Abstract
Vesicular transport of peptide hormones from the cell body to the plasma membrane for activity-dependent secretion is important for endocrine function, but how it is achieved is unclear. Here we uncover a mechanism in which the cytoplasmic tail of transmembrane carboxypeptidase E (CPE) found in proopiomelanocotin (POMC)/ACTH vesicles interacts with microtubule-based motors to control transport of these vesicles to the release site in pituitary cells. Overexpression of the CPE tail in live cells significantly reduced the velocity and distance of POMC/ACTH- and CPE-containing vesicle movement into the cell processes. Biochemical studies showed that the CPE tail interacted with dynactin, which, in turn, recruited microtubule plus-end motors kinesin 2 and kinesin 3. Overexpression of the CPE tail inhibited the stimulated secretion of ACTH from AtT20 cells. Thus, the CPE cytoplasmic tail interaction with dynactin-kinesin 2/kinesin 3 plays an important role in the transport of POMC vesicles for activity-dependent secretion.
PEPTIDE HORMONES AND neuropeptides are synthesized at the cell body and must be transported to the plasma membrane for activity-dependent secretion to mediate various endocrine functions. Peptide hormones such as ACTH and β-endorphin are synthesized as larger precursors [proopiomelanocortin (POMC)], and packaged along with their processing enzymes [prohormone convertases and carboxypeptidase E (CPE)] into secretory vesicles/granules at the trans-Golgi network (TGN).
Studies have shown that post-Golgi transport of newly synthesized peptide hormone-containing secretory vesicles to the plasma membrane in endocrine cells is microtubule dependent (1,2). Various microtubule motors are involved in vesicle transport (1,3,4,5). These include the microtubule plus-end-directed motor kinesin, which is the major conveyer for anterograde transport toward the release site, and cytoplasmic dynein, a minus-end-directed motor, which is responsible for retrograde transport to the cell body (6).
Although much is known about the motor/microtubule transport system and some of the cytosolic proteins, such as dynactin and Rab GTPase (7,8), which associate indirectly with the vesicle to facilitate post-Golgi vesicle transport, how the vesicles are anchored to the microtubule system is not known. Similar to constitutive secretory pathway vesicles (e.g. amyloid precursor protein-containing vesicles (9,10), it is likely that the cytoplasmic tail of vesicular transmembrane proteins are also required to directly anchor peptide hormone vesicles to the transport system. A recent study suggests that CPE may mediate the trafficking of vesicles from the inner segment of the retina where the cell bodies of the photoreceptors are located, to the outer plexiform layer where synaptic terminals reside (spherules) (11). Hence, CPE is a candidate for mediating peptide hormone vesicular transport.
CPE is known to be present in peptidergic secretory vesicles in (neuro)endocrine cells and exists in a soluble and a membrane form (12,13). The soluble form of CPE functions as an exopeptidase to remove paired basic residues from endoproteolytically cleaved proneuropeptides within the lumen of the vesicle to yield biologically active peptides (14,15). A luminal domain of the membrane form of CPE has been demonstrated to bind to a motif of POMC (16) and brain-derived neurotrophic factor (13) to sort these precursors at the TGN into vesicles of the regulated secretory pathway in pituitary cells and hippocampal neurons, respectively (see supplemental Fig. S1, published as supplemental data on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Membrane CPE is associated with lipid rafts and can exist in a transmembrane orientation in secretory granules (17). Molecular modeling studies revealed that the C-terminal 25-amino-acid region of CPE (CPEC25), contains an atypical transmembrane domain that assumes an α-helical structure at acidic pH (5.5–6.5 found in TGN and secretory granules) and a cytoplasmic tail of about 10 amino acids (17,18). The transmembrane orientation is supported by previous studies showing that the CPE cytoplasmic tail is required for interaction with an activated form of the cytoplasmic small GTPase, ADP-ribosylation factor 6 (Arf6), to recycle the enzyme from the plasma membrane to the TGN (see Fig. S1D) in Neuro2A cells (19). Deletion of the final six amino acids (−SETLNF477) or point mutations (S472A and E473A) of CPE resulted in loss of interaction with Arf6 and inhibition of its Arf6-dependent recycling from the plasma membrane (19). Furthermore, the CPE cytoplasmic tail was shown to inhibit endocytosis of eosinophil cationic protein, a CPE interacting protein, when the recycling defective mutants of CPE (S472A and E473A) were expressed in neuroendocrine cells (20).
In this study, we have investigated the role of the cytoplasmic tail of vesicular CPE in post-Golgi transport of POMC/ACTH vesicles. We show that the cytoplasmic tail of CPE controls transport of POMC/ACTH vesicles to the secretion sites in pituitary cells by recruiting onto the vesicles dynactin that binds kinesin 2 and kinesin 3. Thus we have uncovered a novel CPE tail-driven vesicle transport mechanism that is essential for activity-dependent secretion of POMC-derived peptides to mediate important endocrinological functions such as such as alleviation of stress and pain (21) and regulation of appetite and energy balance (22).
RESULTS
Overexpression of the CPE Cytoplasmic Tail Reduces the Localization of Endogenous POMC/ACTH Vesicles in the Processes of AtT20 Cells
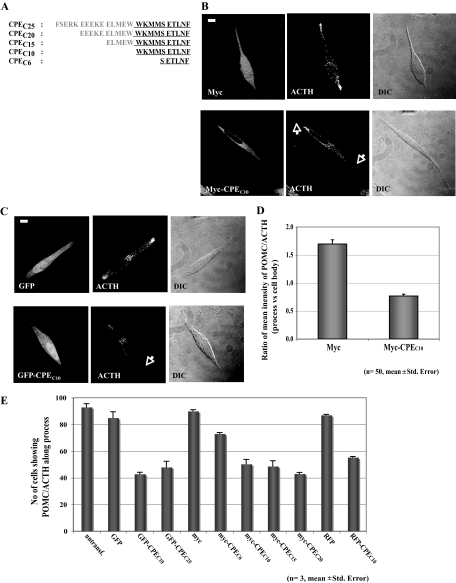
To examine the role of the cytoplasmic tail of CPE in microtubule-based transport of POMC/ACTH vesicles, we subcloned cDNA sequences that correspond to amino acids from the C terminus of CPE into mammalian expression vectors pCMV-Myc, pEGFP, and pDsRed2 (Fig. 1A). If the CPE cytoplasmic tail is necessary for the vesicle transport mechanism, its overexpression without a signal peptide to allow accumulation in the cytoplasm would be expected to displace the interaction of endogenous CPE tails with microtubule motors and interfere with POMC/ACTH vesicle movement. We compared the protein level of transfected exogenous CPE tail, green fluorescent protein (GFP)-CPEC10, to that of total endogenous CPE in AtT20 cells, a pituitary cell line, and found a 1:1 ratio (n = 3, supplemental Fig. S2). Data from our previous study (18) indicate that about 50% of total CPE in AtT20 cells is in POMC vesicles, and about 40% of vesicular CPE is lipid raft-associated. If all of these raft-associated CPE molecules in POMC vesicles were in a transmembrane orientation, we estimate that the exogenous CPE tail would be present in at least an approximately 2.5-fold excess compared with endogenous membrane CPE cytoplasmic tails in our studies. The intracellular distribution of endogenous immunoreactive POMC/ACTH in cell processes was analyzed in AtT20 cells expressing Myc alone, GFP alone, red fluorescent protein (RFP) alone, Myc-tagged CPE C termini containing 6, 10, 15, or 20 residues (CPEC6, CPEC10, CPEC15, and CPEC20), GFP-tagged 10- and 25-amino-acid residues (CPEC10 and CPEC25), or RFP-tagged 10-amino-acid residues of the C terminus of CPE (CPEC10) as an indicator of POMC/ACTH vesicle transported into processes at steady state (Fig. 1, B and C). Cells expressing Myc (Fig. 1B) or GFP (Fig. 1C) showed distribution of immunoreactive POMC/ACTH in the cytoplasm, Golgi complex, and along and at the tips of processes, similar to untransfected cells (data not shown). The same pattern of distribution was observed in cells expressing RFP alone (see Fig. 2A). However, cells overexpressing Myc- or GFP-CPEC10 showed a significant reduction in the level of POMC/ACTH localization in the processes (Fig. 1, B and C) without causing abnormal cell morphology or cell death (data not shown). The mean intensity of POMC/ACTH immunostaining in the processes relative to the cell body was determined in AtT20 cells expressing either Myc alone or Myc-CPEC10 (Fig. 1D). In 50 cells overexpressing Myc alone, the average ratio of POMC/ACTH intensity in the processes vs. the cell body was 1.75, whereas in cells overexpressing Myc-CPEC10, the ratio was 0.75, representing a more than 50% reduction. We observed a similar extent of reduction in the ratio in cells expressing GFP/RFP-CPEC10 compared with GFP/RFP (see Fig. 2D). Quantitative analysis of the number of cells showing POMC/ACTH immunostaining in the processes of AtT20 cells overexpressing Myc, GFP, RFP, Myc-CPEC6, Myc-CPEC10, Myc-CPEC15, Myc-CPEC20, GFP-CPEC10, GFP-CPEC25, or RFP-CPEC10 is shown in Fig. 1E. Approximately 55% of the cells expressing CPEC10, CPEC15, CPEC20, or CPEC25, showed reduced staining of POMC/ACTH in the processes, whereas about 28% of cells expressing CPEC6 and only 10% of control cells (Myc-, GFP-, or RFP-expressing cells) lacked POMC/ACTH in the processes (Fig. 1E). These results indicate that the entire cytoplasmic tail of CPE, consisting of the last 10 residues at the C terminus, is necessary for efficient steady-state localization of POMC/ACTH in the processes of AtT20 cells.
