Abstract
The relationship between mental exercise and mental aging is a controversial issue. People generally believe the so-called mental-exercise hypothesis, that is, the age-related decline in cognitive function is less pronounced for people who are mentally active, yet there is insufficient scientific evidence supporting this hypothesis. Previous randomized controlled trial studies showed convincing beneficial effects of cognitive training on directly targeted cognitive functions. In this study, we performed a single-blind, randomized controlled trial on cognitive intervention in 124 community-dwelling seniors (age range, 70 to 86) and estimated the beneficial effects of non-targeted cognitive functions. As for cognitive intervention, the subjects were asked to solve systematized basic problems in reading and arithmetic every day for 6 months. Neuropsychological measures were determined prior to and 6 months after the intervention (post-test) by mini-mental state examination (MMSE), frontal assessment battery at bed side (FAB), and digit-symbol substitution test (DST) of WAIS-R. The FAB and DST scores showed a statistically significant (p<0.001 and p<0.01, respectively) improvement in the post-test compared with the pre-test, such improvement was maintained up to 6 months of follow-up tests in only the experimental group. The transfer effect of cognitive intervention by reading and solving arithmetic problems on non-targeted cognitive functions was demonstrated in this study. This study shows that daily mental training can improve cognitive functions in normal adults. Although general interests in brain training have been increasing in the public, evidence for its beneficial effects, particularly the positive transfer effect on non-targeted cognitive function still remains insufficient. Here, we introduce a new cognitive intervention program for normal aged people, the concept of which is derived from the knowledge of both brain science and clinical studies. We performed a carefully designed single-blind, randomized controlled study, and the results of this study showed convincing evidence that cognitive training provides the beneficial transfer effect.
Keywords: Prefrontal cortex, Daily intervention, Randomized controlled study, Reading, Arithmetic
Introduction
It has been argued that the age-related decline in a wide variety of cognitive measures begins at the ages of 20s or 30s (Salthouse 2006), but most people become aware of losses in cognitive functions when they are in their 60s (Fillit et al. 2002; Ritchie et al. 2001). The exception to this pattern is for variables with a large knowledge component, such as measures of vocabulary (Salthouse 2006). Recently, cognitive training has generally received increasing interest as a solution to such an age-related cognitive decline. Although the general public’s interest in cognitive or brain training is increasing, scientists doubt the effect of cognitive training, particularly the generalizing or transfer effect of such training (see Editorial comment, Nature Neurosci 10:263, 2007).
Salthouse (2006) reviewed previous studies of cross-sectional comparisons between the amount of mental activity and mental aging and summarized that the preventive effect of mental stimulation on age-related cognitive decline is not very consistently observed in these studies. However, the results of previous randomized controlled trial studies suggest that cognitive training improves not only directly targeted cognitive functions but also daily cognitive functions. Mahncke et al. (2006) asked community-dwelling people to perform an hour-long training in auditory recognition, discrimination, and memory tasks for 5 days a week for 8 to 10 weeks using computer programs at home. Those in the experimental group showed improvement in cognitive functions in directly trained tasks as well as non-targeted global memory test after the training. Willis and colleagues performed a randomized controlled study named ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly) with the largest sample size. In their study, 2,832 independently living persons were asked to perform ten sessions of memory, reasoning, or processing speed tasks, and four sessions of booster training 11 and 35 months after the initial training. They found that targeted cognitive functions improved with each session (Ball et al. 2002), that such an improved performance continued until, at least, 5 years later (Willis et al. 2006), and that only the training reasoning resulted in less functional decline in self-reported instrumental activities of daily living (Willis et al. 2006). The results of the two studies showed convincing evidence that cognitive training has beneficial effects, at least, on cognitive functions related to training tasks. Nevertheless, it remains unclear whether cognitive training has a positive transfer effect on other cognitive functions necessary for improving performance in real-life tasks and independent living.
