Abstract
The electrosensory lateral line lobe (ELL) of the electric fish Apteronotus leptorhynchus is a layered medullary region receiving electroreceptor input that terminates on basal dendrites of interneurons and projection (pyramidal) cells. The molecular layer of the ELL contains two distinct glutamatergic feedback pathways that terminate on the proximal (ventral molecular layer, VML) and distal (dorsal molecular layer) apical dendrites of pyramidal cells. Western blot analysis with an antibody directed against mammalian Ca2+/calmodulin-dependent kinase 2, α subunit (CaMK2α) recognized a protein of identical size in the brain of A. leptorhynchus. Immunohistochemistry demonstrated that CaMK2 α expression in the ELL was restricted to fibers and terminals in the VML. Posttetanic potentiation (PTP) could be readily elicited in pyramidal cells by stimulation of either VML or DML in brain slices of the ELL. PTP in the VML was blocked by extracellular application of a CaMK2 antagonist (KN62) while intracellular application of KN62 or a CaMK2 inhibitory peptide had no effect, consistent with the presynaptic localization of CaMK2 α in VML. PTP in the dorsal molecular layer was not affected by extracellular application of KN62. Anti-Hebbian plasticity has also been demonstrated in the VML, but was not affected by KN62. These results demonstrate that, while PTP can occur independent of CaMK2, it is, in some synapses, dependent on this kinase.
Short-term enhancement (STE) of synaptic transmission is commonly observed in many central and peripheral synapses of both vertebrates and invertebrates (1, 2). Three types of enhancement: facilitation, augmentation and posttetanic potentiation (PTP) have been described; the kinetics of these forms of enhancement differ, ranging from <500 ms (facilitation), <10 s (augmentation) to >1 min (PTP), but numerous studies have demonstrated that they all depend on a sustained presynaptic increase in intracellular Ca2+ (the residual calcium hypothesis; refs. 2 and 3). Recent data has demonstrated that the amount of STE is linearly related to the level of residual Ca2+ (4) and that the time course of STE may be due to the kinetics of Ca2+ influx, buffered diffusion, release from intracellular stores, and extrusion (5, 6). Quantitative electrophysiological and imaging studies have also shown that the degree and time course of PTP cannot be accounted for by the direct action of residual free Ca2+ on the release process, but are consistent with Ca2+ binding to a high affinity intermediary protein(s) distinct from the release site (3, 4, 7–10). These studies are consistent with the idea that the putative Ca2+ binding protein(s) is a Ca2+-dependent second messenger and that the phosphorylation of proteins involved in vesicle targeting or release might be obligatory steps in the processes leading to PTP.
Pyramidal cells of the electrosensory lateral line lobe (ELL) receive glutamatergic feedback input onto the spines of their apical dendritic tree (11–14). The input to the proximal apical dendrites enters the ELL as a band of myelinated fibers [stratum fibrosum (StF) fibers to ventral molecular layer (VML)] is probably related to attentional mechanisms (15–17), while the parallel fiber input to distal apical dendrites underlies gain control (18–22). PTP and anti-Hebbian plasticity can be elicited by in vivo or in vitro activation of the StF pathway (15, 16). In this study we analyze the role of Ca2+/calmodulin-dependent kinase 2 (CaMK2), subunit α (CaMK2α) in synaptic plasticity in the ELL and demonstrate that CaMK2 kinase-dependent and -independent PTP can exist in morphologically similar glutamatergic synapses terminating on dendritic spines of the same pyramidal cells.
MATERIALS AND METHODS
Immunoblotting.
Western blot analysis of samples of rat cortex and electric fish brain were carried out as described (23). Briefly, samples were disaggregated (10 ml/mg tissue of 100 mM Tris⋅H3PO4, pH 6.8/100 mM EGTA/2% SDS/15% glycerol). The mixture was sonicated, heated to 100°C for 5–10 min, aliquoted (10 μl) and stored at −80°C. The proteins were separated on SDS/PAGE (24) and transferred by electrophoresis to polyvinylidene difluoride membranes (0.2 μm, Bio-Rad) overnight (4°C, 20 V; ref. 25). The polyvinylidene difluoride membranes were washed (distilled H2O) and immunoreacted with an antibody directed against mammalian CaMK2 α (Boehringer Mannheim catalog no. 1481 703 diluted 1:1,000 in PBS with 0.1% Tween). After treatment with the secondary antibody [Amersham catalog no. NA9310 anti-mouse Ig horseradish peroxidase linked F(ab′)2 fragment diluted 1:10,000 in PBS containing 0.01% Tween 20 (TPBS)], the membranes were immersed in chemiluminescent reagent (Amersham enhanced chemiluminescence kit, RPN 2108) for 60 s. The immunoreacted membranes were then exposed in a cassette to Hyperfilm-enhanced chemiluminescence (Amersham, RPN2103) and developed.
Immunohistochemistry.
