Cerebral injury is a frequent complication of cardiac surgery and has been associated with high mortality, morbidity, hospital costs, and an increased likelihood of admission to a secondary care facility after hospital discharge, and impaired quality of life.47,71,78,86 There are a variety of manifestations of perioperative cerebral injury including ischemic (or, less commonly, hemorrhagic) stroke that occurs in 1.5% to 5.2% of patients, encephalopathy affecting 8.4% to 32% of patients, and neurocognitive dysfunction affecting 20% to 30% of patients one month after surgery.47,71,78,86 The range in reported incidences between studies is likely due to different patient populations (e.g., patient age and risk status, types of procedures), diagnostic definitions, and the intensity of clinical surveillance. Contemporary studies using sensitive brain MRI with diffusion weighted imaging report that as many as 45% of patients who have undergone cardiac surgery have new ischemic brain lesions that are often clinically undetected.47,63
The prevailing hypothesis, though not definitively proven, is that all forms of injury associated with cardiac surgery (i.e., stroke, encephalopathy, and neurocognitive dysfunction) have a similar etiology and that the manifestations depend on the extent and location of brain injury (e.g., motor cortex vs. areas subserving cognition). Many earlier studies that have described long-term neurocognitive changes after cardiac surgery have failed to include a non-surgical control group.47,79 In a longitudinal study of patients with coronary artery disease undergoing either percutaneous coronary interventions or coronary artery bypass grafting (CABG), there were no differences in cognitive measures 36 months after either procedure.88 These data imply that the effects of cardiac surgery on cognition may be short-lived (i.e., ~ 3 mo) and that progression of inherent cerebral vascular disease is a more important determinant of long-term cognitive decrements. These results further underscore the low sensitivity and specificity of psychometric testing for detecting cerebral injury in elderly populations with a high prevalence of preexisting cognitive impairment.47
In this paper, we will examine postulated mechanisms for cerebral injury from cardiac surgery. Most emphasis has been placed in the past on the intraoperative interval as being the period of highest cerebral vulnerability. Many clinical cerebral events, however, occur in the postoperative period. We have reported, in fact, that > 20% of clinical strokes occur after recovery from surgery and anesthesia.45,71 Thus, patients must be considered vulnerable to cerebral injury any time during the perioperative period.
Cerebral Embolism
Cerebral injury from cardiac surgery is primarily ischemic, secondary to embolism and/or cerebral hypoperfusion.47 Primary intra-cerebral hemorrhage is found on brain imaging in < 1% to 2% of patients, depending on the type of surgery.11 Neuronal injury is likely exacerbated by inflammatory processes resulting from cardiopulmonary bypass (CPB) and ischemia/reperfusion.47,59 Between 30–50% of perioperative strokes detected with brain imaging are due to cerebral macroembolism likely arising from the ascending aorta.10,28,45,64,101 Encephalopathy and neurocognitive dysfunction are believed to result primarily from cerebral microembolism..3,18,20,47,57,73,82,84,92 Microemboli are either gaseous or particulate in composition. Gaseous emboli can arise form an open left-sided cardiac chamber or from air entrained into the CPB circuit.47 The view that microemboli are the proximate cause of neurocognitive dysfunction is based in part on indirect data from retinal angiographic evaluations, autopsy studies, and animal experiments where cognitive end-points were not assessed.47,9,19 A more direct link is suggested by studies showing that the number of transcranial Doppler detected arterial embolic signals during CPB is related to cognitive impairment after CABG surgery.10 It is increasingly apparent, though, that postoperative cognitive dysfunction is not solely explained by this mechanism and is likely multifactorial.1,15,30,99 For example, in studies of open-chamber cardiac valve surgery cognitive decline was not correlated with the number of Doppler cerebral microembolic signals, and reducing cerebral microembolism (e.g., with “off-pump” surgery) does not significantly improve cognitive outcomes.13,99
Cerebral Hypoperfusion
Blood pressure during CPB is often maintained at a MAP of 50 to 70 mmHg based on data showing that cerebral blood flow (CBF) autoregulation is maintained using α-stat pH management.76 In brief, α-stat pH management accepts physico-chemical changes that occur with cooling of the blood that result in an increased solubility of CO2 and thus increases in pH. The rationale is that this type of response is physiologic in reptiles and that it maintains optimal functioning of enzymes during hypothermia. From a practical perspective, with α-stat pH management, the arterial blood gases are performed and interpreted at 37°C (regardless of actual body temperature). Thus, targets for pH and PaCO2 are the same as with normothermia (i.e., not corrected to the patients temperature). In contrast, with pH-stat arterial blood gas management, pH and PaCO2 performed at 37°C, are corrected to the patient’s body temperature (automatically with a computer, or with a nomogram). Thus, to maintain normocarbia, CO2 is often added to the CPB circuit leading to cerebral vasodilation and loss of autoregulation.