Figure 1.
Overexpression of the CPE Cytoplasmic Tail Disrupts POMC/ACTH Vesicle Localization in AtT20 Cell Processes
A, Amino acid sequences of the different C-terminal constructs of CPE used for in vitro and in vivo studies. Amino acids in the cytoplasmic tail are shown in bold. B, Immunocytochemistry showing the intracellular distribution of POMC/ACTH in AtT20 cells expressing Myc (top panels) or Myc-CPEC10 (bottom panels). POMC/ACTH and the Myc tag were detected as described in Materials and Methods. Differential interference contrast (DIC) images for Myc/Myc-CPEC10-expressing cells showed no major difference in cell morphology (DIC). Scale bar, 5 μm. C, Immunocytochemistry showing the intracellular distribution of POMC/ACTH in AtT20 cells expressing GFP (top panels) or GFP-CPEC10 (bottom panels). Differential interference contrast (DIC) images for GFP/GFP-CPEC10-expressing cells showed no major difference in cell morphology (DIC). Scale bar, 5 μm. D, Bar graph showing the ratio of the mean immunostaining intensity of POMC/ACTH in the processes relative to the cell body, in cells overexpressing GFP-CPEC10 or GFP alone. The intensity of POMC/ACTH immunostaining in the processes and cell body of 50 AtT20 cells in each case was quantified using Metamorph software. E, Bar graph showing the quantification of the number of cells with accumulation of punctate POMC/ACTH immunostaining in the processes in untransfected cells (untransf.) and cells transfected with GFP alone, Myc alone, RFP alone, GFP-CPEC10, GFP-CPEC25, Myc-CPEC6, Myc-CPEC10, Myc-CPEC15, Myc-CPEC20, or RFP-CPEC10. A total of 100 cells expressing each of the constructs was scored in three independent experiments.
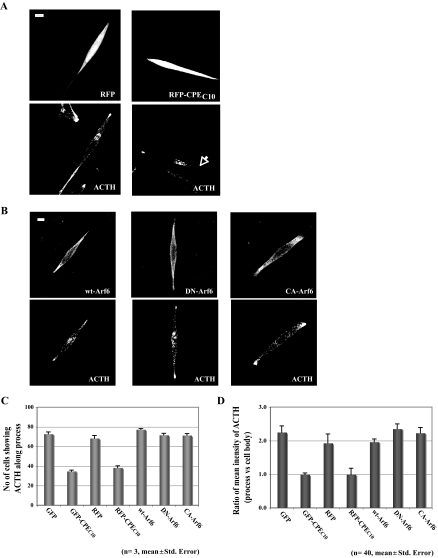
Figure 2.
Overexpression of Different Arf6 Forms Do Not Affect ACTH Vesicle Transport into the Processes of AtT20 Cells
A, Immunocytochemistry showing the intracellular distribution of ACTH in AtT20 cells expressing RFP (top panels) or RFP-CPEC10 (bottom panels). Scale bar, 5 μm. B, Immunocytochemistry showing the intracellular distribution of ACTH in AtT20 cells expressing HA-tagged wt/DN/CA-Arf6. ACTH and HA-tagged Arf6s were detected as described in Materials and Methods. Scale bar, 5 μm. C, Bar graph showing the quantification of the number of cells with accumulation of punctate ACTH immunostaining in the processes in untransfected cells (untransf.) and cells transfected with wt-Arf6, DN-Arf6, CA-Arf6, GFP, or GFP-CPEC10. A total of 100 cells expressing each of the constructs was scored in three independent experiments. D, Bar graph showing the ratio of the mean immunostaining intensity of ACTH in the processes relative to the cell body in untransfected cells (untransf.) or cells overexpressing wt/DN/CA-Arf6, GFP/GFP-CPEC10, or RFP/RFP-CPEC10 (n = 40). The intensity of ACTH immunostaining in the processes and cell body of AtT20 cells in each condition was quantified using Metamorph software.
Overexpression of Different Forms of Arf6 Have No Significant Effect on POMC/ACTH Localization in the Processes of AtT20 Cells
Previously, the cytoplasmic tail of CPE was shown to interact directly with an active form of Arf6 in K+/Ba2+-stimulated Neuro2A cells and was shown to play a role in recycling of CPE from the plasma membrane to the Golgi (19). To rule out the involvement of Arf6 in CPE cytoplasmic tail-driven ACTH vesicle transport to the processes of AtT20 cells, we quantitatively analyzed the steady-state distribution of ACTH in cells expressing hemagglutinin (HA)-tagged wild-type (wt-Arf6), dominant-negative (T27N; DN-Arf6), or constitutively active (Q67L; CA-Arf6) forms of Arf6. Mouse anti-ACTH antibody was used instead of rabbit anti-POMC/ACTH, in conjunction with the rabbit anti-HA antibody. Figure 2B shows that the distribution of ACTH along the processes was not perturbed by either wt-Arf6, DN-Arf6, or CA-Arf6 compared with untransfected cells (data not shown) and GFP- or RFP-expressing cells; however, with GFP-CPEC10 or RFP-CPEC10 expression (Fig. 2A), the levels were decreased to about 50% of control (Fig. 2C).
To quantitatively compare the amount of ACTH in the processes between cells expressing different Arf6 forms and cells expressing GFP, RFP, RFP-CPEC10, or GFP-CPEC10, the ratio of intensities of ACTH immunostaining in the processes relative to the cell body was calculated. Similar to the results observed in Fig. 1E (tag alone vs. tagged CPEC10), the ratio of processes to cell body staining was reduced by 44% in GFP-CPEC10-expressing cells (0.99) and by 50% in RFP-CPEC10-expressing cells (0.99) compared with GFP (2.25) or RFP (1.93), respectively, whereas expression of wt-Arf6 (1.96), DN-Arf6 (2.35), and CA-Arf6 (2.23) showed no significant reduction (Fig. 2D). This indicated that overexpression of any form of Arf6 does not significantly affect the normal localization of ACTH in the processes. Thus, from these results, we conclude that Arf6 does not participate in CPE tail-driven post-Golgi ACTH vesicle transport to the process.
Overexpression of CPEC10 Interferes with Real-Time Trafficking of POMC/ACTH Secretory Vesicles in Live AtT20 and Primary Anterior Pituitary Cells
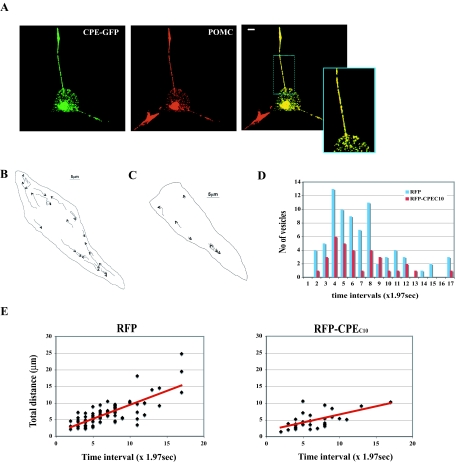
To determine whether the decrease in the steady-state localization of POMC/ACTH punctate staining in the processes of AtT20 cells was due to inhibition of post-Golgi POMC/ACTH vesicle transport, real-time trafficking of secretory vesicles was monitored in live AtT20 cells expressing either RFP alone or RFP-CPEC10. This live cell imaging was used to visualize the effect of overexpression of the CPE tail on vesicle movement. To visualize the vesicles within the cells, full-length CPE tagged with GFP at its C terminus (CPE-GFP) was used as a marker that, when expressed in AtT20 cells, showed a punctate distribution in the cell body and along the processes that overlapped with immunoreactive endogenous POMC/ACTH (Fig. 3A; colocalization correlation r = 0.76 ± 0.01). CPE-RFP expressed in AtT20 cells also showed a similar distribution and colocalization with POMC/ACTH (data not shown, colocalization correlation r = 0.59 ± 0.02) and verified that either CPE-GFP or CPE-RFP can be used as a marker for endogenous POMC/ACTH secretory vesicles in these live cells. Therefore, CPE-GFP was used here in conjunction with RFP-CPEC10 for live cell imaging.
Figure 3.
Overexpression of RFP-CPEC10 Disrupts Transport of Secretory Vesicles Containing Fluorescence-Tagged CPE and POMC in Live AtT20 Cells
A, Images of AtT20 cells expressing CPE-GFP (green) and immunostained with anti-POMC/ACTH (red). Note the colocalization of CPE-GFP and POMC/ACTH in vesicles (yellow punctate staining in merged images: colocalization correlation r = 0.76 ± 0.01) in the cell processes (see high-magnification inset). Scale bar, 5 μm. B, Tracks of each CPE-GFP vesicle movement in cells expressing RFP alone (supplemental Fig. 3 video). Scale bar, 5 μm. C, Tracks of each CPE-GFP vesicle movement in cells expressing RFP-CPEC10 (supplemental Fig. 3 video 2). Scale bar, 5 μm. D, Bar graph showing the number of vesicles that moved and the duration of their movement. Note the reduction in the total number of vesicles that moved and the reduced duration of the vesicles that did move in the RFP-CPEC10-expressing AtT20 cells compared with RFP alone. E, Graphs showing analyses of live cell images of in vivo movement of CPE-GFP secretory vesicles in AtT20 cells (RFP, 81 vesicles, four cells; RFP-CPEC10, 33 vesicles, six cells) (B, supplemental Fig. 3 videos 1 and 2). Each image was taken at a time interval of 1.97 sec and the total distance calculated. Both the overall velocity and duration of vesicle movement were decreased by overexpression of RFP-CPEC10.
