Abstract
Local estrogen biosynthesis is a major factor in the pathogenesis of endometriosis. Aberrant expression of steroidogenic acute regulatory protein (StAR) and aromatase in endometriotic tissue leads to an up-regulation of estrogen production. The transcription factor steroidogenic factor-1 (SF-1) activates the promoters of both StAR and aromatase in endometriotic tissue. We investigated differences in SF-1 expression in endometriotic tissue and normally located endometrium to elucidate the mechanism underlying increased StAR and aromatase activities in endometriosis. Serial deletion and site-directed mutants of the SF-1 promoter showed that an E-box sequence was critical for its activity in endometriotic stromal cells. EMSAs showed that the upstream stimulatory factor (USF) 1 and 2 in nuclear extracts from endometrial and endometriotic stromal cells bound to the E-box. Chromatin-immunoprecipitation-PCR assay, however, demonstrated in intact cells that binding activity of USF2 to the SF-1 promoter was strikingly higher than that of USF1 in endometriotic stromal cells and that USF1 or USF2 binding activity was hardly detectable in endometrial stromal cells. Moreover, knockdown of USF2 but not USF1 resulted in robust and consistent down-regulation of SF-1 and its target genes StAR and aromatase in endometriotic stromal cells. USF2 but not USF1 mRNA and protein levels were significantly higher in endometriotic vs. endometrial stromal cells. In vivo, USF2 mRNA and immunoreactive USF2 levels in endometriotic tissues were strikingly higher than those in endometrium. Taken together, the elevated levels of USF2 in endometriosis account for, in part, the aberrant expression of SF-1 and its target gene StAR and aromatase.
ENDOMETRIOSIS IS A chronic and recurrent disease characterized by the presence and proliferation of endometrial tissue outside the uterine cavity (1,2). It is estimated to affect 6–10% of women of reproductive age, and its incidence has increased in recent years (3). In addition to potentially impairing fertility, endometriosis is associated with dysmenorrhea, dyspareunia, and chronic nonmenstrual pain. Endometriosis is considered to be an estrogen-dependent disease, and several studies have established the role of aberrant estrogen biosynthesis in promoting endometrial growth and malignancy.
Biologically active estrogen, estradiol, is produced from cholesterol in a series of six enzymatic conversions (4). The two rate-limiting steps in this pathway include the entry of cholesterol into the mitochondrion, which is facilitated by steroidogenic acute regulatory protein (StAR), and the conversion of androstenedione to estrone by aromatase (4,5). We and others previously demonstrated significantly higher mRNA levels and activities of StAR and aromatase in endometriotic tissue compared with eutopic endometrium (5,6,7). The introduction of aromatase inhibitors as potential therapy for endometriosis underscores the importance of aromatase and estrogen overproduction in the development of the disease (8,9). Based on these observations, it is widely believed that aberrant StAR and aromatase expression accounts for the increase in local biosynthesis of estrogen that promotes the growth of endometriotic lesions.
Steroidogenic factor 1 (SF-1) belongs to the orphan nuclear receptor family and regulates the expression of many steroidogenic genes in the adrenals and gonads by binding to a nuclear receptor half-site in its target gene promoters (10,11,12). SF-1 is the key transcription factor that transactivates the promoters of both StAR and aromatase (13). In earlier studies, we reported aberrant SF-1 expression in endometriotic stromal cells compared with eutopic endometrial stromal cells (13). We have also shown previously that treatment of endometrial stromal cells with prostaglandin E2 (PGE2) increases StAR and aromatase expression and activity in endometriotic tissues (6). Conversely, PGE2 did not induce StAR or aromatase in eutopic endometrial tissues. Taken together, these data suggest that stimulation of SF-1 expression in endometriosis by PGE2 mediates increased estrogen formation via induction of the StAR and aromatase promoters.
The mechanism for SF-1 expression in endometriosis is not well understood. The SF-1 proximal promoter region (−110 bp 5′-flanking region) is highly conserved among many mammalian species and contains at least three cis-acting elements that are essential for its activity species (14,15,16,17,18,19). The first element is an E-box, which is recognized by the transcription factors upstream stimulatory factor (USF) 1 and 2. These factors are thought to regulate many developmental processes, including muscle differentiation, neurogenesis, hematopoiesis, and sex determination (20,21). The second element is a CCAAT box recognized by nuclear factors YA, YB, and YC species (14). The third is a GA-rich element, which binds the transcription factor Sp1 (14).
We hypothesized that aberrant SF-1 expression in endometriosis is regulated by changes in transactivation of specific cis-acting elements in the SF-1 proximal promoter region. Here, we identified the key promoter elements and transcription factors involved in SF-1 expression and suggest that the relative availability of these transcription factors regulates the differential expression of SF-1 in eutopic endometrium and endometriotic tissue. Our findings provide further insight into the molecular mechanisms underlying local estradiol production and the pathogenesis of endometriosis.
RESULTS
Differential SF-1 in Endometriotic and Endometrial Stromal Cells
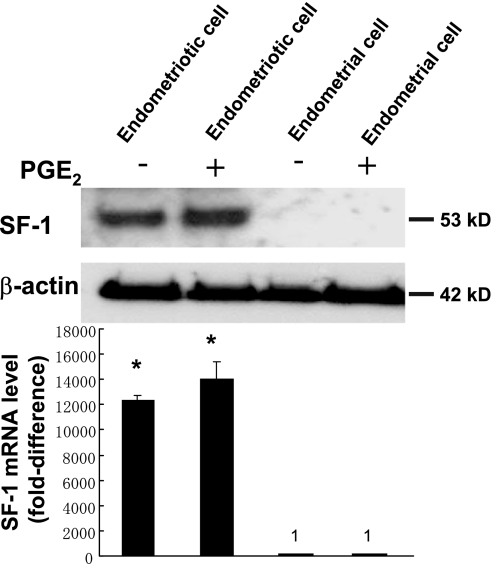
Real-time RT-PCR and immunoblotting analyses were performed to measure SF-1 expression in endometriotic and endometrial stromal cells. SF-1 mRNA levels in endometriotic cells were 12,352 times those found in endometrial stromal cells (Fig. 1, A and B). Treatment with PGE2 did not change SF-1 mRNA and protein levels in either endometrial or endometriotic stromal cells. These results were reproducibly observed in three independent experiments.
