Abstract
GH binds dimerized GH receptors (GHRs) to form a trimolecular complex and induces downstream signaling events. The mechanism by which GH binding converts the inactive predimerized GHR to its active signaling conformation is uncertain. GH has no axis of symmetry. Its interaction with GHR is mediated by two asymmetric binding sites on GH, each with distinct affinity. Site 1 is of high affinity and is thought to mediate the first binding step. Mutation of binding site 2 (as in the human GH mutant, G120R) disrupts the second binding but leaves site 1 binding intact. G120R is a GH antagonist; it binds only one GHR and thus fails to signal, and it prevents productive GHR binding by normal GH. We previously demonstrated that prolactin receptor signaling was achieved by a dimeric version of a prolactin antagonist. We now employ assays of cellular signaling and receptor conformational changes to examine whether GH molecules harboring two site 1 regions can trigger GHR activation. We used recombinantly produced GH-GH and G120R-G120R dimers in which monomers in tandem are connected by a short linker peptide. Rabbit GHR-expressing human fibrosarcoma cells (C14) were treated with GH, G120R, GH-GH, or G120R-G120R. As expected, GH and GH-GH, but not G120R, induced GHR disulfide linkage, as assessed by anti-GHR blotting of cell extracts resolved by SDS-PAGE under nonreducing conditions. Disulfide linkage of GHRs reflects attainment of the active signaling conformation. Likewise, GH and GH-GH, but not G120R, caused Janus kinase 2 (JAK2) and signal transducer and activator of transcription 5 (STAT5) activation. Notably, G120R-G120R, despite its lack of an intact site 2 in either dimer partner, also promoted GHR disulfide linkage and JAK2 and STAT5 activation, albeit less potently than either GH or GH-GH. Time-course responses of the three agonists were similar in terms of JAK2 and STAT5 activation. Pretreatment of cells with our conformation-sensitive inhibitory monoclonal antibody, anti-GHRext-mAb, prevented ligand-induced receptor activation for all three agonists. GHR was also rendered less immunoprecipitable by anti-GHRext-mAb after treatment with these agonists. These results are important in that they indicate that a ligand with two intact binding sites 1 causes GHR to adopt similar conformational changes as does GH and thus triggers activation of JAK2 and downstream signaling. Furthermore, we infer that there is substantial flexibility in the GHR extracellular domain, such that it productively accommodates GH dimers that are much larger than GH.
THE DIVERSE PHYSIOLOGICAL activities of GH are initiated by its binding to the GH receptor (GHR) on the surface of target cells (1). Signal transduction in response to GH-GHR interaction is rapid; among other signals, robust tyrosine phosphorylation of the GHR, the receptor-associated kinase Janus kinase 2 (JAK2), and the transcription factor, signal transducer and activator of transcription 5 (STAT5) are detected within minutes (1,2,3).
Mechanisms by which GH binding promotes receptor activation have been intensely studied but are as yet uncertain. GH's interaction with GHR is mediated by two asymmetric binding sites (1 and 2) on GH, each with distinct affinity. In early models, it was suggested that GH induces sequential dimerization of GHR monomers in a scenario in which site 1 of GH binds a GHR monomer with higher affinity and is followed by a somewhat lower affinity binding of site 2 of the same GH molecule to a second GHR monomer (4,5,6); thus, the GH-(GHR)2 complex observed in co-crystallization studies was taken to represent the activated GHR conformation. Indeed, mutated GH molecules with disruption of binding site 2 (as in the human GH mutant, G120R) were developed as GH antagonists and were even more effective when combined with additional mutations that enhanced binding at site 1 (e.g. pegvisomant) (6,7). The antagonism of G120R was believed indicative of its ability to bind to only one GHR and thereby prevent productive binding by normal GH to a second GHR. This view fit well with the sequential dimerization model of GH action.
More recent studies suggest that the GHR is likely dimerized even in the absence of the ligand, much as observed for other class I cytokine receptors with topological similarity to the GHR (8,9,10,11,12,13). This predimerization may be mediated by the receptor transmembrane domain, perhaps in concert with elements of the extracellular domain (9,10,13). In comparison with the sequential dimerization model's presumed mechanism of activation (bringing together two GHR monomers and their associated JAK2 kinases, thereby activating them), it is less clear how GH binding to predimerized GHRs leads to JAK2 activation. Brown et al. (10) postulated that GH binding might result in a clockwise rotation of the receptor's JAK2 binding region (via transmembrane domain/juxtamembrane torsion) that brings the JAK2 molecules into proximity for kinase transphosphorylation. In this model, it is not clear the degree to which GH's occupancy of the GHR extracellular domain is constrained to allow such rotation. However, in recent studies, we replaced the native GHR transmembrane domain with that of the low-density lipoprotein receptor (which is several residues shorter than the GHR transmembrane domain) and found that GH signaling was unaffected, suggesting that there is some flexibility in the structure of the receptor extracellular/transmembrane domain in terms of its ability to achieve the active signaling conformation in response to GH (13).
In this study, we further probe the mechanisms by which GHR engagement leads to activation of intracellular signal transduction. In particular, we ask whether dimeric GH molecules can be accommodated by the receptor to trigger signaling. We used recombinantly produced GH-GH and G120R-G120R dimers in which monomers in tandem are connected by a short linker peptide. By performing concentration dependence and time-course experiments, we found that G120R-G120R, despite its lack of an intact site 2 in either dimer partner, promoted JAK2 and STAT5 activation, albeit less potently than either GH or GH-GH. Furthermore, we employed several of our novel biochemical assays of GHR conformational change in intact cells and arrived at the interesting conclusion that a ligand with two intact binding sites 1 causes GHR to adopt similar conformational changes as does GH. These studies lead to the important inference that, contrary to expectations, there is substantial flexibility in the GHR extracellular domain, such that it accommodates GH dimers that are much larger than GH.
