Abstract
Oxidative stress has been documented in tissues and biofluids of subjects with sporadic Alzheimer disease (AD) and mild cognitive impairment (MCI). The aim of this study was to determine whether (a) salivary protein carbonyls are elevated in AD and MCI subjects, (b) salivary protein carbonyl contents in these groups exhibit diurnal variation, and (c) apolipoprotein E ɛ4 (apoE ɛ4) carrier status impacts salivary carbonyl concentrations or rhythmicity in the AD and MCI cohorts. Unstimulated saliva was collected at fixed intervals between 8 AM and 10 PM from 15 AD subject , 21 MCI subjects, and 30 cognitively-intact controls. Salivary protein carbonyl concentrations were measured by ELISA. ApoE genotyping was performed on the AD and MCI individuals. For all groups, mean protein carbonyl contents were significantly elevated at 2 PM relative to other time points surveyed. Mean salivary protein carbonyl concentrations did not differ among the diagnostic groups. ApoE ɛ4 carriers exhibited less temporal variation in salivary protein carbonyls relative to noncarriers. Thus, protein carbonyl content exhibits diurnal variation in adult human saliva. ApoE ɛ4 carrier status may impact oropharyngeal disease expression by attenuating the inherent diurnal variability in salivary redox homeostasis. Salivary protein carbonyls do not differentiate AD and MCI from normal individuals. In conclusion, oxidative stress has been documented in tissues and biofluids of subjects with sporadic AD and MCI. This article demonstrates that levels of protein carbonyls, a marker of oxidative stress, exhibit robust diurnal variation in the saliva of normal elderly, MCI, and AD subjects. Apolipoprotein E ɛ4 allele carrier status may attenuate this temporal variability in salivary redox homeostasis and thereby impact the natural history of oropharyngeal diseases.
Keywords: Alzheimer disease, Apolipoprotein E, Diurnal rhythm, Mild cognitive impairment, Oxidative stress, Protein carbonyls, Saliva
Introduction
Sporadic (nonfamilial) Alzheimer disease (AD) is the most common cause of aging-related dementia, affecting approximately 5–10% of North Americans aged 65 and as many as 30–50% of those who survive to the end of their ninth decade. It is a neurodegenerative condition characterized by progressive neuronal loss, gliosis, and the accumulation of intracellular inclusions (neurofibrillary tangles) and extracellular deposits of amyloid (senile plaques) in the basal forebrain, hippocampus, and association cortices (Selkoe 1991). Mild cognitive impairment (MCI) is a diagnosis ascribed to elderly persons who experience gradual cognitive decline (usually memory) of at least 6 months duration that fails to meet the clinical criteria for AD or other dementia. A significant proportion of MCI subjects will progress to probable AD within a 3–5 year follow-up period (Chertkow et al. 2001).
Although the etiology of sporadic AD remains uncertain, oxidative stress and free radical damage have been heavily implicated in the pathogenesis of this condition. Evidence of the latter include augmented oxidative modification of lipids, proteins, and nucleic acids, as well as aberrant antioxidant enzyme expression profiles and altered concentrations of low-molecular weight antioxidant compounds in the brains of AD and MCI subjects relative neurohistologically-normal control tissues (Schipper et al. 2006). A burgeoning literature indicates that perturbed redox homeostasis is not confined to the AD/MCI-affected brain and cerebrospinal fluid (CSF), but is also manifest in various peripheral tissues and biofluids, including blood plasma and leukocytes, peripheral fibroblasts, and urine (Maes et al. 2006a; b). These observations have provided novel insight into the systemic manifestations of AD and have fuelled interest in the development of redox-based peripheral biomarkers to assist in the diagnosis and management of the illness (Hensley et al. 1995; Schipper 2007).
