Abstract
One of the most important clinical problems in caring for elderly patients is treatment of pressure ulcers. One component of normal wound healing is the generation of an inflammatory reaction, which is characterized by the sequential infiltration of neutrophils, macrophages and lymphocytes. Neutrophils migrate early in the wound healing process. In aged C57BL/6 mice, wound healing is relatively inefficient. We examined the effects of neutrophil numbers on wound healing in both young and aged mice. We found that the depletion of neutrophils by anti-Gr-1 antibody dramatically delayed wound healing in aged mice. The depletion of neutrophils in young mice had less effect on the kinetics of wound healing. Intravenous G-CSF injection increased the migration of neutrophils to the wound site. While the rate of wound repair did not change significantly in young mice following G-CSF injection, it increased significantly in old mice.
Keywords: Aging, Neutrophil, Wound healing
Introduction
One of the most important clinical problems in caring for elderly patients is treatment of pressure ulcers. Pressure ulcers are caused by compression, friction, infection and/or malnutrition. The ability to heal pressure ulcers declines with age (Swift et al. 2001). To understand the mechanism underlying the impaired ability of older patients to repair pressure ulcers, we established studies to analyze wound healing in aged mice.
The wound repair process is a highly ordered sequence of events that encompasses hemostasis, inflammatory cell infiltration, tissue regrowth, and remodeling. First, thrombocytes generate a clot, which stops the bleeding, and serves as a temporary barrier and a source of chemotactic factors. Subsequently, attracted leukocytes initiate an inflammatory response before fibroblasts and endothelial cells migrate to the wound to regenerate tissue that contracts the wound margins. Finally, epithelial cells complete the repair process by covering the denuded wound surface (Martin 1997).
The inflammatory reaction is a critical component of normal wound healing and is characterized by the sequential infiltration of neutrophils, macrophages, and lymphocytes (Ross and Benditt 1962a, b). Neutrophils normally begin arriving at the wound site within minutes of injury, continuing for several days. Neutrophils are themselves phagocytosed by tissue macrophages. Although the primary role of neutrophils is to clear contaminating bacteria, neutrophils are also a source of pro-inflammatory cytokines, including interleukins 1 alpha and beta (IL-α and β) and tumor necrosis factor alpha (TNF-α) which provide some of the earliest signals activating local fibroblasts and keratinocytes (Hubner et al. 1996). Thus, it is well accepted that neutrophils play important roles in wound healing. However, previous studies have also shown adverse effects of neutrophils in wound healing. Classical experiments in the 1970’s directly tested neutrophil and macrophage function by depleting them by antisera in a guinea pig wound model. These experiments showed that antisera depletion of neutrophils seemed not to perturb tissue repair, but depletion of macrophages with antisera and steroids resulted in a failure of debridement (Leibovich and Ross 1976; Leibovich and Ross 1975; Simpson and Ross 1972). More recent neutrophil knockdown experiments in mice using specific anti-mouse neutrophil antibodies support the depletion studies conducted in the 1970s. In fact, they go further, showing that repair is even more rapid in the mice receiving rabbit anti-mouse neutrophil serum than in the mice receiving control serum provided conditions are sterile (Dovi et al. 2003). These studies have been done using young mice.
It is well-known that neutrophil functions are altered with aging (Fulop et al. 2004), which could profoundly influence wound healing in aging patients. Therefore, we asked in this report, whether neutrophils impede or accelerate wound healing processes in aged mice compared to young mice.
Materials and methods
Mice
Two-month-old C57BL/6 (C57BL/6J) male mice were originally purchased from SLC Japan and maintained at the Animal Research Facility at Nagoya University Graduate School of Medicine under specific pathogen-free conditions and used according to institutional guidelines. Aged mice were maintained there, and no abnormalities were found at 20 months of age.
Excision wound preparation and macroscopic examination
After shaving and extensive cleaning with 70% ethanol, the dorsal skin was picked up at the midline and was punched through two layers of skin with a sterile disposable biopsy punch (diameter 3 mm; Kai Industries, Tokyo, Japan). This is an aseptic model of wound, which is completely different from pressure ulcer. This procedure generated two excisional full-thickness wounds with one on each side of the midline. The same procedure was repeated three times, generating six wounds on each animal. Each wound site was digitally photographed at the indicated time intervals, and wound areas were determined on photographs using PhotoShop (version 7.0; Adobe Systems). Changes in wound areas were expressed as the proportion of the initial wound areas. In some experiments, wounds and their surrounding areas (including the scab and epithelial margins) were cut for further analyses with a sterile disposable biopsy punch with a diameter of 6 mm (Kai Industries) at the indicated time points.
