Abstract
We investigated the inter-relationship between two downstream effectors of vascular endothelial growth factor (VEGF), the serine-threonine kinase Akt (or protein kinase B) and the transcription factor ETS1, in tubulogenesis. We show that VEGF upregulates ETS1 transcription through an Akt-dependent pathway in primary endothelial cells. Activation of Akt also results in tubule formation in vitro, a process requiring ETS1 activity. In vivo, the Drosophila ETS1 is required for cell motility per se while Akt is responsible for organized cell movement. Thus, ETS1 and Akt control different aspects of cell migration that are integrated in the regulation of vascular tubule formation.
Keywords: Akt, ETS1, angiogenesis, Drosophila, tubulogenesis, tracheal development
Angiogenesis is the mechanism by which new blood vessels are formed from the pre-existing vascular network (Shiojima and Walsh 2002). It is widely accepted that vascular endothelial growth factor (VEGF) is the main activator of angiogenesis, inducing basement membrane degradation, endothelial cell proliferation and cell motility (Merenmies et al. 1997). Among the cellular mechanisms that underlie angiogenesis, cell motility is the least understood. Angiogenic tubulogenesis requires, a priori, migratory potential of the vascular cells. However, the cell migration events that lead to blood vessel formation needs to be both organized and directional. In other words, simple mobilization of vascular cells does not necessarily lead to tubule formation. How organized cell movement is regulated remains unresolved. Since VEGF is sufficient for inducing tube-like structures in three dimensional cell culture systems (Nehls and Drenckhahn 1995; Papapetropoulos et al. 1997), this model provides a good approximation for dissecting the complex mechanisms involved in organized cell migration. VEGF can activate many different signaling pathways, depending on cellular context and environmental cues (Veikkola et al. 2000). Among the various responses VEGF can elicit, Akt and ETS1 have emerged as two important down-stream regulators of angiogenic cell movement.
Upon growth factor stimulation, phosphatidylinositol-3-kinase (PI3K) is activated which leads to recruitment of Akt to the plasma membrane where it binds phosphoinositol lipids via its pleckstrin homology domain (Chan et al. 1999). PI3K-Akt-mediated signaling has been implicated in many aspects of cellular functions, including cell survival and cell size control, which are regulated by other growth factors such as EGF and the insulin family of proteins (Brazil et al. 2002). These Akt functions are evolutionarily conserved at least in Drosophila (Scanga et al. 2000; Potter et al. 2002; Radimerski et al. 2002). Recently PI3K and by extension, Akt, have been implicated in upregulation of angiogenesis in vivo and tubule formation in vitro (Jiang et al. 2000; Morales-Ruiz et al. 2000). Although the inductive effect of PI3K on angiogenesis has been ascribed to promoting cell migration, no studies have clearly differentiated general migration versus the organized migration that is required for tubule formation.
On the other hand, the effect of ETS1 on cell migration is much better documented. Endothelial cell growth factors such as VEGF and FGF specifically upregulate the activity of ETS1 in primary endothelial cells, although the exact mechanism is not known (Chen et al. 1997). This induction results in the activation of downstream target genes, such as metalloproteinases that are important for matrix degradation and cell migration (Vu and Werb 2000). In addition, antisense ETS1 can block the proangiogenic function of VEGF in cultured endothelial cells (Iwasaka et al. 1996; Chen et al. 1997; Nakano et al. 2000). Thus, ETS1 appears to be a major regulator of migratory potential in response to chemotactic growth factors, a function that is evolutionarily conserved. In the Drosophila tracheal system pointed (pnt), the homologue of ETS1, is expressed at the tips of the migrating tracheal tubes and is required for appropriate branching morphogenesis (Samakovlis et al. 1996).
The Drosophila tracheal system arises from 10 clusters of 80 to 100 cells, called tracheal placodes, located on either side of the developing embryo. Each cluster forms six primary branches via cell migration and subsequent cell elongation (reviewed in Metzger and Krasnow 1999; and Krasnow and Nelson 2002). At no point after the tracheal placodes are set aside do the tracheal cells proliferate, nor are other cells recruited into the network. Since tubule formation by HUVEC in Matrigel are also due to changes in cellular migration and not to changes in proliferation, in both our model systems cellular mechanisms that specifically govern cell motility can be analyzed unambiguously.