Figure 1.
SF-1 mRNA and Protein Is Highly Expressed in Endometriotic Stromal Cells
Immunoblot analysis was performed to detect SF-1 protein (53 kDa) expression in the presence (+) or absence (−) of PGE2 treatment. Actin was used as a loading control. Representative results from three independent experiments are shown. Real-time RT-PCR analysis was performed to measure SF-1 mRNA expression in the presence (+) or absence (−) of PGE2 treatment. Summary data for three independent experiments are shown. Results are expressed as the mean ± sem. *, P < 0.0001, endometriotic vs. endometrial cells.
Deletion Analysis of the SF-1 Promoter
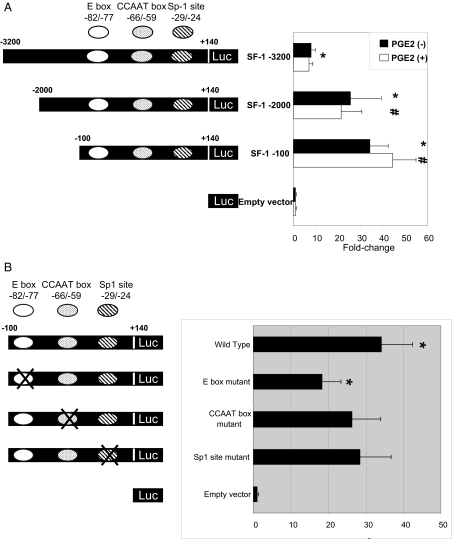
To identify cis-acting elements within the SF-1 promoter, we used a series of luciferase reporter constructs that contained 5′ deletions of the SF-1 promoter at nucleotides (nt) −3200, −2000, and −100, with a common 3′-terminus at +140. The promoter activities of the 5′-deletion mutants were assessed by luciferase assay after transient transfection into endometriotic stromal cells. The −3200/+140 construct showed low but significant promoter activity compared with empty vector (7.9-fold), whereas the −2000/+140 and −100/+140 constructs had markedly high activities compared with empty vector (25.8-fold and 34.2-fold, respectively) and with the −3200/+140 construct (3.3-fold and 4.3-fold, respectively) (Fig. 2A). Although PGE2 treatment induced StAR and aromatase expression in endometriotic stromal cells in previous studies, there was no significant difference in SF-1 deletion mutant promoter activities in the presence or absence of PGE2 (5,6). When the SF-1 deletion constructs were transfected into NIH-3T3 fibroblasts, which are nonsteroidogenic and do not express endogenous SF-1, no promoter activity was observed (data were not shown). These results indicated that the region between −100 and +140 of the SF-1 promoter contains cis-acting elements required for SF-1 expression in endometriotic stromal cells.
Figure 2.
An E-Box cis-Acting Element within the −100/+140 Region of the SF-1 Promoter Is Essential for SF-1 Expression in Endometriotic Stromal Cells
A, Serial deletion analysis was performed to localize the functional regions of the SF-1 promoter. A schematic representation of the 5′-flanking region of the SF-1 gene is shown. The SF-1 promoter/luciferase reporter constructs were named according to the nucleotide positions of their 5′-termini. All constructs were transiently transfected into endometriotic stromal cells. Luciferase assays were performed a minimum of three times. Mean luciferase activity of each construct is given relative to the promoterless vector (pGL3). Summary data for three independent experiments are shown. Results are expressed as the mean ± se. *, P < 0.01 vs. promoterless vector; #, P < 0.05 vs. −3200/+140 plasmid construct. B, Mutational analysis of the SF-1 promoter was performed to functionally identify cis-acting regulatory elements. Substitution mutations within the E-box, CCAAT box, and Sp-1 site were made in the SF-1 −100/+140 plasmid construct. Constructs were transiently transfected into endometriotic stromal cells, and luciferase activities were measured. Mean luciferase activity of each construct is given relative to the promoterless vector (pGL3). Results shown are the mean ± se of three independent experiments. *, P < 0.05 vs. wild-type construct.
Identification of cis-Acting Elements in the SF-1 Promoter
To identify specific cis-acting regulatory elements within the −100/+140 region of the SF-1 promoter, we generated a series of reporter constructs containing substitution mutations and transiently transfected them into endometriotic stromal cells. These mutations of potential regulatory sites were chosen based on previously published data (14,15,16,17,18,22). Mutations in two different regions, from nt −65 to −58 (a potential CCAAT box) and nt −30 to −25 (a potential Sp-1 site), did not reduce promoter activities significantly, whereas mutation within nt −81 to −76 (a potential E box) decreased promoter activity significantly by 47% compared with the wild-type construct (Fig. 2B). These results suggested that the E-box located between −81 and −76 of the SF-1 promoter is a critical cis-acting element regulating SF-1 expression in endometriotic stromal cells.