CPE-GFP vesicles in live AtT20 cells expressing RFP alone showed various modes of movements, including those moving on trajectory routes in the cell body and processes (Fig. 3B; supplemental Fig. 3 video 1). A group of CPE-GFP vesicles in the cell body moved toward the proximity of the processes, whereas vesicles at the entry point of a process proceeded along the process toward the tip. Additionally, another group of vesicles appeared to move back from the tip of the processes toward the cell body. The movement of the CPE-GFP vesicles was completely stopped upon microtubule depolymerization by treatment of the cells with 33.3 μm nocodazole, demonstrating that it was microtubule based (data not shown). In contrast to cells expressing RFP alone, cells overexpressing RFP-CPEC10 exhibited a 75% decrease in the overall number of CPE-GFP vesicles moving with a trajectory toward the processes per cell (Fig. 3, C and D; supplemental Fig. 3 video 2), noting an even fewer number of the vesicles moving for longer than 13.76 sec (7 × 1.97 sec, Fig. 3D).
To examine which aspect of CPE-GFP vesicle movement was affected by excess RFP-CPEC10, we monitored the change in the total distance each vesicle moved during the observed time intervals (see Materials and Methods). The CPE-GFP vesicles in the RFP-CPEC10-expressing cells moved at a slower velocity for a shorter duration compared with the cells expressing RFP (Fig. 3, D and E). We also quantified the average velocity and the distances moved by each vesicle in cells expressing either RFP alone or RFP-CPEC10. We quantified the velocity and distance without discriminating between anterograde and retrograde movements. CPE-GFP vesicles in RFP-expressing cells showed a mean velocity of 0.53 ± 0.02 μm/sec and an average distance moved of 6.83 ± 0.44 μm (Table 1). In contrast, the mean velocity of CPE-GFP vesicles in cells expressing RFP-CPEC10 was decreased to 0.41 ± 0.03 μm/sec, and their distances moved were shorter (5.76 ± 0.80 μm) (Table 1), which is consistent with the data shown in Fig. 3, D and E. These results indicate that the C terminus of CPE containing the CPEC10 cytoplasmic tail plays a critical role in the processive trafficking of a population of POMC/ACTH vesicles in live AtT20 cells, especially evident in a fast-moving pool of vesicles.
Table 1.
Effect of Overexpression of the CPE Tail on the Distance and Velocity of CPE Vesicle Movement in Anterior Pituitary Cells
| Cell Type | Distance (μm) | P Value | Velocityavg (μm/sec) | P Value | ||
|---|---|---|---|---|---|---|
| AtT20 | 6.83 ± 0.02 (RFP) | 5.076 ± 0.80 (RFP-CPEC10) | <0.05 | 0.53 ± 0.02 (RFP) | 0.41 ± 0.03 (RFP-CPEC10) | <0.001 |
| Prim. AP | 5.03 ± 0.33 (GFP) | 2.42 ± 0.22 (GFP-CPEC25) | <0.001 | 0.44 ± 0.02 (GFP) | 0.30 ± 0.01 (GFP-CPEC25) | <0.001 |
AtT20 cells were transfected with CPE-GFP and either RFP or RFP-CPEC10, and primary anterior pituitary cells (Prim. AP) with CPE-RFP and either GFP or GFP-CPEC25. Real-time images of movement of CPE-FP-containing vesicles in live cells were taken at an interval of 1.97 sec (AtT20) and 0.98 sec (primary anterior pituitary). The distance and mean velocity of each CPE-GFP/RFP vesicle movement was calculated using Metamorph software. Average ± sem was calculated from the distances and mean velocities of vesicle movements in each condition and cell type. Student's t test was performed to determine the level of significance in changes observed in different conditions. The numbers of CPE-FP-containing vesicles evaluated were as follows: AtT20 (RFP = 81; RFP-CPEC10 = 33) and primary anterior pituitary (GFP = 43; GFP-CPEC25 = 28).
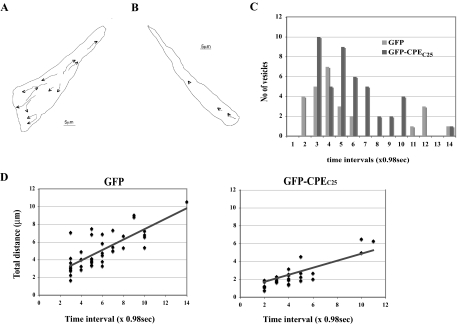
Because AtT20 cells are derived from a corticotropic tumor cell line and could be physiologically different from cells in the pituitary gland, we investigated whether overexpression of CPEC25, which had a similar negative effect on POMC/ACTH localization into processes (Fig. 1E), interferes with movement of CPE-RFP vesicles in primary cell cultures of mouse anterior pituitary gland (Fig. 4, supplemental Fig. 4 videos 1 and 2). Similar to AtT20 cells, primary pituitary cells showed a decrease in the number of moving vesicles and the duration of vesicle movement (Fig. 4). GFP-CPEC25 also decreased both the velocity and the duration of CPE-RFP vesicle movement (Fig. 4D). As shown in Table 1, CPE-RFP vesicles in the cells expressing GFP moved at a velocity of 0.44 ± 0.02 μm/sec and an average distance of 5.03 ± 0.33 μm. Overexpression of GFP-CPEC25 significantly reduced the velocity and distance of CPE-RFP vesicle movement to 0.30 ± 0.01 μm/sec and 2.42 ± 0.02 μm, respectively (Table 1). These results indicate that the involvement of the CPE cytoplasmic tail in the transport of POMC/ACTH vesicles is not unique to the AtT20 corticotropic cell line but also extends to primary cells of the anterior pituitary gland.
Figure 4.
Overexpression of GFP-CPEC25 Disrupts Transport of Secretory Vesicles Containing Fluorescence-Tagged CPE and POMC in Live Anterior Pituitary Cells
A, Tracks of each CPE-RFP vesicle movement in cells expressing GFP alone (supplemental Fig. 4 video 1). Scale bar, 5 μm. B, Tracks of each CPE-RFP vesicle movement in cells expressing GFP-CPEC25 (supplemental Fig. 4 video 2). Scale bar, 5 μm. C, Bar graph showing the number of vesicles that moved and the duration of their movement. Note the reduction in the total number of vesicles that moved and the reduced duration of the vesicles that did move in the GFP-CPEC25-expressing cells compared with GFP alone. D, Graphs showing analyses of live cell images of in vivo movement of CPE-RFP secretory vesicles in primary cultures of anterior pituitary cells transfected with GFP-CPEC25, which has a similar competitive behavior as CPEC10 (see Fig. 1D). The number of cells analyzed was 43 vesicles in two cells for GFP and 28 vesicles in 5 cells for GFP-CPEC25 (supplemental Fig. 4 videos 1 and 2). Each image was taken at a time interval of 0.984 sec and the total distance calculated. Both the overall velocity and duration of vesicle movement were decreased by overexpression of GFP-CPEC25.
CPE Tail Interacts with Endogenous Microtubule-Based Motors in AtT20 Cell Extracts
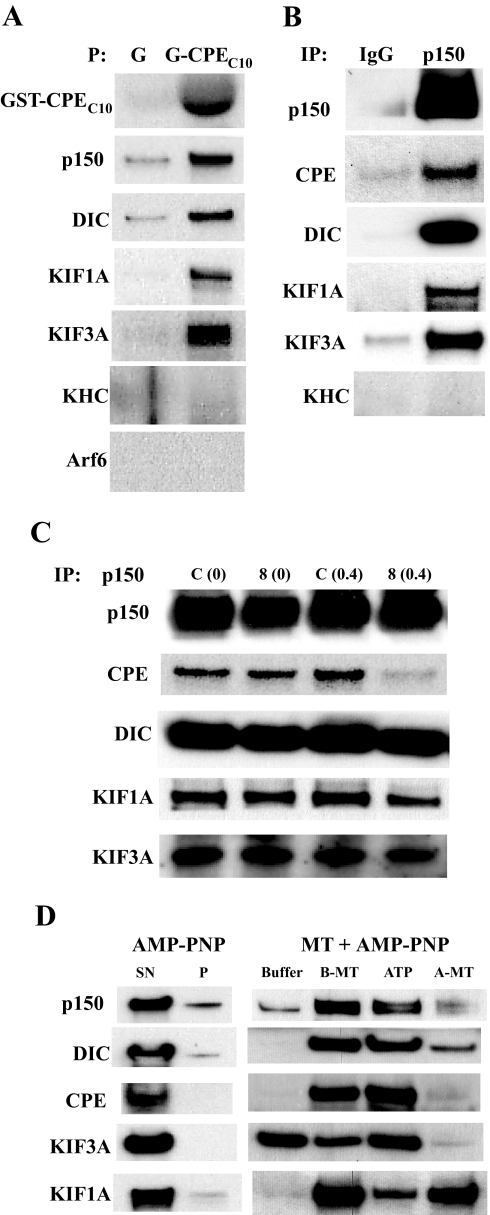
Our analysis of POMC/ACTH vesicle movement in live cells indicated that the cytoplasmic tail of CPE may interact directly or indirectly with a microtubule-based motor system to confer high speed and processivity onto POMC/ACTH vesicle trafficking. Therefore, we performed different types of biochemical assays to identify motor or motor-related proteins that might interact with the cytoplasmic tail of CPE. Recombinant glutathione S-transferase (GST)-tagged CPEC10 was used for coprecipitation assays. Bacterially expressed GST tag alone or GST-CPEC10 was added to cytosol from unstimulated AtT20 cells to pull down proteins that interact with the CPE cytoplasmic tail. CPEC10 pulled down a significant amount of dynactin (p150), dynein intermediate chain (DIC), kinesin family member 3A (KIF3A; kinesin 2), and KIF1A (kinesin 3), but not kinesin heavy chain (KHC; kinesin 1) from AtT20 cell cytosol (Fig. 5A). Silver staining of the SDS gel that contained the separated coprecipitated proteins from AtT20 cells showed minimal pull-down of nonspecific proteins by control GST-tag alone (supplemental Fig. S3A).