We set out to test whether VEGFR, Akt and ETS1 form a linear signaling pathway that promotes tubule formation, cross-referencing human cell culture and the in vivo Drosophila systems. Rather than a simple epistatic relationship, we uncovered a complex interplay between Akt and ETS1. We show, for the first time, that during the early phase of VEGF induction, activated Akt leads to increased ETS1 transcription but not to an increase in phosphorylation per unit of protein. Activated Akt and ETS1 in turn regulate different aspects of cell migration during tubule formation: ETS1 is necessary for cell movement while Akt confers directionality of the movement.
Results and Discussion
VEGF stimulates transcription of ETS1, not changes in its phosphorylation
As shown in Fig. 1A, stimulation of human umbilical vein endothelial cells (HUVEC) with VEGF causes an upregulation of ETS1 message within 3 hours that is maintained for at least 24 hours. Treatment of HUVEC with the global transcription inhibitor actinomycin D blocks the upregulation of ETS1 in response to VEGF stimulation (Fig. 1B), indicating that VEGF affects the transcription of ETS1. A direct effect on ETS1 transcription is confirmed by nuclear run-on assays. As shown in Fig. 1C, VEGF induces a 2.5-fold increase in the promoter strength of ETS1.
Figure 1.
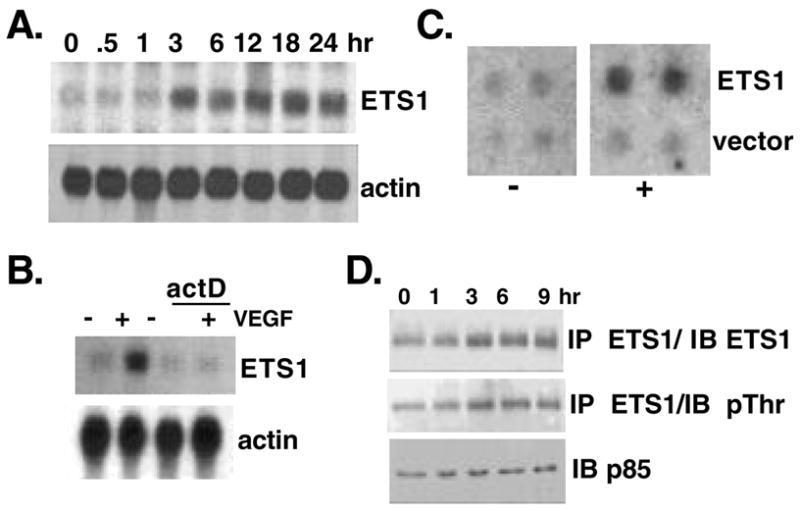
VEGF stimulates the expression of ETS1 without changes in its phosphorylation. (A) Northern blot analysis of total RNA from HUVEC treated with or without VEGF for the times indicated and probed for ETS1 and actin. (B) Northern blot analysis of HUVEC treated with or without actinomycin D (10ng/ml) and stimulated with VEGF (20ng/ml) for 3 hours. (C) Nuclear run-on analysis of nuclei isolated from HUVEC treated (+) or untreated (−) with VEGF for 3 hours and pulse labeled with γ–32PUTP. Isolated nascent RNA was used to probe dot blots containing either vector alone or ETS1 cDNA (1cg/dot). (D) Western blot analysis of total protein isolated from HUVEC treated with VEGF for the times indicated, immunoprecipitated with anti-ETS1 antibody and immunoblotted with antibodies against either ETS1 or phospho-threonine. An immunoblot for the p85 subunit of PI3K serves as loading control.
In other cellular systems ETS1 activity is controlled by phosphorylation of Thr38 (Yordy and Muise-Helmericks 2000). We therefore tested whether VEGF treatment also causes changes in the phosphorylation of ETS1. As shown in Fig. 1D, the threonine-specific phosphorylation level of ETS1 closely mirrors the overall increase in protein level, suggesting that VEGF does not induce marked changes in the phosphorylation of threonine residues beyond what is proportional to the increased ETS1 protein level.
PI3K-Akt signaling mediates the upregulation of ETS1
Since VEGF can activate both the ERK and the PI3K-Akt pathways, we sought to identify which of these two pathways are responsible for ETS1 upregulation. Two commonly used pharmacological inhibitors were first tested for their specificity. As shown in Fig. 2A, wortmannin (WTM) and PD098059 (PD) specifically block phosphorylation of Akt and Erk1 and 2, respectively, without altering the protein levels. These inhibitors were then used to block ETS1 upregulation by VEGF. As shown in Fig. 2B, inhibition of PI3K by wortmannin blocked the induction of ETS1 by VEGF whereas inhibition of MAP kinase by the MEK1 inhibitor, PD098059, caused a 3-fold increase of ETS1 mRNA in the absence of growth factor and a super-induction of ETS1 RNA in the presence of VEGF. The significance of this inhibitory cross-talk is currently unclear.
