Abstract
Balance problems are often related to a loss of plantar-sensitivity in elderly people. The purpose of this study was to explore the contribution of plantar cutaneous inputs induced by a spike support surface to the control of stance. Nineteen elderly (mean age 69.0 years, range 62–80) and 19 young adults (mean age 25.9 years, range 21–32) were instructed to stand (standing session) or to walk (walking session) for 5 min with sandals equipped with spike insoles (spike condition). Both sessions also involved a no spike condition in which participants stood or walked for 5 min without these insoles (no spike condition). In all conditions, postural responses were assessed during unperturbed stance and were performed (1) immediately after putting the spike or the no spike insoles, and (2) 5 min after standing or walking with them. Sway parameters, such as centre of foot pressure mean location, surface area, mean speed, root mean square and median frequency on the antero-posterior and medio-lateral axes, were calculated. As postural performances are often related to plantar-surface sensitivity, cutaneous sensitivity threshold was also evaluated with Semmes–Weinstein monofilaments. Although no immediate effect of the spike insoles was found, results indicated that standing or walking for 5 min with sandals equipped with spike insoles led to a significant improvement of quiet standing in the elderly. Balance improvement was also observed in young adults. The results provided evidence that wearing sandals with spike insoles can contribute, at least temporarily, to the improvement of unperturbed stance in elderly people with relatively intact plantar cutaneous sensation. Further research is needed to assess the effects of longer and discontinuous stimulations with spike insoles on postural control.
Keywords: Elderly people, Foot sole stimulation, Plantar-surface sensitivity, Spike surface, Unperturbed stance
Introduction
It is now well established that plantar cutaneous information participates, among other sensory inputs (Tremblay et al. 2005; Vuillerme et al. 2007), to balance control (Maki et al. 1999; Nurse and Nigg 1999; Perry et al. 2000, 2001; Kavounoudias et al. 2001). Different types of mechanoreceptors [plantar-surface (PS) and deep receptors] are involved and are widely distributed under the foot sole (Kennedy and Inglis 2002). As the feet interface directly with the ground, cutaneous cues provide very detailed spatial and temporal information about the support surface properties, and about the variations of pressure under the feet that directly result from a shift of the centre of foot pressure (CoP) displacements (Maurer et al. 2001; Perry 2006). The plantar sole is a “dynamometric map” (Kavounoudias et al. 1998).
Experimentally, cooling (Eils et al. 2002; Eils et al. 2004), anesthetising (Do et al. 1990; Horak et al. 1990; Meyer et al. 2004a, b) or ischeming (Diener et al. 1984) the foot soles leads to a degradation of stability. Clinically, patients suffering from peripheral neuropathy (e.g. diabetics) generally exhibit an increase of postural sway. Several studies have also demonstrated that tactile sensation is age- and location-related (Wells et al. 2003; Perry 2006). Perry (2006) concluded that both vibratory and touch detection thresholds decline with age: The loss of cutaneous sensation correlated with an impaired control of balance and an increased likelihood of falling. Other authors showed that applying vibration (Dhruv et al. 2002; Priplata et al. 2003; Priplata et al. 2006) or rotary plantar massages (Bernard-Demanze et al. 2004) to the foot soles enhances both cutaneous sensation (Dhruv et al. 2002) and balance control (Maurer et al. 2001; Bernard-Demanze et al. 2004). Placing a raised edge underneath the perimeter of the plantar foot surface also facilitates postural stability (Maki et al. 1999).
Whereas reducing/suppressing or stimulating the plantar afferents are two relevant methods to explore the role of tactile messages in postural control, a third approach consists of changing the characteristics of the supporting surface (Watanabe and Okubo 1981; Maki et al. 1999; Maurer et al. 2001). The aim of the study was to reproduce, to some extent, the effects of a massage of the plantar sole, via a “mechanical” and continuous system that does not require a therapeutic intervention. An indented surface composed of spikes directly in contact with the foot sole may involve a somewhat “active” change of pressure distribution under the feet because of the reduction of the supporting surface directly in contact with the plantar soles. It is still unknown whether the spike insoles available on the market may benefit to postural stability, especially in the elderly. We particularly investigated: (1) the immediate and temporary effects of wearing these insoles; (2) whether the stimulation had a similar effect on postural control when participants were standing (i.e. with no movement of the spikes) or walking (i.e. with a movement of the spikes that could be assimilated to a massage); and (3) whether the stimulation had an effect on PS sensitivity. It was hypothesised that these insoles may improve both cutaneous sensation and postural stability in elderly.