Figure 5.
The Cytoplasmic Tail of CPE Interacts with Endogenous Dynactin, KIF3A, KIF1A, and Dynein from AtT20 Cell Cytosol
A, Immunoblots showing proteins pulled down by GST-tagged CPEC10 or GST alone from AtT20 cell cytosol. G, GST alone; P, precipitation. B, Immunoblot showing proteins coimmunoprecipitated with dynactin (p150). Anti-p150 antibody or rabbit IgG was added to cytosol from AtT20 cells for coimmunoprecipitation. IP, Immunoprecipitation; p150, anti-p150 antibody. C, Immunoblot showing proteins coimmunoprecipitated with anti-p150 in the presence of CPEC8 peptide (8) or control peptide (C) added to the cytosol of AtT20 cells; 0 or 0.4 μg/ml of each peptide was used. D, Immunoblot of proteins bound to microtubules and released by ATP. Microtubule co-pelleting assays were performed to examine the microtubule-binding nature of CPE, motor proteins, and their associated proteins in AtT20 cell cytosol. Buffer indicates wash with buffer alone, and ATP indicates wash with buffer containing ATP. A-MT, Microtubule pellet washed with ATP buffer; B-MT, microtubule pellet washed with buffer; P, pellet; SN, supernatant.
As a counter pull-down experiment, co-immunoprecipitation was performed using an antibody against p150, a core subunit of dynactin. Dynactin was immunoprecipitated by anti-p150 antibody along with endogenous CPE from AtT20 cell cytosol (Fig. 5B). Dynein, KIF3A, and KIF1A were also found to co-immunoprecipitate with dynactin. Again, KHC was not detected in the co-immunoprecipitate, which also verifies the specificity of our pull-down studies. Coomassie blue staining of the SDS gel that contained the separated immunoprecipitated proteins showed minimal pull-down of nonspecific proteins by anti-p150 rabbit antibody and control rabbit antibody (supplemental Fig. S3B). These results confirm an interaction between dynactin and CPE, which was shown in the coprecipitation studies in Fig. 5A. Additionally, KIF1A, KIF3A, and dynein also interacted with dynactin, whereas KHC did not.
Because CPEC10 pulled down dynactin, dynein, KIF3A, and KIF1A (Fig. 5A), the presence of excess CPEC10 would be expected to interfere with the interaction between endogenous CPE and dynactin or other motors. To test this possibility, an excess amount of a 99.5% pure CPEC8 peptide representing 80% of the amino acids in the CPE tail was added to AtT20 cell cytosol before co-immunoprecipitation using anti-p150 antibody. As shown in Fig. 5C, the amount of dynactin pulled down was not different when the control peptide or the CPEC8 peptide was added. However, the amount of endogenous CPE pulled down by antidynactin antibody was significantly decreased in the presence of 0.4 μg/ml CPEC8 compared with the control scrambled peptide (Fig. 5C). This indicates that the CPE cytoplasmic tail interacts with dynactin. In contrast, CPEC8 did not significantly affect dynein, KIF3A (kinesin 2), and KIF1A (kinesin 3) binding to dynactin, indicating that these motor proteins do not interact with CPE tail directly.
The CPE-Dynactin-KIF1A-KIF3A-Dynein Complex Binds Microtubules in an ATP-Dependent Manner
Because microtubule-based motors or their associated proteins bind microtubules in an ATP-dependent manner (23,24), microtubule co-pelleting assays (25) were performed to test whether the CPE binds microtubules in a similar manner to dynactin. Exogenously polymerized bovine microtubules were added into AtT20 cell cytosol along with 4 mm AMP-PNP, a nonhydrolyzable ATP analog that promotes association of motor proteins with microtubules during co-pelleting (26). After ultracentrifugation through a double sucrose cushion, the microtubules were washed either with buffer alone or with 10 mm ATP, which displaces motors and their associated proteins from microtubules. Dynactin and dynein that were bound to microtubules in the presence of AMP-PNP were efficiently released from microtubules by the ATP wash (Fig. 5D). Because it was possible that endogenous CPE might be pelleted at high-speed ultracentrifugation used for microtubule co-pelleting, the AtT20 cell cytosol was subjected to the centrifugation without microtubules but with AMP-PNP. However, no CPE and microtubule motors were found in the pellet (Fig. 5D). CPE from AtT20 cells was dissociated from microtubules with the ATP wash in a similar way to dynactin and dynein. Only a minor pool of KIF3A was found in the same microtubule-bound pool as CPE, dynactin, and dynein, whereas the rest did not bind to microtubules in an ATP-dependent manner. This indicates that only a subset of kinesin 2 is associated with the CPE-dynactin complex, for which binding to microtubules depends on ATP. KIF1A that bound to microtubules was only partially released by the ATP wash. This may be due to the K loop-based strong affinity of KIF1A to microtubules (27). Coomassie blue staining of the SDS gel that contained proteins pelleted by microtubules and AMP-PNP, and subsequently washed off by either buffer or ATP, showed that the proteins released from microtubules by ATP were not due to major depolymerization of microtubules (supplemental Fig. S3C). These observations taken together indicate that the CPE tails associate with microtubules via dynactin and that the CPE-dynactin complex contains dynein, a subset of kinesin 2, and a small amount of kinesin 3.
The CPE-Dynactin-KIF1A-KIF3A-Dynein Complex Is Associated with Secretory Vesicles
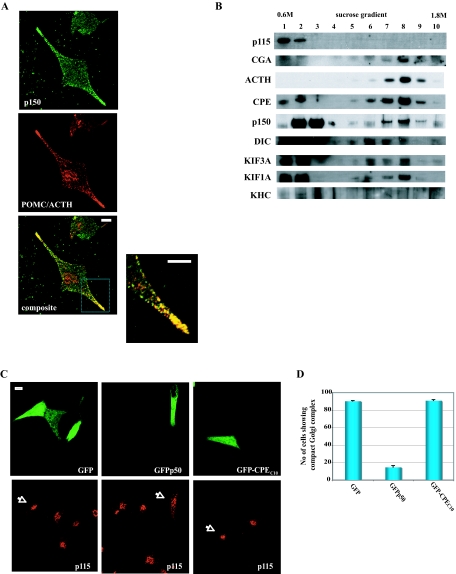
To verify that the dynactin is associated with secretory vesicles, immunocytochemistry of AtT20 cells was carried out. Figure 6A shows that dynactin (p150) is associated with POMC/ACTH vesicles as revealed by the yellow punctate staining pattern in the processes of the cells (n = 4, colocalization correlation r = 0.83 ± 0.02). As controls, we measured the colocalization correlation R of CPE-GFP vs. p115: 0.29 ± 0.08 (data not shown). Other motor proteins could not be visualized because available antibodies against them were not useful for immunocytochemistry. We also examined whether GFP-CPEC10 could interfere with the colocalization between dynactin (p150) and POMC/ACTH vesicles seen in Fig. 6A. Cy3 was used to label p150, and Cy5 was used to label POMC/ACTH in GFP-expressing cells (supplemental Fig. S4). Although the overall correlation R values for the Cy3 and Cy5 fluorophores were smaller than that for FITC (488 nm) and TexasRed (568 nm) used in Fig. 6A, overexpression of CPEC10 decreased the value for colocalization of dynactin (Cy3) and POMC/ACTH (Cy5) in cell processes from 0.44 ± 0.03 to 0.15 ± 0.04. Further demonstration of the association of the motors with secretory vesicles was carried out by subcellular fractionation studies. A postnuclear supernatant after centrifugation at 1000 × g was loaded onto 0.6–1.8 m sucrose gradient and centrifuged at approximately 100,000 × g for 16 h. In Fig. 6B, marker for Golgi: p115 (Golgi) stayed in the light/floating membrane fractions (nos. 1 and 2), whereas CGA (dense core granule marker) was fractionated in the heavy membrane pools at nos. 7–9 (peak at no. 8). Consistent with regulated secretory pathway granule localization, ACTH was found enriched in these fractions along with CPE, p150, DIC, KIF3A, and KIF1A. KHC was not found in the dense-core vesicle fraction. The immunocytochemistry and subcellular fraction results taken together indicate association of dynactin as well as the motors dynein, kinesin 2, and kinesin 3 with secretory vesicles.
Figure 6.
Microtubule Motors Are Associated with ACTH Vesicles, and Overexpression of GFP-CPEC10 Does Not Affect Dynactin Integrity
A, Immunocytochemistry showing the distribution of endogenous dynactin and POMC/ACTH in AtT20 cells. Dynactin (green) and POMC/ACTH (red) were detected as described in Materials and Methods. The composite images (bottom panel and inset, colocalization correlation r = 0.83 ± 0.019), showing yellow punctate staining in the processes and tips, indicate overlap and association of cytosolic dynactin with POMC/ACTH vesicles. Scale bar, 5 μm. B, Immunoblot of proteins in light/floating and heavy membrane fractions. Subcellular fractionation of AtT20 cells was carried out by performing 0.6–1.8 m sucrose equilibrium centrifugation, and 1.5% of each fraction was analyzed on the gel. C, Morphology of the Golgi complex in AtT20 cells expressing GFP, GFP-p50, or GFP-CPEC10. Arrows indicate the Golgi complex in cells expressing GFP proteins. D, Bar graph showing the quantification of the number of cells with either compact or dispersed Golgi complex in different conditions. A total of 100 cells expressing each of the constructs was scored in three independent experiments.