Figure 2.
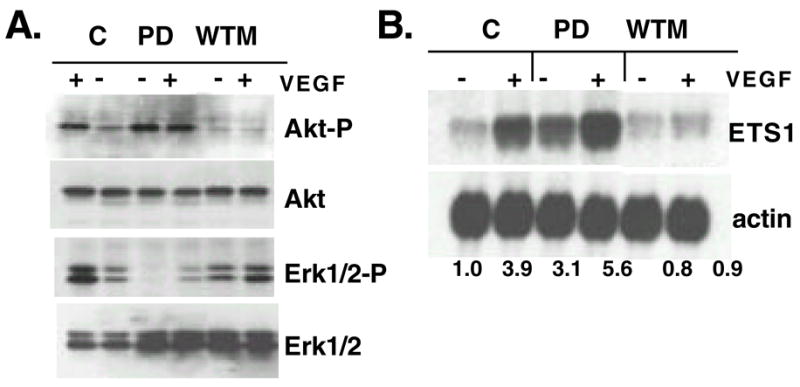
Pharmacological inhibition of VEGF-induced ETS1 expression. (A) Western blot analysis of total protein isolated from serum and growth factor starved HUVEC that are pretreated with DMSO (C), PD098059 (PD) or wortmannin (WTM), then with (+) or without (−) VEGF for 10min. Duplicate blots were probed with antibodies against phosphorylated Akt (Akt-P) and Erk1 and 2 (p42/p44-P). Blots were reprobed with antibodies against total Akt and Erk 1 and 2. (B) Northern blot analysis of total RNA isolated from serum and growth factor starved HUVEC that are pretreated with PD098059 (PD) or wortmannin (WTM) then with (+) or without (−) VEGF for 3 hours and probed for ETS1 and reprobed with actin. Numbers are normalized relative densitometric units.
To show a direct link between the activation of Akt and the upregulation of ETS1 we transiently expressed a myristylated form of Akt (myrAkt) in HUVEC. myrAkt includes an N-terminal Src myristylation signal that targets it to the membrane. This form of Akt is constitutively active (Kennedy et al. 1997). The kinase activity of Akt is first monitored by a nonradioactive in vitro kinase assay using GST-GSK3β as an exogenous substrate (Fig. 3A). The resultant Western blot analysis shows a 3-fold increase of GSK3β phosphorylation in the presence of myrAkt. This level of induction by myrAkt is similar to that induced by VEGF (Gerber et al. 1998). Interestingly, treatment of cells expressing myrAkt with VEGF results in a slight reduction of GSK3β phosphorylation, indicating a potential negative regulatory loop. Importantly, induction of ETS1 transcription exactly parallels the Akt kinase activity (Fig. 4A). To further demonstrate that the myrAkt effect on ETS1 expression is a direct consequence of increased Akt kinase activity and not due to over-expression of the Akt protein, both the normal cellular form of Akt (cAkt) and a kinase-dead mutant (K-Akt) were expressed in HUVEC. Neither cAkt nor K-Akt is able to significantly induce the expression of ETS1 in the absence VEGF (Fig. 3B), although their respective protein levels are comparable to the level of myrAkt that confers induction of ETS1 (Fig. 3C).
Figure 3.