In a randomized study targeting a MAP on CPB of 50 to 60 mmHg or 80 to 100 mmHg, there were fewer combined myocardial infarctions and strokes in the “high” vs “low” MAP group (P=0.026).34 Many cardiac surgery patients might have abnormal CBF-blood pressure autoregulation, or an have an autoregulatory relationship that is displaced towards higher blood pressures.47 Further, episodes of hypotension are common perioperatively for many reasons including cardiac arrhythmias, impaired left ventricular function, or low systemic vascular resistance. Resulting cerebral hypoperfusion might be a primary cause of cerebral injury or it might exacerbate injury due to embolism (e.g., delayed embolism “washout”, impaired perfusion of ischemic penumbra, the area of at-risk brain tissue surrounding an acute infarct).65 This form of injury might be of increasing importance in an aging surgical population.
Contemporary studies using brain magnetic resonance imaging indicate that as many as 50% of elderly patients have old strokes, white matter lesions, and/or lacunar infarcts prior to cardiac surgery.35,47,84 These brain lesions may not be detected clinically but they are associated with cerebral vascular disease that increases the risk for neurological complications. Further, 75% of patients undergoing CABG surgery were found to have cerebral perfusion abnormalities using SPECT imaging before surgery.74 Finally, 27–43% of patients have been reported to have cerebral O2 desaturations during CPB indicating cerebral O2 demand/delivery mismatch.23,44 Further support for the cerebral hypoperfusion hypothesis was provided in 2 recent studies.36,37 In an analysis of data of patients who had diffusion-weighted MRI for stroke after cardiac surgery, 68% were found to have “watershed” strokes occurring at the borderzone between cerebral vascular territories (Figure 1). A reduction in MAP during CPB of ≥ 10 mmHg from baseline was associated with a 4.05 times increased odds (95% CI 1.03, 15.98) for bilateral watershed strokes. In-hospital mortality was higher for patients with bilateral watershed strokes compared with those with other types of cerebral infarctions (17% vs. 4%, p=0.04). In a separate series of patients, a decrease in MAP during CPB from baseline was found to predict short-term worsening in cognitive performance36.
Figure 1.
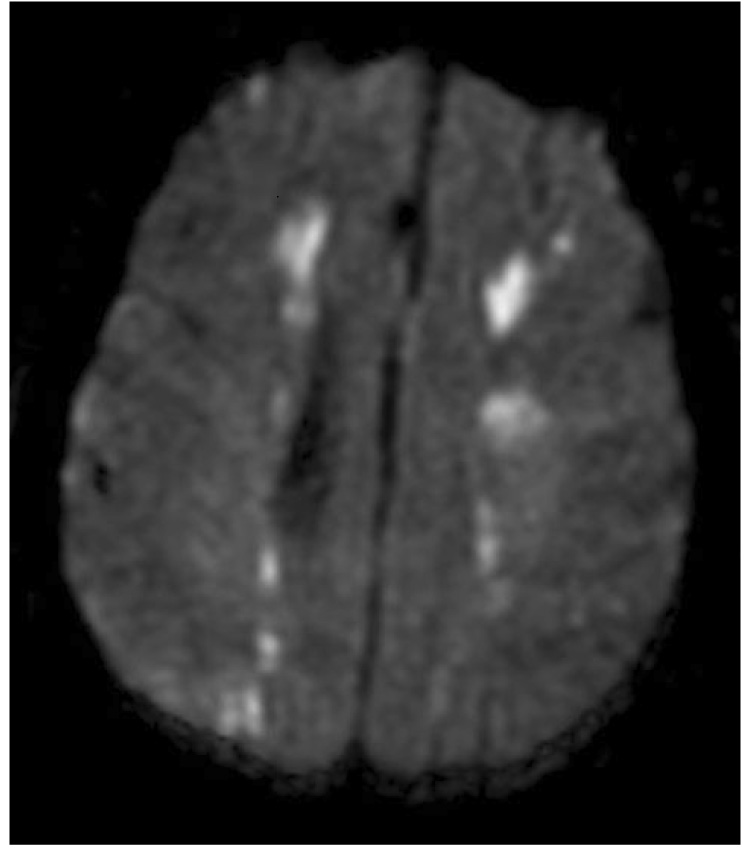
Diffusion weighted brain magnetic resonance image of a patient with stroke after cardiac surgery. The bright images represent brain ischemic injury. The location of the injury in the end vascular territory of the anterior and middle cerebral arteries and the middle and posterior cerebral arteries is consistent with a watershed infarction due to cerebral hypoperfusion.