Overexpression of CPEC10 Does Not Disrupt Dynactin Integrity
Overexpression of GFP-p50, a dynactin subunit that connects the sidearm (p150) to the backbone (Arp1 minifilament) of dynactin, is known to dissociate this connection, which causes dispersion of the Golgi apparatus (28). Because we could not rule out a possibility that overexpression of CPEC10 might affect dynactin integrity, we compared the effect of overexpression of CPEC10 vs. GFP-p50 on Golgi integrity. We monitored Golgi morphology in cells expressing GFP, GFP-CPEC10, or GFP-p50. As shown in Fig. 6, C and D, only overexpression of GFP-p50 disrupted the Golgi complex in up to 90% of the cells compared with less than 10% by GFP or GFP-CPEC10, indicating that the dynactin complex is intact in cells expressing GFP-CPEC10. Thus, the CPE cytoplasmic tail did not disrupt dynactin integrity.
Overexpression of Myc-CPEC10 Inhibits Activity-Dependent Secretion of ACTH from AtT20 Cells
Microtubule-based transport of POMC/ACTH vesicles to secretion sites is a prerequisite for activity-dependent secretion of POMC-derived peptides, such as ACTH. Therefore, we examined the effect of overexpression of Myc-CPEC10 on the secretion of ACTH from AtT20 cells.
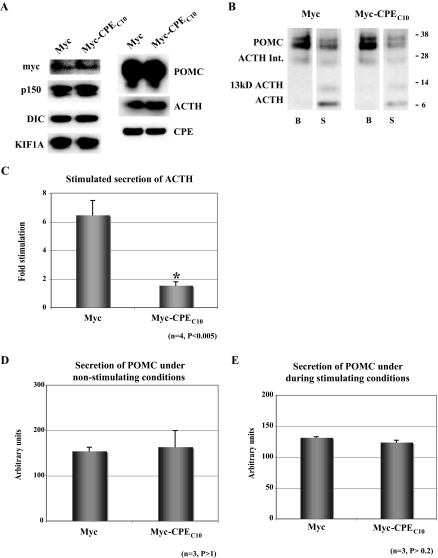
Before proceeding with the secretion assays, we determined whether overexpression of Myc-CPEC10 has any effect on the protein levels of cytoplasmic and intravesicular proteins in AtT20 cells. The level of cytoplasmic proteins, such as tubulin, dynactin (p150), dynein (DIC), and KIF1A in AtT20 cells was not affected by overexpression of Myc-CPEC10 compared with cells expressing Myc alone (Fig. 7A). Similarly, secretory vesicle proteins, such as CPE, POMC, and ACTH, in AtT20 cells expressing Myc-CPEC10 also did not show any significant differences in their levels compared with cells expressing Myc alone.
Figure 7.
Overexpression of Myc-CPEC10 Diminishes Regulated Secretion of ACTH from AtT20 Cells
A, Western blot of AtT20 cell lysate from cells expressing Myc- or Myc-CPEC10. Equal amounts of protein (50 μg) from either Myc-transfected (Myc) or Myc-CPEC10-transfected AtT20 cells were separated on NuPAGE gels and proteins detected by immunoblotting. B, Western blot analysis of POMC/ACTH proteins secreted from Myc- and Myc-CPEC10-transfected AtT20 cells in response to stimulation. A 30-min basal medium (B) and a 30-min stimulated medium (S; 2 mm BaCl2 plus 50 mm KCl) were analyzed. Secreted POMC-related proteins were detected by immunoblotting. Myc-CPEC10 expression significantly diminished stimulated secretion of 13-kDa ACTH and ACTH but not POMC or a POMC-derived ACTH intermediate (Int). C, Bar graph showing fold stimulation of ACTH and 13-kDa ACTH secretion from Myc- and Myc-CPEC10 transfected AtT20 cells. Fold stimulation was calculated from four independent secretion assays (mean ± sem). D and E, Bar graphs showing constitutive secretion of POMC (in arbitrary units) from Myc- and Myc-CPEC10-transfected AtT20 cells without (lanes B above) and during (lanes S above) stimulation. The data shown are from three independent secretion assays (mean ± sem).
For secretion studies, cells transiently transfected with either Myc or Myc-CPEC10 were incubated for 30 min (Fig. 7B, lanes B) in DMEM containing 0.01% BSA to determine basal release, followed by a 30-min stimulation with 50 mm KCl and 2 mm BaCl2 in DMEM. Although 30 min is longer than needed to obtain a stimulated secretory response, it was necessary to obtain sufficient levels of POMC, as a marker for constitutive secretion, so that a comparison between unstimulated and stimulated conditions could be made for both proteins in the same experiment. Furthermore, stimulated secretion of ACTH is desensitized after 5 min in these cells; therefore, a 30-min stimulation time point for ACTH is very similar to a 10-min time point (unpublished data). Figure 7B shows that the 4.5- and the 13-kDa ACTH (glycosylated form of 4.5-kDa ACTH) were released in a stimulated manner (compare lanes B and S) in Myc-transfected cells. In contrast, the level of stimulated secretion of ACTH from Myc-CPEC10-expressing cells was very low (compare lanes B and S). Quantification of the 4.5- and 13-kDa ACTH bands in the secretion medium B and S (Fig. 7C) revealed a 6.47 ± 1.03-fold (n = 4) stimulation in secretion of ACTH in Myc-expressing cells, whereas there was essentially no stimulated release of ACTH (1.54 ± 0.25-fold, n = 4) from Myc-CPEC10 expressing cells. Both Myc and Myc-CPEC10 expressing cells showed similar levels of basal (constitutive) secretion of POMC without and during stimulation (Fig. 7, B–E), characteristic to some degree of the leakiness of these cells (29). This result indicates that CPE tail is not involved in transport of constitutive secretory pathway vesicles.
DISCUSSION
The CPE Cytoplasmic Tail Is Required for POMC/ACTH Vesicle Transport for Activity-Dependent Secretion
In this study, we have investigated a mechanism required for post-Golgi transport of POMC/ACTH vesicles to the plasma membrane for activity-dependent secretion. The mechanism necessitates the interaction of the cytoplasmic tail of transmembrane CPE in POMC vesicles with dynactin, which then recruits the microtubule-based motors kinesin 2 and kinesin 3 to transport these organelles to the release sites.
Competition studies with overexpressed CPE C-terminal fragments in both steady-state immunolocalization assays and live-cell imaging demonstrated that the last 10 amino acids of CPE (CPEC10), which constitutes the entire cytoplasmic tail, was required for post-Golgi transport of POMC/ACTH along processes of pituitary cells (Figs. 1–4 and supplemental Fig. 3 videos and Fig. 4 videos). Overexpression of a CPE tail fragment containing only the last six residues did not significantly block localization of POMC/ACTH vesicle in the processes, indicating that this shorter domain was not sufficient to compete with the endogenous CPE tail for microtubule-based proteins (Fig. 1C). Live-cell imaging studies revealed that movement of a population of POMC/ACTH vesicles was significantly diminished by overexpression of CPEC10 in the cytosol (Fig. 3 and Table 1). Furthermore, overexpression of CPEC10 resulted in the lack of stimulated release of ACTH from pituitary AtT20 cells upon depolarization with high K+ (Fig. 7). Hence, the cytoplasmic tail of CPE consisting of the last 10 amino acid residues at the C terminus is specifically required for anchoring POMC/ACTH vesicles to the transport system for activity-dependent secretion of ACTH from pituitary cells. Additionally, our microscopic analyses clearly demonstrated that various forms of Arf6, such as wt-Arf6, DN-Arf6, and CA-Arf6, did not affect ACTH localization in the processes (Fig. 2). These forms of Arf6 did not decrease the number of cells showing normal ACTH localization in the processes (Fig. 2, B and C), and the intensity ratio of immunostained ACTH in the processes compared with cell body (Fig. 2D) remained unperturbed, in contrast to the results obtained with GFP- or RFP-CPEC10-expressing cells. Hence, interaction of Arf6 with the CPE cytoplasmic tail is necessary only for initiation of recycling of CPE at the plasma membrane upon stimulation (19) but distinctly not required for microtubule-based vesicle transport from the cell body to the processes.