Materials and methods
Participants
A total of 19 healthy elderly people (8 men and 11 women; mean age 69.0 years, range 62–80; mean height 167 ± 2 cm; mean weight= 74.4 ± 1.5 kg) and 19 healthy young adults (10 men and 9 women; mean age 25.9 years, range 21–32; mean height 171 ± 2 cm; mean weight 66.2 ± 2.6 kg) volunteered for this study. They were naive to the purpose of the study. Informed consent was obtained from each participant as required by the Helsinki declaration (1964) and the Local Ethics Committee. All elderly subjects were ambulatory and lived at home. They self-reported to be free from (1) any diagnosed neurological or musculoskeletal diseases (diseases potentially associated with a central or peripheral neuropathy), (2) any history of falls for the last 6 months, (3) any known balance impairment, and (4) any current use of medication that could affect their PS sensitivity or their balance.
Procedures
Participants were exposed to two testing sessions of 45 min that were performed at least 2 days apart: a standing and a walking session. Both sessions included two conditions—spike and no spike—which were counterbalanced across subjects.
-
Standing session
In the so-called standing session, subjects were instructed to stand (1) 5 min upright on the force-plate with sandals equipped with spike insoles (spike condition), and (2) 5 min with the sandals but without the spike insoles (no spike condition). In this latter condition, thin and flexible insoles were placed into the sandals to avoid the cutaneous contact with the spikes: These insoles (3 mm thick) without spikes did not modify the flexibility of the sandals. The 5-min duration was determined on the basis of pre-tests already showing an improvement after 5 min of wearing the spike insoles, and was chosen to minimise presumable effects of fatigue, especially in the elderly people.
-
Walking session
The same protocol as in the standing session was used except that participants walked between the different measures of postural stability.
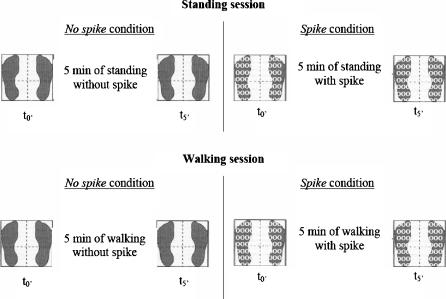
The sway tests were performed during unperturbed stance for both sessions. Participants stood on a force-plate (Equi+, model PF01; Aix les Bains, France) with eyes closed, arms at their sides and feet abducted at 30° with the medial borders of the heels separated by 5 cm. As vision is a predominant sensory system in the elderly (Perrin et al. 1997), and as no significant effect of plantar cutaneous inputs could be seen when vision was available (Meyer et al. 2004a), this information was suppressed in order to explore the influence of the cutaneous cues alone. Participants were asked to sway as little as possible. In each condition, three trials of 32 s with 15 s of standing rest inbetween were recorded (64 Hz sampling frequency) immediately after participants put the spike insoles on (t0’) and after 5 min (t5’) of standing or walking. The no spike condition was included to control that the temporary effects were not solely due to the action of standing or walking. To minimise fatigue or a decrease of attention, a sitting rest of 10 min was imposed between each condition (Fig. 1).
Fig. 1.
Diagram of the experimental protocol.  : measure of postural stability
: measure of postural stability
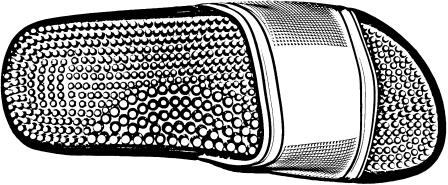
The footwear consisted of the Arena® NewMarco sandals (designed for pool activities). The entire insole was covered with an array of spikes made with semi-rigid PVC (density: 4 spikes/cm2; height of a spike: 5 mm; diameter: 3 mm) and uniformly distributed under the feet except on the medial arch where the spikes were bigger (density: 2 spikes/cm2; height: 1 cm; diameter: 5 mm) (Fig. 2). According to the manufacturer, the spikes enable the plantar soles to be massaged.