Cerebral hypoperfusion might be particularly important as a source of cerebral injury during “off-pump” CABG surgery. During the latter, the heart is displaced to gain exposure of lateral or posterior coronary arteries. This leads to the combination of systemic hypotension and cerebral venous hypertension from traction on the superior vena cava.30 Cerebral hypoxemia inferred from EEG and cerebral oximetry monitoring was found in 15% of 550 patients undergoing “off-pump” CABG surgery.80
Atherosclerosis of the Aorta
Clinical investigations and autopsy reports have clearly linked atherosclerosis of the thoracic aorta and cerebral injury after cardiac surgery.10,101 Observational studies have shown, for example, that atherosclerosis of the ascending aorta detected at the time of surgery is an independent risk factor for stroke.45,86,101 Atherosclerotic disease of the aorta has been further linked to a higher number of TCD detected cerebral microembolic signals during CABG surgery and to greater rates of postoperative neurocognitive dysfunction compared with patients without severe atherosclerosis.66 As many as 1/3 of moderate and severe aortic atheromas may not be detected by direct palpation of the aorta by the surgeon. Epiaortic ultrasound has been established as the most sensitive means for detecting atherosclerosis of the ascending aorta.47,101 Several systems for classification of atherosclerotic lesions have been proposed (Table 1 and Figure 2).24,54,85,95 Regardless of the detection method (transesophageal or epiaortic echocardiography), a relationship between the frequency of neurological events and the severity of aortic atherosclerotic disease is well established.
Table 1.
Classification Systems of Atherosclerosis of the Ascending Aorta
| Davila-Roman et al.24 | |
| Normal | No atheroma |
| Mild | <3 mm |
| Moderate | >3 mm |
| Severe | >3 mm with mobile or ulcerative lesions |
| Tunick et al.95 | |
| Grade I (Insignificant) | Intimal thickening of < 2mm |
| Grade II (Moderate) | Intimal thickening of 2–5 mm |
| Grade III (Severe) | > 5 mm or mobile protrusion |
| Katz et al.54 | |
| Grade 1 | Normal |
| Grade 2 | Extensive intimal thickening |
| Grade 3 | Protrudes < 5 mm into aortic lumen |
| Grade 4 | Protrudes > 5 mm into aortic lumen |
| Grade 5 | Mobile atheroma |
Figure 2.
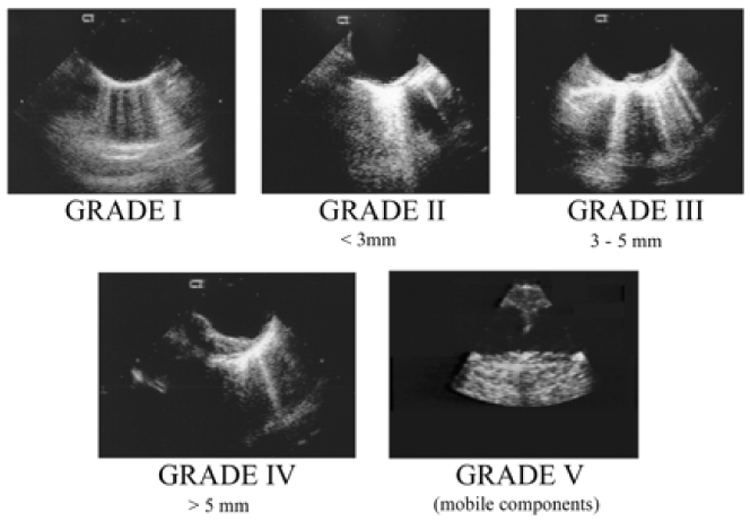
Transesophageal echocardiographic images demonstrating increasing severity of atherosclerosis of the aorta using incremental 5point grading system.
From Fox JA, Formanek V, Friedrich A, et al. Intraoperative Echocardiography. In: Cohn LH and Edmunds, LH Jr., eds. Cardiac Surgery in the Adult, 2nd Edition. New York: McGraw-Hill, 2003:283-314 with permission.
It is widely postulated that the proximate cause of atheroembolism is disruption of atheroma during surgical manipulations such as for aortic cannulation, aortic cross-clamping, or proximal coronary artery anastamosis. These interventions are associated with increases in Doppler detected cerebral embolic signals, but the composition of these emboli cannot be determined.5,92 Disruption of atherosclerotic lesions was verified in a study of 472 patients who underwent epiaortic ultrasound before and after CPB, with new mobile lesions of the ascending aorta identified in 10 (3.4%) of patients after surgery at sites of aortic clamping or cannulation96, and stroke occurring in 3 of these 10 patients. A smaller study by Ribakove et al85 revealed a similar rate of stroke (3 out of 10) in patients with identified mobile lesions. More recently, Swaminathan et al93 demonstrated the potential of atheroma disruption due to the “sandblasting” effect of CPB at the site of the aortic cannula. Injury involving atherosclerotic lesions not only can result in emboli during surgery but may also expose lipid-laden, pro-thrombotic material that could conceivably promote thrombus formation postoperatively after heparin neutralization. Finally, atherosclerosis of the ascending aorta identifies patients likely to have severe atherosclerosis of cerebral arteries who are prone to cerebral injury from hypoperfusion. For this reason, avoidance of significant hypotension during and after surgery may be prudent to avoid neurologic injury.