USF1/USF2/E-Box Binding Activity in Endometriotic and Endometrial Stromal Cells
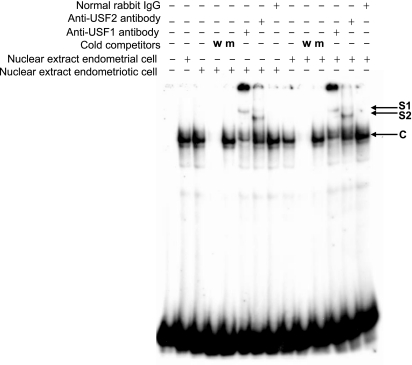
We performed EMSA to demonstrate binding activities of USF1 and USF2 to the E-box motif. Protein/E-box complexes were detected in both endometriotic and eutopic endometrial stromal nuclear extracts, and these complexes could be competed with the addition of cold probe (Fig. 3). Addition of USF1 and USF2 antibodies resulted in supershifted complexes using nuclear extracts from both endometriotic and endometrial stromal cells.
Figure 3.
EMSA Analysis Reveals USF1 and USF2 Binding to the E-Box Element Using Nuclear Extracts from Endometriotic Stromal Cells and Eutopic Endometrial Stromal Cells Under Cell-Free Conditions
Competitive EMSA was performed with an E-box radiolabeled probe (identical to the sequence in the SF-1 promoter) and nuclear extract. A 200-fold molar excess of unlabeled wild-type (w) probe and a mutant (m) probe were used in the competition reactions. Supershift experiments were performed with the wild-type or mutant probe, nuclear extracts, and antibodies against USF1 or USF2. Representative results from three independent experiments are shown. The first lane includes the labeled free probe only. C, Specific complex; S1, complex supershifted by USF1 antibody; S2, complex supershifted by USF2 antibody.
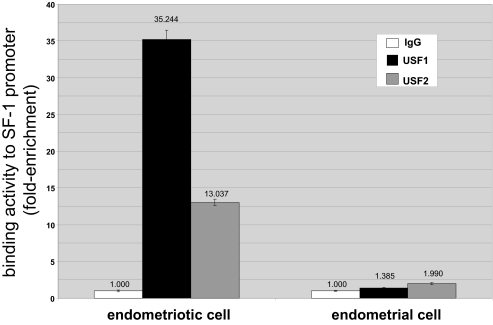
Because EMSA is a cell-free technique and is not quantitative, we then used an in vivo method, namely, chromatin immunoprecipitation (ChIP) followed by real-time PCR to quantify chromatin at the promoter region of SF-1 enriched as a result of hybridization with USF1 or USF2 antibodies. The results were normalized to input DNA and expressed as fold enrichment in relation to nonspecific IgG (Fig. 4). Both USF1 and USF2 showed significant in vivo binding activity to the SF-1 promoter containing the E-box (Fig. 4). In contrast, binding activities of either USF1 or USF2 to the SF-1 promoter was drastically lower in endometrial stromal cells.
Figure 4.
ChIP Analysis Reveals that in Vivo USF1 and USF2 Binding Activities to the E-Box Element in Endometriotic Stromal Cells Are Strikingly Higher Compared with Those in Endometrial Stromal Cells
We performed ChIP assays using endometriotic and endometrial stromal cells. Sonicated cell supernatant was used as input DNA (positive control, not shown). Precleared chromatin was used for immunoprecipitation reactions with a rabbit polyclonal antibody directed against human USF1 and USF2, normal rabbit IgG, and no antibody. Binding activity was expressed as enrichment of chromatin fragments hybridization with USF1 or USF2 antibodies and expressed as fold enrichment in relation to the rabbit IgG. All real-time PCR values (IgG, USF1, and USF2) were first normalized to input DNA. Representative results from five independent experiments are shown.
Endogenous USFs Regulate SF-1 Gene Expression in Endometriotic Stromal Cells
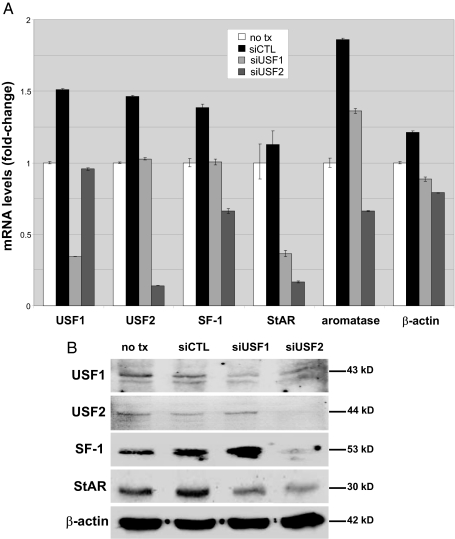
Small interfering RNA (siRNA) technology was used to determine whether endogenous USF transcription factors in endometriotic stromal cells regulate SF-1 expression. As shown in Fig. 5, transfection of endometriotic stromal cells with either USF1 or USF2 siRNA drastically reduced endogenous USF1 or USF2 mRNA levels (P < 0.0001) and significantly reduced the endogenous SF-1 mRNA levels (P < 0.01). More importantly, USF2 siRNA also reduced mRNA levels of SF-1 target genes StAR and aromatase, whereas only StAR mRNA levels decreased in response to USF1 knockdown. Transfection with a nonspecific control siRNA slightly increased expression of all genes tested (Fig. 5A). We calculated fold change in relation to nontransfected endometriotic cells.
Figure 5.
Endogenous USFs Regulate SF-1 Gene Expression in Endometriotic Stromal Cells
Endometriotic stromal cells were transfected with control siRNA (siCTL), USF1 siRNA, or USF2 siRNA and cultured for an additional 24 h. A, USF1, USF2, SF-1, StAR, aromatase, and β-actin mRNA levels were analyzed by real-time RT-PCR; B, USF1 (43 kDa), USF2 (44 kDa), SF-1 (53 kDa), StAR (30 kDa), and β-actin (42 kDa) protein levels in lysates from the same cells were analyzed by immunoblotting. Actin was used as a loading control. Representative results from six independent experiments are shown.