Fig. 2.
Spike sandals used in the study
Touch test
Fine-touch sensitivity was assessed by probing both feet at four different foot sole locations [great toe, first metatarsal head (MT), fifth MT, heel]. The six Semmes–Weinstein monofilaments (Touch test monofilaments, foot kit; Biomedix laboratories, France) used to determine the sensitivity threshold of the slow adapting receptors (Merkel cells and Ruffini endings) were 2.83, 3.61, 4.31, 4.56, 5.07 and 6.65 in evaluator size. The Meissner and the Pacinian corpuscles were not tested. The test was performed before (pre-test sensitivity) and after (post-test sensitivity) the 5 min spike condition of the standing session. Both assessments were done without insoles. Participants were seated blindfolded and were informed about the beginning of the testing period and stimulation localisation. Whereas the ascending and descending method of limits was used, they were instructed to indicate whenever stimulation was perceived. A maximum of three stimulations per monofilament was applied on each area. The sensitivity threshold was determined by the thinnest monofilament that was detected at least once.
Dependent variables
CoP motion was processed through mean CoP location (in mm), surface area (in mm2), mean speed (in mm.s-1), root mean square (RMS in mm) and median frequency (MF in Hz) on the antero-posterior (AP) and medio-lateral (ML) axes. All sway parameters can illustrate age-related changes in postural control (Melzer et al. 2003; Bernard-Demanze et al. 2004). Touch threshold (in monofilament size) was used to determine subjects’ variation of sensitivity across time.
Statistical analysis
A 2 ages (young adults and elderly) × 2 sessions (standing and walking) × 2 conditions (spike and no spike) × 2 times (t0’ and t5’) analysis of variance (ANOVA) with repeated measures on the last three factors was applied to (1) identify the immediate and temporary effects of the spike insoles, and (2) compare the effects between a standing and a walking stimulation. Post-hoc analyses (Tukey HSD) were used whenever necessary. In addition, Spearman R correlations were calculated to assess whether postural performance was associated with PS sensitivity. The level of significance was set at α = 0.05.
Results
Control of the mean position of the CoP along both medial–lateral and anterior–posterior axes
The analysis revealed neither main effect nor interaction on both medial–lateral (ML) (ps > 0.11) and anterior–posterior (AP) (ps > 0.09) axes, ruling out a possible effect of asymmetric (Genthon and Rougier 2005) or leaning (Rougier et al. 2001) postures.
Immediate and temporary effects of the spike insoles on postural control
Percentages of improvement due to wearing the spike sandals were calculated on all postural variables (except the mean CoP location). This improvement of postural stability was up to 55.8% and was observed in 8–18 young adults and in 10–14 elderly people depending on the postural variable (Table 1).
Table 1.
Improvement in percentage [Imp (%)] due to wearing the sandals, and the number of young adults and elderly persons (Number of subjects out of 19 for both groups) who improved their postural stability in the standing and walking session (means and SD)
| Young | Elderly | |||||||
|---|---|---|---|---|---|---|---|---|
| Standing session | Walking session | Standing session | Walking session | |||||
| Imp (%) | Number of subjects | Imp (%) | Number of subjects | Imp (%) | Number of subjects | Imp (%) | Number of subjects | |
| Surface area (mm2) | 11.6 | 9 | 55.8 | 15 | 26.7 | 14 | 22.7 | 13 |
| SD | 97.5 | 84.6 | 35.3 | 45.9 | ||||
| Speed (mm/s) | -3.1 | 10 | -0.2 | 18 | 10.6 | 10 | 3.9 | 10 |
| SD | 15.2 | 22.1 | 12.2 | 16 | ||||
| AP RMS (mm) | 0.6 | 10 | 9.8 | 15 | 16.3 | 11 | 2.7 | 10 |
| SD | 50.5 | 43.2 | 27.3 | 32.6 | ||||
| ML RMS (mm) | 3.9 | 8 | 9.1 | 13 | 10.6 | 12 | 18.4 | 14 |
| SD | 34 | 38.4 | 23.2 | 33.4 | ||||
| AP MF (Hz) | -1.1 | 10 | 0.5 | 11 | 1.2 | 14 | -0.7 | 14 |
| SD | 15.3 | 20.6 | 15.4 | 13.2 | ||||
| ML MF (Hz) | 3.0 | 11 | 2.1 | 12 | -0.6 | 13 | 1.8 | 12 |
| SD | 30.1 | 35.8 | 25.3 | 20.3 | ||||
A positive percentage involves an improvement, a negative percentage involves a degradation.