Histopathological analyses of wound sites
Wound specimens were fixed in 2% formaldehyde buffered with PBS (pH 7.2), and then embedded with O.C.T compound (Sakura Finetechnical Co., Ltd., Japan). Frozen 5 mm sections were stained with H & E. Other sections were further processed for immunohistochemical analyses to evaluate leukocyte infiltration. They were treated with FITC-labeled anti-mouse Gr-1 or PE-labeled anti-mouse CD11b (BD Pharmingen) or with goat anti-mouse MPO (Santa Cruz Biotechnology), followed by rabbit anti-goat-HRP (Southern Biotechnology Associates. Ins.).
Depletion of Neutrophils in vivo
In order to deplete neutrophils, we used rat anti-mouse Gr-1 monoclonal antibody RB6-8C5 purchased from Beckman Coulter. The mice were intravenously injected with RB6-8C5 mAb (1.4 mg/kg).
G-CSF treatment
Recombinant mouse G-CSF was purchased from R&D. G-CSF (20 μg/kg) was administered intravenously to C57BL/6. The punch biopsy was done 5 days after G-CSF treatment.
Direct injection of neutrophils to wound area
Zymosan A was purchased from Sigma. C57BL/6 mice were injected 0.5mg Zymosan intraperitonealy then the exudates cells were taken from peritoneal cavity by 2.5 ml PBS flash twice. They were cultured for 2 h. in DMEM 10 cm dish with 10%FBS. Then only floating cells were gathered by pipetting. Purity of neutrophils were examined by Gimsa staining. The purified neutrophils were injected directly into wound area.
Statistical analyses
Data were expressed as mean ± standard error of the mean (SE). Statistical comparison was performed by ANOVA followed by Fisher’s post hoc test. Values of p < 0.05 were considered statistically significant.
Results
Delayed wound repair of aged mice
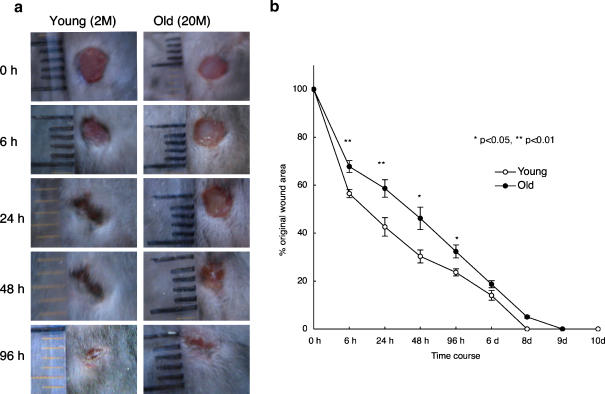
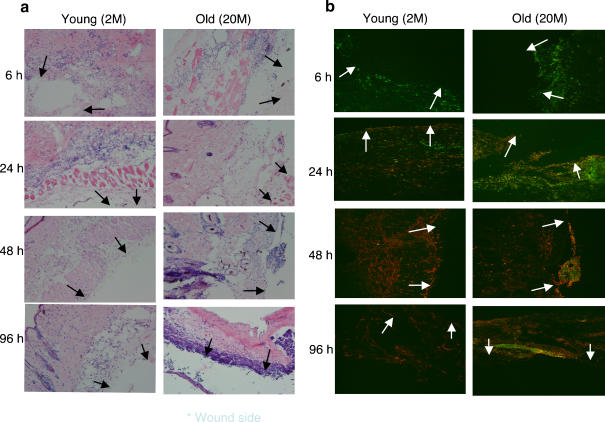
We first examined the time course of wound repair in aged and young mice. As shown in Fig. 1, the duration of wound healing was extended in aged mice (Fig. 1a,b). In young mice, neutrophil infiltration into the wounding site occurred very quickly (the peak of neutrophil number occurred at 6 h, then gradually decreased). In old mice, neutrophils appeared at 6 h but did not peak until 24 h after wounding, after which they gradually decreased in number. Macrophage infiltration was observed at 24 h post operation in both young and old mice and peaked at 48 h. In young mice, they had almost disappeared by 96 h. However, in old mice, their disappearance was more gradual (Fig. 2b).
Fig. 1.
Delayed wound healing of aged C57BL/6 mice. a Macroscopic changes of wound healing processes in two month (young) and 20 month (old) mice. Representative results of four experiments. b Changes in percentages of wound areas at each time point in comparison to the original wound area. Data shown at the mean ratio ± SE of four separate experiments. (*; p < 0.05, **; p < 0.01)
Fig. 2.