Lipid Embolism from Pericardial Suction Aspirate
Small capillary arteriolar dilations (“SCADs”) histologically staining positive for lipid material are found at autopsy in brain microvessels of humans dying after cardiac surgery.73 Experimental studies have suggested that the source of SCADs to be fat arising from the cardiotomy suction aspirate that is returned to the CPB reservoir.14 In a small study, dogs undergoing hypothermic CPB using cardiotomy suction were found to have more SCADs/cm2 tissue (n=5, 46.5±14.5, p=0.04) than dogs undergoing CPB without cardiotomy suction (n=3, 4.54±1.69), right heart only CPB (n=3, 1.77±0.77), or lower extremity CPB (n=2, 4.17±1.65 SCADs/cm2 tissue).14 During dog CPB, arterial line filtration was inefficient at decreasing SCADs during CPB compared with processing of cardiotomy suction aspirate with a cell saver (24±5 vs 11±3 SCADs/cm2 of tissue, p=0.02).56 These data have led to some investigators to advocate either discarding or processing pericardial aspirate with a cell saver. There are conflicting data from humans, though, on the efficiency of this strategy to remove lipids from aspirated pericardial blood. In a study of 20 patients, cell saver processing of blood led to a significant reduction in lipid material compared with filtration of the blood prior to return to the CPB reservoir (87% vs 47% reduction in fat/slide, p=0.0007).50 Others, though, have reported that dual filtration of cardiotomy blood (filters before and after a cardiotomy blood specific reservoir) prior to return to the CPB reservoir led to a greater reduction in blood fat content compared with cell saver processing (p<0.001).55 The risk/benefit ratio of these procedures are not well established. It is unclear whether processing of cardiotomy suction blood improves neurological outcomes in humans. Cell saver processing of large quantities of blood (e.g., unexplained bleeding or reoperative sternotomy) can deplete blood coagulation factors and platelets with ensuing coagulopathy. Platelet transfusions have been associated with worse outcomes including stroke in patients undergoing cardiac surgery.89 Thus the reduction in cerebral injury from one source (lipid emboli) by might be offset by other mechanisms of injury (platelet transfusion, hemodynamic instability due to blood loss).
Cerebral Hyperthermia
Hypothermia reduces the size of experimental brain infarction and leads to improved neurological outcomes and survival in victims of out-of-hospital cardiac arrest.7,40,72 Cerebral benefits occur with modest (2°C to 5°C) temperature reductions. While commonly used during CPB, there is little evidence for the neuroprotective effects of hypothermic CPB.47 Using the Cochrane Controlled Trials Register, Rees et al83 reviewed randomized clinical trials of hypothermia for patients undergoing first time or reoperative CABG surgery. There were no differences in the incidence of non fatal strokes with hypothermia versus normothermia (OR 0.68, 95% CI, 0.43 to 1.05), but there was a trend towards a higher rate of non stroke related perioperative deaths with hypothermia (OR 1.46, 95% CI, 0.9 to 2.37). The reviewers concluded that data was insufficient to make firm conclusions regarding the use of mild hypothermia for the prevention of neurological complications. These findings might be explained by inadvertent cerebral hyperthermia during re-warming from hypothermic CPB as well as in the postoperative period.38 Unrecognized cerebral hyperthermia, which can worsen ischemic brain injury, might occur due to the proximity of the aortic cannula to the cerebral vessels and because brain temperature may be underestimated by standard temperature monitoring.21,72 There are several proposed strategies for reducing cerebral hyperthermia during re-warming and after surgery, most notably the avoidance of full re-warming. Nathan et al77 reported that re-warming from hypothermic CPB (32°C) to a nasopharyngeal temperature of 34°C was associated with fewer cognitive deficits 1 week and 3 months after CABG surgery compared with re-warming to 37°C. In that study of 223 patients, the duration of mechanical ventilation, blood loss, blood transfusion rates, and re-operation rates for bleeding were not different between groups.
Hyperglycemia
Elevated serum glucose is common during cardiac surgery even in non-diabetics in part due to the stress response induced by CPB and hypothermia. Hyperglycemia (i.e., > 140 mg/dL) contributes to cerebral injury via several mechanisms including cellular acidosis, oxidative stress, increased blood–brain barrier permeability, and brain edema.47 Interest in strict perioperative glucose control was fostered by data showing lower infection rates by avoidance of hyperglycemia and by data showing improved outcomes in critically ill patients with intensive insulin infusions.31 98 In surgical ICU patients there was a 34% reduction in mortality (8% vs 4.6%, p=0.04) when glucose was kept between 80 to 110 mg/dL with intensive insulin infusions compared with standard therapy (insulin when glucose > 215 mg/dL).98 Improved outcomes were observed, though, only in patients requiring > 5 days of ICU admission. A subsequent trial using a similar protocol in medical ICU patients found lower morbidity but higher mortality in patients receiving intensive insulin treatment who required < 3 days of ICU admission compared with the standard treatment group.97 Mortality was lower with intensive insulin infusion when ICU length of stay was > 3 days.