RESULTS
GH and G120R Dimers Elicit Signaling in Rabbit GHR-Reconstituted Fibrosarcoma Cells
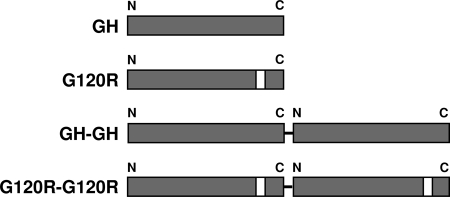
To investigate the requirement for sites 1 and 2 in GHR activation, we recombinantly prepared and purified human GH, human G120R, and versions of each that include two copies of the hormones arranged in tandem within the same polypeptide (as in MATERIALS AND METHODS and depicted in Fig. 1). GH-GH features two human GH proteins arranged as a single fusion protein connected by a two-residue (Gly-Ser) linker such that the carboxy terminus of the first GH moiety is connected to the amino terminus of the second GH moiety. G120R-G120R has the same two-residue linker joining two G120R molecules in the same tandem arrangement as in GH-GH. SDS-PAGE and Coomassie staining revealed the expected migration of each protein (∼22 kDa for GH and G120R and ∼44 kDa for GH-GH and G120R-G120R) and verified their purity and that stocks of equal protein molarities yielded monomers or dimers of expected staining ratios (data not shown).
Figure 1.
GH, G120R, and Their Dimers
Schematic of GH, G120R, GH-GH, and G120R-G120R. Dimers are arranged in tandem and are linked as detailed in Materials and Methods. White bars indicate the presence of mutated site 2.
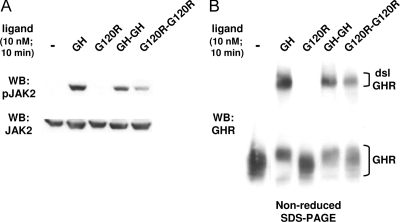
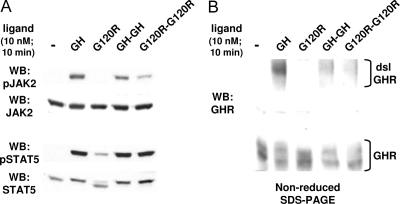
We first asked whether each ligand could stimulate signal transduction, using our well-characterized GH-responsive reconstitution system referred to as C14 cells (14,15,16,17,18). These cells are the result of stable transfection of the GHR- and JAK2-deficient human fibrosarcoma cell line γ2A (19) with the wild-type rabbit GHR and mouse JAK2. C14 cells were treated with equimolar concentrations (10 nm) of each ligand for 10 min, after which detergent-extracted proteins were resolved by SDS-PAGE and immunoblotted with anti-phospho-JAK2 (anti-pJAK2), which recognizes the tyrosine-phosphorylated JAK2 that corresponds to the kinase-active form (Fig. 2A). As expected based on previous data (13,20,21,22), GH, but not G120R, induced marked JAK2 activation. Notably, although less intense than GH, both GH-GH and G120R-G120R also caused detectable JAK2 activation under these conditions.
Figure 2.
GH, GH-GH, and G120R-G120R Induce Signaling and GHR Disulfide Linkage in Rabbit GHR-Reconstituted Human Fibrosarcoma Cells
Serum-starved C14 cells were treated with vehicle (−) or the indicated ligands (GH, G120R, GH-GH, or G120R-G120R; 10 nm) for 10 min. Detergent extracts were resolved by SDS-PAGE under either reducing (A) or nonreducing (B) conditions. Resolved proteins were transferred to nitrocellulose and immunoblotted sequentially with anti-pJAK2 and anti-JAK2 (A) or with anti-GHR (anti-GHRcyt-AL47, directed at the receptor cytoplasmic domain) (B). Positions of the dsl and non-dsl GHR are indicated. The data shown in A and B came from separate experiments and are each representative of three such experiments.
The signaling elicited by GH-GH and, in particular, by G120R-G120R would not necessarily be predicted by existing models of GHR activation. To better understand this, we employed assays to probe receptor conformation. In previous work, we demonstrated that productive GH binding, which leads to the receptor conformational changes required for signaling, is associated with formation of a high-molecular-mass GHR form detectable by anti-GHR immunoblotting of cell extracts resolved under nonreducing conditions (13,16,20,23). This disulfide-linked form of GHR (dsl GHR) results from covalent bonding between receptors mediated by cysteine-241, the only unpaired extracellular cysteine, which resides in the GHR extracellular stem region six residues from the transmembrane domain (20). GHR disulfide linkage is not required for signal transduction but can be used to monitor the attainment of the receptor's activated conformation (20). We stimulated C14 cells with each ligand under the same conditions as in Fig. 2A and blotted proteins resolved by nonreducing SDS-PAGE with anti-GHR (Fig. 2B). As expected, GH promoted appearance of the dsl GHR, whereas G120R failed to do so. In concert with the signaling results, both GH-GH and G120R-G120R caused receptor disulfide linkage, albeit less so than GH. These data suggest that both dimeric ligands can cause the GHR to undergo conformational changes that allow signaling to be triggered.
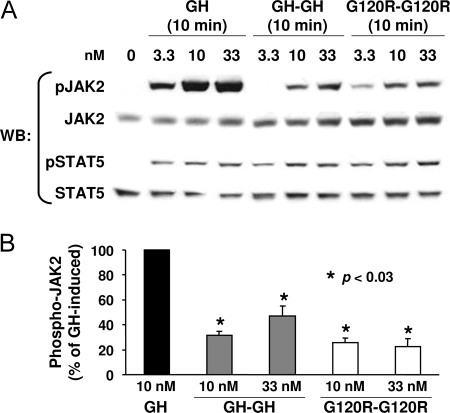
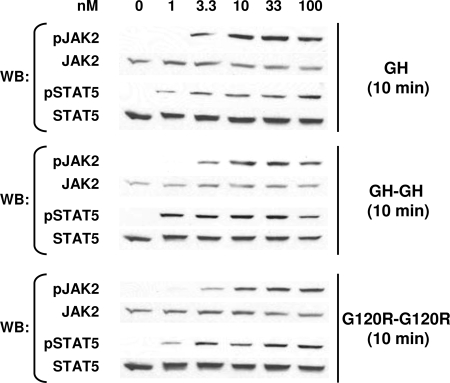
We examined the relative strength of signaling generated by each ligand by performing dose-response experiments. Serum-starved C14 cells were treated for 10 min with GH (3.3–33 nm), GH-GH, or G120R-G120R (1–66 nm for the latter two), and proteins were detergent extracted. Equal aliquots of protein from each sample were resolved on the same gel by SDS-PAGE and blotted under identical conditions sequentially with anti-pJAK2, anti-JAK2, anti-pSTAT5 (which recognizes tyrosine-phosphorylated STAT5), and anti-STAT5 (Fig. 3). This allowed direct comparison of each ligand's relative signaling potency. Both JAK2 and STAT5 phosphorylation were detected with as little as 3.3 nm GH, GH-GH, or G120R-G120R, although the strength of signals achieved for GH was substantially greater than that seen for both dimeric ligands (Fig. 3A). JAK2 activation was densitometrically analyzed for several such experiments and is shown graphically in Fig. 3B. At 10 nm, JAK2 activation by GH-GH and G120R-G120R was about 20–30% that induced by GH. A higher concentration (33 nm) of GH-GH yielded nearly 50% of the JAK2 activation induced by 10 nm GH.