Human saliva is an eminently accessible biofluid with enormous biomarker potential (Kaufman and Lamster 2002). An array of oxidative damage products (e.g protein carbonyls, 8-hydroxy-2′-deoxyguanosine [8-OHdG], 4-hydroxyalkenals, malondialdehyde) and antioxidant enzyme activities (e.g. superoxide dismutase, glutathione peroxidase, catalase) have been detected in human saliva. Importantly, changes in salivary redox homeostasis as manifested by deviations in absolute levels or expression patterns of these indices may reflect the presence and severity of various oral (e.g. periodontitis) and systemic (e.g. diabetes, inflammatory bowel disease) conditions (Arana et al. 2006; Bahar et al. 2007; Celec et al. 2005; Gorelik et al. 2007; Jahanshahi et al. 2004; Kolarzyk et al. 2006; Mashayekhi et al. 2005; Nagler et al. 2000; Reznick et al. 2006; Ryo et al. 2006; Sawamoto et al. 2005; Sculley and Langley-Evans 2003; Sugano et al. 2003; Takane et al. 2002; Tsai et al. 2005). Changes in salivary cortisol secretion patterns (Fiocco et al. 2006; Giubilei et al. 2001; Hatfield et al. 2004; Porter et al. 2002; Scherder et al. 2006; Wolf et al. 2002; Woods and Dimond 2002), amylase activity (Sramek et al. 1995), and acetylcholinesterase activity (Sayer et al. 2004) have been documented in persons with AD or MCI, attesting to the plausibility of exploiting saliva for neurodegenerative disease biomarker discovery. However, we are unaware of any published reports on the redox status of saliva in AD/MCI, and whether salivary redox homeostasis in humans is subject to circadian fluctuations. In this study, we set out to determine whether (a) protein carbonyls, a widely-accepted marker of protein oxidation, are elevated in the saliva of AD and MCI subjects relative to cognitively-intact controls, (b) salivary protein carbonyl contents in these groups exhibit diurnal variation, and (c) apolipoprotein E ɛ4 (apoE ɛ4) carrier status, an AD risk factor (Corder et al. 1993; Fagan and Holtzman 2000; Huang et al. 2004), impacts salivary carbonyl concentrations or rhythmicity in the AD and MCI cohorts.
Materials and methods
Study subjects
The study was approved by the research ethics committee of the S.M.B.D. Jewish General Hospital (JGH). Written informed consent was obtained from all participants or their primary caregivers. A total of 66 subjects were enrolled in this study. Fifteen patients with sporadic (nonfamilial) AD and 21 individuals with MCI were recruited from the JGH/McGill University Memory Clinic, a tertiary care facility for the evaluation of memory loss in Montreal. All AD subjects met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD. MCI individuals exhibited cognitive decline (usually memory) of at least 6 months duration that did not meet dementia criteria and scores of 0.5 on the Clinical Dementia Rating scale (Hughes et al. 1982; Levy 1994). All AD and MCI patients underwent comprehensive neuropsychological testing by Memory Clinic neuropsychologists, as previously described (Schipper et al. 2000). The Folstein Mini-Mental State Examination (MMSE) was administered to all subjects. Thirty cognitively normal controls were recruited among patients and staff at the JGH. Control subjects had no memory complaints and scored 28 or greater (of 30) on the MMSE and within one standard deviation of education-standardized normal values on a series of memory and attention tests. Exposure to vitamin E (400 IU or more/day), ascorbic acid (500 mg or more/day), or other antioxidants (at any dosage) was recorded in the medication records. Smokers, diabetics, and individuals suffering from significant dental, gingival, or chronic systemic inflammatory diseases were excluded from the study.
Apolipoprotein E genotyping
ApoE genotyping was performed on all AD and MCI patients in the Molecular Diagnostics Laboratory of the JGH, as previously reported (Berlin et al. 2004). The PCR primers and assay conditions were identical to those initially described by Hixon and Vernier (Hixson and Vernier 1990).
Saliva collection and preparation
Whole, unstimulated saliva samples were collected at least 30 min after food or liquid ingestion in sterilized salivette tubes (SARSTEDT, Inc., Newton, NC, USA) at 8 AM, 10 AM, 2 PM, 4 PM, and 10 PM. The tubes were sealed immediately after sample collection and refrigerated at 4°C before transport to the laboratory. The saliva was centrifuged at 10,000×g for 20 min at 4°C to diminish salivary residua and viscosity. The supernatants were aliquoted and stored at −80°C prior to analysis.