SD Standard deviation, AP anterior–posterior, ML medial–lateral, RMS root mean square, MF median frequency
The four-way interaction of age × session × condition × time was significant for the surface area (p = 0.027) and the AP RMS (p = 0.007). For the mean speed, results showed a significant three-way interaction of age × condition × time (p = 0.040). A two-way interaction of condition × time was significant for the ML RMS (p = 0.010). Whereas the analysis indicated no effect on the ML MF (ps > 0.07), a main effect of age was observed on the AP MF (p = 0.001) and on the ML RMS (p = 0.006): it was lower in the young adults.
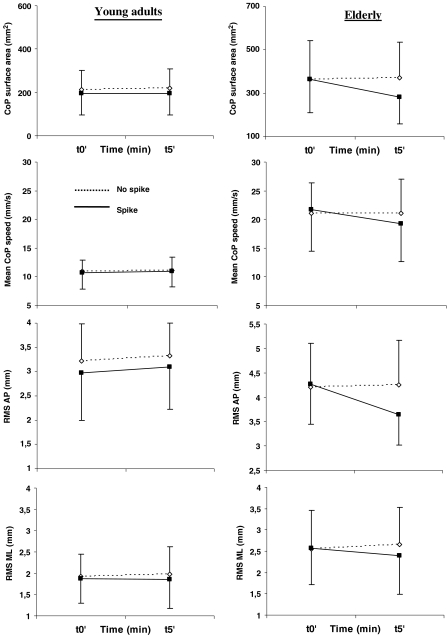
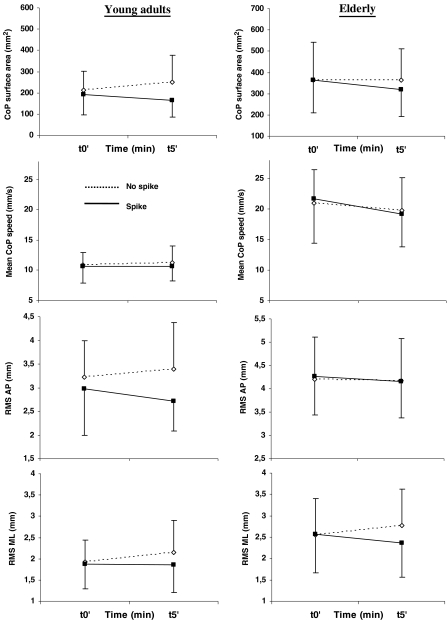
In the elderly, post-hoc analysis showed an improvement of postural control in both sessions. In the standing session, there was a decrease of the CoP surface area and the AP RMS between t0’ and t5’ of the spike condition (p = 0.001, p = 0.003, respectively) and between t5’ of both conditions (p = 0.001, p = 0.004, respectively). The decrease of the CoP surface area and the AP RMS was not significant in the walking session (ps > 0.342 and s > 0.99, respectively). However, and whatever the session, a significant difference of mean speed appeared in the spike condition between t0’ and t5’ of the spike condition (p = 0.005), with the lower values occurring at t5’ (10.6% of improvement in the standing session and 3.9% in the walking session). Lower values of ML RMS were also obtained at t5’ in the spike condition compared to the no spike condition (p = 0.036; 10.6% of improvement in the standing session and 18.4% in the walking session).