Previously, we introduced a new cognitive intervention program for senile dementia, the concept of which is derived from the knowledge of both brain science and clinical studies, named learning therapy (Kawashima et al. 2005). Learning therapy has been developed to stimulate the cognitive functions of the dorsolateral prefrontal cortex, as well as those of the temporal and parietal association cortices. We prepared two tasks in arithmetic and Japanese language, which were systematized basic problems in arithmetic and reading, respectively, for daily training. Results of brain imaging studies indicate that reading sentences or words aloud (Hagoort et al. 1999; Herbster et al. 1997; Miura et al. 2003, 2005; Price et al. 1994; Wingfield and Grossman 2006) and simple arithmetic operations (Burbaud et al. 1995; Dehaene et al. 1996; Kawashima et al. 2004; Menon et al. 2000; Rickard et al. 2000; Roland and Friberg 1985; Rueckert et al. 1996) activate the three association cortices, including the dorsolateral prefrontal cortex, of the bilateral hemispheres of humans. Reading aloud is accomplished by a combination of several cognitive processes, for example, recognition of visually presented words, conversion to phonological representation from graphic representation of words, analysis of the meaning of words, and control of pronunciation. In particular, older adults show bilateral activation of an associated network of brain regions that support working memory when they comprehend long or syntactically complex sentences (Wingfield and Grossman 2006). Solving arithmetic problems is also accomplished by many cognitive processes, for example, recognition of visually presented numbers, arithmetic operations, and control of hand movements, and the bilateral prefrontal cortices are activated even when solving very simple and easy problems. Both reading aloud and solving arithmetic problems require working memory, and this prefrontal stimulation may lead to the positive transfer effect on other cognitive functions. In addition, both reading aloud and solving arithmetic problems can be very simple and easy, so that even people with senile dementia can understand, perform, and continue the tasks prepared. A comparison between the randomly assigned intervention and control groups revealed that not only frontal functions but also functions associated with communication and independence improved in the intervention group (Kawashima et al. 2005). Our results suggest that a daily cognitive intervention by reading and solving arithmetic problems improves non-targeted cognitive functions.
In the present study, we applied a similar daily cognitive training to the study of community-dwelling people to determine the effects of training on cognitive functions, particularly on the function of the prefrontal cortex, by a single-blind randomized controlled trial. The differences between this study and our previous study of daily intervention in senile dementia (Kawashima et al. 2005) are the lowest level of difficulty of problems and place of learning. In the previous study, the lowest levels were set as the counting practice for the arithmetic problems, and reading and writing single syllables for the Japanese language problems, since dementia patients were the subject. In the present study, the lowest levels were set as single-digit addition and reading and writing simple sentences for the arithmetic and language problems, respectively. In the previous study, the daily learning was carried out approximately 5 days a week at a learning center. However, in the present study, the subjects were asked to go to the learning center 1 day a week and to do their homework for 4 days a week at their home. The effects of daily cognitive training intervention by reading and solving arithmetic problems on the cognitive functions of the dorsolateral prefrontal cortex in seniors were determined in this study as the primary goal. No change in task-specific performance, that is, the ability to solve reading and arithmetic problems, was deserved in this study.
Method
Participants
Our study population comprised community-dwelling people who were living in the Tsurugaya district of Sendai, Japan. In the first phase of the survey, we mailed information regarding the present study to all district residents aged 70 years or over (total, 3,136: 1,301 men, and 1,853 women). We invited all these individuals to participate in a comprehensive geriatric assessment, which included the assessment of medical and dental statuses, physical functions and cognitive functions. This assessment program is called the Tsurugaya Study (Hozawa et al. 2004; Koizumi et al. 2005; Taki et al. 2005). To the 962 individuals enrolled in the Tsurugaya Study, we distributed leaflets describing the purpose and plan of this study, among which 269 applied to participate in the present study. The inclusion criteria were a mini-mental state examination (MMSE) (Folstein et al. 1975) score greater than 23 and a Geriatric Depression Scale (GDS) score less than 15. Applicants who had a history of cerebrovascular diseases, who were active in another cognitive-related training, and/or who were unavailable during the study period were excluded. Finally, 124 participants who satisfied the inclusion and exclusion criteria were allocated into either the experimental group (n=62) or the control group (n=62) by random drawing using a computer. In the case that the participants were a couple, each was allocated into the same group. The random allocation sequence was concealed until intervention was assigned. During the intervention period, 11 and 15 participants in experimental and control groups, respectively, withdrew from the study for the following reasons: scheduling conflict, poor health, participation in another active cognitive training program, and lack of interest in continuing. During the 6-month follow-up period after the intervention period, two and one subjects of the experimental and control groups, respectively, withdrew owing to a lack of interest in continuing.