Fish (n = 21) were intracardially perfused with 4% paraformaldehyde, 0.5% glutaraldehyde in PBS (0.1 M, pH 7.4) and subsequently washed in cold sucrose (20%) in buffer overnight. Brain sections (20 μm) were cut through the ELL and routinely processed for immunohistochemistry using the same antibody (1:1,000 in phosphate buffer) and immunoperoxidase staining (secondary antibody, Amersham catalog no. RPN 1001 biotinylated sheep anti-mouse Ig diluted 1:200 in phosphate buffer; tertiary antibody, Amersham catalog no. RPN 1231 streptavidin-horseradish peroxidase conjugate diluted 1:100 in phosphate buffer). Photographs of immunostained sections through ELL were scanned at 1,500 dpi (Polaroid SprintScan) and montaged in Adobe Systems (Mountain View, CA) photoshop without any digital image manipulation; lettering was applied with Adobe Systems illustrator.
Electrophysiology.
ELL slices and intracellular recording from pyramidal cells in the centromedial segment of the ELL was routinely carried out as described (16, 17, 26) using an interface chamber with a flow rate of ≈2 ml/min. Borosilicate glass (1.5 mm) was pulled (Sutter Instruments, Novato, CA; P87) to a resistance of 250–300 MΩ and then bevelled to a final resistance of 80–120 MΩ. Stimulation of StF (VML afferent fibers) or dorsal molecular layer (DML) was via unipolar tungsten electrodes (16) placed on the StF medial to the recording site or in the DML lateral to the recording site, respectively (this is appropriate to the trajectory of these fiber systems; ref. 11); the stimulus intensity (3–10 V) was adjusted so that the posttetanic excitatory postsynaptic potential (EPSP) did not trigger an action potential and typically produced an EPSP of about two-thirds of the maximum response. After a pretetanus baseline was established (average of 10–20 EPSPs at 5-min intervals three times before tetanus), tetanic stimulation carried out at 100 Hz for 100 ms (three times at an interval of 1–3 s). This stimulation protocol mimics the in vivo firing characteristics of StF (27); the discharge patterns of DML afferents is not known and we adopted the same stimulation protocol to facilitate comparison between StF and DML data. Posttetanic testing was done at 5-s intervals (single stimuli) for 2 min; we have shown that PTP after tetanization of StF decays to baseline after 120 s (16), but we only used the EPSP amplitude at 5 s posttetanus for statistical analyses (Student’s t test on raw data). For anti-Hebbian plasticity, tetanic stimulation was paired with intracellular hyperpolarization and testing was initiated 5 min posttetanus (16). Results are presented as mean ± SEM of percent change.
The specific CaMK2 antagonist, KN62 (28, 29) or an inactive structural analog, (KN04; ref. 29), was dissolved in dimethyl sulfoxide, diluted in artificial cerebrospinal fluid (ACSF; ref. 26) to 3.5 μM and applied by pressure ejection to the ELL molecular layer (DML + VML); drops of 150 μl were applied at rates >1 drop/min for 15 min before data collection and tetanization. The slice chamber has a volume of 1 ml so that the minimal concentration expected would be 0.5 μM, which is in excess of the the IC50 of 0.4 μM (29). In our interface chamber application of drugs to the slice surface results in rapid diffusion into the tissue; because the affinity of KN62 for CaMK2 is very high we are confident that higher KN62 concentrations (closer to 3.5 μM) are finally achieved in the tissue. This technique has been successfully used for rapid (<30 s) blockade of Na+ channels (26) and glutamate receptors (17) on cells >200 μM from the slice surface. In a small number of cases (see Results) we also bath applied the same concentration of KN62 or nimodipine (20 μM).
For intracellular application KN62 (10.5 μM; ref. 30) or the CaMK2 inhibitory peptide (1.1 mM; ref. 31) were also applied via the recording pipette; in this case stimulation was commenced after 30–45 min to allow diffusion of these agents up the apical dendrites (≈200 μm to VML).
RESULTS
Western Blot Analysis and Immunohistochemistry.
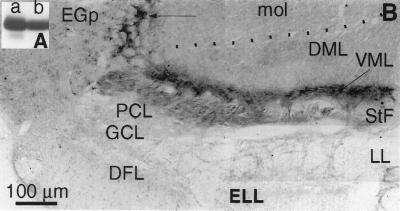
The Western blot analysis of electric fish brain revealed a single band at ≈50 kDa, the expected molecular mass of CaMK2α (Fig. 1A). Immunoreactivity for CaMK2α in the ELL was confined to fibers in the StF and their terminal field-a dense band comprising the VML (Fig. 1B); pyramidal cells and interneurons of the ELL were unlabeled. Numerous small cells in nucleus praeminentialis (the presumed cells of origin of StF; ref. 32) were also labeled (data not shown). Omission of the primary antibody completely eliminated all staining (data not shown).
Figure 1.