Figure 3.
Relative Dose Response for Signaling in C14 Cells in Response to Monomeric and Dimeric Ligands
A, Serum-starved C14 cells were treated with the indicated concentrations of GH, GH-GH, or G120R-G120R for 10 min before extraction, resolution of equal amounts of proteins per sample by SDS-PAGE, Western transfer, and sequential immunoblotting with the indicated antibodies. In each experiment, all samples were evaluated on the same gel and under identical electrophoresis and immunoblotting conditions. B, Several experiments as in A were evaluated by densitometry to quantify the relative JAK2 tyrosine phosphorylation. Within each experiment, the signal generated in response to GH was considered as 100%. Values are expressed as mean ± se; n = 3. *, P < 0.03 compared with control (GH-treated). WB, Western blot.
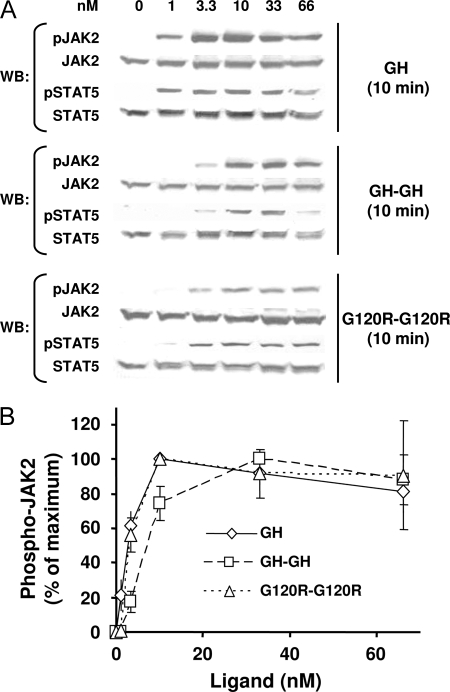
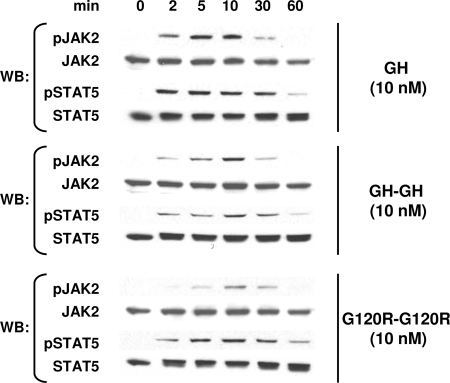
To examine more completely the dose-response profile for each ligand, we treated C14 cells with 1–66 nm GH, GH-GH, or G120R-G120R for 10 min and again monitored JAK2 and STAT5 tyrosine phosphorylation by immunoblotting (Fig. 4). In these experiments, however, samples for each stimulus were processed on separate gels and blots with exposures for each optimized to detect signals (i.e. in contrast to experiments in Fig. 3, no attempt was made to compare the magnitude of signals elicited by each ligand to the other). Representative results are shown in Fig. 4A for STAT5 and JAK2 activation, the profiles of which corresponded well to each other. Quantitative densitometric evaluation of JAK2 activation in several experiments is shown in Fig. 4B, wherein the maximal JAK2 tyrosine phosphorylation signal achieved for each ligand is considered as 100%. This analysis indicated that the dose-response profiles for GH and G120R-G120R were nearly identical, with the maximal signal achieved at 10 nm and the EC50 about 3 nm for each. GH-GH induced maximal JAK2 activation at 33 nm, and its EC50 was about 7 nm, indicating that GH-GH's decreased potency is also accompanied by a relative rightward shift in dose response.
Figure 4.
Dose-Response Profiles for Signaling in C14 Cells in Response to Monomeric and Dimeric Ligands
A, Serum-starved C14 cells were treated with the indicated concentrations of GH, GH-GH, or G120R-G120R for 10 min before extraction, resolution of equal amounts of proteins per sample by SDS-PAGE, Western transfer, and sequential immunoblotting with the indicated antibodies. In each experiment, samples resulting from stimulation with each ligand were evaluated on separate gels, and immunoblotting was optimized for each so as provide comparable exposures for each stimulus and allow comparison of dose-response profiles rather than comparison of relative signal intensities. B, Several experiments as in A were evaluated by densitometry to quantify the relative JAK2 tyrosine phosphorylation. For each ligand, the maximal signal achieved in response to each ligand was considered 100% for each ligand. Values are expressed as mean ± se. For 1 and 66 nm concentrations of each ligand, n = 3. For 3.3, 10, and 33 nm concentrations of each ligand, n = 5. WB, Western blot.
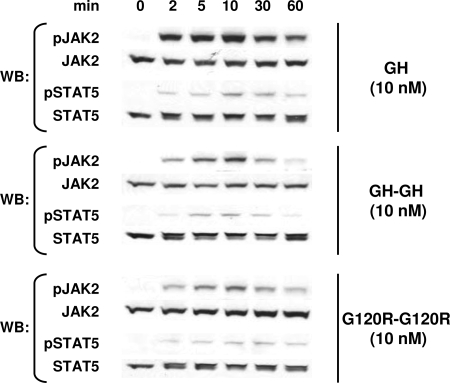
The decreased potency of both dimeric ligands and altered dose response for GH-GH suggested that their ability to bind GHR might be somewhat compromised compared with GH. Indeed, we found in competitive equilibrium binding assays with C14 cells that 20 nm unlabeled GH reduced 125I-labeled human GH (hGH) binding by over 97%, whereas 20 nm unlabeled GH-GH and G120R-G120R reduced [125I]hGH binding by 69 and 79%, respectively (data not shown). Thus, their affinities and/or binding capacities for GHR were somewhat diminished compared with GH. Despite this, we found that the time course of activation of signaling for the dimeric ligands in C14 cells was nearly identical to that observed with GH (Fig. 5). In response to each ligand (10 nm), both JAK2 and STAT5 activation were detected after as little as 2 min and peaked after 10 min.
Figure 5.