In this study, the participants in the control group had no social contact with the experimenters. We did not designate a placebo social contact group, since results of previous intervention studies (Mahncke et al. 2006; Clark et al. 1997) suggest that a placebo group is unnecessary for this type of study since there is no difference in cognitive or functional improvement between the placebo and no-social-contact groups. During 6-month follow-up period, the subjects of the control group underwent exactly the same intervention program as those of the experimental group.
Both the intervention and control groups were assessed at the same time prior to the intervention (pre) and immediately after a 6-month intervention (post). The protocol of this study was approved by the Institutional Review Board of the Tohoku University Graduate School of Medicine. Written informed consent was obtained from each participant.
Cognitive intervention
The method of cognitive intervention was similar to that of the cognitive training for senile dementia patients previously used by our group (Kawashima et al. 2005). The tasks used for the cognitive intervention involved solving arithmetic and Japanese language problems, which were systematized basic problems in arithmetic and reading, respectively. We prepared a wide range of materials, which were used in everyday classes of first- to fourth-grade elementary school students. The problems were printed on both sides of an A4-size paper (210×297 mm). As for the arithmetic problems, the lowest level of difficulty was single-digit addition, and the highest level was three-digit division. As for the Japanese language problems, the lowest level of difficulty was reading and writing simple sentences, and the highest level was reading fairy tales aloud. A week prior to the start of the intervention program, subjects of the experimental group were asked to go to classed in two elementary schools near their place of residence, and the appropriate level of difficulty and workload for all the subjects were assessed by diagnostic tests. The diagnostic tests consisted of 70 arithmetic and 16 language problems. The difficulty levels of the arithmetic problems ranged from single-digit addition to three-digit division. Those of language problems ranged from reading and comprehending Japanese Haiku (17 characters) to that of stories (110 characters). For both types of tests, the percentage of correct answers and the time it took to solve all problems were determined. The subjects were divided into three groups according to their performance determined from these two parameters as follows: group A, 100% correct answers and less than 5 min; group C, less than 90% correct answer or more than 10 min; group B, others. The difficulty levels of the problems for each group are summarized in the Table 1. The difficulty level and workload of each task were set such that each subject was able to solve the problems with ease and without mental stress within 15 min.
Table 1.
Difficulty levels of arithmetic and language problems
| Lowest level (initial) | Maximum level | |
|---|---|---|
| Language problems | ||
| Group A | Sentences (40-50 chars. / page) | Sentences (100-120 chars. / page) |
| Group B | Single sentence (30-35 chars. / page) | Sentences (70-80 chars. / page) |
| Group C | Japanese Haiku (17 chars. / page) | Sentences (40-50 chars. / page) |
| Arithmetic problems | ||
| Group A | Single-digit addition with advancement | Three-digit division |
| Group B | Single-digit addition without advancement | Three-digit subtraction |
| Group C | Single-digit addition without advancement | Two-digit subtraction |
The subjects were divided into three groups according to the results of the diagnosis tests
The cognitive intervention was conducted for 23 weeks from October of 2003 to March of 2004. The participants in the experimental group were asked to go to classes in two elementary schools once a week. They were then instructed to complete five sheets of each task prepared for each individual for that day, which were then assessed by the staff. Mistakes were corrected by the participants themselves. The study period ended when the participants completed each of the problems correctly. The daily learning time for the two tasks was approximately 15 min. The intervention was on individual basis, so that the subjects were able to decide how to use their learning time of 15 min freely. The subjects were also asked to do their homework of two tasks for 4 to 6 days a week. As for their homework, the subjects were asked to complete five sheets of each task prepared for each individual. The subjects were asked to bring back their achievements of homework on the next school day, and the experimenters checked their homework and provided advices when necessary.