(A) Immunoblot of rat cortex (lane a) and A. leptorhynchus whole brain (lane b) homogenates with an antibody directed against mammalian CaMK2α. Strong labeling of a protein with identical molecular weight (49.5 kDa) is evident in electric fish brain. (B) Transverse section through the ELL reveals strong immunolabeling with a CaMK2α antibody confined to afferent fibers in StF and their terminal field in VML. Dotted line indicates boundary between molecular layer of cerebellum (mol) and ELL (DML). Immunoreactive fibers also run dorsally (arrow) into the overlying cerebellum (eminentia granularis posterior) as previously described for anterogradely labeled StF afferents to ELL (14). Note that primary afferents (DFL), cerebellar afferents (DML), pyramidal cells (PCL), and interneurons (GCL) are not labeled. EGp, eminentia granularis posterior (cerebellar granule cells specialized for electroreception; ref. 14); DFL, deep fiber layer containing electroreceptor afferents; DML, containing parallel fibers from granule cells of EGp; GCL, granular cell layer, containing the majority of ELL interneurons (22); LL, lateral lemniscus, containing pyramidal cell efferent fibers (unlabeled); mol, molecular layer of the cerebellum (EGp); PCL, pyramidal cell layer (unlabeled); StF, feedback afferent fibers (labeled); VML, termination zone for StF (strongly labeled).
Electrophysiology.
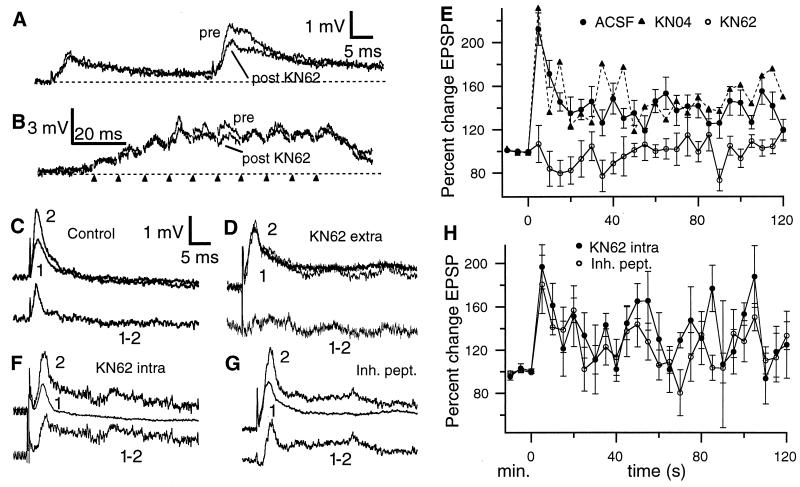
Single pulse stimulation of StF produced a characteristic EPSP (1–3 mV; 5.4 ± 0.1 ms to peak) as described (17). Application of KN62 did not alter baseline StF-evoked EPSPs (Fig. 2A; n = 36). Paired pulse facilitation (PPF) is typically seen with StF stimulation (Fig. 2A; ref. 17). Under control conditions (ACSF or KN04) PPF (50-ms interval) was 146.6% ± 10%; KN62 significantly reduced PPF to 110 ± 9.6% (n = 18, P < 0.05; Fig. 2A). Train stimulation of StF produces a augmenting response due to both temporal summation of EPSPs and the voltage dependent increase in the amplitude of later EPSPs in the train and these effects dominate over PPF (17). Thus after KN62, although there is a noticeable decrease in some EPSPs during the train, the overall response to tetanic stimulation is not greatly affected (Fig. 2B). This demonstrates that KN62 does not reduce PTP (see below) by a gross alteration in the response to train stimulation.
Figure 2.
(A) Paired pulse facilitation of StF (50 ms interval). StF stimulation evokes a rapid EPSP with a slow decay phase; the second pulse evokes a substantially larger EPSP. KN62 does not alter the first EPSP (superimposed trace from the same cell 15 min after drug ejection) but reduces PPF. The stimulus artefact was digitally removed in this and subsequent figures. (B) Effect of KN62 on train stimulation of StF. Although a reduction of some EPSPs does occur under KN62, this is slight due to the strong voltage dependent augmentation of later EPSPs in the train, so that the overall response to tetanic stimulation is not grossly altered. Markers indicate times of stimulation. (C, Upper) Superimposed pre- and posttetanus (5 s) EPSPs under control (ACSF) conditions. (Bottom) Subtraction of pre- from posttetanus EPSP reveals extent of PTP. (D, Upper) superimposed pre- and posttetanus (5 s) EPSPs after application of KN62. (Bottom) Subtraction of pre- from posttetanus EPSP reveals block of PTP. (E) Time course of PTP with ACSF and KN04 (error bars for KN04 plot are omitted for clarity); KN04 cases are indistinguishable from control. KN62 completely blocks PTP at all posttetanus times. (F and G, Upper) Superimposed pre- and posttetanus (5 s) EPSPs after intracellular application of KN62 (F) or a CaMK2α inhibitory peptide (G). (Bottom) subtraction of pre- from posttetanus EPSP reveals normal PTP. (H) The time course of PTP is not altered by intracellular application of CaMK2α antagonists. Note that in E and H the pretetanus time scale is in minutes (pretetanus EPSPs were evoked at 5-min intervals to assess their stability) while the posttetanus scale is in s.