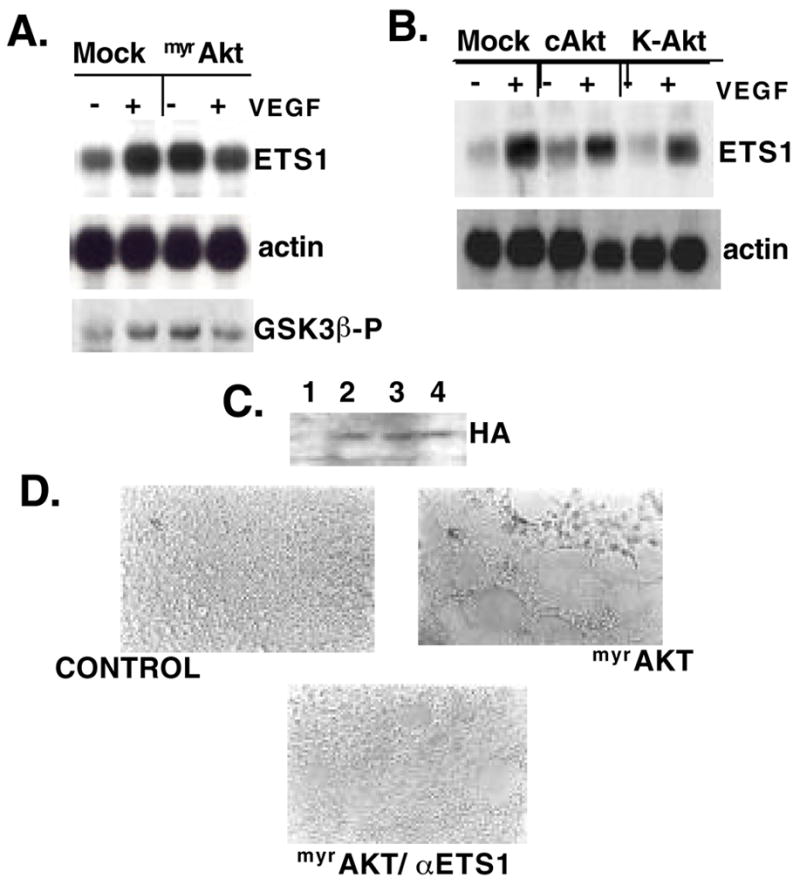
Constitutively active Akt results in upregulation of ETS1 and tubule formation in Matrigel. (A) Northern blot analysis of ETS1 and actin expression in total RNA isolated from HUVEC transfected with myrAkt or empty vector (mock), treated for 3 hours with (+) or without (−) VEGF. Cells treated as above were also tested for in vitro kinase activity using GSK3β as an exogenous substrate. The resultant Western blot analysis for the phosphorylated substrate is shown (GSK3β-P). (B) Northern blot analysis of ETS1 and actin expression in total RNA from HUVEC treated as in A but transfected with expression constructs containing HA-tagged cAKT and kinase-dead Akt (K-Akt). The protein expression levels of these three forms of Akt are monitored in the Western blot shown in (C). (D) HUVEC transfected with empty vector (C), myrAkt or myrAkt plus antisense ETS1 were plated onto Matrigel-coated plates. Matrigel assays were performed in duplicate at least three independent times.
Figure 4.
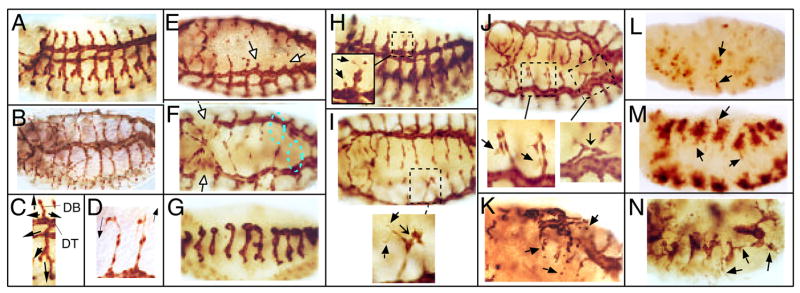
Tracheal defects in Dakt1 and pnt mutants. Embryos were stained with mouse monoclonal anti-β-Gal antibody. In all panels, anterior is to the left. (A) Lateral view of a stage 14 1-eve-1 embryo, representing wild type. (B) Dorsal-lateral view of a stage 16 1-eve-1 embryo, showing the fully formed tracheal system. (C) One wild-type tracheal metameric subunit at stage 14. Migratory paths of the six primary branches are marked by arrows. Two relevant branches are indicated: DB, dorsal branch; DT: dorsal trunk. (D) Close-up view of two wild-type dorsal branches at stage 16. Arrows indicate the directions of the extending cellular processes at the tips of the elongating tubes. (E) Dorsal-lateral view of a stage 15 pntΔ88, 1-eve-1/+ embryo. Empty arrows point to "stunted" dorsal branches in which tracheal cells fail to migrate out. (F) Dorsal view of a stage 16 pntΔ88, 1-eve-1/+ embryo. Open arrows point to the gaps in the dorsal trunks and dashed circles marked positions where dorsal branches are missing. (G) Lateral view of a homozygous stage 15 pntΔ88, 1-eve-1 embryo. Very little branching occurs. (H) Lateral view of a stage 13 Dakt1q, 1-eve-1/+ embryo. Arrows in the enlarged view point to ectopic filopodia and an errand cell sprouting from the main dorsal trunk. (I) Dorsal view of a stage 16 Dakt1q, 1-eve-1/+ embryo. Arrows in the enlarged view point to ectopic filopodia. Sharp arrow points to the ectopic connection between two adjacent subunits. (J) Dorsal view of a homozygous stage 16 Dakt1q, 1-eve-1 embryo. Arrows in the enlarged view point to ectopic filopodia. Sharp arrow points to the ectopic connection between two adjacent subunits. (K) Dorsal view of a homozygous stage 16 Dakt1q, 1-eve-1 embryo. Arrows point to ectopic branches and breakaway tracheal cells. (L) Lateral view of a stage 14 Dakt1q, 1-eve-1/pntΔ88, 1-eve-1 embryo. Arrows point to ectopic branches and breakaway tracheal cells. (M) Ventral view of a stage 13 Dakt1q, 1-eve-1/pntΔ88, 1-eve-1 embryo. Most of the tracheal cells remain within the tracheal placodes while erratic branches are seen sprouting out (arrows). (N) Lateral view of a stage 13 Dakt1q, 1-eve-1/pntΔ88, 1-eve-1 embryo. The tracheal subunits lack stereotypical branching, similar to the phenotype observed in the pnt mutant (G) but erratic small branches are seen sprouting out (arrows).