Assessment
Since the target of our intervention was to improve general brain functions by stimulating the dorsolateral prefrontal cortex, the efficacy of the intervention was measured using the Frontal Assessment Battery at bedside (FAB) (Dubois et al. 2000) and the digit symbol substitution test (DST) of WAIS-R (Wechsler 1981). The FAB consists of six subsets exploring different functions related to the frontal lobes, and originally designed for validating the frontal lobe function in neurodegenerative diseases. The internal consistency (Cronbach’s coefficient alpha) of FAB is 0.78 (Dubois et al. 2000). DST is a sensitive subtest for screening brain dysfunction (Lezak et al. 2004), and the score in this test strongly correlates with the age of subjects (e.g., Hoyer et al. 2004; Wielgos and Cunningham 1999). The first assessment (pre-test) was performed 2 weeks prior to the start of intervention at a community center of their place of residence. Then, the intervention continued for 23 weeks. A week after the end of intervention, the subjects were asked to go to the community center for the second assessment (post-test). All the subjects belonging to both experimental and control groups were assessed at the same time and the place. The assessors did not know whether the participants were allocated to the experimental or control group. A paired t-test for each variable was used to analyze the efficacy of the intervention. Additionally, to confirm the difference between the two groups, a two-sample t-test for each variable was conducted for the pre-test and post-test. Statistical significance was set at p<0.05 for all comparisons. Since the purpose of this study was to determine the effects of our cognitive intervention, the data analysis was focused on comparing neuropsychological measures of the pre- and post-tests for within and between groups of the participants who completed the assessment batteries.
In addition, it has generally been argued that the longevity of changes after the intervention is important in evaluating the effect of the program (Salthouse 2006; Mahncke et al. 2006); thus, we asked the participants in the experimental group as well as the control group to come back 26 weeks after the post-test for follow-up assessment (follow-up). After the 6-month intervention, the subjects in the experimental group had no social contact with the experimenters for 6 months. During the 6-month follow-up period after the intervention period, the subjects in the control group performed the same intervention. This cognitive intervention was conducted for 23 weeks from April 2004 to September 2004.
Results
There were 51 and 47 participants in the experimental and control groups, respectively, who continued participating in further analysis. In the experimental group, 49 (96.0%) participants continued the cognitive intervention for 7 days a week for 6 months; the rest continued for 5 days a week for 6 months. Mean age, sex, the number of years of education, marriage status, and the scores in MMSE, GDS, FAB and DST showed no statistically significant differences between the experimental group and the control group (Table 2).
Table 2.
Neuropsychological characteristics of participants at the pre-test
| Experimental group N=51 | Control group difference N=47 | P value (t-value) | |
|---|---|---|---|
| Mean age | 75.2 (3.8) | 75.6 (4.2) | p=0.62 (t=0.49) |
| Age range | 70 to 85 | 70 to 86 | |
| Women % | 51.0 | 38.3 | p=0.16 (t=1.42) |
| Years of education | 12.9 (3.9) | 12.5 (3.3) | p=0.64 (t=0.46) |
| Married % | 54.9 | 61.8 | p=0.54 (t=0.62) |
| Mean MMSE | 28.9 (1.4) | 28.6 (1.4) | p=0.36 (t=0.92) |
| Mean GDS | 5.34 (2.8) | 6.16(3.4) | p=0.15 (t=1.44) |
| Mean FAB | 13.9 (2.3) | 13.9 (2.4) | p=0.98 (t=0.10) |
| Mean DST | 43.5 (9.8) | 41.6 (8.6) | p=0.30 (t=1.05) |
Numbers in the parenthesis indicate SD. Statistical analysis was done by Student t-test (two-tailed). Degree of freedom is 96 for each t-test
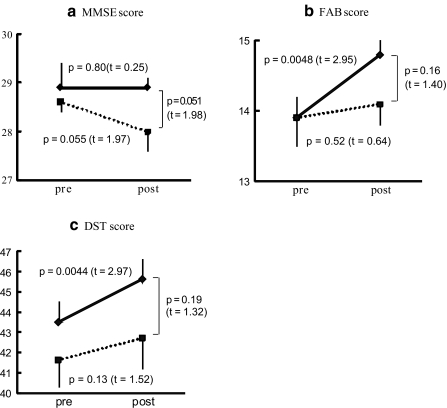
The scores of the neuropsychological characteristics in the pre- and post-tests are summarized in Fig. 1. In the experimental group, the FAB and DST scores showed a statistically significant (p<0.001) increase after the intervention. However, the MMSE score showed no significant changes. In the control group, the FAB and DST scores showed no significant changes. The MMSE score decreased in the post-test compared with in the pre-test. However, the decrease did not reach a statistically significant level (p=0.055). In the post-test, the MMSE score of the experimental group was higher than that of the control group. However, the difference did not reach a statistically significant level (p=0.05). The DST score showed no significant difference between the experimental and control groups in the post-test.