Tetanic stimulation of StF produces PTP (because there were no differences in PTP produced in ASCF or when ACSF + dimethyl sulfoxide was pressure ejected, we pooled these results) of 212.2 ± 14.9% at 5 s posttetanus (n = 23, Fig. 2 C and E) as described (16). We then first induced PTP of StF and, after StF-evoked EPSPs had decayed back to baseline values (>10 min), we applied KN62 to the molecular layer (KN62 was continuously applied for 15 min before retetanizing StF to allow time for tissue penetration). KN62 blocked PTP of StF (106.8 ± 17.1%; Fig. 2 D and E); values of StF-evoked EPSPs at 5 s posttetanus in KN62 were significantly less than predrug controls (n = 9, P < 0.001). In 7 cases KN62 was first applied for the analysis of anti-Hebbian plasticity; after washout (≈45 min) normal PTP (203.8 ± 18.2%) was induced by StF tetanus; in two additional cases we first induced PTP (265%), blocked it with KN62 (113.4%), and then achieved recovery after washout (196.1%). Application of KN04 (following the same protocol as for KN62) did not block the induction of PTP (231 ± 41.4% increase at 5 s posttetanus; Fig. 2E). It has been shown that KN62 (at concentrations of 1–10 μM) can directly block Ca2+ channels in some preparations (33, 34), although this nonspecific effect is not always observed (35, 36). More recently it has been demonstrated that the KN62 block of Ca2+ channels is mimicked by KN04 (37–39); because KN04 failed to mimic the KN62 blockade of PTP subsequent to tetanus of StF, it is unlikely that this blockade is due to a direct action of this drug on Ca2+ channels. Because it has been suggested that L-type Ca channels are most likely to be directly affected by KN62 (34), we also examined the effect of bath applied nimodopine (20 μM); this L-type Ca2+ channel antagonist initiated spike bursting in pyramidal cells (within < 5 min) that made detailed analysis of PPF and PTP difficult; however this drug did not appear block StF-evoked PTP (≈196% PTP at 5 s posttetanus, n = 2; data not shown). These data, together with the lack of effect of KN62 on PTP in DML (see below), strongly suggests that in the ELL KN62 blocks PTP via a specific action on CaMK2.
Pyramidal cells (and interneurons) in ELL are strongly immunoreactive for CaMK2β (L.M., unpublished observations). Therefore to determine whether the effect of KN62 was pre- or postsynaptic, we applied either KN62 (n = 6) or the CaMK2 inhibitory peptide (n = 4) via the recording pipette. Neither treatment diminished StF-induced PTP (KN62, 180.1 ± 26.6%; peptide, 196.6 ± 20.5%; Fig. 2 F–H), suggesting that PTP at StF synapses in the VML is due to a CaMK2α-dependent presynaptic mechanism. We have no independent physiological confirmation that either compound reached the pyramidal cell proximal apical dendrites (≈200 μm from the soma; ref. 11). However biocytin (molecular mass, 372 kDa) takes only 10–30 min to diffuse to the most distal dendrites (>600 μm; refs. 11 and 26) and Lucifer yellow (molecular mass, 521 kDa) takes <5 min for a complete cell fill (N. Berman and L.M., unpublished observations); we therefore expect that KN62 (molecular mass, 722 kDa) would reach the proximal dendrites within 30–45 min.
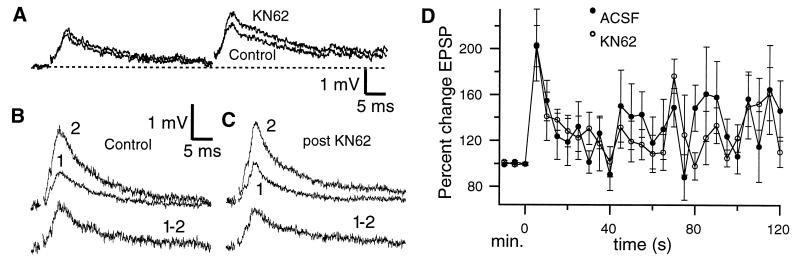
Because CaMK2α is confined to the VML and absent from the parallel fibers in DML (Fig. 1B) while CaMK2β is found throughout the pyramidal cell dendritic tree (L.M., unpublished observations), we attempted a more rigorous control of the specificity and site of action of KN62. Stimulation of the DML evoked an EPSP similar to that evoked from StF (1–3 mV, 8.9 ± 0.3 ms to peak; Fig. 3A); neither single EPSPs nor PPF in DML are affected by application of KN62 (Fig. 3A). Tetanic stimulation of DML also produces PTP (203.1 ± 31.5%, n = 6 at 5 s posttetanus) that decays to baseline in <2 min (Fig. 3 B and D). When KN62 is then applied (after the EPSP has returned to baseline) tetanic stimulation still induces PTP of similar magnitude (201.2% ±18.4%, n = 6; Fig. 3 C and D). Because we were concerned about the effective dose of KN62 in these DML experiments, we redid them (n = 2) by using bath application (3.5 μM) of the drug; we again found no effect on PTP (230%) or PPF. These experiments strongly suggest that the CaMK2β within pyramidal cells does not regulate STE.
