matched 8- to 10-wk-old wild-type and FABP-VP-hPXR transgenic mice (26) were used. Mice were housed in a pathogen-free animal facility under a standard 12-h light, 12-h dark cycle with free access to water and food. For treatment, mice received 1 ml of 5 mm LCA enema or vehicle (corn oil) twice a day for 5 wk before being killed. Colon tissues were harvested and processed for paraffin sections and stained for TUNEL or hematoxylin and eosin. The DMH model was carried out essentially as described by Narisawa and colleagues (41). In brief, 8-wk-old mice were subjected to 18 weekly ip injections of DMH (from Sigma Chemical Co., St. Louis, MO) at the dose of 10 mg/kg body weight. Mice were killed 12 wk after the last dose of DMH, and the entire colon tissues were dissected and inspected for tumor nodules. The use of mice in this study has complied with all relevant federal guidelines and institutional policies.
Western Blot Analysis
Cells from 60-mm culture dishes were lysed with 100 ml Cell Lysis Buffer (catalog no. E153A) from Promega Corp. (Madison, WI). Lysates were resolved by 12% SDS-PAGE gel, and proteins were then transferred to polyvinylidinedifluoride membrane. Membranes were blocked with 5% nonfat dry milk in PBS-Tween 20 at room temperature for 1 h. Immunoblotting of primary antibodies was performed at 4 C overnight. The following day, membranes were washed and incubated with secondary antibody, and signals were detected with enhanced chemiluminescence reagent (catalog no. RPN2209) from Amersham Pharmacia Biotech (Piscataway, NJ). TP53 antibody (catalog no. 9282) and β-actin antibody (catalog no. 4967) were purchased from Cell Signaling Technology (Danvers, MA).
Acknowledgments
We thank Dr. Lin Zhang at the University of Pittsburgh Cancer Institute for his insightful comments and Ying Mu for helping to create PXR cell lines.
Footnotes
This work was supported in part by U.S. National Institutes of Health Grants ES012479 and CA107011 (to W.X.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 20, 2007
Abbreviations: BAG3, Bcl-2-associated athanogene 3; BAK1, Bcl2-antagonist/killer 1; BIRC2, baculoviral inhibitor of apoptosis repeat containing protein 2; CAR, constitutive androstane receptor; CYP3A, cytochrome P450 3A; DCA, deoxycholic acid; DMH, dimethylhydrazine; FABP, fatty acid binding protein; LCA, lithocholic acid; MCL-1, myeloid cell leukemia sequence 1; PXR, pregnane X receptor; RIF, rifampicin; SULT2A1 and SULT2A9, sulfotransferase 2A1 and 2A9; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling; TP53, tumor protein 53; VP16, viral protein 16.
References
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van AR, Brown-Davis C, Marciniak DA, Mayer RJ 1997 Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 112:594–642 [DOI] [PubMed] [Google Scholar]
- Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, Luongo C, Borenstein N, Dove W 1993 Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75:631–639 [DOI] [PubMed] [Google Scholar]
- Prescott SM, Fitzpatrick FA 2000 Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta 1470:M69–M78 [DOI] [PubMed] [Google Scholar]
- Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, Giardina C, Rosenberg DW 2003 Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis 24:307–315 [DOI] [PubMed] [Google Scholar]
- Kushi L, Giovannucci E 2002 Dietary fat and cancer. Am J Med 113(Suppl 9B):S63–S70 [DOI] [PubMed] [Google Scholar]
- Pence BC, Buddingh F 1988 Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis 9:187–190 [DOI] [PubMed] [Google Scholar]
- Reddy BS, Watanabe K 1979 Effect of cholesterol metabolites and promoting effect of lithocholic acid in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res 39:1521–1524 [PubMed] [Google Scholar]
- Baijal PK, Fitzpatrick DW, Bird RP 1998 Comparative effects of secondary bile acids, deoxycholic and lithocholic acids, on aberrant crypt foci growth in the post initiation phases of colon carcinogenesis. Nutr Cancer 31:81–89 [DOI] [PubMed] [Google Scholar]
- Russell DW 2003 The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72:137–174 [DOI] [PubMed] [Google Scholar]
- Chiang JY 2003 Bile acid regulation of hepatic physiology. III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol 284:G349–G356 [DOI] [PubMed] [Google Scholar]
- Kishida T, Taguchi F, Feng L, Tatsuguchi A, Sato J, Fujimori S, Tachikawa H, Tamagawa Y, Yoshida Y, Kobayashi M 1997 Analysis of bile acids in colon residual liquid or fecal material in patients with colorectal neoplasia and control subjects. J Gastroenterol 32:306–311 [DOI] [PubMed] [Google Scholar]
- Bradley BA, Evers BM 1997 Molecular advances in the etiology and treatment of colorectal cancer. Surg Oncol 6:143–156 [DOI] [PubMed] [Google Scholar]
- Bernstein C, Bernstein H, Garewal H, Dinning P, Jabi R, Sampliner RE, McCuskey MK, Panda M, Roe DJ, L'Heureux L, Payne C 1999 Bile acid-induced apoptosis assay for colon cancer risk and associated quality control studies. Cancer Res 59:2353–2357 [PubMed] [Google Scholar]
- Garewal H, Bernstein H, Bernstein C, Sampliner R, Payne C 1996 Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: a potential biological marker for cancer risk. Cancer Res 56:1480–1483 [PubMed] [Google Scholar]
- Qiao D, Gaitonde SV, Qi W, Martinez JD 2001 Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis 22:957–964 [DOI] [PubMed] [Google Scholar]
- Qiao D, Chen W, Stratagoules ED, Martinez JD 2000 Bile acid-induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. J Biol Chem 275:15090–15098 [DOI] [PubMed] [Google Scholar]
- Qiao D, Stratagouleas ED, Martinez JD 2001 Activation and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis 22:35–41 [DOI] [PubMed] [Google Scholar]
- LaRue JM, Stratagoules ED, Martinez JD 2000 Deoxycholic acid-induced apoptosis is switched to necrosis by bcl-2 and calphostin C. Cancer Lett 152:107–113 [DOI] [PubMed] [Google Scholar]
- Yui S, Saeki T, Kanamoto R, Iwami K 2005 Characteristics of apoptosis in HCT116 colon cancer cells induced by deoxycholic acid. J Biochem (Tokyo) 138:151–157 [DOI] [PubMed] [Google Scholar]