The CPE Cytoplasmic Tail Interacts with Dynactin that Recruits Microtubule-Based Motor Proteins Kinesin 2 and Kinesin 3 for Anterograde Vesicle Transport
Post-Golgi transport of POMC/ACTH vesicles is dependent on microtubules because depolymerization of microtubules with nocodazole disrupted movement of these vesicles. Therefore, the cytoplasmic tail of CPE must recruit microtubule-based motors to confer processivity to vesicle movement. Dynactin has been shown to coordinate microtubule-based movements of vesicles toward both plus and minus ends (30,31). It binds microtubules and dynein, a minus-end-directed motor (32), or kinesin (33), a plus-end-directed motor, simultaneously via its p150/p135-dimer sidearm to confer microtubule-based movement. Our in vitro pull-down experiments demonstrated that CPEC10 was able to bind microtubule-associated proteins dynactin, KIF1A and KIF3A, members of the kinesin family (34), and dynein, from cytosol of the pituitary cell line AtT20 (Fig. 5A). Furthermore, dynactin (p150 component) was shown to coimmunoprecipitate with endogenous CPE, KIF1A, KIF3A, and dynein and therefore interacts with these components (Fig. 5B). The interaction between dynactin and KIF3A/B was previously reported by Berezuk and Schroer (33). The CPE binding to dynactin was displaced by excess CPEC8 peptide, suggesting that the CPE tail interacts directly with dynactin (Fig. 5C), although indirect interaction with other proteins cannot be ruled out. Endogenous CPE, as well as dynactin, dynein, KIF1A, and KIF3A from AtT20 cells were co-pelleted with and dissociated from microtubules with an ATP wash, demonstrating that CPE is associated with the dynactin that recruits kinesin 2 (KIF3A), kinesin 3 (KIF1A), and dynein, the microtubule-based motors (Fig. 5D). Immunocytochemistry and subcellular fractionation studies verified that dynactin, KIF1A, KIF3A, and dynein are associated with ACTH vesicles of the regulated secretory pathway and can therefore play a role in transport of these vesicles in vivo (Fig. 6, A and B). This interaction between CPE tail and the motors is specific for transport of regulated secretory pathway ACTH vesicles because constitutive secretion of POMC, which is also dependent on microtubule-based transport, was not adversely affected (Fig. 7D). Furthermore, absence of CPE in hippocampal neurons of cpe−/− mice had no effect on constitutive secretion of brain-derived neurotrophic factor (13). Therefore, the CPE tail is necessary only for transport of the regulated secretory pathway POMC/ACTH vesicles but not other CPE-independent constitutive vesicle transport.
Because CPEC10 interacted significantly with dynactin in AtT20 cell cytosol in vitro, we considered the possibility that overexpressed CPEC10 in our in vivo studies might disrupt the integrity of the dynactin complex, thereby interfering with overall dynactin functions, such as the organization of the Golgi complex and microtubules (28,35). However, we found no obvious effect of overexpression of GFP-CPEC10 on morphology of the Golgi complex (Fig. 6, C and D) in AtT20 cells, unlike overexpression of GFP-p50, which disrupted the Golgi complex (Fig. 6, C and D). Therefore, overexpression of GFP-CPEC10 in cells did not affect the integrity of dynactin but perturbed only the function of dynactin as an ACTH vesicle transporter.
Thus, all the data taken together strongly indicate that the CPE cytoplasmic tail associates with microtubules via the dynactin to facilitate transport of regulated secretory pathway POMC vesicles from the cell body to the release site. Furthermore, this study has revealed for the first time that dynactin is an adaptor not only for kinesin 2 (KIF3A) but also kinesin 3 (KIF1A). Kinesin 3 was known to be involved in microtubule-based transport of regulated secretory vesicles but was previously speculated to bind vesicles directly via its PH domain (36).
The CPE Cytoplasmic Tail: An Essential Anchor Linking Peptide Hormone Secretory Vesicles to the Transport System in Endocrine Cells
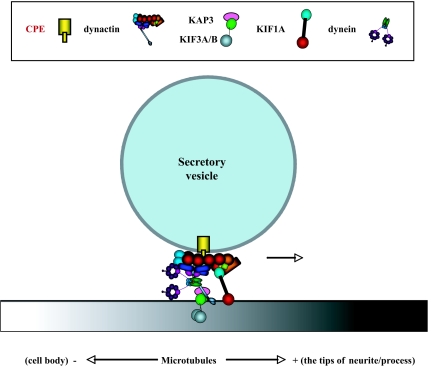
The findings in this study have provided evidence for a novel mechanistic model (Fig. 8 and Fig. S1B) of how regulated secretory pathway peptidergic vesicles (e.g. ACTH) are anchored via the cytoplasmic tail of CPE to the microtubule-based transport system for delivery to the release site for activity-dependent secretion. In this model, the cytoplasmic tail of transmembrane CPE, specifically found in regulated secretory pathway peptidergic vesicles of neurons and neuroendocrine cells, interacts with and recruits dynactin on to the vesicles. In turn, kinesin 2 and kinesin 3, the motor proteins, bind simultaneously to dynactin and microtubules to effect plus-end microtubule-based delivery of the secretory vesicles to the release site for activity-dependent secretion (Fig. 8). Genetic evidence from studies in Caenorhabditis elegans corroborates a role of KIF1A in anterograde microtubule-based transport of CPE-containing vesicles because mutation of the unc-104 gene, a nematode ortholog of KIF1, caused a defect in anterograde movement of neuroendocrine secretory vesicles containing egl-3 (prohormone convertase 2) and egl-21 (CPE) (37). Kinesin 2, the heterotrimer of KIF3A/KIF3B/KAP3, is known to mediate vesicle transport in various kinds of cells including neurons (38,39,40). However, until our study, there has been no evidence reported previously regarding a role of kinesin 2 in vesicle transport in the regulated secretory pathways of endocrine and neuroendocrine cells.
Figure 8.
Model Showing the Mechanism for Transport of POMC/ACTH Vesicles
The cytoplasmic tail of transmembrane CPE in these POMC vesicles recruits dynactin that associates with and confers processivity to KIF1A (kinesin 3) and KIF3A (kinesin 2). KIF1A and KIF3A, plus-end microtubule-based motors, simultaneously bind dynactin and microtubules to mediate delivery of these vesicles to the release site for activity-dependent secretion of ACTH from endocrine cells. Dynein, a minus-end-directed motor, also binds dynactin and mediates return of secretory vesicles from the process terminal back to the cell body under nonstimulated conditions in neurons (see text).
Dynein also interacts with dynactin but would bind only to microtubules for retrograde transport, because this microtubule-based motor is associated with minus-end movement. A recent live-cell imaging study showed that excess neuropeptide-containing vesicles are transported from the site of biogenesis in the soma to the synaptic terminus, but if not trapped at the synapse for release during stimulation, the unused vesicles are transported back to the cell body in a retrograde fashion (41). Dynein's interaction with dynactin in the CPE tail-dynactin complex can serve such a role in retrograde transport of vesicles in nonstimulated neurons, presumably by switching the microtubule association of kinesin 2 to dynein by a yet unknown mechanism. Indeed, we have shown that overexpression of CPEC10 also reduced retrograde movement of peptidergic vesicles in hippocampal neurons (our unpublished data), although how all of the different motors interact with dynactin-CPE tail in an orchestrated manner to switch anterograde motors to retrograde ones, or vice versa, remains to be elucidated. The CPE tail-dynactin-dynein interaction may serve a role in the retrograde transport of POMC/ACTH vesicles from the synapse to the cell body of POMC neurons in the arcuate nucleus of the hypothalamus (42). Additionally, we cannot rule out the possibility that the disturbance of retrograde transport might be secondary to the negative effect on anterograde transport.
In conclusion, this study has uncovered a new molecular mechanism that controls post-Golgi transport of peptidergic vesicles in the regulated secretory pathway of neuroendocrine cells. We show that the cytoplasmic tail of CPE plays a critical role in directly anchoring POMC/ACTH vesicles to the microtubule-based transport system via interaction with dynactin and kinesin 2/kinesin 3 for delivery to the secretion site. Such a transport mechanism may be generally used in (neuro)endocrine cells that contain CPE to deliver vesicles containing neuropeptides and peptide hormones for activity-dependent secretion necessary to mediate various (neuro)endocrinological functions.
MATERIALS AND METHODS
DNA Constructs
To generate GST-tagged CPE tail fragments, 5′-EcoRI-XhoI-3′ digests of PCR products for CPEC10 (Fig. 1A) were subcloned into pGEX4T-2 (Amersham Pharmacia, Piscataway, NJ). GFP or RFP was cloned to the N-terminal end of CPEC10 or CPEC25 by subcloning a 5′-XhoI-PstI-3′ digest of the PCR product amplified from full-length CPE cDNA into pEGFP-1 C or pDsRed2, respectively (BD Bioscience, San Diego, CA). Myc tag was cloned to the N-terminal end of CPEC6, CPEC10, CPEC15, or CPEC20 by inserting the 5′-XhoI-EcoRI-3′ digests of PCR products from full-length CPE mouse cDNA into pCMV-Myc (BD Bioscience). GFPp50 was a gift from Dr. Trina Schroer (Johns Hopkins University, Baltimore, MD). CPE-GFP was generated in our laboratory (Zhang, C. F., unpublished). The full-length CPE cDNA was excised from pEGFP-N1 and inserted into pDsRed-Express-N1 (BD Bioscience) to generate CPE-RFP. HA-tagged wt, DN (T27N), and CA (Q67L) forms of Arf6 were gifts from Dr. Julie Donaldson (National Institute of Heart, Lung, and Blood, NIH, Bethesda, MD).
Antibodies
The goat antibodies to KHC and p150Glued, the rabbit antibodies to p150Glued, and the mouse antibodies to DIC (74.1) and Arf6 were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse antibodies to β-tubulin were from Sigma Chemical Co. (St. Louis, MO); the mouse antibodies to the Myc tag were from BD Bioscience, and the mouse antibodies to p115, CPE, KIF3A, or KIF1A were from Transduction Laboratories (San Jose, CA). Mouse antibody to ACTH was from Abcam (Cambridge, MA). The rabbit antibody to POMC (DP4) (43) and the rabbit antibody to the C terminus of CPE (16) were generated in our laboratory. For immunocytochemistry, goat antimouse and goat antirabbit secondary antibodies (Vector Laboratories, Burlingame, CA) were used. For immunoblotting, horseradish peroxidase-conjugated antimouse/antirabbit (Amersham Pharmacia) or donkey antigoat (Amersham Pharmacia) secondary antibodies were used. ECL plus Western Blotting Detection System (Amersham Pharmacia) or SuperSignal West Dura Stable Peroxide System (Pierce Co., Rockford, IL) was used to detect protein bands on polyvinylidene difluoride (PVDF) membranes.