USF1 or USF2 siRNA also reduced USF1 and USF2 protein (Fig. 5B). USF-1 level was reduced by approximately 70% after the knockdown of its mRNA, whereas USF-2 was undetectable after the knockdown of USF-2 mRNA. USF2, but not USF1, knockdown gave rise to decreases in protein levels of SF-1 and its target gene StAR (Fig. 5B).
USFs mRNA and Protein Are Differentially Expressed in Eutopic Endometrial and Endometriotic Stromal Cells
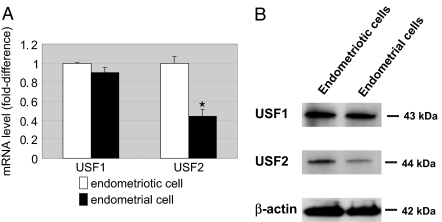
Real-time RT-PCR and immunoblotting analyses were performed to characterize the expression patterns of USFs in eutopic endometrial and endometriotic stromal cells. As shown in Fig. 6, A and B, both USF1 and USF2 were strongly expressed in endometriotic stromal cells, whereas USF2 expression was significantly lower in eutopic endometrial stromal cells. These results were reproducibly observed in three independent experiments.
Figure 6.
USF2 mRNA and Protein Expression Is Lower in Endometriotic Stromal Cells Compared with Endometrial Stromal Cells
A, Real-time RT-PCR analysis was performed to measure USF1 and USF2 mRNA levels. Summary data from simultaneously obtained endometriotic and endometrial cells from three subjects are represented in this graph. Results are expressed as the mean ± sem. *, P < 0.05. B; Immunoblot analysis was performed to measure USF1 (43 kDa) and USF2 (44 kDa) protein expression. Actin was used as a loading control. Representative results from three independent experiments are shown.
Cellular Localization of USFs in Tissues of Endometriosis and Endometrium
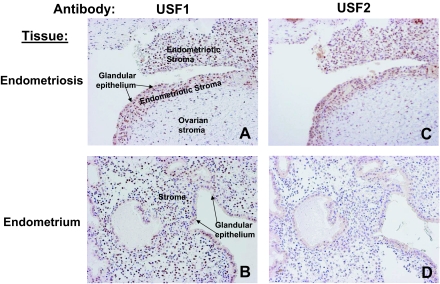
Immunohistochemistry was used to characterize the distribution of USFs in endometriotic and endometrial tissue. Consistent with our real-time RT-PCR and immunoblot analyses on endometriotic and endometrial stromal cells, USF1 was expressed ubiquitously in both tissues (Fig. 7, A and B), whereas USF2 was predominantly detected in endometriotic tissue (P < 0.05; labeling index in endometriotic tissue of 27.4 ± 7.5 vs. labeling index in eutopic endometrium of 11.0 ± 6.7; Fig. 7, C and D). There was no significant correlation between USF1 and USF2 expression in endometriotic and endometrial tissue (data not shown). These immunohistochemical analyses were performed on paired endometriotic and endometrial tissues obtained simultaneously from 25 subjects.
Figure 7.
USF1 Localizes to Both Eutopic Endometrium and Endometriotic Tissue, whereas USF2 Is Detected Primarily in Endometriotic Tissue
Immunohistochemistry for USF1 and USF2 was performed using serial paired endometriotic and endometrial samples from 25 subjects. A and C, Serial sections of a representative endometriotic tissue in an ovary. The nuclei of endometriotic stromal cells show immunoreactive USF1 and USF2. B and D, Serial sections of a representative eutopic endometrial tissue. Endometrial stromal cell nuclei exhibit readily visible staining with USF1 but not USF2. Original magnification, ×200.
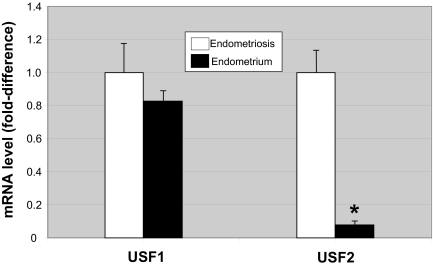
In a separate set of nine paired tissues of endometriosis and endometrium, we performed real-time PCR to quantify USF1 and USF2 mRNA levels. Consistent with the previous in vivo data, we found that USF2 mRNA levels were strikingly higher in endometriotic vs. endometrial tissue (P < 0.001), whereas no significant difference was observed with respect to USF1 mRNA levels.
DISCUSSION
USF1 and USF2 are structurally related to the Myc oncoproteins and belong to the bHLH-Zip family of transcription factors (23,24,25). Although USF1 and USF2 are ubiquitously expressed, they appear to be involved in the regulation of developmental and tissue-specific expression of a number of genes, including SF-1 (22,26,27). The majority of USF1 and USF2 proteins exist as heterodimers in vivo, whereas the homodimers are underrepresented (28,29). Functionally, it has been shown that overexpression of USF1 and USF2 homodimers leads to repression of rRNA gene transcription, whereas the USF1/USF2 heterodimers act as transcriptional activators (30). The relevance of these previously published observations to the transcriptional regulation of the SF-1 gene by USFs, however, is not clear.
We demonstrated that USF1 was expressed in stromal cells of both eutopic endometrium and endometriotic tissue, but USF2 was uniquely expressed in endometriotic stromal cells. In intact endometriotic stromal cells, USF1 binds to the SF-1 promoter, but its knockdown appears to have little effect on expression of SF-1 or the SF-1 target genes StAR and aromatase. On the other hand, USF2 binds to the SF-1 promoter more avidly, and its knockdown significantly reduces mRNA and protein levels of SF-1 and its target genes. These findings are suggestive that USF2 is essential for SF-1 expression in endometriosis and that its homodimers or heterodimers with USF1 may both stimulate SF-1 expression.