- Xie W, Uppal H, Saini SP, Mu Y, Little JM, Radominska-Pandya A, Zemaitis MA 2004 Orphan nuclear receptor-mediated xenobiotic regulation in drug metabolism. Drug Discov Today 9:442–449 [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM 2000 Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439 [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM 2001 An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98:3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA 2001 The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM 2002 Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc Natl Acad Sci USA 99:13801–13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA 2002 Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277:2908–29015 [DOI] [PubMed] [Google Scholar]
- Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, Ren S, Kagan VE, Day BW, Zimniak P, Xie W 2006 Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol 20:279–290 [DOI] [PubMed] [Google Scholar]
- Yokochi T, Robertson KD 2004 Doxorubicin inhibits DNMT1, resulting in conditional apoptosis. Mol Pharmacol 66:1415–1420 [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM 2001 The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med 7:584–590 [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, Blumberg B 2006 Mutual repression between steroid and xenobiotic receptor and NF-κB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchinia N, Sousaa G, Bailly-Maitreb B, Gugenheimc J, Barsd R, Lemairea G, Rahmania R 2005 Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim Biophys Acta 1745:48–58 [DOI] [PubMed] [Google Scholar]
- Takayama S, Xie Z, Reed JC 1999 An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 274:781–786 [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Clem RJ, Miller LK 1994 An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol 68:2521–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels J, Johnson PW, Packham G 2005 Mcl-1. Int J Biochem Cell Biol 37:267–271 [DOI] [PubMed] [Google Scholar]
- Sundararajan R, Cuconati A, Nelson D, White E 2001 Tumor necrosis factor-α induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem 276:45120–45127 [DOI] [PubMed] [Google Scholar]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P 1989 Mutations in the p53 gene occur in diverse human tumour types. Nature 342:705–708 [DOI] [PubMed] [Google Scholar]
- Qiao D, Im E, Qi W, Martinez JD 2002 Activator protein-1 and CCAAT/enhancer-binding protein mediated GADD153 expression is involved in deoxycholic acid-induced apoptosis. Biochim Biophys Acta 1583:108–116 [DOI] [PubMed] [Google Scholar]
- Woronicz JD, Calnan B, Ngo V, Winoto A 1994 Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 367:277–281 [DOI] [PubMed] [Google Scholar]
- Moll UM, Marchenko N, Zhang XK 2006 p53 and Nur77/TR3—transcription factors that directly target mitochondria for cell death induction. Oncogene 25:4725–4743 [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD 2005 Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19:1646–1653 [DOI] [PubMed] [Google Scholar]
- Kozoni1 V, Tsioulias G, Shiff S, Rigas B 2000 The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis 21:999–1005 [DOI] [PubMed] [Google Scholar]
- Narisawa T, Fukaura Y, Kotanagi H, Asakawa Y 1992 Inhibitory effect of cryptoporic acid E, a product from fungus Cryptoporus volvatus, on colon carcinogenesis induced with N-methyl-N-nitrosourea in rats and with 1,2-dimethylhydrazine in mice. Jpn J Cancer Res 83:830–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Moore DD, Evans RM, Xie W 2004 A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol 65:292–300 [DOI] [PubMed] [Google Scholar]
- Uppal H, Saini SP, Moschetta A, Mu Y, Zhou J, Gong H, Zhai Y, Ren S, Michalopoulos GK, Mangelsdorf DJ, Xie W 2007 Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology 45:422–432 [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM 2003 Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113:159–170 [DOI] [PubMed] [Google Scholar]
- Yang Q, Yamada A, Kimura S, Peters JM, Gonzalez FJ 2006 Alterations in skin and stratified epithelia by constitutively activated PPARα. J Invest Dermatol 126:374–385 [DOI] [PubMed] [Google Scholar]
- Uppal H, Toma D, Saini SP, Ren S, Jones TJ, Xie W 2005 Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 41:168–176 [DOI] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M 2005 CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42:420–430 [DOI] [PubMed] [Google Scholar]
- Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y 1999 Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 18:6183–6190 [DOI] [PubMed] [Google Scholar]
- LaCasse EC, Baird S, Korneluk RG, MacKenzie AE 1998 The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17:3247–3259 [DOI] [PubMed] [Google Scholar]
- Clohessy JG, Zhuang J, Boer J, Gil-Gomez G, Brady HJ 2006 Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem 281:5750–5759 [DOI] [PubMed] [Google Scholar]
- Sakamuro D, Sabbatini P, White E, Prendergast GC 1997 The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene 15:887–898 [DOI] [PubMed] [Google Scholar]
- Caelles C, Helmberg A, Karin M 1994 p53-Dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370:220–223 [DOI] [PubMed] [Google Scholar]
- Cuconati A, Mukherjee C, Perez D, White E 2003 DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev 17:2922–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X 2003 Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev 17:1475–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas C, White E 2005 BH3-only proteins in control: specificity regulates MCL-1 and BAK-mediated apoptosis. Genes Dev 19:1263–1268 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W 2006 A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281:15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y 2007 Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells. Mol Pharmacol 72:1045–1053 [DOI] [PubMed] [Google Scholar]