Total measurement of protein carbonyls
Total carbonylated proteins were quantified in individual saliva samples using a sensitive ELISA method (Buss et al. 1997; Winterbourn and Buss 1999), as previously performed in our laboratory (Song et al. 2006). Oxidized bovine serum albumin (BSA; Sigma Chemical Co., St. Louis, MO, USA) served as a standard. Oxidized BSA was prepared by reacting natural BSA (50 mg/ml in PBS) with hypochlorous acid (5 mM) for 1 h at 37°C, followed by overnight dialysis against PBS at 4°C. Fully reduced BSA was prepared by reacting natural BSA (5 mg/ml in PBS) with sodium borohydride (1 mg/ml) for 30 min at room temperature, followed by slow neutralization with 2 M HCl. The carbonyl content of BSA was calibrated colorimetrically by absorbance at 375 nm (ɛ = 22,000 M-1.cm-1) (Winterbourn and Buss 1999). The standard curve was constructed by mixing varying proportions of oxidized BSA and fully reduced BSA at a constant total protein concentration (4 mg/ml). Saliva protein levels were measured using Lowry’s method (Bio-Rad Laboratories Inc., Hercules, CA, USA) and adjusted to 4 mg protein/ml by dilution or speed vacuum concentration. The standards and protein samples were incubated with three volumes of 10 mM 2,4-dinitro-phenylhydrazine (2,4-DNP) in 6 M guanidine-HCl, 0.5 M potassium phosphate, pH 2.5, for 45 min at room temperature (mixing every 10–15 min). 5 μl aliquots of DNP-derivatized proteins were diluted in 1 ml PBS. 200 μl of diluted DNP-derivatized proteins were adsorbed to 96-well immunoplates by incubation overnight at 4°C. Nonspecific binding was blocked with 0.1% Tween-20 in PBS. The wells were incubated with primary biotinylated anti-DNP antibody (1:1000 dilution in 0.1% Tween-20/PBS; Molecular Probes, Eugene, OR) for 1 hour at 37°C. Wells were washed and incubated with streptavidin-biotinylated horseradish peroxidase (1:3000 dilution; Amersham Bioscience, Buckinghamshire, UK) for 1 hour at room temperature. After further washing, o-phenylenediamine/peroxide solution was added for color development. The reaction was stopped after 10 min with 2.5 M sulfuric acid, and the absorbance read at 490 nm. A 6-point standard curve was generated for each plate analysis. Specific absorbance for each sample was calculated by subtracting basal absorbance of the DNP reagent from total absorbance. Protein carbonyl concentrations were expressed as nmol per mg of total protein in saliva.
Statistical procedures
Chi-square and Fisher’s exact and unpaired t tests were used to compare differences between groups for categorical (e.g., gender, antioxidants, APOE ɛ4 allele) and continuous variables (e.g., age, years of education, and MMSE score), respectively. The data were presented as means ± standard errors.
The primary analysis was performed to compare protein carbonyl level measures between groups within the 14 h sampling period. Repeated measures analysis was performed using a linear mixed model (SAS PROC MIXED): subjects are random effects, while diagnostic group, time, and their interactions are fixed effects. We included covariates (age, education, and MMSE) as fixed effects. Only age was kept in the final analysis as confounder. To model the quadratic effect of time, we add time*time to the model. We noted that this term was significant (p < 0.0001). The best model was selected based on Akaike’s Information Criterion (AIC). The secondary analysis was performed using ANOVA (PROC GLM) comparing protein carbonyl levels measures between groups at each follow-up time. In addition, we compared the protein carbonyl levels measures between both ɛ4 carrier and noncarrier groups within the 14 h sampling period (SAS PROC MIXED) and at each follow-up time (PROC GLM). The control group was excluded from these analyses. The level of significance was set at 0.05 (two-sided) for the statistical tests. Statistical analyses were performed using SAS software version 9.1.
Results
Demographics, antioxidants, and MMSE scores
Control participants were significantly younger than the MCI and AD groups (p < 0.0001 for each comparison). Differences in mean ages between the AD and MCI groups were not significant (p = 0.47). Mean years of formal education scores were lower for the AD and MCI groups relative to the control cohort (p < 0.0001 for each comparison), as commonly reported in dementia studies (Bennett et al. 2003; Pavlik et al. 2006). Gender distribution was similar among the three groups (p = 0.33), and a trend towards greater antioxidant exposure in the AD and MCI groups vs. control subjects did not achieve statistical significance (p = 0.08). As anticipated, mean MMSE scores were significantly lower in the AD vs. MCI and control groups (p < 0.0001) and in the MCI group relative to control values (p < 0.0001) shown in Table 1.
Table 1.