Fig. 1.
Changes of neuropsychological measures.Solid and dotted lines indicate experimental and control groups, respectively. Pre and Post indicate pre-test and post-test, respectively. The FAB and DST scores showed a statistically significant increase in the post-test compared with in the pre-test only the experimental group. Degree of freedom is 50 and 47 for each t-test of experimental and control groups, respectively
The scores of the individual items of FAB in the pre- and post-tests are summarized in Table 3. In the experimental group, the scores for lexical fluency (mental flexibility) and conflicting instructions (sensitivity to interference) showed statistically significant (p<0.01 and p<0.05, respectively) increases in the post-test compared with the pre-test (paired t-test). In the control group, none of scores showed significant differences between the pre- and post-tests (paired t-test). In the pre-test, none of the scores showed significant differences between the experimental and control groups. In the post-test, the lexical fluency (mental flexibility) score of the experimental group was significantly higher than that of the control group (p=0.058, t=1.91, degrees of freedom=96).
Table 3.
Mean sub scores for FAB
| Experimental group | Control group | |||||
|---|---|---|---|---|---|---|
| Pre-test | Post-test | P-value(t-value) | Pre-test | Post-test | P-value(t-value) | |
| Similarities (conceptualization) | 1.6 (0.9) | 1.9 (0.8) | 0.083 (t=1.77) | 1.7 (0.8) | 1.9 (0.8) 0.086 | (t=1.76) |
| Lexical fluency (mental flexibility) | 2.0 (0.7) | 2.3 (0.6) | 0.0048 (t=2.94) | 1.9 (0.8) | 2.0 (0.7) | 0.44 (t=0.78) |
| Motor seriesz (programming) | 2.6 (0.6) | 2.8 (0.6) | 0.11 (t=1.64) | 2.7 (0.6) | 2.6 (0.7) | 0.72 (t=0.36) |
| Conflicting instructions (sensitivity to interference) | 2.7 (0.7) | 2.9 (0.3) | 0.026 (t=2.29) | 2.8 (0.6) | 2.8 (0.6) | 0.67 (t=0.42) |
| Go-No Go (inhibitory control) | 1.9 (1.0) | 2.0 (1.0) | 0.89 (t=0.14) | 1.9 (1.0) | 1.8 (0.5) | -0.44 (t=0.78) |
| Prehension behavior (environmental autonomy) | 3.0 (0.2) | 3.0 (0.0) | 0.16 (t=1.42) | 3.0 (0.1) | 3.0 (0.0) | 0.32 (t=0.16) |
Numbers in parenthesis are S.D. Statistical analysis was done by paired t-test (two-tailed). Degree of freedom is 50 and 46 for each t-test of experimental and control groups, respectively
In the follow-up, that is, 6 months after the training period, the scores of all neuropsychological measures of the experimental group showed no changes compared with those in the post-test (Table 4). Compared with the pre-test, FAB and DST scores of the experimental group still showed statistically significant (p<0.001) increases in the follow-up. In the control group, the FAB and DST scores showed statistically significant increases in the follow-up (after undergoing the intervention) compared with the pre-test (Table 5).
Table 4.