Cell Culture and Immunocytochemistry
AtT20 cells or primary culture cells from mouse anterior pituitary gland were grown in DMEM (GIBCO-BRL, Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (GIBCO-BRL), and 2 × 104 cells were seeded on 15-mm2 round coverslips and grown for 18–20 h in DMEM (Biosource, Rockville, MD) supplemented with 10% FBS before processing for transfection and/or immunocytochemistry. Transfection of DNA constructs was performed using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Briefly, cells were grown in 35-mm dishes to 50% confluency, washed with Hanks' balanced salt solution buffer (Biosource), and then DNA (4 μg), Lipofectamine (10 μl) in OPTI-MEM I (GIBCO-BRL), and 1.5 ml DMEM were added, followed by incubation for 16–18 h. Transfection efficiencies of 50–60% were routinely obtained for AtT20 cells and about 10% for primary culture cells.
For immunocytochemistry, all steps were performed at room temperature unless otherwise noted. Cells were rinsed with PBS, fixed in 3.5% formaldehyde in PBS for 30 min, and permeabilized in 0.1% Triton X-100 in PBS for 30 min. Cells were then blocked in Tris-buffered saline with 0.1% Tween 20 and 2% BSA, incubated for 30 min in primary antibodies, washed in Tris-buffered saline with 0.1% Tween 20 and 2% BSA (three times for 5 min each), and incubated in Alexa Green (488 nm)/Red (568 nm)/Blue (657 nm) secondary antibody for 15 min. Samples were washed again and mounted on slides in Gel/Mount (Biomeda, Foster City, CA) for analysis.
Microscopy
POMC/ACTH localization in the processes of AtT20 cells was carried out by visual inspection under a Nikon upright Labphot microscope (Nikon, Kanagawa, Japan) and scored on the basis of whether there is any POMC/ACTH accumulation in the processes or none at all. For all conditions and controls, at least 100 overexpressing cells on multiple coverslips were analyzed in two or more independent experiments.
The intensity of POMC/ACTH immunostaining in the processes of AtT20 cells was also quantified. Immunofluorescence microscopy was performed at room temperature using a Zeiss Axiovert 200 M inverted microscope (Carl Zeiss Inc., Thornwood, NY) equipped with ×63 Zeiss plan-apochromat oil, 1.4-NA, differential interference contrast and ×100 Zeiss α-plan fluor oil, 1.45-NA, differential interference contrast objectives. Images were acquired by a Meta detector for spectral imaging (Carl Zeiss) and digitized using LSM 510 Meta software version 3.5 (Carl Zeiss) and quantification performed using Metamorph software (Molecular Devices Co., Downingtown, PA). The LSM510 Meta software was also used to calculate colocalization correlation R.
Golgi morphology was quantitatively analyzed using the Zeiss Axiovert 200 M inverted microscope. Golgi morphology was scored on the basis of whether there is a compacted or dispersed Golgi complex in different conditions.
For time-lapse imaging, AtT20 cells expressing CPE-GFP and RFP/RFP-CPEC10 were maintained in phenol red-free DMEM plus 10% FBS in a temperature-controlled (37 C) Bioptechs Delta T live-cell environmental chamber (Bioptechs Inc., Butler, PA) and imaged using the Zeiss Axiovert 200 M inverted microscope and LSM 510 Meta software (Carl Zeiss) at 1.97-sec exposure per shot for 200 shots. Images were converted to 8-bit movies using LSM 510 Meta software. In multiple experiments, cells were pretreated with 33.3 μm nocodazole (Sigma) for 2 h at 37 C and then maintained in nocodazole-containing medium throughout the observation period. Primary culture cells from mouse anterior pituitary gland were transfected with CPE-RFP and GFP/GFP-CPEC25 and processed as above. Images were taken at 0.984-sec exposure per shot for 200 shots. The distance each CPE vesicle moved was calculated by converting image pixels to micrometers using the LSM 510 Meta software. The velocity of vesicle movement was calculated from distances moved (micrometers) per 1.97 or 0.984 sec. Statistical analysis was carried out using the Student's t test (two-tailed, nonpaired), and the mean values ± sem are reported for the average distances and velocities that the vesicles moved.
Coprecipitation and Coimmunoprecipitation Studies
For coprecipitation studies, bacterially expressed GST or GST-CPEC10 was purified on glutathione (GSH) beads (Amersham Pharmacia) according to manufacturer's protocol. GST-CPE tail (∼0.2 mg/ml protein) was bound to 200 μl GSH beads (Novagen Co., Madison, WI). AtT20 cells from eight T75 flasks at about 90% confluency were harvested and resuspended in 1.2 ml PMEE buffer (pH 7.0; 35 mm KOH, 35 mm PIPES, 5 mm MgSO4, 1 mm EGTA, 1% BSA, and 0.5 mm EDTA) containing 1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride and 1× protease inhibitor cocktail. Cells were then ruptured by passing them through a 27-gauge needle (Becton Dickinson, Franklin Lakes, NJ) 20 times to break down any vesicular structures. Cell debris and nuclear membranes were removed by centrifugation for 5 min at 14,500 × g. The supernatant (1.0 mg/ml protein) was then centrifuged at 192,000 × g for 30 min at 4 C to remove any vesicular compartments by pelleting and obtain a high-speed soluble cytosol fraction. Any lipids that floated to the top of the cell cytosol were removed after ultracentrifugation before additional processing. Three 400-μl aliquots of cell cytosol fraction were each added to 100 μl GSH beads and incubated for 1 h at 4 C to preclear the cytosol. The 400-μl aliquots of precleared cell cytosol were then each mixed with GST-tagged CPEC10 or control (GST tag alone) bound to GSH beads. The mixture was then incubated at 4 C for 18 h on a rotating platform. The agarose beads were washed six times with 0.5 ml PMEE buffer and incubated in 200 μl 10 mm GSH. The eluate from the GSH beads was then boiled in 100 μl SDS loading buffer and loaded onto SDS gel for Western blotting. Proteins were separated by NuPAGE (Invitrogen), transferred to PVDF membrane, and detected by immunoblotting.
For coimmunoprecipitation studies, AtT20 cell cytosol was prepared as described in the coprecipitation experiment, and 0.8 ml of the cytosol fraction (1.0 mg/ml protein) was split into two sets of 400 μl. Each 400 μl of the cytosol was mixed with 100 μl protein A-agarose beads (Sigma) and incubated for 1 h at 4 C on a rotating platform to preclear the cytosol. After removal of the agarose beads, the cytosol was mixed with either 5 μg control rabbit IgGs or rabbit IgGs against dynactin (p150) and incubated for 18 h at 4 C. Then 100 μl protein A-agarose beads were added to the tubes and incubated for 8 h. After incubation, the beads were washed seven times with PMEE buffer and boiled in 100 μl SDS loading buffer. Proteins were separated by NuPAGE (Invitrogen), transferred to PVDF membrane, and detected by immunoblotting. For competition assays in the coimmunoprecipitation study, all procedures were carried out as described above, except that 0 or 0.4 μg/ml of a control peptide (AALALHVG) or CPEC8 (MMSETNLF) was added to an equivalent volume of cytosol before coimmunoprecipitation with dynactin (p150) antibodies. Both control and CPEC8 peptides were custom synthesized by SynPep (Dublin, CA).
Subcellular Fractionation Studies
AtT20 cells were harvested from five 100-mm culture dishes of about 90% confluency. After washing with PBS, the cell pellet was resuspended in 0.8 ml PMEE buffer and passed through a 27-gauge needle three times. A postnuclear supernatant was generated from the cell lysate after centrifugation at 1000 × g for 10 min at 4 C. The postnuclear supernatant was centrifuged on 18 ml of 0.6–1.8 m sucrose gradient at about 100,000 × g for 16 h at 4 C to separate heavy dense-core granules from other membranous components such as plasma membrane, Golgi complex, endoplasmic reticulum, and mitochondria. After ultracentrifugation, 2-ml fractions were taken from the top of the gradient (5%) using an automatic fraction collector (LABCONCO, Kansas City, MO), and 30 μl from each fraction was analyzed by Western blot.
Microtubule Co-Pelleting Assay
Ten T75 flasks at about 90% confluency were incubated in 10% FBS-containing medium for 20 h. Cells were harvested with 0.05% Trypsin-EDTA, washed once in PBS followed by a wash with PMEE buffer. The cells were then resuspended in an equal volume of ice-cold homogenizing PMEE buffer [HG buffer: PMEE buffer plus 1 mm dithiothreitol, 1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 1× protease inhibitor cocktail, and 0.5 mm ATP] and passed through a 27-gauge needle (Becton Dickinson). The cell lysate was centrifuged at 14,500 × g for 10 min at 4 C in a microcentrifuge. The supernatant was then ultracentrifuged at 192,000 × g for 30 min at 4 C to obtain a high-speed cell cytosol fraction (1.0 mg/ml protein), and 20 μm taxol and 1 mm GTP were added to 600 μl high-speed cell cytosol, which was then mixed with taxol/GTP-stabilized bovine brain microtubules (0.3 mg tubulin protein) and 4 mm AMP-PNP. The microtubule-binding reaction was incubated, with frequent inverting, at room temperature for 20 min under light, and 500 μl of the binding reaction was layered over 200 μl of 12.5%/25% sucrose cushions in a 700-μl ultracentrifuge tube and spun at 192,000 × g at 25 C for 45 min. As a negative control, a 4 C high-speed cell cytosol without exogenous microtubules/GTP/taxol but with AMP-PNP was also spun on the sucrose cushion at 192,000 × g at 25 C for 45 min. An aliquot of the supernatant was saved, and the rest of supernatant and sucrose cushion were removed in a stepwise manner by vacuum suction and cotton swab wiping, until only the microtubule pellet was left. The microtubule pellet was subsequently resuspended in 160 μl 25 C HG buffer containing 20 μm taxol, 1 mm GTP, and 10 mm MgSO4 and without (buffer wash) or with 10 mm ATP (ATP wash). The resuspended pellet was incubated at 25 C for 20 min and then centrifuged at 192,000 × g at 25 C for 30 min. The supernatant was saved, and the microtubule pellet was resuspended in 160 μl HG buffer containing 20 μm taxol, 1 mm GTP, and 5 mm MgSO4 at 25 C. Both the supernatant and pellet were analyzed by Western blotting.