Activation of Akt induces in vitro tubule formation
Upon transfection of myrAkt, as described above, we noticed changes in HUVEC morphology (data not shown) in the absence of exogenously added VEGF. The significance of these morphological changes was tested in simulated extracellular matrix such as Matrigel. 14–18 hr after myrAkt- or mock-transfection, HUVEC were plated onto the Matrigel-coated culture wells in serum-free media. Within 3 hr of plating, myrAkt-transfected HUVEC formed striking tube-like structures (Fig. 3D), similar to those induced by VEGF (Neihls and Drenckhahn 1995; Papapetropoulos et al. 1997; and data not shown). At 3hr in the absence of myrAkt and serum, HUVEC remained an even monolayer.
Since our data suggest that ETS1 expression is downstream of Akt in HUVEC and inhibition of ETS1 is known to prevent in vitro angiogenesis and cellular migration (Chen et al. 1997), we asked whether the morphological effect of Akt activation required the expression of ETS1. As shown in Fig. 3D, co-transfection of an antisense ETS1 construct with myrAkt reduces the ability of the activated kinase to initiate tube formation. Taken together our results indicate that activation of Akt plays a central role in the cellular reorganization essential for the generation of new capillary vessels and that this process requires the activity of ETS1.
Role of Akt and ETS1 in tracheal formation in Drosophila
In the results described above myrAkt can substitute for two VEGF functions in HUVEC, an early event upregulating the expression of ETS1 within 3hr and later events that lead to tubule formation in vitro. These results suggest then a potential linear epistatic pathway in regulating tubulogenesis, VEGFR→Akt→ETS1→tubule formation. We sought to test this possibility in an in vivo model: the Drosophila tracheal system in which both homologs of Akt (Dakt1) and ETS1 (pointed) play important roles.
The chemotactic cues for tracheal tube formation are provided by the Drosophila FGF that is produced in cells just distal to the migrating tubule and is received by the Drosophila FGF receptor on the tracheal cells. The pnt/ETS1 gene is expressed in the migrating tip cells of the tracheal branches. Mutations in pnt result in severe impairment of branching morphogenesis (Samakovlis et al. 1996).
The involvement of Dakt1 in tracheal cell migration has not been described, although it has been shown that Dakt1 is required for cell survival during early embryogenesis (Scanga et al. 2000) as well as early cell fate determination in the tracheal placodes (Jin et al. 2001). However, these early essential functions can be sustained by maternal contributions in the zygotic mutant. As such, Dakt1 mutant embryos resulting from heterozygous crosses can develop to term. This allows for analyses of subtle phenotypes in tracheal branching morphogenesis, a relatively late organogenesis event, in Dakt1 zygotic mutants.
To better visualize the tracheal system, we utilized a reporter transgenic line 1-eve-1 that expresses lacZ in all tracheal cells (Perrimon et al. 1991). The lacZ insertion does not cause tracheal defects (Fig. 4A–D). The 1-eve-1 marker was then combined with Dakt1 and pnt alleles and tracheal defects were visualized by staining with anti-β-Gal antibody. Consistent with previous reports, both heterozygous and homozygous pnt mutants (pntΔ88) show lack of tubule migration of different degrees of severity. In milder phenotypes, found mostly in the heterozygotes, the overall tracheal network is formed but with missing branches and breakage in the main tracheal tube (dorsal trunk; Fig. 4E–F). In severe cases found in both heterozygotes and homozygotes, very few branches are formed (Fig. 4G). These phenotypes are consistent with the general notion that ETS1 confers migratory potential to vascular cells.