Changes of neuropsychological characteristics of experimental group (N=49)
| P-value (t-value) | |||||
|---|---|---|---|---|---|
| Pre-test | Post-test | Follow-up | vs. Pre | vs. Post | |
| MMSE | 28.9 (S.D. 1.4) | 28.9 (S.D. 1.6) | 28.7 (S.D. 1.5) | 0.39 (t=0.87) | 0.44 (t=0.78) |
| FAB | 13.9 (S.D. 2.3) | 14.9 (S.D. 1.7) | 15.1 (S.D.1.7) | 0.00028 (t=4.50) | 0.37 (t=0.91) |
| DST | 43.3 (S.D. 9.9) | 45.2 (S.D. 11.9) | 46.0 (S.D. 10.3) | 0.0045 (t= 4.21) | 0.26 (t=1.14) |
P-value is estimated by paired t-test (two-tailed) compared with follow-up versus pre-test or Post-test. Degree of freedom is 48 for each t-test
Table 5.
Changes of neuropsychological characteristics of control group (N=46)
| P-Value (t-value) | |||||
|---|---|---|---|---|---|
| Pre-test | Post-test | Follow-up | vs. Pre | vs. Post | |
| MMSE | 28.7 (S.D. 1.3) | 28.3 (S.D. 1.5) | 28.4 (S.D. 1.6) | 0.18 (t=-1.35) | 0.85 (t=0.19) |
| FAB | 14.1 (S.D. 2.1) | 14.3 (S.D. 2.2) | 14.8 (S.D.1.9) | 0.013 (t=2.57) | 0.057 (t=1.95) |
| DST | 42.2 (S.D. 7.7) | 43.2 (S.D. 9.5) | 44.5 (S.D. 10.8) | 0.0042 (t=3.02) | 0.11 (t=1.62) |
P-value is estimated by paired t-test (two-tailed) compared with follow-up versus pre-test or post-test. Degree of freedom is 45 for each t-test
Discussion
The results of this single-blind randomized controlled trial showed convincing immediate beneficial effects of a daily training program involving reading and solving arithmetic problems on the speed of processing (as measured by DST) and executive function (as measured by FAB) that are not directly tied to the intervention. Such improvements were maintained 6 months after the training.
In this study, a significant improvement in neuropsychological measures was found in terms of the FAB and DST scores of the experimental group. FAB is a cognitive and behavioral battery of tests for assessing frontal lobe functions (Dubois et al. 2000). According to a recent study of an Italian population (Appollonio et al. 2005), the mean FAB scores of 70–79 and 80–89 years old were 15.5 and 13.8, respectively. Our FAB scores seem to be consistent with these data. In this study, significant increases were observed in lexical fluency and sensitivity to interference subscores of FAB in the experimental group. The lexical fluency task requires a self-organized retrieval from semantic memory. The improvement in lexical fluency could be a direct effect of cognitive training, since continuous training by reading problems increases semantic knowledge. The sensitivity to an interference task requiring behavioral self-regulation under verbal commands is in conflicts with sensory information. This task is similar to the Stroop test in which the executive function is a key cognitive function to perform.
The DST of WAIS-R (Wechsler 1981) is a sensitive subtest for screening general brain dysfunction (Lezak et al. 2004). In previous studies, the performance in DST has a strong negative correlation with the age of normal subjects (e.g., Salthouse 2006; Hoyer et al. 2004; Wielgos and Cunningham 1999). Studies examining correlations between DST score and those of neuropsychological measurements suggested that perceptual speed is the key function for executing DST (Joy et al. 2003; Kreiner and Ryan 2001) and working memory also plays a subsidiary role (Joy et al. 2003). Since perceptual speed and working memory are not directly trained by our intervention, our results support evidence of the improvement in non-targeted cognitive functions.