POMC/ACTH Secretion Assays
AtT20 cells in six-well plates were grown to about 60% confluency in DMEM/10% FBS. Cells were then transfected with either Myc or Myc-CPEC10 DNA (4 μg), using Lipofectamine [10 μl in OPTI-MEM I (GIBCO-BRL) and 1.5 ml DMEM] for 16–18 h. Each well was rinsed twice and then incubated with 2 ml DMEM containing 0.01% BSA for two 30-min periods in a 37 C incubator maintained at 5% CO2. Each medium (basal) was collected from individual wells and centrifuged at 1000 × g for 3 min to remove cell debris, after which 1.5 ml of the supernatant was transferred to a 1.5-ml microtube, and 160 μl 100% ice-cold trichloroacetic acid (Sigma) and 50 μg BSA were added to precipitate the proteins. The cells were then incubated with 2 ml DMEM containing 0.01% BSA/50 mm KCl/2 mm BaCl2 for 30 min. Each medium (stimulated) was collected from individual wells and processed in a similar manner as the basal medium samples. The trichloroacetic acid-precipitated proteins were collected by centrifugation, rinsed with ice-cold acetone, and then reconstituted in SDS sample buffer for Western blot analysis. Basal secretion was analyzed for the first 30-min period without stimulation. Stimulated secretion of ACTH was analyzed by comparing ACTH in medium during the second 30-min period in the basal condition vs. stimulated condition. The protein band intensity on the immunoblot was quantified using the NIH Image 1.62′ program. The mean band intensity ± sem was calculated from four independent secretion assays.
Supplementary Material
Acknowledgments
We thank Dr. Bai Lu [National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH)], Dr. Joel Rosenbaum (Yale University), Dr. Trina Schroer (Johns Hopkins University), and Dr. Jennifer Lippincott-Schwartz (NICHD, NIH) for their suggestions and critical reading of the manuscript. We also thank Drs. Hong Lou, Guhan Nagappan, and Hisatsugu Koshimizu (all from NICHD) for technical assistance and helpful discussions. We thank Dr. Julie Donaldson (National Heart, Lung, and Blood Institute, NIH) for the Arf6 constructs. We thank Dr. Vincent Schram and Chip Dye in the NICHD Microscopy Imaging Core for their technical support. This research was supported by the Intramural Research Program of the NICHD, NIH.
Footnotes
This research was supported by the Intramural Research Program of the NICHD, NIH.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 17, 2008
Abbreviations: AMP-PNP, 5′-Adenylylimidodiphosphate; Arf6, ADP-ribosylation factor 6; CA, constitutively active; CPE, carboxypeptidase E; CPEC25, C-terminal 25-amino-acid region of CPE; DIC, dynein intermediate chain; DN, dominant-negative; FBS, fetal bovine serum; GFP, green fluorescence protein; GHS, glutathione; GST, glutathione S-transferase; HA, hemagglutinin; KHC, kinesin heavy chain; KIF3A, kinesin family member 3A; POMC, proopiomelanocortin; PVDF, polyvinylidene difluoride; RFP, red fluorescent protein; TGN, trans-Golgi network; wt, wild type.
References
- Alexander K, Nikodemova M, Kucerova J, Strbak V 2005 Colchicine treatment differently affects releasable thyrotropin-releasing hormone (TRH) pools in the hypothalamic paraventricular nucleus (PVN) and the median eminence (ME). Cell Mol Neurobiol 25:681–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Salm T, Rustom A, Gerdes HH 2001 Dynamics of immature secretory granules: role of cytoskeletal elements during transport, cortical restriction, and F-actin-dependent tethering. Mol Biol Cell 12:1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Da Costa S, Yang T, Wei X, Gierow JP, Mircheff AK 1997 Cholinergic stimulation of lacrimal acinar cells promotes redistribution of membrane-associated kinesin and the secretory protein, β-hexosaminidase, and increases kinesin motor activity. Exp Eye Res 64:141–156 [DOI] [PubMed] [Google Scholar]
- Senda T, Yu W 1999 Kinesin cross-bridges between neurosecretory granules and microtubules in the mouse neurohypophysis. Neurosci Lett 262:69–71 [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL 2007 A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci 10:980–989 [DOI] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z 2000 Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci 23:39–71 [DOI] [PubMed] [Google Scholar]
- Schroer TA 2004 Dynactin. Annu Rev Cell Dev Biol 20:759–779 [DOI] [PubMed] [Google Scholar]
- Jordens I, Marsman M, Kuijl C, Neefjes J 2005 Rab proteins, connecting transport and vesicle fusion. Traffic 6:1070–1077 [DOI] [PubMed] [Google Scholar]
- Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N 2003 A scaffold protein JIP-1b enhances amyloid precursor protein phosphorylation by JNK and its association with kinesin light chain 1. J Biol Chem 278:22946–22955 [DOI] [PubMed] [Google Scholar]
- Taru H, Iijima K, Hase M, Kirino Y, Yagi Y, Suzuki T 2002 Interaction of Alzheimer's β-amyloid precursor family proteins with scaffold proteins of the JNK signaling cascade. J Biol Chem 277:20070–20078 [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu K, Rife L, Cawley NX, Brown B, Adams T, Teofilo K, Lillo C, Williams DS, Loh YP, Craft CM 2005 Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J Neurochem 95:1351–1362 [DOI] [PubMed] [Google Scholar]
- Fricker LD, Das B, Angeletti RH 1990 Identification of the pH-dependent membrane anchor of carboxypeptidase E (EC 3.4.17.10). J Biol Chem 265:2476–2482 [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP 2005 Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron 45:245–255 [DOI] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH 1982 Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci USA 79:3886–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VY, Loh YP 1984 Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci USA 81:2776–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP 1997 Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88:73–83 [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP 2002 Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry 41:52–60 [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Loh YP 2000 Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem 275:29887–29893 [DOI] [PubMed] [Google Scholar]
- Arnaoutova I, Jackson CL, Al-Awar OS, Donaldson JG, Loh YP 2003 Recycling of Raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6. Mol Biol Cell 14:4448–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CM, Chang HT, Chang MD 2004 Membrane-bound carboxypeptidase E facilitates the entry of eosinophil cationic protein into neuroendocrine cells. Biochem J 382:841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F 1977 β-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science 197:1367–1369 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Johnson KA 1998 The structural and mechanochemical cycle of kinesin. Trends Biochem Sci 23:429–433 [DOI] [PubMed] [Google Scholar]
- Sakato M, King SM 2004 Design and regulation of the AAA+ microtubule motor dynein. J Struct Biol 146:58–71 [DOI] [PubMed] [Google Scholar]
- Park JH 2003 Roles for the dynactin complex in the glucocorticoid receptor signaling pathway. PhD thesis, Johns Hopkins University, Baltimore, MD [Google Scholar]
- Bingham JB, King SJ, Schroer TA 1998 Purification of dynactin and dynein from brain tissue. Methods Enzymol 298:171–184 [DOI] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N 2000 Mechanism of the single-headed processivity: diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc Natl Acad Sci USA 97:640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA 1999 Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol 147:321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CJ, Haugwitz M, Eaton B, Moore HP 1997 Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell 8:2171–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI 2003 Dynactin is required for bidirectional organelle transport. J Cell Biol 160:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell KR 2003 Dynactin polices two-way organelle traffic. J Cell Biol 160:291–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA 2000 Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol 2:20–24 [DOI] [PubMed] [Google Scholar]
- Berezuk MA, Schroer TA 2007 Dynactin enhances the processivity of kinesin-2. Traffic 8:124–129 [DOI] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N 1995 The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81:769–780 [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB 1997 Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol 139:469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD 2004 The lipid binding pleckstrin homology domain in UNC-104 kinesin is necessary for synaptic vesicle transport in Caenorhabditis elegans. Mol Biol Cell 15:3729–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM 2003 The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci 23:2122–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N 1994 KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol 125:1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N 1995 KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol 130:1387–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N 1996 Cloning and characterization of KAP3: a novel kinesin superfamily-associated protein of KIF3A/3B. Proc Natl Acad Sci USA 93:8443–8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Levitan ES 2006 Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci 9:896–900 [DOI] [PubMed] [Google Scholar]
- Cheung S, Hammer Jr RP 1995 Gonadal steroid hormone regulation of proopiomelanocortin gene expression in arcuate neurons that innervate the medial preoptic area of the rat. Neuroendocrinology 62:283–292 [DOI] [PubMed] [Google Scholar]
- Loh YP, Parish DC, Tuteja R 1985 Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem 260:7194–7205 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.