Demographics, antioxidant exposure, MMSE scores, and apoE status of the study groups
| Variable | Controls | MCI | AD |
|---|---|---|---|
| Subjects | 30 | 21 | 15 |
| Age in years (SEM) | 69.20 (1.26) | 81.14 (0.73)* | 82.40 (0.70)* |
| Gender M/F (%) | 13 (43.33) 17 (56.67) | 10 (42.62) 11 (52.38) | 10 (66.67) 5 (33.33) |
| Education in years (SEM) | 15.03 (0.33) | 11.00 (0.27)* | 11.87 (0.48)* |
| MMSE score/30 (SEM) | 29.03 (0.13) | 27.53 (0.16)* | 24.00 (0.40)*,** |
| Antioxidants (%) | 3 (10.00) | 7 (33.33) | 5 (33.33) |
| APOE ɛ4 allele carrier (%) | – | 5 (26.32) | 3 (27.27) |
Two tailed p-value for chi-square, Fisher’s exact and unpaired t statistics. MCI mild cognitive impairment, AD Alzheimer disease
*p < 0.0001 vs. control group
**p < 0.0001 vs. MCI group
Salivary protein carbonyl levels
Diagnosis and sampling time
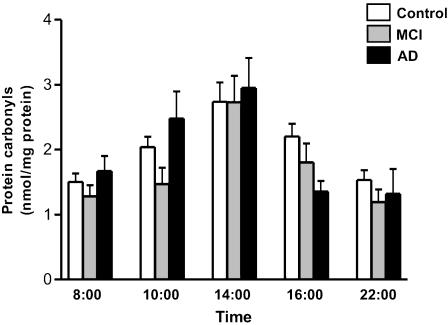
Figure 1 depicts mean salivary protein carbonyl levels in the control, MCI, and AD subjects as a function of sampling time over the course of a 14-hour period. For all three diagnostic groups, mean protein carbonyl contents were greatest at 2 PM (controls = 2.74 ± 0.32, MCI = 2.73 ± 0.37, AD = 2.95 ± 0.48 nmol/mg protein). The lowest mean protein carbonyl levels were observed at 8 AM (control = 1.50 ± 0.13, MCI = 1.28 ± 0.19, AD = 1.67 ± 0.20 nmol/mg protein) and at 10 PM (control = 1.54 ± 0.17, MCI = 1.19 ± 0.23, AD = 1.32 ± 0.27 nmol/mg protein). Mean salivary protein carbonyls were significantly lower in the MCI group (1.47 ± 0.27 nmol/mg protein) than in the AD subjects (2.48 ± 0.30 nmol/mg protein) at 10 AM (p = 0.017 in GLM). Otherwise, there were no statistically significant differences among the diagnostic groups with respect to salivary protein carbonyl concentrations (p > 0.05).
Fig. 1.
Salivary protein carbonyl levels as a function of diagnosis and sampling time. Depicted are protein carbonyl concentration (nmol/mg protein) in control, MCI, and AD subjects over a 14-hour sampling period. Results shown in Figs. 1 and 2 are averaged from ELISA run in triplicate. n = 30 per control group, n = 21 per MCI, and n = 15 per AD group. For Figs. 1 and 2, statistical significance values are provided in the text
In repeat multivariate analyses of the data (PROC MIXTE, SAS) adjusting for age, mean salivary protein carbonyl levels were found to differ significantly as a function of collection time over the 14-hour sampling period (p < 0.0001) independently of diagnostic category. Overall mean salivary protein carbonyl concentrations for the control, MCI, and AD groups were, respectively, 1.80 ± 0.15, 1.93 ± 0.19, and 2.16 ± 0.22 nmol/mg protein (p = 0.44).
ApoE status
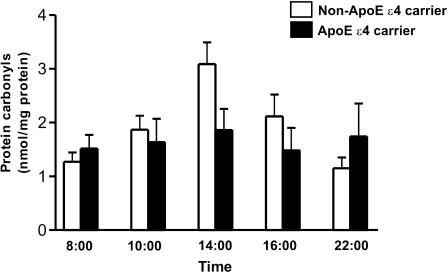
One or two apoE ɛ4 alleles were present in 27.3% of the AD and 26.3% of the MCI subjects. Salivary protein carbonyls for the ApoE ɛ4 allele carriers (n = 8) and noncarriers (n = 22) for the 14-hour collection period are shown in Fig. 2. Average salivary carbonyl contents exhibited peak values at 2 PM for both ɛ4 carriers (1.86 ± 0.62 nmol/mg protein) and noncarriers (3.09 ± 0.37 nmol/mg protein), with a trend towards higher levels in the noncarriers that did not reach statistical significance (p = 0.10 in GLM). Of note, the ɛ4 carriers exhibited considerably less temporal variation in salivary protein carbonyls relative to the noncarriers over the 14-hour sampling period. Age was not a confounder in the final Apo E analysis (non-ApoE ɛ4 carriers: 81.73 ± 6.48 years vs. ApoE ɛ4 carriers: 79.34 ± 8.61; p = 0.08) and was not associated with the outcome (p = 0.99).
Fig. 2.
Salivary protein carbonyl levels as a function of apoE status. Protein carbonyl concentration (nmol/mg protein) in ApoE ɛ4 allele carriers (n = 8) and non-carriers (n = 22) over a 14-hour sampling period
In repeat multivariate analyses (PROC MIXTE, SAS), overall mean protein carbonyl concentrations were not different (p = 0.45) between the ApoE ɛ4 carriers (1.71 ± 0.32 nmol/mg protein) and noncarriers (2.0 ± 0.20 nmol/mg protein). However, salivary carbonyl levels again varied significantly (p < 0.0001) as a function of collection time, with peak values at 2 PM.