In the young adults, post hoc analysis indicated a small improvement of postural stability in the standing session. In fact, there was no significant effect on (1) the surface area (ps > 0.93), (2) the mean speed (ps > 0.99), or (3) the AP RMS (ps > 0.39). As for the elderly, there was solely a significant decrease of the ML RMS with lower values occurring at t5’ in the spike condition compared to the t5’ of the no spike condition (p = 0.036; 3.9% of improvement in the standing session and 9.1% in the walking session). More benefits appeared in the walking session with a decrease of (1) the CoP surface area between t5’ of both conditions (p = 0.001), (2) the AP RMS between t0’ and t5’ of the spike condition (p = 0.027) and between t5’ of both conditions (p = 0.001) ,and (3) the ML RMS (see above).
Comparison between the standing and the walking effect on postural control
When the four-way interaction appeared to be significant, the values of the standing and walking sessions observed at t5’ in the spike condition were compared to explore whether standing might be as beneficial as walking. As indicated above, no effect of session appeared for the mean speed and the ML RMS. For the surface area, the post hoc test showed no difference for both young adults (p = 0.795) and elderly (p = 0.581). However, whereas no significant difference was established for the AP RMS in the young adults (p = 0.278), the standing session led to lower AP RMS values than the walking session in elderly (p = 0.023) (Figs. 3 and 4).
Fig. 3.
Immediate and temporary effects of the spike insoles (i.e. 5 min) in the standing session. Left and right panels represent the evolution of the CoP surface area, mean speed, AP and ML RMS for the young adults and the elderly, respectively (means and SD). Refer to text to see significant effects or interactions. For illustration purposes, the ordinate scale of the graphs has been adapted so that its amplitude remains the same for the two populations
Fig. 4.
Immediate and temporary effects of the spike insoles (i.e. 5 min) in the walking session. Left and right panels represent the evolution of the CoP surface area, mean speed, AP and ML RMS for the young adults and the elderly, respectively (means and SD). Refer to text to see significant effects or interactions. For illustration purposes, the ordinate scale of the graphs has been adapted so that its amplitude remains the same for the two populations
Fine-touch sensitivity evolution in the standing session
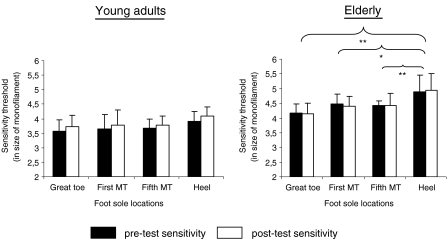
A 2 ages (young and old) × 2 times (pre-test and post-test) × 4 foot soles areas (great toe, first and fifth MT and heel) ANOVA with repeated measures on the last two factors was used to compare cutaneous sensitivity between the pre- and post-test in both populations. Although the two-way interaction of age × time was significant (p = 0.002), the post-hoc analysis revealed no difference between the overall pre-test and post-test sensitivity in the young adults (3.7 ± 0.4 and 3.8 ± 0.4, respectively; p = 0.187) or in the elderly (4.5 ± 0.6 and 4.5 ± 0.5, respectively; p = 0.991). Differences were observed between both populations at t0’ (p = 0.014) and was almost significant at t5’ (p = 0.075). Results also showed a significant two-way interaction of age × foot sole area (p = 0.046) and post-hoc analysis indicated that the heel was less sensitive than the great toe (p = 0.001), the first (p = 0.036) and the fifth MT (p = 0.033) in elderly, whatever the time: The mean sensitivity of the heel was 3.90 ± 0.35 for the young adults and 4.88 ± 0.60 for the elderly (Fig. 5).
Fig. 5.
Changes of sensation between the pre-test and post-test sensitivity for the young adults and the elderly. Note that the threshold values are log-transformed: the 2.83, 3.61, 4.31, 4.56, 5.07 and 6.65 monofilaments correspond respectively to a pressure perception threshold of 0.1, 0.4, 2.1, 3.6, 11.7 and 446.7 g. The higher the size of the monofilament, the smaller the fine-touch sensitivity. The significant values are reported (*p < 0.05, **p < 0.01)
Additionally, the relationship between the pre-test, post-test sensitivity (mean threshold of the four areas) and all postural variables was explored with a Spearman R correlation analysis in the standing session for each population. Results revealed that postural stability was not correlated with PS sensitivity, whatever the age group (ps > 0.06).