The benefits of “tight” glucose control during cardiac surgery, particularly with regards to neurological outcomes, is less clear. In a prospective randomized trial, there were no differences in the frequency of neurological complications 6 weeks and 6 months after CABG surgery for patients receiving intraoperative insulin when glucose was > 100 mg/dL compared with insulin when glucose was >200 mg/dL.16 Serum glucose was difficult to maintain in that study, thus, leaving unanswered the question of whether euglycemia during cardiac surgery might improve neurological outcomes. In a randomized trial of 400 patients undergoing cardiac surgery, the frequency of the composite outcome of death, cardiac morbidity, stroke, or renal failure was higher for patients given an insulin infusion to maintain intraoperative glucose between 80–100 mg/dL compared with conventional treatment of insulin when glucose was > 200 mg/dL (p=0.02).32 While “tight glycemic control” may have other benefits for reducing infection, there are little data to support this strategy as a means for improving neurological outcomes from cardiac surgery.
Perioperative Anemia
Observational studies in humans suggest a link between low hematocrit on CPB and mortality after cardiac surgery.25,29,41 Habib et al41 further reported that hematocrit <22% on CPB was independently associated with stroke and other complications. In an analysis of data from 10,949 patients, Karkouti et al53 confirmed this finding showing that the odds of stroke increased 10% for each 1% decrease in hematocrit during CPB. Anemia may result in cerebral injury by reducing tissue oxygen delivery and/or by increasing embolic load due to higher cerebral blood flow. The brain compensates for reduced arterial oxygen content by increasing oxygen extraction and cerebral blood flow.22 In dogs, compensatory responses are sufficient to meet cerebral metabolic demand to a hematocrit of 15% at 28°C and a hematocrit of 18% at 28°C.22 Ischemic cerebral tissue, however, might be unable to increase oxygen extraction when hematocrit is < 30%.26 The limited data on transfusion triggers in adults recovering from CABG surgery do not address neurological outcomes.51 12 Mathew et al67 randomized 107 patients undergoing CABG surgery to a minimal CPB hematocrit of 27% or 15% to 17% (presented as an abstract). The study was interrupted by the data safety monitoring committee due to higher adverse event rates in the low hematocrit group, including greater cognitive decline 6 weeks after surgery in the lower hematocrit groups. While anemia during CPB is associated with risk for cerebral injury, whether red blood cell transfusion reduces this risk is unclear.
Atrial Fibrillation
Atrial fibrillation is common after cardiac surgery, affecting > 30% of patients with even higher rates reported after cardiac valve surgery and in older patients.46,69 This arrhythmia may occur any time perioperatively but it typically occurs on postoperative day 2 or 3. Multiple studies have documented an independent association between postoperative atrial fibrillation and postoperative stroke.46 A relation between atrial fibrillation and postoperative neurocognitive dysfunction has been further demonstrated.90 In a study of 2630 patients undergoing CABG surgery, Lahtinen et al60 found that atrial fibrillation preceded stroke in 19 of 52 (36.5%) patients with a cerebral ischemic event. These patients experienced an average of 2.5 episodes of atrial fibrillation before the stroke. In a multicenter observational study of 4657 CABG surgery patients, Mathew et al69 emphasized the importance of delineating the timing of postoperative atrial fibrillation in relation to cerebral events and other adverse outcomes. Atrial fibrillation was not associated with risk for postoperative stroke, but it was an independent predictor of encephalopathy and of a composite outcome that included changes in Mini-Mental State Exam score and increases in the National Institutes of Health Stroke Scale (higher score indicates worsening neurological function). Treatment with aspirin early after surgery might explain the lack of an association between atrial fibrillation and postoperative stroke in this cohort. While there have been numerous randomized trials of interventions to decrease the incidence of atrial fibrillation following cardiac surgery, a consistent reduction in stroke or encephalopathy rates is not demonstrated in these studies.