Discussion
A major observation in the current study was the robust diurnal variation in salivary protein carbonyl levels in well-ascertained cohorts of individuals with normal cognition, MCI, and sporadic AD. In all three groups, serial measurements performed over a 14-hour (waking) period revealed peak salivary carbonyl concentrations at 2 PM. The study design and multivariate analyses indicated that this temporal variability in salivary protein oxidation was independent of factors which could theoretically have influenced the data viz., age, gender, oral hygiene, chronic systemic illness, smoking, salivary secretagogues, recent food intake, or antioxidant exposure (Bahar et al. 2007; Hershkovich et al. 2007; Nagler et al. 2000; Sculley and Langley-Evans 2003; Zloczower et al. 2007).
Circadian rhythms for salivary cortisol secretion and chromogranin A levels have previously been described in human saliva (Den et al. 2007; Fiocco et al. 2006; Ice et al. 2004). To our knowledge, this study is the first to report significant diurnal fluctuations in a marker of salivary redox homeostasis. This observation is consistent with the fact that, across the phylogenetic spectrum and in a wide range of tissues, numerous antioxidant enzymes (e.g. blood superoxide dismutase), low molecular weight antioxidant compounds (e.g. plasma uric acid, melatonin, reduced thiols), and oxidatively-generated substrates (e.g. plasma malondialdehyde, urinary 8-OHdG) exhibit strong circadian or other temporal fluctuations in amount or activity (Bridges et al. 1992a; b; Hardeland et al. 2003; Kanabrocki et al. 2002, 2004, 2000; Luo et al. 1997; Niklowitz et al. 2006; Reiter et al. 1996; Valencia et al. 2001). Whether the afternoon peak in salivary protein carbonyls (present study) is due to diurnal deficiencies in salivary gland / orophayngeal antioxidant defenses or augmented ROS production remains unclear. Future studies assessing potential rhythmic fluctuations in a broad range of salivary (and, if feasible, salivary gland) antioxidant defenses, ROS, and oxidatively-modified protein, lipid, and nucleic acid substrates may resolve this issue. It may also be of considerable interest to determine whether susceptibility to specific oral cavity pathologies is temporally-associated with salivary redox status. From a practical point of view, the data presented herein have important implications for the design and interpretation of salivary biomarker studies. For example, diurnal variations in salivary redox homeostasis may have confounded the data sets in several published reports on the relationship of periodontal disease (and its therapy) (Sculley and Langley-Evans 2003), cigarette smoking (Nagler et al. 2000), oral cancer (Bahar et al. 2007), and diabetes (Zloczower et al. 2007) to salivary redox indices if the timing of specimen procurement was not standardized.
As described in the introduction, free radical-mediated substrate modifications have been amply demonstrated in affected brain and in various systemic tissues (blood cells, fibroblasts) and biofluids (plasma, serum, CSF) derived from patients with sporadic AD relative to age-matched individuals without cognitive impairment (Smith et al. 1991, 1996). Similar, and occasionally even more robust, evidence of neural and systemic oxidative stress has been garnered in subjects with MCI, a frequent preclinical harbinger of incipient AD (Schipper et al. 2006). Contrary to this literature, salivary protein carbonyls showed no significant variations in overall levels or temporal expression patterns among the normal, MCI, and AD persons enrolled in the current study. Thus, salivary protein carbonyls per se do not appear to express the enhanced state of oxidative stress experienced by AD and MCI subjects. These negative findings argue against the use of salivary protein carbonyls as a potential biomarker of this disease. Whether AD and MCI will register in the oxidative modification(s) of other chemical substrates in saliva, or whether salivary redox homeostasis is entirely unaffected in this disease, remains to be determined.
The presence of one or two apoE ɛ4 alleles, a risk factor for the development of sporadic AD (Corder et al. 1993; Fagan and Holtzman 2000; Huang et al. 2004), did not significantly impact average salivary protein carbonyl levels in the AD and MCI cohorts. However, the temporal variation in salivary protein carbonyl concentrations over the 14-hour sampling period appeared to be dampened in ɛ4 carriers relative to the noncarriers. If confirmed in larger studies, these findings would implicate apoE genotype as a novel and potentially important regulator of temporal protein oxidation profiles in human saliva. Such a conclusion would not be altogether surprising given the known influence of apoE status on redox homeostasis in a host of mammalian neural and systemic tissues (Biswas et al. 2005; Chan and Shea 2006; Folkmann et al. 2007; Jofre-Monseny et al. 2007; Mazur-Kolecka et al. 2006). In this regard, it may prove interesting to determine whether the development or severity of oral pathologies linked to oxidative damage in humans, such as periodontitis (Mashayekhi et al. 2005; Sawamoto et al. 2005; Sculley and Langley-Evans 2003; Takane et al. 2002; Tsai et al. 2005) or local neoplasms (Bahar et al. 2007; Hershkovich et al. 2007), are influenced by the apoE genotype.