We should note that we observed a modest, but consistent, nonspecific stimulatory effect of control siRNA transfection on SF-1 and its target genes StAR and aromatase (Fig. 5). Nonetheless, when USF-2 siRNA-transfected cells were compared with cells transfected with control siRNA or untransfected cells, we observed that knockdown of USF-2 strikingly and consistently reduced mRNA and/or protein levels of SF-1, StAR, and aromatase.
We recently showed that hypomethylation of SF-1 promoter in endometriosis may in part be responsible for SF-1 expression in endometriosis and its absence in endometrium (31). Methylation, however, does not account for the lack of SF-1 expression in endometrial cells because treatment of endometrial cells with a demethylating agent increased SF-1 expression to a level that represents a small fraction of what is observed in endometriotic cells (31). Neither did demethylation give rise to a significant level of SF-1, which could induce StAR or aromatase in PGE2-treated endometrial stromal cells (31). Thus, although hypomethylation of SF-1 promoter may contribute to its expression in endometriotic cells, this is not sufficient to explain its extremely high levels in endometriosis. The aberrant expression of USF2 in endometriotic cells may be a more important mechanism, because its knockdown effectively down-regulated SF-1 protein and SF-1-target genes that encode the key steroidogenic proteins StAR and aromatase.
Our in vivo findings represented in Figs. 4–8 make the most important point in this manuscript. In vivo expression of USF2 and its binding to the SF-1 promoter in endometriotic stromal cells emerge as a key mechanism for aberrant SF-1 expression in this tissue. The aberrant overexpression of the nuclear receptor SF-1 in endometriosis is arguably the most striking abnormality because its mRNA levels are over 10,000-fold higher in primary endometriotic vs. endometrial cells, and more importantly, SF-1 target genes, e.g. StAR and aromatase, give rise to local estrogen production that makes this tissue independent of circulating estrogen levels. This pertains because postmenopausal endometriosis is dependent for growth on estrogen produced via local aromatase activity, and treatment with an aromatase inhibitor is the only current medical option for postmenopausal endometriosis (8,32). As discussed above, hypomethylation of the SF-1 promoter in endometriosis accounted for a relatively small aspect of the overall in vivo mechanism indicative of the presence of other factors responsible for SF-1 expression in endometriotic stromal cells. We suggest that differential overexpression of USF2 in endometriosis vs. its normal counterpart endometrium is a key molecular abnormality for local steroidogenesis. This was verified by decreases in SF-1-target steroidogenic genes StAR and aromatase upon USF2 knockdown.
Figure 8.
Summary of Real-Time PCR Quantification of mRNA for USF1 and USF2
Paired frozen endometriotic and endometrial tissues from nine subjects were used. Results are expressed as the mean ± sem. *, P < 0.001.
Additional studies are required to understand the mechanisms responsible for aberrant USF2 expression in endometriosis. Moreover, pathological roles of USF2 other than steroidogenesis should be explored.
MATERIALS AND METHODS
Tissue Preparation
Thirty-four normal cycling human endometria (proliferative phase, 14 cases; secretory phase, 20 cases) and 50 ovarian endometriotic cyst walls were obtained from the surgical pathology files of Northwestern University Prentice Hospital (Chicago, IL) and Tohoku University Hospital (Sendai, Japan). A large portion of these specimens (normally located intrauterine endometrial tissues and ovarian endometrioma cyst walls) were obtained simultaneously from the same subjects (n =34) during surgery. All of the patients examined had not received irradiation or chemotherapy before surgery. Human tissues were collected according to a research protocol approved by the Institutional Review Board of Northwestern University and the Ethics Committee at Tohoku University School of Medicine, and required informed consent was obtained.
Plasmids and Site-Directed Mutagenesis
Three SF-1 promoter-luciferase reporter plasmids (−3200-, −2000-, and −100/+140-bp deletion series) using the pGL3 vector were kindly provided by Dr. Y. Sadovsky (Washington University, St. Louis, MO). Isolation of the mouse SF-1 genomic clone (strain 129Sv) has been reported (33), and construction of the SF-1 promoter-luciferase reporter plasmids was carried out as described previously (14). Constructs containing site mutations within the E-box, CCAAT box, and Sp-1 site were constructed using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. E-box (−81/−76 bp), CCAAT box (−65/−58 bp), and Sp-1 site (−30/−25) sequences were changed as follows: E-box, 5′-GAGTCACGTGGGG-3′ to 5′-GAGTCcatTGGGG-3′; CCAAT box, 5′-ACCAATTGGGCC-3′ to 5′-ACCAATctGGCC-3′; and Sp-1 site, 5′-CGAGGGGAGGA −3′ to 5′-CGAGGGagGGA-3′. Lowercase nucleotides indicate mutation sites and underlined nucleotides indicate the core element sequence. The mutations and the orientation of insert were confirmed by direct sequencing. Plasmids used in transfection experiments were purified using an EndoFree plasmid isolation kit (QIAGEN, Valencia, CA), and their purity was verified by spectrophotometry and agarose gel electrophoresis.