Time Courses for Signaling in C14 Cells in Response to Monomeric and Dimeric Ligands
Serum-starved C14 cells were treated for the indicated durations with 10 nm GH, GH-GH, or G120R-G120R before extraction, resolution of equal amounts of proteins per sample by SDS-PAGE, Western transfer, and sequential immunoblotting with the indicated antibodies. In each experiment, samples resulting from stimulation with each ligand were evaluated on separate gels, and immunoblotting was optimized for each so as provide comparable exposures for each stimulus and allow comparison of time courses rather than comparison of relative signal intensities. The experiments shown are representative of two such experiments. WB, Western blot.
Collectively, the data in Figs. 2–5 indicate that GH-GH and G120R-G120R, although their binding and signaling are less robust than GH, clearly cause the rabbit GHR to undergo disulfide linkage and trigger signal transduction. Furthermore, the similarity of the dose-response profiles and achievement of disulfide linkage argue that the conformational changes in the GHR induced by GH and the dimeric ligands are qualitatively similar (more below).
Endogenous GHR in Mouse Preadipocytes Is Activated by GH and G120R Dimers
Because the studies in Figs. 2–5 were carried out in a reconstitution system characterized by high levels of (rabbit) GHR and JAK2, we sought independent verification in a separate model system. 3T3-F442A cells are mouse fibroblasts that can be differentiated into adipocytes in response to GH and other growth factors (24). In the preadipocyte form, 3T3-F442A expresses endogenous GHR and JAK2 and responds to GH stimulation with activation of the JAK2/STAT5 and other pathways (22,25,26,27,28,29).
We tested the ability of the dimeric ligands to activate JAK2 and STAT5 signaling via the endogenous mouse GHR in 3T3-F442A cells. First, serum-starved cells were treated with 10 nm GH, G120R, GH-GH, or G120R-G120R for 10 min before detergent lysis and immunoblotting with anti-pJAK2, anti-JAK2, anti-pSTAT5, and anti-STAT5 (Fig. 6A). As expected, GH strongly activated JAK2 and STAT5 in these cells. G120R failed to elicit detectable JAK2 tyrosine phosphorylation but, interestingly, caused a small degree of STAT5 tyrosine phosphorylation, suggesting that this hGH antagonist may not be completely unable to trigger signaling via the murine receptor. In contrast to this very modest effect of G120R, GH-GH and G120R-G120R both caused substantial JAK2 and STAT5 activation, much as seen in C14 cells. Accordingly, both dimeric ligands also promoted GHR disulfide linkage in 3T3-F442A cells (Fig. 6B), which was not discernable in response to G120R.
Figure 6.
GH, GH-GH, and G120R-G120R Induce Signaling and GHR Disulfide Linkage in Mouse 3T3-F442A Cells
Serum-starved 3T3-F442A cells were treated with vehicle (−) or the indicated ligands (GH, G120R, GH-GH, or G120R-G120R; 10 nm) for 10 min. Detergent extracts were resolved by SDS-PAGE under either reducing (A) or nonreducing (B) conditions. Resolved proteins were transferred to nitrocellulose and immunoblotted sequentially with anti-pJAK2 and anti-JAK2 (A) or with anti-GHR (anti-GHRcyt-AL47, directed at the receptor cytoplasmic domain) (B). Positions of the dsl and non-dsl GHR are indicated. The experiments shown are representative of two such experiments. WB, Western blot.
Dose-response and time-course experiments were then carried out in 3T3-F442A cells, and representative results are shown in Figs. 7 and 8, respectively. Notably, the dose-response profiles for JAK2 and STAT5 activation by GH-GH and G120R-G120R were remarkably similar to that observed for GH, although the signals elicited by G120R-G120R were of less magnitude than by GH-GH. For each ligand, STAT5 activation was easily detected after 10 min stimulation with as low as 1 nm concentration (Fig. 7). The time courses of activation by GH, GH-GH, and G120R-G120R were also remarkably similar to each other in 3T3-F442A cells. For each ligand (10 nm), maximal JAK2 tyrosine phosphorylation was observed after 10 min and dropped dramatically thereafter. STAT5 activity was more sustained but also declined after 30 min for each ligand. The results with 3T3-F442A cells in Figs. 6–8 indicate that endogenous mouse GHR, like reconstituted rabbit GHR in C14 cells, binds both GH-GH and G120R-G120R and that both GH dimers trigger acute intracellular signaling in a pattern quite similar to that observed for normal GH. Furthermore, the data in 3T3-F442A cells make clear that the results in C14 cells were not a consequence of overexpression of GHR and/or JAK2.
Figure 7.
Dose-Response Profiles for Signaling in 3T3-F442A Cells in Response to Monomeric and Dimeric Ligands
Serum-starved 3T3-F442A cells were treated with the indicated concentrations of GH, GH-GH, or G120R-G120R for 10 min before extraction, resolution of equal amounts of proteins per sample by SDS-PAGE, Western transfer, and sequential immunoblotting with the indicated antibodies. In each experiment, samples resulting from stimulation with each ligand were evaluated on separate gels, and immunoblotting was optimized for each so as to provide comparable exposures for each stimulus and allow comparison of dose-response profiles rather than comparison of relative signal intensities. The experiments shown are representative of three such experiments. WB, Western blot.
Figure 8.
Time Courses for Signaling in 3T3-F442A Cells in Response to Monomeric and Dimeric Ligands
Serum-starved 3T3-F442A cells were treated for the indicated durations with 10 nm GH, GH-GH, or G120R-G120R before extraction, resolution of equal amounts of proteins per sample by SDS-PAGE, Western transfer, and sequential immunoblotting with the indicated antibodies. In each experiment, samples resulting from stimulation with each ligand were evaluated on separate gels, and immunoblotting was optimized for each so as to provide comparable exposures for each stimulus and allow comparison of time courses rather than comparison of relative signal intensities. The experiments shown are representative of three experiments. WB, Western blot.