Figure 3.
(A) Individual EPSPs and paired pulse facilitation of DML afferents is not altered by application of KN62; in this case there was a slight (not significant) increase in PPF after application of the drug. (B and C) PTP in DML. (Upper) Superimposed pre- and posttetanus (5 s) EPSPs under control (B) or KN62 (C) conditions. (Bottom) Subtraction of pre- from posttetanus EPSP reveals that PTP in DML is unaffected by KN62. (D) The time course of PTP in DML is not altered by extracellular application of KN62. Time scale as in Fig. 2 D and G.
Pairing tetanic stimulation of StF with hyperpolarization of pyramidal cells produces an increase in StF-evoked EPSPs lasting 10–15 min (anti-Hebbian plasticity) in vivo (15) and in vitro (16). KN62 did not block this form of anti-Hebbian plasticity of VML afferents (control:137.7 ± 6.8% increase at 5 min posttetanus, n = 4; post-KN62: 125.7 ± 7.0%, n = 6; p = 0.47).
DISCUSSION
The major conclusions of this study are: (i) CaMK2α in the ELL is confined to a specific glutamatergic fiber system (VML); CaMK2α is not detectable (by immunohistochemistry) in other glutamatergic inputs to ELL (primary afferents and DML; ref. 13; these inputs also lack CaMK2β: L.M., unpublished observations) or in γ-aminobutyric acid-ergic interneurons (22). (ii) PTP can be elicited in both VML and DML, but can be blocked (presynaptically) by a CaMK2 antagonist only in VML, even though both afferent systems terminate on dendritic spines of the same pyramidal cells. Although we attribute the KN62 blockade of StF-induced PTP to CaMK2α, it is also possible that CaMK1 and/or CaMK4 might also be located in VML and involved in the regulation of PTP, because CaMK1 is known to phosphorylate synapsin 1 (40) and CaMK4 is antagonized by KN62 (41). Further work will be required to determine whether additional CaMK isoforms are present in ELL.
While it had been generally accepted that PPF and PTP are caused by presynaptic mechanisms (3), recent evidence suggests the involvement of postsynaptic processes in STE at some synapses. In Aplysia sensorimotor synapses, both pre- and postsynaptic mechanisms appear to be required for the induction of PTP, although its expression is presynaptic (42). In hippocampal neurons PPF appears to be regulated by postsynaptic influx of Ca2+ and activation of CaMK2 and IP3 and ryanodine receptors (43, 44). ELL pyramidal cells contain CaMK2β (unpublished observations) and ryanodine receptors (45) and CaMK2 can phosphorylate and regulate ryanodine receptors (46–49). It is therefore possible that our results might be due to involvement of postsynaptic CaMK2. However in hippocampal neurons postsynaptic CaMK2 appears to act by phosphorylating α–amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, increasing their desensitization and subsequently decreasing PPF (44), which is opposite to our results (blockade of CaMK2 reduces PPF and PTP); further, although pyramidal cell dendrites in DML also contain CaMK2β (unpublished observations) and ryanodine receptors (45), KN62 does not affect either PPF or PTP at these synapses. Thus, although we cannot rule out a postsynaptic involvement in STE in ELL pyramidal cells, it is unlikely that any putative postsynaptic mechanism involves CaMK2.