Genetic Predisposition
It has been suggested that genetic predisposition might explain the marked variability in individual susceptibility for cerebral injury from cardiac surgery despite similar risk. Tardiff et al94 was the first to show a relationship between risk of postoperative neurocognitive dysfunction presence of the apolipoprotein E ε4 allele. The apolipoprotein E (APOE) ε4 genotype is a plausible candidate gene since it has been shown to increase risk for Alzheimer’s disease and cognitive decline after cerebral injury.42,49,52,100 Apolipoprotein E is encoded by three alleles, ε2, ε3, and ε4 that occur respectively in 8%, 75%, and 17% of individuals of European decent..33 Apolipoprotein E has multiple functions in the central nervous system that might predispose to cerebral injury when the lipoprotein is abnormal.6,61,62,81 The mechanism by which apolipoprotein ε4 might increase risk for cognitive impairment is not known but it may be due in part to its relationship with advanced atherosclerosis.48 Data are inconsistent regarding the role of the apolipoprotein ε4 genotype in perioperative cerebral injury.2,4,43,87,91,94
Other investigators have examined for a relationship between polymorphisms of genes involved in pathways regulating coagulation, cell adhesion, and inflammation with perioperative cerebral injury. Grocott et al39 found that the combination of 2 minor alleles of C-reactive protein (CRP; 3′UTR 1846C/T) and interleukin-6 (IL-6; -174G/C) were significantly associated with stroke after cardiac surgery (odds ratio, 3.3; 95% CI, 1.4 to 8.1; P=0.0023). Mathew et al68 found that the PlA2 variant of the platelet glycoprotein IIIa was independently associated with decline from baseline on the mini-mental status examination after cardiac surgery. The C-reactive protein minor allele 1059G/C SNP (odds ratio, 0.37, 95% CI, 0.16 to 0.78; p = 0.013) and the P-selectin allele SELP 1087G/A allele (odds ratio, 0.51, 95% CI, 0.30 to 0.85; p = 0.011) were further found to be associated with decline in cognition 6 weeks after CABG surgery.70 These data suggest that risk stratification for perioperative cerebral injury based on genotype may become possible after validation of putative genes in diverse patient populations.75
Off-Pump CABG Surgery
Performing CABG surgery “off-pump” has been advocated to improve neurological outcomes by avoiding the hemodynamic, inflammatory, and microembolic perturbations associated with CPB. Support for this approach came initially from multiple non-randomized case series. In a prospective randomized trial comparing “on” versus “off” CPB CABG surgery, Van Dijk et al99 found no differences in neurocognitive outcomes early after surgery and after 1 year. In this trial of 281 patients, the mean age was nearly 61 years and the patients were generally at low risk for operative complications. In a follow-up study these investigators reported that cognitive outcomes at 5 years remained no different between patients randomized to surgery with or without CPB.27 In a meta-analysis of prospectively randomized clinical trials of “on” versus “off” pump CABG surgery, Cheng et al17 concluded that there are no reductions in the risk for stroke with off-pump CABG surgery, but there is insufficient evidence on whether the rates of neurocognitive dysfunction might be affected. Randomized trials thus far enrolled only low risk patients leaving unanswered the question of whether there are benefits to avoiding CPB in patients at high risk for cerebral injury. Non-randomized studies, indeed, suggest that stroke risk is lowered in such high risk patients by performing off-pump CABG surgery.8 Despite the theoretical advantages of off-pump CABG surgery, embolization of atheroma is still possible during aortic manipulations for proximal bypass graft anastamosis.58 Further, systemic hypotension combined with venous hypertension during cardiac manipulations for enhancing coronary artery exposure can lead to reduced cerebral perfusion pressure inadvertently predisposing to cerebral hypoperfusion. Consequently, the potential benefits of avoiding CPB might be offset by other mechanisms of cerebral injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. Acta Anaesthiol Scan. 2000;44:1246. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 2.Abildstrome H, Christiansen M, Siersma VD, et al. Apolipoprotein E genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology. 2004;101:855. doi: 10.1097/00000542-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JV, Blauth CI, Smith PL, et al. Demonstration of cerebral microemboli occurring during coronary artery bypass graft surgery using fluorescein angiography. J Audiovisual Media Med. 1990;13:87–90. doi: 10.3109/17453059009055108. [DOI] [PubMed] [Google Scholar]
- 4.Askar FZ, Cetin HY, Kumral E, et al. Apolipoprotein E epsilon 4 allele and neurobehavioral status after on-pump coronary artery bypass grafting. J Card Surg. 2005;20:501. doi: 10.1111/j.1540-8191.2005.2004138.x. [DOI] [PubMed] [Google Scholar]
- 5.Barbut D, Hinton RB, Szatrowski TP, et al. Cerebral emboli detected during bypass surgery are associated with clamp removal. Stroke. 1994;25:2398. doi: 10.1161/01.str.25.12.2398. [DOI] [PubMed] [Google Scholar]