The data reported herein provide evidence for the existence of robust temporal variations in levels of salivary protein oxidation in human saliva over a 14-hour (waking) period. These observations raise the intriguing possibility that various oral pathologies and their responsiveness to therapeutic interventions may be impacted by diurnal (possibly circadian) fluctuations in salivary redox homeostasis. Absolute concentrations and temporal expression patterns of salivary protein carbonyls did not differ among AD, MCI, and cognitively-normal subjects thereby discounting salivary protein carbonyls as a viable biomarker of this common neurodegenerative disorder. Finally, apoE ɛ4 status affected the temporal rhythmicity of salivary protein oxidation, raising the possibility that the former may bear on the natural history of redox-related oropharyngeal lesions.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research and internal JGH funds (to HMS).
References
- Arana C, Cutando A, Ferrera MJ, Gomez-Moreno G, Worf CV, Bolanos MJ, Escames G, Acuna-Castroviejo D (2006) Parameters of oxidative stress in saliva from diabetic and parenteral drug addict patients. J Oral Pathol Med 35:554–559 [DOI] [PubMed]
- Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM (2007) Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer 109:54–59 [DOI] [PubMed]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60:1909–1915 [DOI] [PubMed]
- Berlin D, Chong G, Chertkow H, Bergman H, Phillips NA, Schipper HM (2004) Evaluation of HFE (hemochromatosis) mutations as genetic modifiers in sporadic AD and MCI. Neurobiol Aging 25:465–474 [DOI] [PubMed]
- Biswas SK, Newby DE, Rahman I, Megson IL (2005) Depressed glutathione synthesis precedes oxidative stress and atherogenesis in Apo-E(−/−) mice. Biochem Biophys Res Commun 338:1368–1373 [DOI] [PubMed]
- Bridges AB, Fisher TC, Scott N, McLaren M, Belch JJ (1992a) Circadian rhythm of white blood cell aggregation and free radical status in healthy volunteers. Free Radic Res Commun 16:89–97 [DOI] [PubMed]
- Bridges AB, Scott NA, McNeill GP, Pringle TH, Belch JJ (1992b) Circadian variation of white blood cell aggregation and free radical indices in men with ischaemic heart disease. Eur Heart J 13:1632–1636 [DOI] [PubMed]
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC (1997) Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med 23:361–366 [DOI] [PubMed]
- Celec P, Hodosy J, Celecova V, Vodrazka J, Cervenka T, Halcak L, Bozek P, Kopani M, Kudela M (2005) Salivary thiobarbituric acid reacting substances and malondialdehyde—their relationship to reported smoking and to parodontal status described by the papillary bleeding index. Dis Markers 21:133–137 [DOI] [PMC free article] [PubMed]
- Chan A, Shea TB (2006) Dietary and genetically-induced oxidative stress alter tau phosphorylation: influence of folate and apolipoprotein E deficiency. J Alzheimers Dis 9:399–405 [DOI] [PubMed]
- Chertkow H, Bergman H, Schipper HM, Gauthier S, Bouchard R, Fontaine S, Clarfield AM (2001) Assessment of suspected dementia. Can J Neurol Sci 28(suppl 1):S28–S41 [DOI] [PubMed]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923 [DOI] [PubMed]
- Den R, Toda M, Nagasawa S, Kitamura K, Morimoto K (2007) Circadian rhythm of human salivary chromogranin A. Biomed Res 28:57–60 [DOI] [PubMed]
- Fagan AM, Holtzman DM (2000) Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo. Microsc Res Tech 50:297–304 [DOI] [PubMed]
- Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ (2006) Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: relation to cognitive functioning. Stress 9:143–152 [DOI] [PubMed]
- Folkmann JK, Loft S, Moller P (2007) Oxidatively damaged DNA in aging dyslipidemic ApoE−/− and wild-type mice. Mutagenesis 22:105–110 [DOI] [PubMed]
- Giubilei F, Patacchioli FR, Antonini G, Sepe Monti M, Tisei P, Bastianello S, Monnazzi P, Angelucci L (2001) Altered circadian cortisol secretion in Alzheimer’s disease: clinical and neuroradiological aspects. J Neurosci Res 66:262–265 [DOI] [PubMed]
- Gorelik S, Kohen R, Ligumsky M, Kanner J (2007) Saliva plays a dual role in oxidation process in stomach medium. Arch Biochem Biophys 458:236–243 [DOI] [PubMed]