However, in both heterozygous and homozygous Dakt1q (a kinase-dead mutation; Staveley et al. 1998; Scanga et al. 2000), we observed ectopic migration, instead of the expected lack of migration. In moderate phenotypes, dorsal branches from adjacent subunits are frequently linked, rather than connecting with the counterparts across the embryonic midline (Fig. 4I–J). At higher magnification, ectopic filopodia-like cellular projections can be detected at the migrating tracheal tips (Fig. 4H–J). In severe cases, the overall tubule network is disrupted with obvious ectopic branching and “breakaway” tracheal cells (Fig. 4K). These phenotypes suggest that the normal function of Dakt1 in tubule formation is to restrict the movement of migrating cells. We note that there is no obvious change of cell size in the Dakt1 mutant trachea (Fig. 4H–K) nor did we observe increased apoptosis (data not shown).
Opposite actions of Dakt1 and pnt/ETS1 in tracheal cell migration
How can the above interpretation be reconciled with the data obtained in HUVEC? It is possible that tubule formation requires two opposing functions, one to mobilize the cells (the ETS1 function) and one to confer the restricted directionality of the cell movement (the Akt function). To test this model, we performed a genetic epistasis analysis in Drosophila. Since both Dakt1q and pntΔ88 heterozygotes exhibited moderate but significant penetrance, in trans-heterozygous combination, we should be able to detect rescued or exacerbated phenotypes. We reasoned that if pnt/ETS1 confers cell motility while Dakt1 restricts it, pnt mutation should improve the Dakt1 phenotypes because with reduced migratory potential (in pnt mutant) there should be less ectopic migration (in Dakt1 mutant). This is indeed the case. To avoid complications from the cell-fate determination prospect of the Dakt1 and pnt/ETS1 functions, we tabulated only the phenotypes observed during the branching phase of tracheal development, that is, from embryonic stage 13 to 16 (see Fig. 4A–B). As shown in Table 1, the Dakt1-like and the pnt-like phenotypes described in Fig. 4 can be clearly distinguished in the trans-heterozygotes. There is a 60% reduction of the phenocopy number of Dakt1-like phenotypes in Dakt1q/pntΔ88 trans-heterozygotes as compared to the Dakt1q heterozygotes. On the other hand, there is some but less significant reduction of the pnt-like phenotypes in the trans-heterozygotes as compared to the pntΔ88 heterozygotes. Interestingly, in the trans-heterozygotes occasionally a new class of mutants was observed, which we interpret as having dual characteristics of pnt and Dakt1 phenotypes. In these mutants, primary branches do not form but breakaway cells are detected around the remnants of tracheal placodes (Fig. 4L); or small, erratic branches sprout from the otherwise clustered tracheal subunits (Fig. 4M–N). These data suggest that Pointed/ETS1 and Dakt1 do not function in a linear pathway; rather, they control different aspects of the cell motility that ultimately result in organized tubule network. This interpretation can explain why blocking ETS1 function with antisense ETS1 can counteract the action of constitutively active Akt in promoting tubule formation (Fig. 3D), since there can be no organized cell migration (the Akt function) without a priori the intrinsic ability of the cells to migrate (the ETS1 function).
Table 1.
Antagonist genetic interaction between Dakt1 and pnt/ETS1.
| Dakt1q, 1-eve-1/1-eve-11 (n=159) | PntΔ88, 1-eve-1/1-eve-12 (n=170) | Dakt1q, 1-eve-1/pntΔ88, 1-eve-13 (n=90) | |
|---|---|---|---|
| normal | 41.5% | 84.1% | 60.0% |
| Dakt1-like | 58.5% | — | 23.3% |
| pnt-like | — | 15.9% | 11.1% |
| dual4 | — | — | 5.6% |
Progeny of Dakt1q, 1-eve-1/TM3, P{ry+t7.2=HZ2.7}DB2, Sb1 × 1-eve-1; the heterozygosity is recognized by lack of lacZ expression in maxillary segment conferred by the DB2 transgene.
Progeny of pntΔ88, 1-eve-1/TM3, P{ry+t7.2=HZ2.7}DB2, Sb1 × 1-eve-1
Progeny of Dakt1q, 1-eve-1/TM3, P{ry+t7.2=HZ2.7}DB2, Sb1 × pntΔ88, 1-eve-1/TM3, P{ry+t7.2=HZ2.7}DB2, Sb1
Embryos exhibiting both Dakt1 and pnt phenotypes.