- 6.Barger SW, AD H. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 8.Biancari F, Mosorin M, Rasinaho E, et al. Postoperative stroke after off-pump versus on-pump coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:169. doi: 10.1016/j.jtcvs.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Blauth CI, Arnold JV, Schulenberg WE, et al. Cerebral microembolism during cardiopulmonary bypass. Retinal microvascular studies in vivo with fluorescein angiography. J Thorac Cardiovasc Surg. 1988;95:668. [PubMed] [Google Scholar]
- 10.Blauth CI, Cosgrove DM, Webb BW, et al. Atheroembolism from the ascending aorta: an emerging problem in cardiac surgery. J Thorac Cardiovasc Surg. 1992;103:1104. [PubMed] [Google Scholar]
- 11.Borger MA, Ivanov J, Weisel RD, et al. Decreasing incidence of stroke during valvular surgery. Circulation. 1998;98:II137. [PubMed] [Google Scholar]
- 12.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 13.Brækken SK, Reinbang I, Russell D, et al. Association between intraoperative cerebral microembolic signals and postoperative neuropsychological deficit: comparison between patients with cardiac valve replacement and patients with coronary artery bypass grafting. J Neurol Neurosurg Psychiatry. 1998;65:573–576. doi: 10.1136/jnnp.65.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 15.Browne SM, Halligan PW, Wade DT, et al. Postoperative hypoxia is a contributing factor to cognitive impairment after cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1061. doi: 10.1016/s0022-5223(03)00616-0. [DOI] [PubMed] [Google Scholar]
- 16.Butterworth J, Wagenknecht LE, Legault C, et al. Attempted control of hyperglycemia during cardiopulmonary bypass fails to improve neurologic or neurobehavioral outcomes in patients without diabetes mellitus undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2005;130:1319. doi: 10.1016/j.jtcvs.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Cheng DCBD, Martin JE, Novick RJ. Evidence-Based Perioperative Clinical Outcomes Research Group.: Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology. 2005;102:188. doi: 10.1097/00000542-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Blauth CI. Macroemboli and microemboli during cardiopulmonary bypass. Ann Thorac Surg. 1995;59:1300. doi: 10.1016/0003-4975(95)00105-t. [DOI] [PubMed] [Google Scholar]
- 19.Cook DJ, Huston J, III, Trenerry MR, et al. Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 21.Cook DJ, Orszulak TA, Daly RC, et al. Cerebral hyperthermia during cardiopulmonary bypass in adults. J Thorac Cardiovasc Surg. 1996;111:268. doi: 10.1016/S0022-5223(96)70425-7. [DOI] [PubMed] [Google Scholar]
- 22.Cook DJ, Orszulak TA, RC D. Minimum hematocrit at differing cardiopulmonary bypass temperatures in dogs. Circulation. 1998;98:III170. [PubMed] [Google Scholar]
- 23.Croughwell ND, Newman MF, Blumenthal JA, et al. Jugular bulb saturation and cognitive dysfunction after cardiopulmonary by pass. Ann Thorac Surg. 1994;58:1702. doi: 10.1016/0003-4975(94)91666-7. [DOI] [PubMed] [Google Scholar]
- 24.Davila-Roman VG, Murphy SF, Nickerson NJ, et al. Atherosclerosis of the ascending aorta is an independent predictor of long-term neurologic events and mortality. J Am Coll Cardiol. 1999;33:1308. doi: 10.1016/s0735-1097(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 25.DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Ann Thorac Surg. 2001;71:769. doi: 10.1016/s0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 26.Dexter F, BJ H. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modeling study. Br J Anaesth. 1997;79:346. doi: 10.1093/bja/79.3.346. [DOI] [PubMed] [Google Scholar]
- 27.Diederik van Dijk M, PhD, Monique Spoor, MS, Ron Hijman, PhD, Hendrik M. Nathoe, MD, PhD, Cornelius Borst, MD, PhD, Erik WL Jansen, MD, PhD, Diederick E Grobbee, MD, PhD, Peter PT de Jaegere, MD, PhD, Cor J Kalkman., MD, PhD for the Octopus Study Group Cognitive and Cardiac Outcomes 5 Years After Off-Pump vs On-Pump Coronary Artery Bypass Graft Surgery. J Am Med Assoc. 2007;297:701. doi: 10.1001/jama.297.7.701. [DOI] [PubMed] [Google Scholar]
- 28.Djaiani G, Fedorko L, Borger M, et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356. doi: 10.1161/01.STR.0000138783.63858.62. [DOI] [PubMed] [Google Scholar]
- 29.Fang WC, Helm RE, Krieger KH, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96:II194. [PubMed] [Google Scholar]
- 30.Fong HK, Sand LP, JM L. The role of postoperative analgesia in delerium and cognitive decline in eldelry patients: A systematic review. Anesth Analg. 2006;102:1255. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 31.Furnary AP, Wu Y, SO B. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedrues: the Portland Diabetic Project. Endocr Pract. 2004;10:21. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi FY, Nuttall GA, Abel MD, et al. Intensive Intraoperative Insulin Therapy versus Conventional Glucose Management during Cardiac Surgery. Ann Intern Med. 2007;146:233. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 33.Gerdes LU, Klausen IC, Sihm I, et al. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations aroudn the world. Genet Epidemiol. 1992;9:155. doi: 10.1002/gepi.1370090302. [DOI] [PubMed] [Google Scholar]
- 34.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass: a randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 35.Goto T, Baba T, Honma K, et al. Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2001;72:137. doi: 10.1016/s0003-4975(01)02676-5. [DOI] [PubMed] [Google Scholar]