Histochemical and immunohistochemical analyses of neutrophils and macrophages recruited into skin excisional wound sites in young and old mice. The samples were obtained at six h to 96 h after three mm punch biopsies. Frozen sections were stained with H & E (a) and with FITC-labeled anti-Gr-1 and PE-labeled anti-CD11b. Arrowhead indicates the site of punch biopsy
Neutrophil depletion delayed wound repair in aged mice
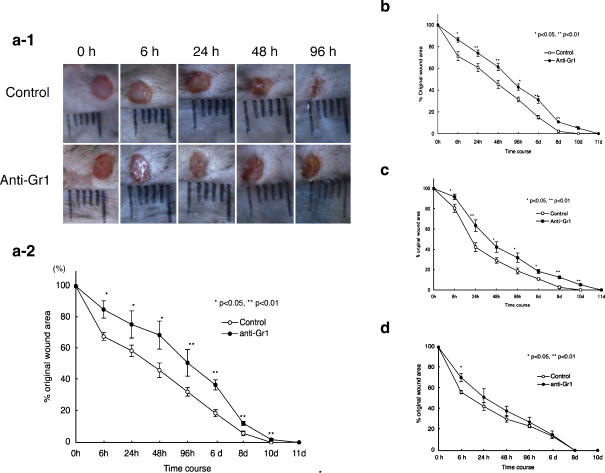
To investigate the role of neutrophils in wound repair, mice were intravenously injected with a mAb against Gr-1 (RB6-8C5mAb) to deplete the neutrophils. We performed punch biopsies on the backs of 2 (Fig. 3d) , 6 (Fig. 3c), 10 (Fig. 3b), 20 (Fig. 3a-1,a-2) month old mice 24 h after the injection of RB6-8C5mAb. We found that the intravenous injection of RB6-8C5mAb delayed wound repair in old mice (Fig. 3a-1, a-2). The delay of wound healing was gradually decreased by younger age. To confirm the lack of neutrophil infiltration into the wound site following intravenous injection of anti-Gr-1, we examined the wound site by anti-Gr-1 staining (Fig. 4a,b). We also used anti-MPO with different specificity to neutrophils. (Fig. 4c). Both in young and old mice, following injection of anti-Gr-1 antibody, the infiltration of neutrophils into the wound site was dramatically decreased for 24 h after punch biopsy. However, neutrophils infiltration into the wound site was gradually observed at later times (Fig. 4a,b). The depletion of neutrophils was confirmed by another neutrophil specific antibody (anti-MPO: Fig. 4c). As a control we injected anti-CD4 antibody, which had no significant differences with PBS injection both in young and old mice (data not shown).
Fig. 3.
Effect of anti-Gr-1 treatment on wound repair in young and aged mice. Anti-Gr-1 monoclonal antibody (1.4 mg/kg) was administered intravenously to young or old C57BL/6 mice. Twenty-four h after injection, punch biopsies on the backs of these mice were performed. Macroscopic wound repair was photographically recorded (a-1: 20M old C57BL/6mice). Changes in percentages of wound areas at each time point in comparison to the original wound area (a-2:20M b: 10M c: 6M d: 2M). Data shown at the mean ratio ± SE of four separate experiments
Fig. 4.