Discussion
The results provided evidence that wearing sandals with spike insoles can contribute, at least temporarily, to the improvement of unperturbed stance in the elderly with relatively intact plantar cutaneous sensation, and also in young adults. Immediately after participants put the spike insoles on they exhibited no adaptation. Interestingly, standing or walking for 5 min with these spike sandals led to a significant improvement of balance in both groups for the AP and ML planes. In the elderly, the effects were more pronounced in the standing than in the walking session. But the comparison of percentage of improvement showed moderate differences between both sessions except for the ML RMS (Table 1). Although the magnitude of the effects appeared to be small, some similar modest differences have already been shown to be predictive of falling risk (Maki et al. 1999). In young adults, modest benefits were observed in the standing session. However, the decrease of the surface area up to 55.8% and the moderate improvement of 9% of the AP and ML RMS observed in the walking session showed that they could also benefit from the spikes. Previous studies already indicated an improvement of postural control in young and elderly people when the foot soles were stimulated (Maki et al. 1999; Priplata et al. 2003). These results supported the hypothesis that the spikes provide relevant tactile information about body position in reference to verticality. As slow adapting receptors code the continuous pressures applied to their field (Kennedy and Inglis 2002), it can be suggested that the spikes are another indented surface that increased the body awareness and improved the spatial representation of the pressure distribution under the feet sole (Wu and Chiang 1997; Kavounoudias et al. 1998).
Since several studies demonstrated the relationship between PS sensitivity and postural control (Do et al. 1990; Maki et al. 1999; Eils et al. 2004), we expected that wearing spike insoles would enhance cutaneous sensation as well as postural stability in elderly people. The absence of correlation between PS sensitivity and postural control suggested that the spikes stimulated other receptors such as the deep ones. As indicated by Maurer (2001), PS receptors are mainly involved in the evaluation of the support surface whereas deep receptors contribute to the continuous control of CoP displacements. As we only tested the Merkel cells and Ruffini endings, vibratory testing at different frequencies is needed to check this assumption.
In conclusion, the present main findings suggested that standing or walking with the spike sandals enhance postural control in both young adults and the elderly. Further research is needed to investigate the evolution of postural sway while wearing regularly these insoles. As daily activities include standing, walking and also resting periods, it remains to determine whether being discontinuously stimulated by the spikes may have the same effect than continuous stimulation. In other words, we can wonder whether the benefits are lost immediately after taking off the sandals or whether these benefits remain over a longer period of time.
Limitations and perspectives
Potential limitations of the experiment pertain to the subjects’ selection, the quantification of postural performances, and statistical analyses. No advanced loss of sensitivity was observed in the elderly and may be an explanation for the lack of correlation between PS sensitivity and postural stability. There is a need to assess elderly people with loss of sensitivity.
The quantification of postural responses was only assessed during unperturbed stance. Although these measures give information about balance strategies, they do not provide clues about postural responses induced by perturbations. Using induced-sway tests in future studies may help to determine whether the spike insoles facilitate balance recovering. Additionally, it remains to determine whether the effects observed persist (1) when vision is available (although this blindfolded condition appears firstly not to be a real-life situation, it is close to situations with poor environmental lighting or with visual impairment), and (2) when normal shoes are used, especially during walking. Finally, the statistical methodology, by involving multiple comparisons with a relative small sample size, did not totally prevent from false positives. Even though the criterion level was set at α = 0.05, most of the probabilities of error were smaller than 0.01. Further research is probably needed to assess the generalization of the results.
Acknowledgments
The authors would like to thank all participants.