Our intervention program is based on a daily training system. Other previous cognitive intervention studie used a relatively short-term intervention compared with the present study, and also found a beneficial effect on cognitive functions during long-term monitoring. Previous intervention studies by Mahncke et al. (2006) and the ACTIVE trial (2002) showed the convincing beneficial effect of improving cognitive functions. There is no doubt that the brain has a lifelong capacity for plasticity, and results of many clinical studies of humans and experimental studies of animals support the idea that a continuous stimulation of the brain causes changes in the brain (Merzenich and Jenkins 1993). Therefore, it is reasonable to induce changes in the brain networks using such a type of daily intervention program. On the other hand, other cognitive intervention studies have used a relatively short-term intervention, and also found a beneficial effect on cognitive functions during long-term monitoring. For example, in the Seattle Longitudinal Study (Wills and Schaie 1986), seniors received just 5 h of training in mental tasks, and a beneficial effect on targeted cognitive functions was reported 7 years after the intervention. This result could not be directly related to brain plasticity through cognitive training, and can probably be caused by changes in lifestyle through knowledge gained through training. The sustained beneficial effects on non-directly targeted cognitive functions found in this study and in other intervention studies (Mahncke et al. 2006; Willis et al. 2006) may be related to the same mechanism. Although, we found a sustained high cognitive activity 6 months after the intervention, further estimation of the longevity of the intervention-induced improvement is necessary.
In conclusion, we introduced a new cognitive intervention program for normal aged people, and demonstrated that our daily training program involving reading and arithmetic problems was effective for the improvement of cognitive functions of community-dwelling elderly. The results of this study are convincing evidence that cognitive training has the beneficial effects of maintaining and improving cognitive functions.
Acknowledgements
We thank Professor Ichiro Tsuji at the Graduate School of Medicine, Tohoku University, for suggestion and collaboration for experimental setup and design. We also thank all stuff members involved in “Tsurugaya Project”, and Kumon Learning Center c/o for technical support for cognitive measures and intervention. This study was supported by RISTEX, JST, and a collaborative study between Tohoku University and Sendai City (Gakuto collaboration research project).
References
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005) The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci 26:108–116 [DOI] [PubMed]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL (2002) Effects of cognitive training interventions with older adults. JAMA 288:271–2281 [DOI] [PMC free article] [PubMed]
- Burbaud P, Degreze P, Lafon P, Franconi JM, Bouligand B, Bioulac B, Caille JM, Allard M (1995) Lateralization of prefrontal activation during internal mental calculation: a functional magnetic resonance imaging study. J Neurophysiol 74:194–2200 [DOI] [PubMed]
- Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, Hay J, Josephson K, Cherry B, Hessel C, Palmer J, Lipson L (1997) Occupational therapy for independent-living older adults: a randomized controlled trial. JAMA 278:1321–1326 [DOI] [PubMed]
- Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B (1996) Cerebral activations during number multiplication and comparison: a PET study. Neuropsychol 34:1097–1106 [DOI] [PubMed]
- Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB. A frontal assessment battery at bedside. Neurol 55:1621–1626 [DOI] [PubMed]
- Fillit HM, Butler RN, O’Connell AW, Albert MS, Birren JE, Cotman CW, Greenough WT, Gold PE, Kramer AF, Kuller LH, Perls TT, Sahagan BG, Tully T (2002) Achieving and maintaining cognitive vitality with aging. Mayo Clinic Proceedings 77:681–696 [DOI] [PubMed]
- Folstein MF, Folstein S, Mchugh PR (1975) Mini Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 121:189–198 [DOI] [PubMed]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ (1999) The neural circuitry involved in the reading of German words and pseudowords: a PET study. J Cognit Neurosci 11:383–398 [DOI] [PubMed]