GH and G120R Dimers Cause Similar Conformational Changes in GHR as Does GH
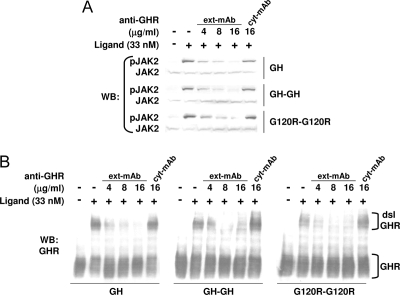
Our signaling data and assessment of ligand-induced GHR disulfide linkage indicate that GH (with normal site 1 and site 2), GH-GH (which possesses two copies each of both site 1 and site 2 within the same molecule), and G120R-G120R (which has two copies of site 1, but no normal site 2) each cause the receptor to adopt a signaling-competent conformation. We sought to explore this issue further by using a conformation-sensitive monoclonal GHR antibody. Anti- GHRext-mAb is a mouse monoclonal antibody we previously raised against the rabbit GHR extracellular domain, which cross-reacts with human, but not mouse, GHR; its epitope is contained within subdomain 2 of the extracellular domain, a region distinct from that which primarily engages GH (16,20,30). This antibody interacts with GHR on the surface of intact cells and exhibits conformational sensitivity (16,20). Pretreatment of cells with anti-GHRext-mAb only modestly decreases GH binding but substantially inhibits subsequent GH-induced activation of JAK2 and STAT5 signaling (16), suggesting that the antibody exerts inhibition by locking the GHR in an inactive state and preventing it from undergoing conformational changes rather than simply inhibiting GH binding. Thus, we employed anti-GHRext-mAb to compare GH and the dimers with regard to induction of an activated receptor conformation.
In the experiments shown in Fig. 9, serum-starved C14 cells were incubated with the indicated concentrations of anti-GHRext-mAb for 10 min at 37 C before treatment without (−) or with (+) GH, GH-GH, or G120R-G120R (33 nm in each case) as indicated. As a negative control, cells were pretreated with anti- GHRcyt-mAb, a different GHR monoclonal antibody that reacts with the intracellular domain (13,16,20). Detergent extracts were resolved by SDS-PAGE and blotted sequentially with anti-pJAK2 and anti-JAK2 (Fig. 9A). As previously observed (13,16), anti- GHRext-mAb dose-dependently inhibited GH-induced JAK2 activation, whereas anti-GHRcyt-mAb had no effect. Notably, both GH-GH- and G120R-G120R-induced JAK2 activation were similarly specifically inhibited by anti-GHRext-mAb pretreatment. Consistent with these signaling findings, GHR disulfide linkage induced by GH, GH-GH, and G120R-G120R was inhibited by anti-GHRext-mAb pretreatment with very similar dose dependencies (Fig. 9B). Thus, the acquisition of the active receptor signaling conformation promoted by all three ligands is prevented by the binding of anti-GHRext-mAb, consistent with the idea that each ligand promotes similar conformational changes to achieve activation of the GHR.
Figure 9.
Signaling in Response to GH, GH-GH, or G120R-G120R Is Inhibited by Anti-GHRext-mAb Pretreatment
Serum-starved C14 cells were pretreated with the indicated concentrations of anti-GHRext-mAb or anti-GHRcyt-mAb, as in Materials and Methods, before treatment with 33 nm GH, GH-GH, or G120R-G120R for 10 min. Detergent extracts were resolved by SDS-PAGE under either reducing (A) or nonreducing (B) conditions. Resolved proteins were transferred to nitrocellulose and immunoblotted sequentially with anti-pJAK2 and anti-JAK2 (A) or with anti-GHR (B). Positions of the dsl and non-dsl GHR are indicated. The experiments shown are representative of three such experiments. WB, Western blot.
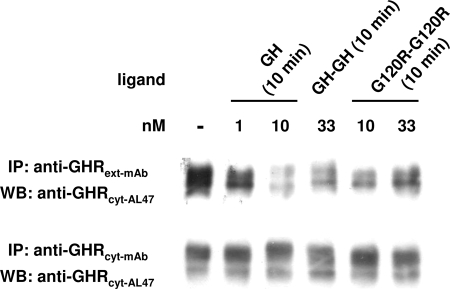
Anti-GHRext-mAb can also immunoprecipitate the GHR after detergent extraction. Notably, GHR from cells previously stimulated by GH and then detergent extracted is less immunoprecipitated by anti-GHRext-mAb in comparison with receptor from unstimulated cells, again reflecting the conformational sensitivity of this antibody (20). Thus, immunoprecipitation with this antibody can also be used to track ligand-induced receptor conformational change. In the experiment shown in Fig. 10, C14 cells were treated for 10 min with the indicated concentrations of GH, GH-GH, or G120R-G120R, after which cellular proteins were detergent extracted. For each sample, extracts were divided into two aliquots; one was subjected to immunoprecipitation with anti-GHRext-mAb and the other with anti-GHRcyt-mAb. Eluates from each precipitate were immunoblotted with anti-GHRcyt-AL47, the same polyclonal antibody directed at the cytoplasmic domain that was used for blotting in Figs. 2, 6B, and 9B. GH caused loss of precipitation of the GHR by anti-GHRext-mAb but not by anti-GHRcyt-mAb. Thus, the decreased precipitation by anti-GHRext-mAb could not be explained by either GH-induced down-regulation of GHR abundance or by other loss of material in the extract. Although the effects were blunted in comparison with GH, both GH-GH and G120R-G120R treatments before extraction also led to decreased precipitation of GHR by anti-GHRext-mAb after extraction. Thus, the change in receptor conformation induced by each ligand similarly led to decreased reactivity with anti-GHRext-mAb thereafter, suggesting that these ligands promote the same active GHR conformation.
Figure 10.
GHR Immunoprecipitation by Anti-GHRext-mAb in Cells Treated with GH, GH-GH, or G120R-G120R
Serum-starved C14 cells were pretreated with the indicated concentrations of GH, GH-GH, or G120R-G120R for 10 min. Detergent extracts were divided equally and immunoprecipitated with either anti-GHRext-mAb or anti-GHRcyt-mAb, as in Materials and Methods. Eluates were resolved by SDS-PAGE, and resolved proteins were transferred and immunoblotted with anti-GHRcyt-AL47. The experiments shown are representative of four such experiments. IP, Immunoprecipitation; WB, Western blot.
DISCUSSION
Activation of receptors that encode intrinsic tyrosine kinase activity, such as the insulin receptor, the epidermal growth factor receptor, and other tyrosine kinase growth factor receptors, is believed to involve the ligand-induced trans-autophosphorylation of closely apposed kinase domains within a dimeric receptor assemblage (31,32,33,34). Among the many tyrosine kinase growth factor receptors, diverse mechanisms are employed to achieve the dimeric conformations that bring the kinase domains into proximity. In some instances, the ligand is itself dimeric; in others, the receptor is a preformed dimer that undergoes conformational changes in response to the ligand (35).