- Hardeland R, Coto-Montes A, Poeggeler B (2003) Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20:921–962 [DOI] [PubMed]
- Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH (2004) Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 127:1061–1074 [DOI] [PubMed]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM et al (1995) Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem 65:2146–2156 [DOI] [PubMed]
- Hershkovich O, Shafat I, Nagler RM (2007) Age-related changes in salivary antioxidant profile: possible implications for oral cancer. J Gerontol A Biol Sci Med Sci 62:361–366 [DOI] [PubMed]
- Hixson JE, Vernier DT (1990) Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 31:545–548 [PubMed]
- Huang Y, Weisgraber KH, Mucke L, Mahley RW (2004) Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci 23:189–204 [DOI] [PubMed]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572 [DOI] [PubMed]
- Ice GH, Katz-Stein A, Himes J, Kane RL (2004) Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology 29:355–370 [DOI] [PubMed]
- Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M (2004) Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci 49:1752–1757 [DOI] [PubMed]
- Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G (2007) Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun 357:319–324 [DOI] [PMC free article] [PubMed]
- Kanabrocki EL, Third JL, Ryan MD, Nemchausky BA, Shirazi P, Scheving LE, McCormick JB, Hermida RC, Bremner WF, Hoppensteadt DA, Fareed J, Olwin JH (2000) Circadian relationship of serum uric acid and nitric oxide. JAMA 283:2240–2241 [DOI] [PubMed]
- Kanabrocki EL, Murray D, Hermida RC, Scott GS, Bremner WF, Ryan MD, Ayala DE, Third JL, Shirazi P, Nemchausky BA, Hooper DC (2002) Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int 19:423–439 [DOI] [PubMed]
- Kanabrocki EL, Ryan MD, Hermida RC, Ayala DE, Scott GS, Murray D, Bremner WF, Third JL, Johnson MC, Foley S, Van Cauteren J, Shah F, Shirazi P, Nemchausky BA, Hooper DC (2004) Altered circadian relationship between serum nitric oxide, carbon dioxide, and uric acid in multiple sclerosis. Chronobiol Int 21:739–758 [DOI] [PubMed]
- Kaufman E, Lamster IB (2002) The diagnostic applications of saliva-a review. Crit Rev Oral Biol Med 13:197–212 [DOI] [PubMed]
- Kolarzyk E, Pietrzycka A, Stepniewski M, Lyszczarz J, Mendyk A, Ostachowska-Gasior A (2006) Micronutrients and macronutrients and parameters of antioxidative ability in saliva of women: inhabitants of Krakow (Poland) in the course of uncomplicated singleton pregnancy. Biol Trace Elem Res 114:73–84 [DOI] [PubMed]
- Levy R (1994) Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr 6:63–68 [DOI] [PubMed]
- Luo H, Guo H, Xiao J, Xue Z (1997) Circadian variations of plasma SOD and MDA in health subjects. Hua Xi Yi Ke Da Xue Xue Bao 28:401–403 [PubMed]
- Maes OC, Kravitz S, Mawal Y, Su H, Liberman A, Mehindate K, Berlin D, Sahlas DJ, Chertkow HM, Bergman H, Melmed C, Schipper HM (2006a) Characterization of alpha1-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiol Dis 24:89–100 [DOI] [PubMed]
- Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM (2006b) Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol Aging 28:1795–1809 [DOI] [PubMed]
- Mashayekhi F, Aghahoseini F, Rezaie A, Zamani MJ, Khorasani R, Abdollahi M (2005) Alteration of cyclic nucleotides levels and oxidative stress in saliva of human subjects with periodontitis. J Contemp Dent Pract 6:46–53 [PubMed]
- Mazur-Kolecka B, Dickson D, Frackowiak J (2006) Induction of vascular amyloidosis-beta by oxidative stress depends on APOE genotype. Neurobiol Aging 27:804–814 [DOI] [PubMed]
- Nagler R, Lischinsky S, Diamond E, Drigues N, Klein I, Reznick AZ (2000) Effect of cigarette smoke on salivary proteins and enzyme activities. Arch Biochem Biophys 379:229–236 [DOI] [PubMed]
- Niklowitz P, Andler W, Menke T (2006) Coenzyme Q10 concentration in plasma and blood cells: what about diurnal changes? Biofactors 28:47–54 [DOI] [PubMed]
- Pavlik VN, Doody RS, Massman PJ, Chan W (2006) Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord 22:367–377 [DOI] [PubMed]