Roles of Akt and ETS1 in angiogenic tubule formation
Combining the results from the in vitro and in vivo assays we suggest that Akt has two effects, an early one that results in the expression of ETS1 and a more long-term effect that results in the control of cellular changes required for tubule formation. We note that in Drosophila, the endogenous pnt/ETS1 RNA level only showed a 10% reduction in Dakt1q homozygotes and whole-mount RNA in situ for pnt/ETS1 did not show obvious reduction in the Dakt1 mutant tracheal cells, either (data not shown). This may be due to maternal contribution of the Dakt1 function or reflect the difference between the human and the Drosophila systems. On the other hand, Dakt1 does have an early function in tracheal development. It promotes the formation of tracheal placodes through activation of the Hif-1-like factor Trachealess (Jin et al. 2001). Whether this function is mediated by pnt/ETS1 is not clear. In the mammalian system it is also not clear whether Akt is required for the maintenance or establishment of vascular cell fate.
Our data show that activated Akt is a central regulator of migration and branching morphogenesis by both an in vitro system (HUVEC) and an in vivo system (Drosophila tracheal development), neither of which require cellular proliferation. Our comparative analyses using these two systems indicate that simple over-expression of gene functions or epigenetic knockouts commonly employed in cell culture studies, although informative, could potentially be misleading. Thus, by cross-referencing the HUVEC culture and the Drosophila tracheal systems, we provide novel insights into the cell migration events underlying capillary tubule formation, which requires the seemingly opposite but coordinated functions of ETS1 and Akt. Although the involvement of Akt in angiogenesis has been noted (Chan et al. 1999; Jiang et al. 2000; Morales-Ruiz et al. 2000; Shiojima and Walsh 2002), to our knowledge, this report is the first to indicate that the Akt function in tubulogenesis is an essential but restrictive one. The exact cellular mechanism of this aspect of the Akt function remains to be elucidated. One important clue may lie in the Akt action on cell adhesion and cytoskeletal rearrangement. Akt phosphorylation sites have been found in the Rho family of proteins (Higuchi et al. 2001; Sachdev et al. 2002). These GTPases are known to play important roles in the control of cell shape, adhesion, movement and actin cytoskeleton organization. Modulating these GTPases may underlie the mechanisms of directed cell migration. Indeed, while Rac1 is known to promote cell migration, the phosphorylation of Rac1 by Akt may be inhibitory (Kwon et al. 2000). The PI3K-Akt pathway may also direct restricted migration through integrin engagement (Danen and Yamada 2001). Thus, the molecular mechanisms controlled by PI3K and Akt are likely to be complex and involve multiple players. Elucidation of these complex mechanisms will help unravel the process of tubulogenesis.
Materials and methods
Cells, growth factors and inhibitors
Pooled, multiple donor HUVEC and media were purchased from Clonetics and maintained in endothelial basal medium (EBM) supplemented with 12μg/ml bovine brain extract, 10ng/ml hEGF, 1mg/ml hydrocortisone, and 2% fetal bovine serum in procedures described by Clonetics. For serum/growth factor starvation, 80 to 90% confluent monolayers were incubated in RPMI 1640 supplemented with 0.5% fetal calf serum (Gibco BRL) and 2mM glutamine for 10 to 12 hours and stimulated with 20ng/ml VEGF165 (R and D Systems). Pharmacological inhibitors used are PD098059 and wortmannin (Biomol) at concentrations of 50μM and 100nM, respectively. Subsequent cell extract preparation and Northern and Western analyses followed standard procedures (Muise-Helmericks et al. 1998)
Antibodies
The antibodies used for Western blot analysis are as follows: anti-p85 subunit of PI3K (Upstate Biotechnology), anti-ETS1 (Transduction Labs), anti-Akt (Santa Cruz), anti-phosphospecific (Ser473) Akt (Cell Signaling), anti-phosphospecific p42/p44 (Promega), p42/p44 Erk1/2, anti-HA and anti-phosphospecific threonine (Santa Cruz). The ETS1 antibody used for immunoprecipitation was purchased from Santa Cruz.
Transfection and Akt kinase assays
Expression constructs containing hemagglutinin tagged cAkt, myrAkt, and kinase dead (K-) Akt expressed from the CMV promoter were provided by P. Tsichlis (Thomas Jefferson U.) and A. Bellacosa (Fox Chase Cancer Center). All transfections were performed using Lipofectin (Invitrogen). Akt kinase assay kit was purchased from Cell Signaling, Inc.