Presynaptic mechanisms of transmission involve a number of steps including mobilization of vesicles from a reserve pool into an immediately releasable pool, docking of vesicles at the active zone, and vesicular fusion with the plasma membrane (3). Numerous proteins involved in these processes have been identified (50, 51) but it has been difficult to link residual Ca2+ to specific target proteins (although some of these proteins do bind Ca2+) and thus to mechanistically explain STE. One candidate high affinity Ca2+ sensor is CaMK2α, a serine/threonine kinase abundant in the brain (52) where it is enriched at pre- and postsynaptic sites (53) and bound to synaptic vesicles (54). It has been shown that presynaptic CaMK2 can enhance transmitter release (55, 56). Further, genetic manipulations that reduced CaMK2α activity resulted in greatly reduced STE in both fly (facilitation, augmentation and PTP; ref. 57) and mouse models (PPF; ref. 58). Other studies have however found that PTP was maintained (pharmacology in crayfish; ref. 59) or enhanced (mutant mice; ref. 60) after loss of CaMK2 activity. A recent study has directly addressed the issue of diversity in the regulation of STE (61): frequency facilitation was found to be far more prominent in the mossy fiber input to hippocampal pyramidal cells as compared with the commissural-associational input. Further, in agreement with our results, mossy fiber (but not commissural-associational) frequency facilitation could be greatly reduced by application of KN62. It has also been suggested that CaMK2α phosphorylation of synapsin 1 may be required for mobilization of reserve vesicles to the immediately releasable pool and that this might underlie some forms of STE (51, 62). Maler and Mathieson (63) have shown that, in electroreceptor primary afferent terminals in ELL, recruitment of vesicles from a reserve pool to the active zone is dependent on nerve activity; because CaMK2α (and CaMK2β; unpublished observations) is lacking in primary afferents, this implies that vesicular mobilization need not depend on this kinase. Taken together these results imply that there is diversity in the molecular mechanisms that regulate vesicular trafficking and STE.
Although CaMK2α is an abundant kinase it is not universally expressed in neurons (64). The question therefore arises as to the molecular basis of PTP in synapses utilizing CaMK2α vs. those lacking this enzyme. One possibility is that (i) PTP requires binding of residual Ca2+ to a protein(s) directly involved in vesicle recruitment or docking, (ii) at least one such protein has isoforms either having or lacking CaMK2α phosphorylation sites (as is the case for synapsin 1 vs. synapsin 2; 251), and (iii) the activity of the isoform containing CaMK2α phosphorylation sites depends on phosphorylation at these sites and is thus amenable to activity-dependent regulation while, in this scenario, the CaMK2α-independent isoform would support constitutive PTP. Numerous proteins involved in vesicular trafficking are phosphorylated by CaMK2α (51, 54, 65–69) and some have multiple isoforms (synaptotagmin; ref. 70); detailed biochemical/physiological studies will be required to test this hypothesis. CaMK2 might also act via phosphorylation of voltage gated Ca2+ channels leading to a sustained increase in Ca2+ influx (35, 71, 72) or altered interactions of channel and SNARE proteins (73); either scenario might lead to STE. It is also possible that, in neurons lacking CaMK2, other kinases may be found that can regulate STE.
The feedback input to VML has been hypothesized to be a “searchlight” for enhancing electrolocation of small objects (17, 22, 27, 74) and PTP at these synapses to be involved in increasing the gain of this searchlight (16). The feedback input to DML is part of a more global gain control mechanism (18–22) that can also be enhanced by PTP; both protein kinase A and C are present in DML (L.M., unpublished observations) but it not known whether these kinases can modulate PTP. It is not clear from the in vivo physiology of the ELL feedback pathways (20, 27) why only StF feedback afferents utilize CaMK2α for regulating PTP. It has been proposed (52, 75, 76) that the cooperative autophosphorylation of CaMK2α can lead to a threshold spike frequency at which this enzyme becomes highly activated. This leads to the hypothesis that presynaptic CaMK2α will lead to a frequency threshold for PTP; consistent with this hypothesis, we have found that PTP in VML is not elicited by stimulation frequencies <100 Hz (16). A threshold for PTP of the StF feedback pathway is reasonable given its putative searchlight role (17): salient objects that activate StF sufficiently to induce PTP will subsequently be more readily detected. PTP of DML (putative gain control) feedback presumably lack a CaMK2-dependent frequency threshold and would therefore be predicted to simply scale linearly with activity. Tests of this hypothesis may provide important insights into the role of both presynaptic CaMK2α and of PTP in temporal signal processing (2, 77).
Acknowledgments
We would like to thank Dr. N. Berman for helpful discussions and W. Ellis for skilled technical assistance. This work was supported by a grant from the Medical Research Council (Canada) to L.M.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ACSF, artificial cerebrospinal fluid; CaMK2, Ca2+/calmodulin-dependent kinase 2; CaMK2α, CaMK2, α subunit; ELL, electrosensory lateral line lobe; DML, dorsal molecular layer; PPF, paired pulse facilitation; PTP, posttetanic potentiation; STE, short-term enhancement; StF, stratum fibrosum; VML, ventral molecular layer; EPSP, excitatory postsynaptic potential.