Effect of anti-Gr-1 treatment on neutrophil and macrophage infiltration into the wound site. Frozen sections of the wound areas were stained with FITC-labeled anti-Gr-1, and PE-labeled anti-CD11b (a Young mice, b Old mice). c Frozen sections were also stained with goat anti-mouse MPO followed by rabbit anti-goat HRP in old mice. Arrowhead indicates the wound edge of punch biopsy. The anti-MPO positive cells were stained as brown color
Effect of intravenous injection of G-CSF and the direct injection of neutrophils into the wound area
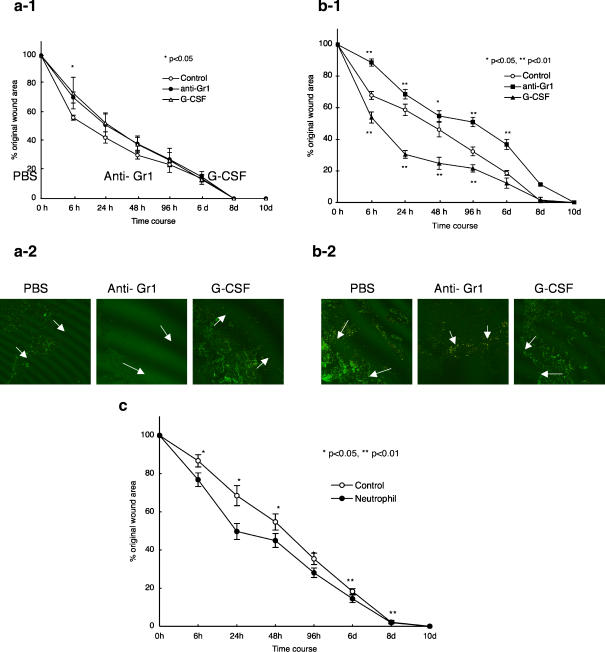
Because the depletion of neutrophils delayed wound repair in old mice, we asked whether increased neutrophil infiltration might increase the rate of wound repair. We injected recombinant G-CSF intravenously into young and old mice. Five days after G-CSF injection, punch biopsies were performed on the backs of young and old mice. We observed a robust infiltration of neutrophils under these conditions (Fig. 5b-1, b-2). The rate of wound repair did not change significantly in young mice (Fig. 5a-1), while in old mice the rate of wound repair was significantly improved by G-CSF injection (Fig. 5b-1). The neutrophils (1 × 106), taken from the peritoneal cavity after injection of Zymozan A , were injected into wound area directly. As shown in Fig. 5c, we found the acceration of wound repair in the neutrophil-injected mice.
Fig. 5.
Effect of intravenous injection of G-CSF and the direct injection of neutrophils into the wound area G-CSF (20 μg/kg) was administered intravenously to young (a) or old (b) C57BL/6 mice. Five days after injection, dorsal punch biopsies were performed. Changes in percentages of wound areas at each time point in comparison to the original wound area (a-1, young mice; b-1, old mice). Frozen sections were stained with FITC labeled-anti-Gr-1, and PE- labeled anti-CD11b. c. Neutrophils taken from 6 M C57BL/6 mice by the methods descrived in Materials and Methods were stained by Gimsa. More than 95% cells were neutrophils. 1 × 106 neutrophils were injected into one wound area of 19 M old C57BL/6 mice. Data shown at the mean ratio ± SE of four separate experiments. (*; p < 0.05, **; p < 0.01)
Discussion
This study demonstrates that the rate of healing of sterile wounds in aged mice declined compared to that seen in young mice. Wound healing is a critical process in the skin and is known to decline with increasing age (Ashcroft et al. 2002; Ballas and Davidson 2001). Delayed wound healing in the elderly has been well described (Grove and Kligman 1983). It was recently reported that older skin required more time than younger skin to recover from UVA-mediated deactivation of catalase (Hellemans et al. 2003). The works done by Roth’s group have clearly shown that macrophage functional decline contribute to the slowing of wound repair in middle-aged and aged mice compared to young mice (Cohen et al. 1987. Danon et al. 1989). Here, we showed that neutrophils have positive effects on skin wound healing, at least in aged mice. Neutrophils likely contribute to the induction of inflammation. However, some reports suggest that neutrophils possess anti-inflammatory capacities. For example, Schroder AK et al. (Immunology 2006) established a cytokine profile of pure human neutrophils. The cells selectively produced anti-inflammatory interleukin-1 receptor antagonist (IL-1ra), but failed to produce pro-inflammatory cytokines IL-1β, IL-6 and TNFα. IL-1ra regulates the cytokine environment by interfering with the pro-inflammatory IL-1 system. Another report, which favors the anti-inflammatory effects of neutrophils, showed that IL-1RII was expressed by neutrophils and scavenges IL-1β by internalization (Bourke et al. J. Immunol. 2003). From their results, it is suggested that the secretion of IL-1ra by neutrophils in wound tissues modulates the inflammatory process by preventing chronic progression and assisting in down-regulating the inflammatory response.
We have shown that neutrophil depletion had less effects on wound healing in young mice. In young mice, the findings are quite similar to those done using anti-neutrophil serum (ANS) (Simpson and Ross 1972). Why, then, does neutrophil depletion delay wound healing in old mice? Aging causes multiple defects in PMN function, notably increased production of ROS, reduced chemotaxis (Fulop et al. 2004) and less efficient protection from apoptosis by pro-inflammatory mediators (Larbi et al. 2005). All these alteration might be caused by alterations of signal transduction pathways, which may be related to membrane fluidity (Fulop et al. 2004). It has been shown that changes in membrane fluidity affect PMN functions, such as chemotaxis and superoxide anion production (Yuli et al. 1982).