GHR exemplifies a large class of receptors, the cytokine receptors, that encode no enzymatic activity but rather couple noncovalently to cytoplasmic Janus kinases to propagate signals (1,3,36). It is now believed that even in the absence of GH binding to the extracellular domain, a substantial fraction of cell surface GHR is dimeric and likely associated via the proximal cytoplasmic domain Box 1 region in a 2:2 stoichiometry with JAK2 (8,9,10). Despite these preformed GHR-JAK2 associations in the unliganded state, GH binding clearly effects a change in the assemblage to cause JAK2 activation and downstream signaling, presumably by bringing the receptor-associated JAK2 molecules into proximity of each other and allowing trans-autophosphorylation. Notably, we and others have observed that co-immunoprecipitation of JAK2 with GHR is enhanced in cells treated with GH, suggesting that ligand binding increases either the affinity of JAK2-GHR binding or the JAK2:GHR stoichiometry (20,23,27).
The determinants of GH-GHR binding that lead to intracellular signaling are incompletely known, but investigation of the activation mechanisms is critical for a comprehensive understanding of GH action. In the current study, we further explored determinants of GH-GHR interaction that trigger receptor activation. Our prior studies demonstrated the unexpected finding that dimers (covalently linked tandem copies) of prolactin (PRL) could activate PRL receptor (PRLR) in breast cancer cells; furthermore, dimers of a mutant PRL molecule defective in site 2 binding and inactive as a monomer could also cause PRLR signaling (37).
Inspired by these findings, we tested GHR activity in response to GH dimers and dimers of the site 2 mutant GH antagonist G120R in C14 and 3T3-F442A cells that bear only GHR and not PRLR (38,39). We found that each dimer caused JAK2 and STAT5 activation, albeit less potently than GH. Although the diminished potency likely in part relates to a somewhat diminished binding capacity of the dimers relative to native GH, we note that the dose-response profile in C14 cells for G120R-G120R was remarkably similar to GH and that for GH-GH was only modestly right-shifted. Furthermore, the time courses for signaling were essentially indistinguishable between GH and both dimers in both rabbit GHR-reconstituted cells (C14) and endogenous mouse GHR-expressing cells (3T3-F442A). We interpret these findings collectively to indicate that binding of the lower-affinity site 2 per se is not required to trigger GHR signaling that in many respects mimics that promoted by normal GH. This conclusion is important in that it is in striking contrast to previous interpretations. However, consistent with extensive previous findings, G120R alone produced little (in 3T3-F442A) or no (in C14) downstream signaling and functioned as an antagonist of signaling by GH or by the GH dimers (not shown); thus, despite the lack of requirement of site 2 in the dimers, a single site 1 in the context of a mutated site 2 is insufficient to efficiently trigger signaling.
How do the dimers yield receptor activation? In the absence of structural resolution of the receptor-dimer complexes, we addressed this question using biochemical approaches to examine the conformations adopted by the activated GHR. We found that GH, GH-GH, and G120R-G120R, but not the GH antagonist alone (20), promoted GHR disulfide linkage in both C14 and 3T3-F442A cells. This extends the correlation between achievement of disulfide linkage and initiation of signaling that we previously established (13,16,20,23) for a variety of GHR mutants and suggests that each ligand similarly causes approximation of cysteine-241 (the only extracellular domain unpaired cysteine) in the stem regions of (presumably dimeric) GHRs that engage the ligand. Likewise, the ability of the dimeric ligands to promote receptor disulfide linkage and trigger signaling was inhibited by pretreatment of cells with the conformation-sensitive extracellular GHR antibody anti-GHRext-mAb in a fashion indistinguishable from the antibody's effects on normal GH actions. Furthermore, treatment with the dimers, like that with GH, rendered GHR less precipitable by anti-GHRext-mAb, indicating that each ligand promoted similar conformational changes in the receptor.
Our findings with anti-GHRext-mAb are especially notable because we previously observed that subtle differences in conformation of GHR mutants capable of signaling could be detected with this antibody. For example, a GHR mutant in which the native transmembrane domain is replaced by that of the low-density lipoprotein receptor (which is several residues shorter than the GHR transmembrane domain) signals normally in response to GH and undergoes disulfide linkage but is markedly less sensitive to inhibition by anti-GHRext-mAb pretreatment than is the wild-type GHR (13). Thus, our findings that signaling and disulfide linkage induced by the dimers are equally sensitive to inhibition by anti-GHRext-mAb as those induced by normal GH strengthens our conclusion that the dimers productively engage the GHR very much as does normal GH.
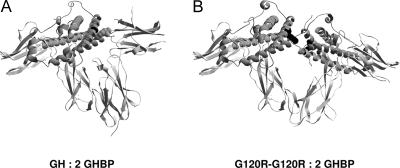
Our data make us consider whether GHR activation by the dimers is achieved by creating higher-order complexes of GHR dimers (e.g. forming dimers/multimers of GHR dimers). That is, could each of the two sites 1 of the GH-GH or G120R-G120R dimer bind two individual preformed GHR dimers, in effect bringing them together (or cross-linking) to enable transphosphorylation of JAK2 molecules between dimers rather than within a dimer? Although our data do not exclude such possibilities, we think instead that they favor that the GHR activation is the result of the dimeric ligand binding to one preformed receptor dimer as schematically represented in Fig. 11, especially in light of our findings with the conformation-sensitive antibody.
Figure 11.
Schematic Representations of GH and G120R-G120R Complexed with a Portion (Residues 32–237) of the GHR Extracellular Domain (GHBP)
Structure of GH bound to two GHBP molecules (42) (A) indicates that Gly 120 on helix 3 of GH (black) interacts with Trp 104 of the second receptor. We envision that the presence of a second binding site 1 in G120R-G120R enables it to interact with the second receptor (G120R-G120R: 2 GHBP) (B), allowing the adoption of an active signaling conformation and consequent signal transduction. Schematics were produced using DeepView/Swiss-PdbViewer.