References
- 1.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 2.Fischer S A, Fischer T M, Carew T J. Trends Neurosci. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- 3.Zucker R S. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 4.Delaney K R, Zucker R S, Tank D W. J Neurosci. 1989;9:3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tank D W, Regehr W G, Delaney K R. J Neurosci. 1995;15:7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Zucker R S. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 7.Regehr W G, Delaney K R, Tank D W. J Neurosci. 1994;14:523–527. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delaney K R, Tank D W. J Neurosci. 1994;14:5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blundon J A, Wright S N, Brodwick M S, Bittner G D. Proc Natl Acad Sci USA. 1993;90:9388–9392. doi: 10.1073/pnas.90.20.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winslow J L, Duffy S N, Charlton M P. J Neurophysiol. 1994;72:1769–1793. doi: 10.1152/jn.1994.72.4.1769. [DOI] [PubMed] [Google Scholar]
- 11.Maler L. J Comp Neurol. 1979;183:323–363. doi: 10.1002/cne.901830208. [DOI] [PubMed] [Google Scholar]
- 12.Maler L, Sas E K, Rogers J. J Comp Neurol. 1981;195:87–139. doi: 10.1002/cne.901950107. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Maler L. Brain Res. 1994;653:215–222. doi: 10.1016/0006-8993(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 14.Sas E, Maler L. Anat Embryol. 1987;177:55–79. doi: 10.1007/BF00325290. [DOI] [PubMed] [Google Scholar]
- 15.Bastian J. J Neurophysiol. 1996;76:2497–2507. doi: 10.1152/jn.1996.76.4.2497. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Maler L. J Neurophysiol. 1997;78:1882–1889. doi: 10.1152/jn.1997.78.4.1882. [DOI] [PubMed] [Google Scholar]
- 17.Berman N J, Plant J, Turner R, Maler L. J Neurophysiol. 1997;78:1869–1881. doi: 10.1152/jn.1997.78.4.1869. [DOI] [PubMed] [Google Scholar]
- 18.Bastian J. J Neurosci. 1986;6:553–562. doi: 10.1523/JNEUROSCI.06-02-00553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastian J. J Comp Physiol A. 1986;158:505–515. doi: 10.1007/BF00603796. [DOI] [PubMed] [Google Scholar]
- 20.Bastian J, Bratton B. J Neurosci. 1990;10:1226–1240. doi: 10.1523/JNEUROSCI.10-04-01226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastian J. J Comp Physiol A. 1995;176:63–73. doi: 10.1007/BF00197753. [DOI] [PubMed] [Google Scholar]
- 22.Maler L, Mugnaini E. J Comp Neurol. 1994;345:224–252. doi: 10.1002/cne.903450206. [DOI] [PubMed] [Google Scholar]
- 23.Berman N J, Hincke M T, Maler L. J Comp Neurol. 1995;361:512–24. doi: 10.1002/cne.903610313. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4954. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner R W, Maler L, Deerinck T, Levinson S R, Ellisman M H. J Neurosci. 1994;14:6453–6471. doi: 10.1523/JNEUROSCI.14-11-06453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratton B, Bastian J. J Neurosci. 1990;10:1241–1253. doi: 10.1523/JNEUROSCI.10-04-01241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tukumitsu H, Chijiwa T H, M, Mizutani A, Terasawa M, Hidaka H. J Biol Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- 29.Ishikawa N, Hashiba Y, Hidaka H. J Pharmacol Exp Ther. 1990;254:598–602. [PubMed] [Google Scholar]
- 30.Stanton P K, Gage A T. J Neurophysiol. 1996;76:2097–2011. doi: 10.1152/jn.1996.76.3.2097. [DOI] [PubMed] [Google Scholar]
- 31.Malinow R, Schulman H, Tsien R W. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 32.Sas E, Maler L. J Comp Neurol. 1983;221:127–144. doi: 10.1002/cne.902210202. [DOI] [PubMed] [Google Scholar]
- 33.Sihra T S, Pearson H A. Neuropharmacology. 1995;34:731–741. doi: 10.1016/0028-3908(95)00051-7. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Hidaka H, Wollheim C B. Mol Pharmacol. 1992;42:489–498. [PubMed] [Google Scholar]
- 35.Williams C L, Porter R A, Phelps S H. Biochem Pharmacol. 1995;50:1979–1985. doi: 10.1016/0006-2952(95)02096-9. [DOI] [PubMed] [Google Scholar]
- 36.Wylie D J A, Nicoll R A. Neuron. 1994;13:635–643. doi: 10.1016/0896-6273(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 37.Marley P D, Thompson K A. Biochem Biophys Res Commun. 1996;221:15–18. doi: 10.1006/bbrc.1996.0536. [DOI] [PubMed] [Google Scholar]
- 38.Maurer J A, Wenger B W, McKay D B. J Neurochem. 1996;66:105–113. doi: 10.1046/j.1471-4159.1996.66010105.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui M, Yanagihara N, Fukunaga K, Minami K, Nakashima Y, Kuroiwa A, Miyamoto E, Izumi F. J Neurochem. 1996;66:2517–2522. doi: 10.1046/j.1471-4159.1996.66062517.x. [DOI] [PubMed] [Google Scholar]