- Herbster AN, Mintun MA, Nebes RD, Becker JT (1997) Regional cerebral blood flow during word and nonword reading. Hum Brain Map 5:84–92 [DOI] [PubMed]
- Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P (2004) Adult age and digit symbol substitution performance: a meta-analysis. Psychol Aging 19:211–214 [DOI] [PubMed]
- Hozawa A, Ebihara S, Ohmori K, Kuriyama S, Ugajin T, Koizumi Y, Suzuki Y, Matsui T, Arai H, Tsubono Y, Sasaki H, Tsuji I (2004) Increased plasma 8-isoprostane levels in hypertensive subjects: the Tsurugaya Project. Hypertension Res 27:557–561 [DOI] [PubMed]
- Joy S, Fein D, Kaplan E (2003) Decoding digit symbol: speed, memory, and visual scanning. Assessment 10:56–65 [DOI] [PubMed]
- Kawashima R, Taira M, Okita K, Inoue K, Tajima N, Yoshida H, Sasaki T, Sugiura M, Watanabe J, Fukuda H (2004) A functional MRI study of simple arithmetic: a comparison between children and adults. Cognit Brain Res 18:225–238 [DOI] [PubMed]
- Kawashima R, Okita K, Yamazaki R, Tajima N, Yoshida H, Taira M, Iwata K, Sasaki T, Maeyama K, Usui N, Sugimoto K (2005) Reading aloud and arithmetic calculation improve frontal function of people with dementia. J Gerontol A Biol Sci Med Sci 60A:426–431 [DOI] [PubMed]
- Koizumi Y, Awata S, Kuriyama S, Ohmori K, Hozawa A, Seki T, Matsuoka H, Tsuji I (2005) Association between social support and depression status in the elderly: results of a 1-year community-based prospective cohort study in Japan. Psychiatry Clin Neurosci 59:563–569 [DOI] [PubMed]
- Kreiner DS, Ryan JJ (2001) Memory and motor skill components of the WAIS-III Digit Symbol-Coding subtest. Clin Neuropsychol 15:109–113 [DOI] [PubMed]
- Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment, 4th edn. Oxford University Press, New York
- Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM (2006) Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Nat Acad Sci USA 103:12523–12528 [DOI] [PMC free article] [PubMed]
- Menon V, Rivera SM, White CD, Glover GH, Reiss AL (2000) Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 12:357–365 [DOI] [PubMed]
- Merzenich MM, Jenkins WM (1993) In: Andersen P (ed) Memory concepts. Elsevier, Amsterdam, pp 437–453
- Miura N, Iwata K, Watanabe J, Sugiura M, Akitsuki Y, Sassa Y, Ikuta N, Okamoto H, Watanabe Y, Riera J, Maeda Y, Matsue Y, Kawashima R (2003) Cortical activation during reading aloud of long sentences: an fMRI study. Neuroreport 14:1563–1566 [DOI] [PubMed]
- Miura N, Watanabe J, Iwata K, Sassa Y, Riera J, Tsuchiya H, Sato S, Horie K, Kawashima R (2005) Cortical activation during reading of ancient versus modern Japanese texts: fMRI study. Neuroimage 26:426–431 [DOI] [PubMed]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS (1994) Brain activity during reading. The effects of exposure duration and task. Brain 117:1255–1269 [DOI] [PubMed]
- Rickard TC, Romero SG, Basso G, Wharton C, Flitman S, Grafman J (2000) The calculating brain: an fMRI study. Neurophsycol 38:325–335 [DOI] [PubMed]
- Ritchie K, Artero S, Touchon J (2001) Classification criteria for mild cognitive impairment: a population-based validation study. Neurol 56:37–42 [DOI] [PubMed]
- Roland PE, Friberg L (1985) Localization of cortical areas activated by thinking. J Neurophysiol 53:1219–1243 [DOI] [PubMed]
- Rueckert L, Lange N, Partiot A, Appollonio I, Litvar I, Le Bihan D, Grafman J (1996) Visualizing cortical activation during mental calculation with functional MRI. Neuroimage 3:97–103 [DOI] [PubMed]
- Salthouse TA (2006) Mental exercise and mental aging. Perspect Psychol Sci 1:68–87 [DOI] [PubMed]
- Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, Goto R, Uchida S, Tsuji I, Arai H, Kawashima R, Fukuda H (2005) Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord 88:313–320 [DOI] [PubMed]
- Wechsler D (1981) Manual for the Wechsler adult intelligence scale-revised. The Psychological Corporation, New York
- Willis SL, Schaie KW (1986) Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging 1:239–247 [DOI] [PubMed]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E (2006) Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 296:2805–2854 [DOI] [PMC free article] [PubMed]
- Wielgos CM, Cunningham WR (1999) Age-related slowing on the Digit Symbol task: longitudinal and cross-sectional analyses. Exp Aging Res 25:109–120 [DOI] [PubMed]
- Wingfield A, Grossman M (2006) Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol 96:2830–2839 [DOI] [PubMed]