References
- Bernard-Demanze L, Burdet C, Berger L, Rougier P (2004) Recalibration of somesthesic plantar information in the control of undisturbed upright stance maintenance. J Integr Neurosci 3:433–451 [DOI] [PubMed]
- Dhruv NT, Niemi JB, Harry JD, Lipsitz LA, Collins JJ (2002) Enhancing tactile sensation in older adults with electrical noise stimulation. Neuroreport 13:597–600 [DOI] [PubMed]
- Diener HC, Dichgans J, Guschlbauer B, Mau H (1984) The significance of proprioception on postural stabilization as assessed by ischemia. Brain Res 296:103–109 [DOI] [PubMed]
- Do MC, Bussel B, Breniere Y (1990) Influence of plantar cutaneous afferents on early compensatory reactions to forward fall. Exp Brain Res 79:319–324 [DOI] [PubMed]
- Eils E, Nolte S, Tewes M, Thorwestern L, Völker K, Rosenbaum D (2002) Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech 35:1307–1313 [DOI] [PubMed]
- Eils E, Behrens S, Mers O, Thorwestern L, Völker K, Rosenbaum D (2004) Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20:54–60 [DOI] [PubMed]
- Genthon N, Rougier P (2005) Influence of an asymmetrical body weight distribution on the control of undisturbed upright stance. J Biomech 38:2037–2049 [DOI] [PubMed]
- Horak FB, Nashner LM, Diener HC (1990) Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82:167–177 [DOI] [PubMed]
- Kavounoudias A, Roll R, Roll J-P (1998) The plantar sole is a ‘dynamometric map’ for human balance control. Neuroreport 9:3247–3252 [DOI] [PubMed]
- Kavounoudias A, Roll R, Roll J-P (2001) Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol 532:869–878 [DOI] [PMC free article] [PubMed]
- Kennedy PM, Inglis JT (2002) Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J Physiol 538:995–1002 [DOI] [PMC free article] [PubMed]
- Maki BE, Perry SD, Norrie RG, McIlroy WE (1999) Effects of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. J Gerontol A Biol Sci Med Sci 54:M281–287 [DOI] [PubMed]
- Maurer C, Mergner T, Bolha B, Hlavacka F (2001) Human balance control during cutaneous stimulation of the plantar soles. Neurosci Lett 302:45–48 [DOI] [PubMed]
- Melzer I, Benjuya N, Kaplanski J (2003) Effects of regular walking on postural stability in the elderly. Gerontology 49:240–245 [DOI] [PubMed]
- Meyer PF, Oddsson LI, De Luca CJ (2004a) The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156:505–512 [DOI] [PubMed]
- Meyer PF, Oddsson LI, De Luca CJ (2004b) Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res 157:526–536 [DOI] [PubMed]
- Nurse MA, Nigg BM (1999) Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech 14:667–672 [DOI] [PubMed]
- Perrin PP, Jeandel C, Perrin CA, Bene MC (1997) Influence of visual control, conduction, and central integration on static and dynamic balance in healthy older adults. Gerontology 43:223–231 [DOI] [PubMed]
- Perry SD (2006) Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci Lett 392:62–67 [DOI] [PubMed]
- Perry SD, McIlroy WE, Maki BE (2000) The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res 877:401–406 [DOI] [PubMed]
- Perry SD, Santos CS, Patla AE (2001) Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res 913:27–34 [DOI] [PubMed]
- Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ (2003) Vibrating insoles and balance control in elderly people. Lancet 362:1123–1124 [DOI] [PubMed]
- Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ (2006) Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol 59:4–12 [DOI] [PubMed]
- Rougier P, Burdet C, Farenc I, Berger L (2001) Backward and forward leaning postures modelled by an fBm framework. Neurosci Res 41:41–50 [DOI] [PubMed]
- Tremblay F, Mireault AC, Dessureault L, Manning H, Sveistrup H (2005) Postural stabilization from fingertip contact II. Relationships between age, tactile sensibility and magnitude of contact forces. Exp Brain Res 164:155–164 [DOI] [PubMed]
- Vuillerme N, Pinsault N, Chenu O, Boisgontier M, Demongeot J, Payan Y (2007) How a plantar pressure-based, tongue-placed tactile biofeedback modifies postural control mechanisms during quiet standing. Exp Brain Res 181:547–554 [DOI] [PubMed]
- Watanabe I, Okubo J (1981) The role of the plantar mechanoreceptor in equilibrium control. Ann NY Acad Sci 374:855–864 [DOI] [PubMed]
- Wells C, Ward LM, Chua R, Inglis JT (2003) Regional variation and changes with ageing in vibrotactile sensitivity in the human footsole. J Gerontol A Biol Sci Med Sci 58:680–686 [DOI] [PubMed]
- Wu G, Chiang JH (1997) The significance of somatosensory stimulations to the human foot in the control of postural reflexes. Exp Brain Res 114:163–169 [DOI] [PubMed]