- 40.Picciotto M R, Czernik A J, Nairn A C. J Biol Chem. 1993;268:26512–26521. [PubMed] [Google Scholar]
- 41.Enslen H, Sun P, Brickey D, Soderling S H, Klamo E, Soderling T R. J Biol Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- 42.Bao J X, Kandel E R, Hawkins R D. Science. 1997;275:969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
- 43.Wang J H, Kelly P T. J Neurophysiol. 1997;78:2707–2716. doi: 10.1152/jn.1997.78.5.2707. [DOI] [PubMed] [Google Scholar]
- 44.Wang J H, Kelly P T. J Neurophysiol. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]
- 45.Zupanc G K H, Airey J A, Maler L, Sutko J L, Ellisman M H. J Comp Neurol. 1992;325:135–151. doi: 10.1002/cne.903250202. [DOI] [PubMed] [Google Scholar]
- 46.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- 47.Hohenegger M, Suko J. Biochem J. 1993;296:303–308. doi: 10.1042/bj2960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takasawa S, Ishida A, Nata K, Nakagawa K, Noguchi N, Tohgo A, Fujisawa H, Okamoto H. J Biol Chem. 1995;270:30257–30259. doi: 10.1074/jbc.270.51.30257. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Best P M. Nature (London) 1992;359:739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- 50.Goda Y. Proc Natl Acad Sci USA. 1997;94:769–772. doi: 10.1073/pnas.94.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greengard P, Valtorta F, Czernik A J, Benfenati F. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 52.Hanson P J, Schulman H. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 53.Ouimet C C, McGuinness T, Greengard P. Proc Natl Acad Sci USA. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benferati F, Valtorta F, Rubenstein J L, Gorelock F S, Greengard P, Czernik A J. Nature (London) 1992;459:417–419. doi: 10.1038/359417a0. [DOI] [PubMed] [Google Scholar]
- 55.Nichols R A, Silva T S, Czernik A J, Nairn A C, Greengard P. Nature (London) 1990;343:647–650. doi: 10.1038/343647a0. [DOI] [PubMed] [Google Scholar]
- 56.Llinas R, McGuinness T L, Leonard C S, Sugimori M, Greengard P. Proc Natl Acad Sci USA. 1985;82:3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Renger J J, Griffith L C, Greenspan R J, Wu C. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 58.Silva A J, Stevens C F, Tonegawa S, Wang Y. Science. 1992;257:201–205. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 59.Kamiya H, Zucker R S. Nature (London) 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- 60.Chapman P F, Frenguelli B G, Smith A, Chen C, Silva A J. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 61.Salin P A, Scanziani M, Malenka R C, Nicoll R A. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pieribone V A, Shupalikov O, Brodin L, Hilfiker-Rothenfluh S, Czernik A J, Greengard P. Nature (London) 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 63.Maler L, Mathieson W B. Cell Mol Neurobiol. 1985;5:373–387. doi: 10.1007/BF00755402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones E G, Huntley G W, Benson D L. J Neurosci. 1994;14:611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fykse E M, Li C, Sudhof T C. J Neurosci. 1995;15:2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahler M, Klein R L, Wang J K T, Benfenati F, Greengard P. J Neurochem. 1991;57:423–430. doi: 10.1111/j.1471-4159.1991.tb03769.x. [DOI] [PubMed] [Google Scholar]
- 67.Rubenstein J L, Greengard P, Czernik A J. Synapse. 1993;13:161–172. doi: 10.1002/syn.890130207. [DOI] [PubMed] [Google Scholar]
- 68.Hirling H, Scheller R H. Proc Natl Acad Sci USA. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Popoli M. FEBS Lett. 1993;317:85–88. doi: 10.1016/0014-5793(93)81496-m. [DOI] [PubMed] [Google Scholar]
- 70.Berton F, Ibbora C, Boudier J, Seagar M J, Marquez B. J Neurosci. 1997;17:1206–1216. doi: 10.1523/JNEUROSCI.17-04-01206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson M E, Braun A P, Schulman H, Premack B A. Circ Res. 1994;75:854–861. doi: 10.1161/01.res.75.5.854. [DOI] [PubMed] [Google Scholar]
- 72.Lu H K, Fern R J, Nee J J, Barrett P Q. Am J Physiol. 1994;267:F183–F189. doi: 10.1152/ajprenal.1994.267.1.F183. [DOI] [PubMed] [Google Scholar]
- 73.Yokoyama C T, Sheng Z, Catterall W A. J Neurosci. 1997;17:6929–6938. doi: 10.1523/JNEUROSCI.17-18-06929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maler L, Mugnaini E. J Comp Physiol A. 1993;173:667–670. [Google Scholar]
- 75.Hanson P I, Meyer T, Stryer L, Schulman H. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 76.DeKonnick P, Schulman H. Science. 1998;279:27–230. [Google Scholar]
- 77.Gabbiani F, Metzner W, Wessel R, Koch C. Nature (London) 1996;384:564–567. doi: 10.1038/384564a0. [DOI] [PubMed] [Google Scholar]