Matrigel tube formation assays
HUVEC were transfected with empty pcDNA3 vector, myrAkt, or both myrAkt and an antisense construct against the full-length p42 ETS1 isoform cDNA. The amounts of myrAkt and of antisense ETS1 were 2μg each. The total amount of DNA (4μg) was kept constant by addition of empty vector. Cells were plated onto Matrigel matrix (BD Laboratory) at1.6×104cells/50ul per well of a 96-well plate 14 to 18 hours after transfection. Assays were performed in duplicate and repeated three independent times.
Drosophila strains and immunostaining
1-eve-1 (N. Perrimon, Harvard Medical School) is a homozygous viable enhancer trap line that contains a β-galactosidase-expressing P-element in the trachealess gene. pntΔ88 is a null deletion allele (Bloomington Stock Center) and Dakt1q is a kinase-dead allele (A.S. Manoukian, U. of Toronto). Embryo collection and whole-mount immunohistochemistry has been described (Bagni et al. 2002), using mouse monoclonal antibody against β-Gal (Sigma) and biotinylated goat anti-mouse IgG secondary antibody and the Elite and DAB substrate kits (Vector).
Acknowledgments
This work was supported by grants from National Cancer Institute (CA78582) and National Center for Research Resources (P20 RR16434) to RCMH, and from National Cancer Institute (CA095888 and CA78582) to TH, and an Abney Foundation Fellowship to KRL. We thank A. Manoukian for a kind gift of the Dakt1 flies and invaluable suggestions, and L. Obeid for critical reading of the manuscript.
References
- Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- Bagni C, Bray S, Gogos JA, Kafatos FC, Hsu T. The Drosophila zinc finger transcription factor CF2 is a myogenic marker downstream of MEF2 during muscle development. Mech Dev. 2002;117:265–268. doi: 10.1016/s0925-4773(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/KB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Ann Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fisher RJ, Riggs CW, Rhim JS, Lautenberger JA. Inhibition of vascular endothelial growth factor-induced endothelial cell migration by ETS1 antisense oligonucleotides. Cancer Res. 1997;57:2013–2019. [PubMed] [Google Scholar]
- Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Masuyama N, Fukui Y, Suzuki A, Gotoh Y. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr Biol. 2001;11:1958–1962. doi: 10.1016/s0960-9822(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase- type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–531. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Anthopoulos N, Wetsch B, Binari RC, Isaac DD, Andrew DJ, Woodgett JR, Manoukian AS. Regulation of Drosophila tracheal system development by protein kinase B. Dev Cell. 2001;1:817–827. doi: 10.1016/s1534-5807(01)00090-9. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes & Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Krasnow MA, Nelson WJ. Tube morphogenesis. Trends Cell Biol. 2002;12:351. doi: 10.1016/s0962-8924(02)02332-2. [DOI] [PubMed] [Google Scholar]
- Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J Biol Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- Merenmies J, Parada LF, Henkemeyer M. Receptor tyrosine kinase signaling in vascular development. Cell Growth Differ. 1997;8:3–10. [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- Nakano T, Abe M, Tanaka K, Shineha R, Satomi S, Sato Y. Angiogenesis inhibition by transdominant mutant Ets-1. J Cell Physiol. 2000;184:255–262. doi: 10.1002/1097-4652(200008)184:2<255::AID-JCP14>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Noll E, McCall K, Brand A. Generating lineage-specific markers to study Drosophila development. Dev Genet. 1991;12:238–252. doi: 10.1002/dvg.1020120309. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- Radimerski T, Montagne J, Rintelen F, Stocker H, van der Kaay J, Downes CP, Hafen E, Thomas G. dS6K-regulated cell growth is dPKB/dPI(3)K-independent, but requires dPDK1. Nat Cell Biol. 2002;4:251–255. doi: 10.1038/ncb763. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Zeng L, Wang LH. Distinct role of phosphatidylinositol 3-kinase and Rho family GTPases in Vav3-induced cell transformation, cell motility, and morphological changes. J Biol Chem. 2002;277:17638–17648. doi: 10.1074/jbc.M111575200. [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- Scanga SE, Ruel L, Binari RC, Snow B, Stambolic V, Bouchard D, Peters M, Calvieri B, Mak TW, Woodgett JR, Manoukian AS. The conserved PI3′K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene. 2000;19:3971–3977. doi: 10.1038/sj.onc.1203739. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- Staveley BE, Ruel L, Jin J, Stambolic V, Mastronardi FG, Heitzler P, Woodgett JR, Manoukian AS. Genetic analysis of protein kinase B (AKT) in Drosophila. Curr Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes & Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]