Cell Culture
Tissues (human eutopic endometrium and endometrial cysts) were cultured using a protocol previously reported by Ryan et al. (34) with minor modifications. The cells were studied at passages 3–5. Tissues were rinsed with sterile phosphate saline solution, minced finely, and digested with collagenase B (1 mg/ml) and deoxyribonuclease I (0.1 mg/ml) at 37 C for 1–2 h. Epithelial cells were separated from stromal cells by filtration through a 25-μm pore size sieve. Stromal cells were then suspended in DMEM/F-12 (GIBCO/BRL, Grand Island, NY) containing 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin (250 ng/ml; growth medium). Fresh suspensions of stromal cells were plated in culture dishes and incubated at 5% CO2 and 37 C. Media was changed at 72-h intervals until the cells became 70–80% confluent. Confluent cells were serum deprived for 16 h in serum-free, phenol red-free DMEM/F-12 before being subjected to the following treatments: 1) serum-free, phenol red-free medium as the baseline control and 2) serum-free, phenol red-free medium with PGE2 (0.1 μm) (Sigma-Aldrich Corp., St. Louis, MO). Stromal cells were used in transient transfection and luciferase assays, ChIP assays, isolation of total RNA extracts for real-time RT-PCR, isolation of whole-cell protein extracts for immunoblotting, and isolation of nuclear extracts for EMSA.
Transient Transfection and Luciferase Assay
The day before transfection, cells were plated into six-well tissue culture plates at a density such that the cells reached 70–80% confluence by the time of transfection. Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), following the protocol provided by the manufacturer. Each transfection was performed using 2 μg firefly luciferase reporter construct containing serial deletion and site-directed mutants of the SF-1 promoter and 1 ng of an internal control Renilla luciferase reporter plasmid pRL-TK (Promega Corp., Madison, WI). Four hours after transfection, medium was removed by aspiration, DMEM/F-12 (2 ml) containing 10% fetal bovine serum was added, and the plates were returned to the incubator for 48 h. Then medium was removed, wells were rinsed with PBS to remove detached cells and residual growth medium, 250 μl 1× passive lysis buffer was added per well, and 20 μl of supernatant was separated for use in the dual-luciferase reporter assay system (Promega). Luciferase activities were determined using the Beckman Coulter LD400 luminometer (Beckman Coulter, Inc., Fullerton, CA). Firefly luciferase activities were normalized to Renilla luciferase activity in each well. These measurements were performed in triplicate and repeated in three independent experiments.
Isolation of Total RNA and Real-Time RT-PCR
Total RNA from eutopic endometrial and endometriotic stromal cells were isolated using TRI reagent (Sigma-Aldrich). Concentration and quality of total RNA were determined spectrophotometrically and by electrophoresis on denaturing formaldehyde-agarose gels.
Reverse-transcription reactions were carried out using Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen), according to the manufacturer's instructions. The expression levels of mRNA for USF1, USF2, and SF-1 were measured by real-time RT-PCR using a standard TaqMan PCR kit protocol on an Applied Biosystems 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Primers and probes were obtained from the ABI TaqMan Gene Expression Assay catalog (USF1, Hs00273038_m1; USF2, Hs00231528_m1; SF-1, Hs00610436_m1; StAR, Hs00264912_m1; aromatase, Hs00903413]lowen-m1; and β-actin, Hs99999903_m1; Applied Biosystems). The probes contained a 6-carboxy-fluorescein phosphoramidite (FAM dye) label at the 5′ end and a minor groove binder and nonfluorescent quencher at the 3′ end and were designed to hybridize across exon junctions. For each sample, triplicates were run for each gene in a 384-well format plate. Template cDNA and TaqMan gene expression assays, which contain PCR primers and probes, were added to TaqMan Universal PCR Mastermix (Applied Biosystems) to a final volume of 20 μl. The reactions were incubated at 50 C for 2 min and 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. The threshold cycle (CT) is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. TaqMan CT values were converted into absolute copy numbers. All RNA samples were normalized based on the TaqMan gene expression assays for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control (Hs99999905_m1; Applied Biosystems).
Immunoblotting
Total protein was extracted from whole cells using M-PER mammalian protein extraction reagent (Pierce, Rockford, IL), following the protocol suggested by the manufacturer. Protein concentration was measured using a BCA protein assay kit (Pierce), according to the manufacturer's instructions. The lysate (20 μg total protein) was mixed with 2× Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) and fractionated on an SDS-PAGE gel (4% stacking gel, 10% resolving gel) at 40 mA for 1 h. Protein samples were then electroblotted to the PVDF-Plus transfer membrane (0.45 μm) (GE Osmonics Inc., Minnetonka, MN). The membranes were then incubated with antibodies directed against either USF1 (at 1:500 dilution), USF2 (1:500), or SF-1 (1:2000) (Santa Cruz Biotechnology, Santa Cruz, CA) in blocking buffer at 4 C overnight. Donkey antirabbit IgG horseradish peroxidase-linked F(ab′)2 fragment (Amersham Pharmacia Biotech Inc., Piscataway, NJ) and goat antirabbit IgG horseradish peroxidase conjugate (Upstate Biotechnology, Waltham, MA) were used as secondary antibodies and were diluted in blocking buffer at 1:160,000 and 1:10,000, respectively. Proteins were detected using an ECL kit (Amersham) following the manufacturer's protocol and exposed to BioMax ML x-ray film (Kodak, Rochester, NY) for less than 1 min. Band intensity was quantitated using the Molecular Analyst version 1.5 software (Bio-Rad).