The work herein leads us to the important conclusion that the GHR extracellular domain, at least in the context of the receptor dimer, exhibits substantially greater flexibility than generally believed such that it accommodates GH dimers that are much larger than GH itself. This novel view may shed light on an interesting feature of GH action. It has long been appreciated that a bell-shaped GH concentration dependence is often observed experimentally in that very high GH concentrations can result in a diminished signal outcome (6). Before it was appreciated that GHR forms dimers even in the absence of its ligand, this bell-shaped dose-response curve was interpreted to indicate that at very high concentrations, GH binds to available GHR molecules via site 1 in unproductive monomeric interactions and thereby self-antagonizes signaling (1,8). In light of our findings that GHR dimers can activate signaling and the fact that GHR is likely a dimer already when it binds GH, we wonder whether even normal GH, under certain conditions such as at very high concentrations, may engage its receptor in a 2:2 ratio more akin to the binding of the covalently coupled GH-GH (and G120R-G120R) dimers we have studied herein. If so, one might anticipate that signaling under these circumstances would proceed but, like that seen with the covalent dimers, might be dampened somewhat. Such a mechanism might more rationally explain the bell-shaped curve. Future studies may indicate the degree to which our findings with covalent GH and GH antagonist dimers will yield better mechanistic understanding of how normal GH triggers the GHR and, by extension, how other similar cytokines activate their receptors.
MATERIALS AND METHODS
Materials
Recombinant hGH (referred to as GH), G120R, hGH-hGH (referred to as GH-GH), and G120R-G120R were produced and purified as previously described (37). Briefly, the cDNA of GH, G120R, GH-GH, or G120-G120R were cloned into NdeI and XhoI sites of the bacterial expression vector pET22b. GH-GH and G120R-G120R were recombinantly engineered by gene fusion. The cDNAs encoding GH or G120R were ligated together via a small linker (GGATCC). The constructs were expressed in bacteria, and the ligands were subsequently purified. Routine reagents were purchased from Sigma Aldrich Corp. (St. Louis, MO) unless otherwise noted. Zeocin and hygromycin B were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA). G418 was from Mediatech (Herndon, VA). Fetal bovine serum, gentamicin sulfate, penicillin, and streptomycin were purchased from BioFluids (Rockville, MD).
Antibodies
The rabbit polyclonal antiserum, anti-GHRcytAL-47, was raised against a bacterially expressed N-terminally histidine-tagged fusion protein incorporating human GHR residues 271–620 (the entire cytoplasmic domain) and has been previously described (21). Anti-JAK2AL33 (directed at residues 746-1129 of murine JAK2) polyclonal serum has been described (40). Anti-GHRcyt-mAb is a mouse monoclonal antibody directed against a bacterially expressed glutathione-S-transferase fusion protein incorporating human GHR residues 271–620 (20). Anti-GHRext-mAb is a mouse monoclonal antibody directed against a bacterially expressed glutathione-S-transferase fusion protein incorporating rabbit GHR (41) residues 1–246; its generation and purification have been described previously (16,20,28,30). Monoclonal anti-STAT5 (W-17) and monoclonal anti-Myc (9E10) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-pSTAT5 affinity-purified rabbit polyclonal antibody (recognizing the tyrosine-phosphorylated form (Tyr-694) of STAT5a and STAT5b) was purchased from Zymed Laboratories Inc. (San Francisco, CA). Polyclonal anti-pJAK2 (recognizing phosphorylated Tyr-1007 and Tyr-1008) were purchased from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase-conjugated antirabbit (1:15,000) or antimouse (1:15,000) were from Pierce Chemical Co. (Rockford, IL).
Cells and Cell Culture
γ2A is a JAK2-deficient human fibrosarcoma cell line (19) kindly provided by Dr. G. Stark (Cleveland Clinic Foundation, Cleveland, OH). C14 is a stable γ2A cell line expressing rabbit GHR and mouse JAK2, as previously described (14). C14 cells were maintained in DMEM (1 g/liter glucose) (Mediatech) supplemented with 10% fetal bovine serum, 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, 100 μg/ml streptomycin, 200 μg/ml G418, 100 μg/ml hygromycin B, and 100 μg/ml zeocin. 3T3-F442A cells (24), kindly provided by Drs. H. Green (Harvard University, Boston, MA) and C. Carter-Su (University of Michigan, Ann Arbor, MI), were cultured in DMEM containing 4.5 g/liter glucose (Mediatech) supplemented with 10% calf serum, 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Cell Stimulation, Protein Extraction, Immunoprecipitation, Electrophoresis, and Immunoblotting
Serum starvation of all cell lines was accomplished by substitution of 0.25% (wt/vol) BSA (fraction V; Roche, Indianapolis, IN) for serum in their respective culture media for 16 h before experiments. All the stimulations were carried out at 37 C in binding buffer [consisting of 25 mm Tris-HCl (pH 7.4), 120 mm NaCl, 5 mm KCl, 1.2 mm MgCl2, 0.1% (wt/vol) BSA, and 1 mm dextrose] unless noted otherwise. Stimulations were terminated by washing cells twice with ice-cold PBS in the presence of 0.4 mm sodium orthovanadate (PBS-vanadate). Cells were solubilized in lysis buffer [150 mm NaCl, 10% (vol/vol) glycerol, 50 mm Tris-HCl (pH 7.4), 50 mm sodium fluoride, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 10 mm 1,10-phenanthroline, 1 μg/ml leupeptin, and 10 μg/ml aprotinin] with 1% (wt/vol) Triton X-100 (0.5% Triton X-100 for immunoprecipitation) on ice for 20 min. The detergent extracts were collected by centrifuging the lysate at 20,000 × g for 15 min at 4 C. In immunoprecipitation experiments, the extracts were incubated with the indicated monoclonal antibody overnight at 4 C on a rolling rack and with protein G-Sepharose for 2 h. The protein G-Sepharose was then collected by centrifugation and washed with the lysis buffer twice and then with PBS twice. Addition of 2× Laemmli SDS-PAGE sample buffer to the Sepharose was followed by boiling samples at 95 C for 5 min.
Extracted proteins or eluates from immunoprecipitation were then resolved by SDS-PAGE and immunoblotted as previously described (13,29). Immunoblotting detection reagents (SuperSignal West Pico chemiluminescent substrate) are from Pierce. Stripping and reprobing of blots was accomplished according to the manufacturer's suggestions.
Densitometric Analysis
Densitometric quantitation of immunoblots was performed using a high-resolution scanner and the ImageJ 1.30 program (developed by W. S. Rasband, Research Services Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD). Pooled data from several experiments are displayed as mean ± se. The significance (P value) of differences of pooled results was estimated by t tests.
Acknowledgments
We appreciate helpful conversations with Drs. Y. Huang, K. He, K. Loesch, L. Deng, J. Cowan, X. Li, and Y. Gan.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant DK58259 (S.J.F.) and in part by NIH Grant DK46395 (S.J.F.).