- Porter RJ, Marshall EF, O’Brien JT (2002) Effects of rapid tryptophan depletion on salivary and plasma cortisol in Alzheimer’s disease and the healthy elderly. J Psychopharmacol 16:73–78 [DOI] [PubMed]
- Reiter RJ, Barlow-Walden L, Poeggeler B, Heiden SM, Clayton RJ (1996) Twenty-four hour urinary excretion of 6-hydroxymelatonin sulfate in Down syndrome subjects. J Pineal Res 20:45–50 [DOI] [PubMed]
- Reznick AZ, Shehadeh N, Shafir Y, Nagler RM (2006) Free radicals related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes mellitus. Arch Oral Biol 51:640–648 [DOI] [PubMed]
- Ryo K, Yamada H, Nakagawa Y, Tai Y, Obara K, Inoue H, Mishima K, Saito I (2006) Possible involvement of oxidative stress in salivary gland of patients with Sjogren’s syndrome. Pathobiology 73:252–260 [DOI] [PubMed]
- Sawamoto Y, Sugano N, Tanaka H, Ito K (2005) Detection of periodontopathic bacteria and an oxidative stress marker in saliva from periodontitis patients. Oral Microbiol Immunol 20:216–220 [DOI] [PubMed]
- Sayer R, Law E, Connelly PJ, Breen KC (2004) Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to cholinesterase inhibitors. Clin Biochem 37:98–104 [DOI] [PubMed]
- Scherder E, Knol D, van Tol MJ, van Someren E, Deijen JB, Swaab D, Scheltens P (2006) Effects of high-frequency cranial electrostimulation on the rest-activity rhythm and salivary cortisol in Alzheimer’s disease: a pilot study. Dement Geriatr Cogn Disord 22:267–272 [DOI] [PubMed]
- Schipper HM (2007) The role of biological markers in the diagnosis of Alzheimer’s disease. Alzheimer’s Dement 3:325–332 [DOI] [PubMed]
- Schipper HM, Chertkow H, Mehindate K, Frankel D, Melmed C, Bergman H (2000) Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology 54:1297–1304 [DOI] [PubMed]
- Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z (2006) Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging 27:252–261 [DOI] [PubMed]
- Sculley DV, Langley-Evans SC (2003) Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 105:167–172 [DOI] [PubMed]
- Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6:487–498 [DOI] [PubMed]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR (1991) Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA 88:10540–10543 [DOI] [PMC free article] [PubMed]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N (1996) Oxidative damage in Alzheimer’s. Nature 382:120–121 [DOI] [PubMed]
- Song W, Su H, Song S, Paudel HK, Schipper HM (2006) Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J Cell Physiol 206:655–663 [DOI] [PubMed]
- Sramek JJ, Cutler NR, Hurley DJ, Seifert RD (1995) The utility of salivary amylase as an evaluation of M3 muscarinic agonist activity in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 19:85–91 [DOI] [PubMed]
- Sugano N, Yokoyama K, Oshikawa M, Kumagai K, Takane M, Tanaka H, Ito K (2003) Detection of Streptococcus anginosus and 8-hydroxydeoxyguanosine in saliva. J Oral Sci 45:181–184 [DOI] [PubMed]
- Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K (2002) New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol 73:551–554 [DOI] [PubMed]
- Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, Hung CC (2005) Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res 40:378–384 [DOI] [PubMed]
- Valencia E, Marin A, Hardy G (2001) Circadian rhythmicity of whole-blood glutathione in healthy subjects. Nutrition 17:731–733 [DOI] [PubMed]
- Winterbourn CC, Buss IH (1999) Protein carbonyl measurement by enzyme-linked immunosorbent assay. Methods Enzymol 300:106–111 [DOI] [PubMed]
- Wolf OT, Convit A, Thorn E, de Leon MJ (2002) Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology 27:777–789 [DOI] [PubMed]
- Woods DL, Dimond M (2002) The effect of therapeutic touch on agitated behavior and cortisol in persons with Alzheimer’s disease. Biol Res Nurs 4:104–114 [DOI] [PubMed]
- Zloczower M, Reznick AZ, Zouby RO, Nagler RM (2007) Relationship of flow rate, uric acid, peroxidase, and superoxide dismutase activity levels with complications in diabetic patients: can saliva be used to diagnose diabetes? Antioxid Redox Signal 9:765–773 [DOI] [PubMed]