ChIP Assay
The ChIP assay was performed with the acetyl-histone H4 immunoprecipitation (ChIP) assay kit (Upstate Biotechnology). A total of 2 × 107 stromal cells of eutopic endometrium and endometriosis were incubated in a final concentration of 1% formaldehyde for 10 min at 37 C to cross-link histones to DNA. The cells were sheared using Sonic Dismembrator model 100 (Fisher Scientific, Hampton, NH) at 70% maximal amplitude (15 sec on, 60 sec off) for four pulses to obtain DNA fragments in the range of 200-1000 bp. The sonicated cell supernatant was diluted 10-fold in ChIP dilution buffer, and 40 μl was used as input DNA (positive control). Precleared chromatin was used for immunoprecipitation reactions with a rabbit polyclonal antibody directed against human USF1 and USF2, normal rabbit IgG (Upstate Biotechnology), or no antibody (negative control). The reactions were incubated on a rotator overnight at 4 C. Chromatin complexes were precipitated with 80 μl salmon sperm DNA/protein agarose slurry for 30 min at 4 C, washed once in low-salt buffer, once in high-salt buffer, once in LiCl immune complex, and finally twice in 10 mm Tris-HCl (pH 8.0), 1 mm EDTA. Precipitated complexes were removed from the beads by incubating for 30 min in 500 μl elution buffer (1% SDS, 0.1 m NaHCO3, and 10 mm dithiothreitol). The protein-DNA cross-links were reversed by heating samples at 65 C for 6 h. DNA was purified by phenol-chloroform extraction. Real-time PCR amplifications were performed under the following conditions: 40 cycles of 30 sec denaturation at 95 C and 30 sec annealing at 60 C. The primers used were SF-1 forward (−83/−64 bp), GAAGGCGAGAGGCCTGCAGA, and SF-1 reverse (49/−30 bp), CGGAGGCCCAATTGGTCTCT. All buffers used in this protocol contained a 1× protease inhibitor cocktail (Sigma-Aldrich).
EMSA
Nuclear protein was extracted from whole cells using NE-PER nuclear and cytoplasmic extraction reagents (Pierce), following the protocol suggested by the manufacturer. Protein concentration was measured with a BCA protein assay kit (Pierce), according to the manufacturer's instructions. EMSA was performed using LightShift chemiluminescent EMSA kit (Pierce) following the protocol suggested by the manufacturer. Biotin end-labeled oligonucleotide probes (see below) were purchased from Sigma-Aldrich and annealed to the complementary oligonucleotides. Twenty femtomoles of labeled probe and 3 μg nuclear extract were incubated for 20 min at room temperature in a reaction mix (total volume, 20 μl) containing 5% (vol/vol) glycerol, 10 mm Tris-HCl, 50 mm NaCl, 1 mm MgCl2, 0.5 mm dithiothreitol, and 2 μg poly(dI-dC). For supershift experiments, 5 μg of antibodies were added, and the incubation was continued for an additional 40 min on ice. For DNA competition EMSA, 4 pmol unlabeled double-stranded oligonucleotide was added simultaneously with labeled probe. Nondenaturing polyacrylamide gels (5%) were pre-electrophoresed and used to resolve protein-DNA complexes with 0.5× Tris-borate-EDTA running buffer at 100 V for 1 h. Protein-DNA was transferred to Biodyne B precut modified nylon membrane (Pierce) at 380 mA for 30 min and cross-linked using a commercial UV-light cross-linker (UVP, Upland, CA). Protein-DNA complexes were detected using a LightShift chemiluminescent EMSA kit (Pierce) and exposed to CL-XPosure Film (Pierce) for less than 20 min.
We used the SF-1 probe 5′-CCTGCAGAGTCACGTGGGGGCAGAGA-3′ that represented the −91/−66-bp regulatory region in the SF-1 gene promoter and contained E-box. The underlined nucleotides indicate the transcription factor binding domain (E-box).
siRNA
One day before transfection, endometriotic stromal cells were trypsinized, counted and plated in a six-well plate in growth medium to achieve 50%–60% confluence at the time of transfection. siRNA-Lipofectamine 2000 complexes were prepared as follows: 1 μl 100 μm USF1 siRNA, USF2 siRNA, or control siRNA oligonucleotide (P/N: USF1 121630, USF2 114348; Ambion, Inc., Austin, TX) was added to 249 μl Opti-MEM I reduced serum medium (Invitrogen) and incubated for 10 min. Ten microliters of Lipofectamine 2000 was combined with 240 μl of Opti-MEM, mixed gently and incubated 5 min at room temperature. The diluted oligonucleotides were combined with the diluted Lipofectamine 2000 (total volume, 500 μl), mixed gently, and incubated for 20 min at room temperature to allow siRNA-Lipofectamine 2000 complexes to form. The growth medium was removed, and the cells were washed once with medium without serum. siRNA-Lipofectamine 2000 complexes were added to each well. The cells were then maintained at 37 C in a CO2 incubator for 6 h. Growth medium containing twice the normal concentration of serum (500 μl) was then added to each dish without removing the transfection mixture. After culture for 24 h, the transfected cells were divided and prepared in either TRI reagent for RNA isolation and analysis in real-time RT-PCR or M-PER reagent for total protein isolation and immunoblot analysis.
Immunohistochemistry
Immunohistochemical analyses were performed with the streptavidin-biotin amplification method using a Histofine kit (Nichirei, Tokyo, Japan) for USF1 and USF2. The dilutions of the primary antibodies used in this study were 1:200. The antigen-antibody complexes were visualized with 3,3′-diaminobenzidine solution [1 mmol/liter 3,3′-diaminobenzidine, 50 mmol/liter Tris-HCl buffer (pH 7.6), and 0.006% H2O2] and counterstained with hematoxylin. Normal rabbit IgG was used instead of the primary antibodies for negative controls. Nonspecific immunoreactivity was detected in these tissue sections (not shown). For quantitative evaluation of USF1 and USF2 expression, we determined the labeling index (the percentage of positive cells) according to the report by Sasano et al. (35).
Statistical Analysis
Statistical analysis was performed by Welch's t test using the StatView 5.0 statistical software package (SAS Institute, Cary, NC). P < 0.05 was considered as significant. All values are given as the mean ± sem.
Acknowledgments
We thank Kiyoshi Ito for collecting tissue samples used in this study.