Parts of this work were presented at the 89th Annual Meeting of The Endocrine Society in Toronto, Canada, 2007.
Disclosure Summary: N.Y., J.F.L., X.W., J.J., W.Y.C., and S.J.F. have nothing to declare.
First Published Online December 20, 2007
Abbreviations: dsl GHR, Disulfide-linked form of GHR; GHR, GH receptor; hGH, human GH; JAK2, Janus kinase 2; pJAK2, phospho-JAK2; PRL, prolactin; PRLR, PRL receptor; STAT5, signal transducer and activator of transcription 5.
References
- Frank SJ, Messina JL 2002 Growth hormone receptor. In: Oppenheim JJ, Feldman M, eds. Cytokine reference on-line. London: Academic Press, Harcourt; 1–21 [Google Scholar]
- Carter Su C, Schwartz J, Smit LS 1996 Molecular mechanism of growth hormone action. Annu Rev Physiol 58:187–207 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Frank SJ 2000 Growth hormone action: signaling via a JAK/STAT-coupled receptor. In: Conn PM, Means A, eds. Principles of molecular regulation. Totowa, NJ: Humana Press; 55–83 [Google Scholar]
- Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA 1991 Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 254:821–825 [DOI] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA 1992 Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA 1992 Rational design of potent antagonists to the human growth hormone receptor. Science 256:1677–1680 [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ 2002 Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 23:623–646 [DOI] [PubMed] [Google Scholar]
- Frank SJ 2002 Receptor dimerization in GH and erythropoietin action: it takes two to tango, but how? Endocrinology 143:2–10 [DOI] [PubMed] [Google Scholar]
- Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ 2002 Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA 99:9858–9863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ 2005 Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 12:814–821 [DOI] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA 1999 Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283:987–990 [DOI] [PubMed] [Google Scholar]
- Remy I, Wilson IA, Michnick SW 1999 Erythropoietin receptor activation by a ligand-induced conformation change. Science 283:990–993 [DOI] [PubMed] [Google Scholar]
- Yang N, Wang X, Jiang J, Frank SJ 2007 Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol 21:1642–1655 [DOI] [PubMed] [Google Scholar]
- He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ 2003 Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol 17:2211–2227 [DOI] [PubMed] [Google Scholar]
- He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ 2005 JAK2 enhances the stability of the mature GH receptor. Endocrinology 145:4755–4765 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ 2004 A conformationally-sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ 2006 JAK2 influences growth hormone receptor metalloproteolysis. Endocrinology 147:2839–2849 [DOI] [PubMed] [Google Scholar]
- Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ 2007 Determinants of growth hormone receptor down-regulation. Mol Endocrinol 21:1537–1551 [DOI] [PubMed] [Google Scholar]
- Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, Ihle JN, Kerr IM 1997 A JAK1/JAK2 chimera can sustain α and γ interferon responses. Mol Cell Biol 17:695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Kopchick JJ, Frank SJ 1999 Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem 274:33072–33084 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ 2001 Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim SO, Yang N, Jiang J, Frank SJ 2004 Physical and functional interaction of GH and IGF-I signaling elements. Mol Endocrinol 18:1471–1485 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Gilliland G, Van Epps C 1994 Treatment of IM-9 cells with human growth hormone (GH) promotes rapid disulfide linkage of the GH receptor. Endocrinology 135:148–156 [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O 1976 Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 7:105–113 [DOI] [PubMed] [Google Scholar]
- Foster CM, Shafer JA, Rozsa FW, Wang X, Lewis SD, Renken DA, Natale JE, Schwartz J, Carter-Su C 1988 Growth hormone promoted tyrosyl phosphorylation of growth hormone receptors in murine 3T3-F442A fibroblasts and adipocytes. Biochemistry 27:326–334 [DOI] [PubMed] [Google Scholar]
- Carter-Su C, Stubbart JR, Wang XY, Stred SE, Argetsinger LS, Shafer JA 1989 Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase. J Biol Chem 264:18654–18661 [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ 1998 Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem 273:2344–2354 [DOI] [PubMed] [Google Scholar]
- Yang N, Huang Y, Jiang J, Frank SJ 2004 Caveolar and lipid raft localization of GH receptor and its signaling elements: impact on GH signaling. J Biol Chem 279:20898–20905 [DOI] [PubMed] [Google Scholar]
- Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ 1998 Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology 139:1927–1935 [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Mohammadi M, Schlessinger J 1998 Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem 273:11987–11990 [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH 2000 Protein tyrosine kinase structure and function. Annu Rev Biochem 69:373–398 [DOI] [PubMed] [Google Scholar]
- Schlessinger J 2000 Cell signaling by receptor tyrosine kinases. Cell 103:211–225 [DOI] [PubMed] [Google Scholar]
- Ward CW, Lawrence MC, Streltsov VA, Adams TE, McKern NM 2007 The insulin and EGF receptor structures: new insights into ligand-induced receptor activation. Trends Biochem Sci 32:129–137 [DOI] [PubMed] [Google Scholar]
- Schlessinger J 1997 Direct binding and activation of receptor tyrosine kinases by collagen. Cell 91:869–872 [DOI] [PubMed] [Google Scholar]
- Bazan JF 1990 Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 87:6934–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenheim JF, Tan D, Walker AM, Chen WY 2006 Two wrongs can make a right: dimers of prolactin and growth hormone receptor antagonists behave as agonists. Mol Endocrinol 20:661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Watling D, Rogers NC, Stark GR 1997 JAK2 and STAT5, but not JAK1 and STAT1, are required for prolactin-induced β-lactoglobulin transcription. Mol Endocrinol 11:1180–1188 [DOI] [PubMed] [Google Scholar]
- Vashdi D, Elberg G, Sakal E, Gertler A 1992 Biological activity of bovine placental lactogen in 3T3-F442A preadipocytes is mediated through a somatogenic receptor. FEBS Lett 305:101–104 [DOI] [PubMed] [Google Scholar]
- Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ 1998 Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem Biophys Res Commun 253:774–779 [DOI] [PubMed] [Google Scholar]
- Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI 1987 Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330:537–543 [DOI] [PubMed] [Google Scholar]
- Sundstrom M, Lundqvist T, Rodin J, Giebel LB, Milligan D, Norstedt G 1996 Crystal structure of an antagonist mutant of human growth hormone, G120R, in complex with its receptor at 2.9 Å resolution. J Biol Chem 271:32197–32203 [DOI] [PubMed] [Google Scholar]