Currently, we hypothesize that neutrophils are necessary for early steps in wound healing. Neutrophils may produce superoxide anions or matrix metalloproteinases, which further degrade the damaged tissue and produce chemokines, which attract additional neutrophils and macrophages. Neutrophils also produce anti-inflammatory mediators such as IL-1ra, which down-regulate the inflammatory response. In young subjects, small numbers of neutrophils may be sufficient for wound repair or other cells may substitute for neutrophils. However, in old subjects, greater numbers of neutrophils may be necessary because the functions of neutrophils decrease and other cells are less able to replace neutrophils because of their own reduced functionality. Further studies, including analysis of the molecular mechanisms of neutrophil functions, and the combined works with bacterial infection in old mice are necessary for the understanding the pressure ulcers of elderly patients.
Acknowledgements
This work was supported by grants from the Ministry of Health, Labor, and Welfare of Japan.
Reference
- Ashcroft GS, Mills SJ, Ashworth JJ (2002) Ageing and wound healing. Biogerontology Rev 3(6):337–345 [DOI] [PubMed]
- Ballas CB, Davidson JM (2001) Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen 9(3):223–237 [DOI] [PubMed]
- Bourke E, Cassetti A, Villa A, Fadlon E, Colotta F, Mantovani A (2003) IL-1 beta scavenging by the type II IL-1 decoy receptor in human neutrophils. J Immunol 170(12):5999–6005 [DOI] [PubMed]
- Cohen BJ, Cutler RG, Roth GS (1987) Accelerated wound repair in old deer mice (Peromyscus maniculatus) and white-footed mice (Peromyscus leucopus). J Gerontol 42(3):302–307 [DOI] [PubMed]
- Danon D, Kowatch MA, Roth GS (1989) Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci USA 86(6):2018–2020 [DOI] [PMC free article] [PubMed]
- Dovi JV, He LK, DiPietro LA (2003) Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol 73:448–455 [DOI] [PubMed]
- Fulop T, Larbi A, Douziech N, Fortin CF, Guerard KP, Lesur O, Khalil A, Dupuis G (2004) Signal transduction and functional changes in neutrophils with aging. Aging Cell 3:217–226 [DOI] [PubMed]
- Grove GL, Kligman AM (1983) Age-associated changes in human epidermal cell renewal. J Gerontol 38:137–142 [DOI] [PubMed]
- Hellemans L, Corstjens H, Neven A, Declercq L, Maes D (2003) Antioxidant enzyme activity in human stratum corneum shows seasonal variation with an age-dependent recovery. J Invest Dermatol 120:434–439 [DOI] [PubMed]
- Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S (1996) Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 8(7):548–556 [DOI] [PubMed]
- Larbi A, Douziech N, Fortin CF, Linteau A, Dupuis G, Fulop T (2005) The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun Ageing 2(1):6 [DOI] [PMC free article] [PubMed]
- Leibovich SJ, Ross R (1975) The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 78(1):71–100 [PMC free article] [PubMed]
- Leibovich SJ, Ross R (1976) A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol 84(3):501–514 [PMC free article] [PubMed]
- Martin P (1997) Wound healing-aiming for perfect skin regeneration. Sci Rev 276(5309):75–81 [DOI] [PubMed]
- Ross R, Benditt EP (1962a) Wound healing and collagen formation. II. Fine structure inexperimental scurvy. J Cell Biol 12:533–551 [DOI] [PMC free article] [PubMed]
- Ross R, Benditt EP (1962b) Wound healing and collagen formation. III. A quantitative radioautographic study of the utilization of proline-H3 in wounds from normal and scorbutic guinea pigs. J Cell Biol 15:99–108 [DOI] [PMC free article] [PubMed]
- Schroder AK, von der Ohe M, Kolling U, Altstaedt J, Uciechowski P, Fleischer D, Dalhoff K, Ju X, Zenke M, Heussen N, Rink L (2006) Polymorphonuclear leucocytes selectively produce anti-inflammatory interleukin-1 receptor antagonist and chemokines, but fail to produce pro-inflammatory mediators. Immunology 119(3):317–327 [DOI] [PMC free article] [PubMed]
- Simpson DM, Ross R (1972) The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest 51(8):2009–2023 [DOI] [PMC free article] [PubMed]
- Swift ME, Burn AL, Gray KL, DiPietro LA (2001) Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117(5):1027–1035 [DOI] [PubMed]
- Yuli I, Tomonaga A, Synderman R (1982) Chemoattractant receptor functions in human polymorphonuclear leukocytes are divergently altered by membrane fluidizers. Proc Natl Acad Sci USA 79(19):5906–5910 [DOI] [PMC free article] [PubMed]