Footnotes
This work was supported by National Institutes of Health Grant HD38961 (to S.E.B.), by a fellowship award (to H.U.) from Japan Menopause Society (Tokyo, Japan), and by funds provided by Friends of Prentice and AVON Foundation.
Disclosure Statement: S.E.B. consulted for Serono and Novartis. He also received lectures fees from Serono. The remaining authors have nothing to disclose.
First Published Online December 28, 2007
Abbreviations: ChIP, Chromatin immunoprecipitation; nt, nucleotides; PGE2, prostaglandin E2; SF-1, steroidogenic factor-1; siRNA, small interfering RNA; StAR, steroidogenic acute regulatory protein; USF, upstream stimulatory factor.
References
- Vessey MP, Villard-Mackintosh L, Painter R 1993 Epidemiology of endometriosis in women attending family planning clinics. BMJ 306:182–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjerulff KH, Erickson BA, Langenberg PW 1996 Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 86:195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S 2005 Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57:359–383 [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Lin CC, Sun HS, and Chan HM 2001 Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab 86:5765–5773 [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE 1997 Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606 [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE 1996 Aromatase expression in endometriosis. J Clin Endocrinol Metab 81:174–179 [DOI] [PubMed] [Google Scholar]
- Takayama K, Zeitoun K, Gunby RT, Sasano H, Carr BR, Bulun SE 1998 Treatment of severe postmenopausal endometriosis with an aromatase inhibitor. Fertil Steril 69:709–713 [DOI] [PubMed] [Google Scholar]
- Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE 2004 Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril 81:290–296 [DOI] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP 1997 Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed] [Google Scholar]
- Morohashi KI, Omura T 1996 Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J 10:1569–1577 [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL 1992 Steroidogenic factor 1, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor 1. Mol Endocrinol 6:1249–1258 [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Michael MD, Bulun SE 1999 Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of SF-1 and COUP-TF to the same cis-acting element. Mol Endocrinol 13:239–253 [DOI] [PubMed] [Google Scholar]
- Woodson KG, Crawford PA, Sadovsky Y, Milbrandt J 1997 Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol Endocrinol 11:117–126 [DOI] [PubMed] [Google Scholar]
- Nomura M, Bartsch S, Nawata H, Omura T, Morohashi K 1995 An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem 270:7453–7461 [DOI] [PubMed] [Google Scholar]
- Scherrer SP, Rice DA, Heckert LL 2002 Expression of steroidogenic factor 1 in the testis requires an interactive array of elements within its proximal promoter. Biol Reprod 67:1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL 2001 Activation of the rat follicle-stimulating hormone receptor promoter by steroidogenic factor 1 is blocked by protein kinase a and requires upstream stimulatory factor binding to a proximal E box element. Mol Endocrinol 15:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett MA, Rice DA, Heckert LL 2000 Expression of steroidogenic factor 1 in the testis requires an E box and CCAAT box in its promoter proximal region. Biol Reprod 62:670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JH, Ingraham HA 2002 Regulation of the orphan nuclear receptor steroidogenic factor 1 by sox proteins. Mol Endocrinol 16:529–540 [DOI] [PubMed] [Google Scholar]
- Caudy M, Vassin H, Brand M, Tuma R, Jan LY, Jan YN 1996 Daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the acheate-scute complex. Cell 55:1061–1067 [DOI] [PubMed] [Google Scholar]
- Mellentin JD, Smith SD, Cleary ML 1989 lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell 58:77–83 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Sawadogo M, Daggett MA, Chen J 2000 The USF proteins regulate transcription of the follicle-stimulating hormone receptor but are insufficient for cell-specific expression. Mol Endocrinol 14:1836–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M, Roeder RG 1985 Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell 43:165–175 [DOI] [PubMed] [Google Scholar]
- Gao E, Wang Y, Alcorn JL, Mendelson CR 1997 The basic helix-loop-helix-zipper transcription factor USF1 regulates expression of the surfactant protein-A gene. J Biol Chem 272:23398–23406 [DOI] [PubMed] [Google Scholar]
- Jaiswal AS, Narayan S 2001 Upstream stimulating factor-1 (USF1) and USF2 bind to and activate the promoter of the adenomatous polyposis coli (APC) tumor suppressor gene. J Cell Biochem 81:262–277 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakayama K, Ito S, Fujii-Kuriyama Y, and Kamataki T 1997 Inhibition of the transcription of CYP1A1 gene by the upstream stimulatory factor 1 in rabbits. Competitive binding of USF1 with AhR/Arnt complex. J Biol Chem 272:30025–30031 [DOI] [PubMed] [Google Scholar]
- Harris AN, Mellon PL 1998 The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 12:714–726 [DOI] [PubMed] [Google Scholar]
- Vallet VS, Henrion AA, Bucchini D, Casado M, Raymondjean M, Kahn A, Vaulont S 1997 Glucose-dependent liver gene expression in upstream stimulatory factor 2 −/− mice. J Biol Chem 272:21944–21949 [DOI] [PubMed] [Google Scholar]
- Sirito M, Lin Q, Maity T, Sawadogo M 1994 Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res 22:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Datta PK, Jacob ST 1997 The dual role of helix-loop–helix-zipper protein USF in ribosomal RNA gene transcription in vivo. Oncogene 14:589–594 [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE 2007 Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- Attar E, Bulun SE 2006 Aromatase inhibitors: the next generation of therapeutics for endometriosis. Fertil Steril 85:1307–1318 [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan IP, Schriock ED, Taylor RN 1994 Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649 [DOI] [PubMed] [Google Scholar]
- Sasano H, Frost AR, Saitoh R, Harada N, Poutanen M, Vihko R, Bulun SE, Silverberg SG, Nagura H 1996 Aromatase and 17β-hydroxysteriod dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab 81:4042–4046 [DOI] [PubMed] [Google Scholar]