- 36.Gottesman RF, Hillis AE, Grega MA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol. 2007;64:E1. doi: 10.1001/archneur.64.8.noc70028. [DOI] [PubMed] [Google Scholar]
- 37.Gottesman RFSP, Grega MA, Yousem DM, Borowicz LM, Jr, Selnes OA, Baumgartner WA, McKhann GM. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 38.Grocott HP, Mackensen GB, Grigore AM, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537. doi: 10.1161/hs0202.102600. [DOI] [PubMed] [Google Scholar]
- 39.Grocott HP, White WD, Morris RW, et al. Genetic polymorphisms and the risk of stroke after cardiac surgery. Stroke. 2005;36:1854. doi: 10.1161/01.STR.0000177482.23478.dc. [DOI] [PubMed] [Google Scholar]
- 40.Group. THACAS: Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 41.Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed. J Thorac Cardiovasc Surg. 2003;125:1438. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 42.Henderson AS, Easteal S, Jorm AF, et al. Apolipoprotein E and m 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- 43.Heyer EJ, Wilson Da, Sahlein DH, et al. APOE-Epsilon 4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurol. 2005;65:1759. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.HL E. Protective effect of neuromonitoring during cardiac surgery. Ann NY Acad Sci. 2005;1053:12. doi: 10.1196/annals.1344.002. [DOI] [PubMed] [Google Scholar]
- 45.Hogue CW, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation. Circulation. 1999;100:642. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 46.Hogue CW, Jr, Creswell LL, Gutterman DD, et al. ACCP guidelines for the prevention and management of postoperative atrial fibrillation: Epidemiology, mechanisms, and risk. Chest. 2005;128:9S. doi: 10.1378/chest.128.2_suppl.9s. [DOI] [PubMed] [Google Scholar]
- 47.Hogue CW, Jr, Palin CA, JE A. Cardiopulmonary Bypass Management and Neurologic Outcomes: An Evidence-Based Appraisal of Current Practices Anesth Analg. 2006;103:21. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 48.Ilveskoski E, Perola M, Lehtimaki T, et al. Age-dependent association of apolipoprotein E genotype with coronary and aortic atherosclerosis in middle-aged men. Circulation. 1999;100:608. doi: 10.1161/01.cir.100.6.608. [DOI] [PubMed] [Google Scholar]
- 49.Isoniemi H, Tenovuo O, Portin R, et al. Outcome of traumatic brain injury after three decades-relationship with ApE genotype. J Neurotrauma. 2006;23:1600. doi: 10.1089/neu.2006.23.1600. [DOI] [PubMed] [Google Scholar]
- 50.Jewell AE, Akowuah EF, Suvarna SK, et al. A prospective randomised comparison of cardiotomy suction and cell saver for recycling shed blood during cardiac surgery. Eur J Cardiothorac Surg. 2003;23:633. doi: 10.1016/s1010-7940(02)00834-5. [DOI] [PubMed] [Google Scholar]
- 51.Johnson RG, Thurer RL, Kruskall MS, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardio-vasc Surg. 1992;104:307. [PubMed] [Google Scholar]
- 52.Jordan BD, Relkin NR, Ravdin LD, et al. Apolipoprotein E epsilon-4 associated with chronic traumatic brain injury in boxing. J Am Med Assoc. 1997;278:136. [PubMed] [Google Scholar]
- 53.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 54.Katz ES, Tunick PA, Rusinek H, et al. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70. doi: 10.1016/0735-1097(92)90139-e. [DOI] [PubMed] [Google Scholar]
- 55.Kaza AK, Cope JT, Fiser SM, et al. Elimination of fat microemboli during cardiopulmonary bypass. Ann Thorac Surg. 2003;75:555. doi: 10.1016/s0003-4975(02)04540-x. [DOI] [PubMed] [Google Scholar]
- 56.Kincaid EH, Jones TJ, Stump DA, et al. Processing scavenged blood with a cell saver reduces cerebral lipid microembolization. Ann Thorac Surg. 2000;70:1296. doi: 10.1016/s0003-4975(00)01588-5. [DOI] [PubMed] [Google Scholar]
- 57.Knipp SCMN, Wilhelm H, et al. Evaluation of brain injury after coronary artery bypass grafting. A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25:791. doi: 10.1016/j.ejcts.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Kotoh KFK, Doi T, Nagura S, Misaki T. Predictors of early postoperative cerebral infarction after isolated off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;83:1679. doi: 10.1016/j.athoracsur.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 59.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 60.Lahtinen J, Biancari F, Salmela E, et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241. doi: 10.1016/j.athoracsur.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 61.Laskowitz DT, Goel S, Bennett ER, et al. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 62.Laskowitz DT, Horsburgh K, AD R. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Leary MCCL. Technology Insight: brain MRI and cardiac surgery-detection of postoperative brain ischemia. Nature Clin Pract: Cardiovasc Med. 2007;4:379. doi: 10.1038/ncpcardio0915. [DOI] [PubMed] [Google Scholar]
- 64.Lilosky DS, Marrin CA, Caplan LR, et al. Determinatino of etiologic mechanism of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 65.Caplan LR. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolims, and ischemic stroke. Arch Neurol. 1998;55:1475. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 66.Mackensen GB, Ti LK, Phillips-Bute B, et al. Cerebral embolization during cardiac surgery: impact of aortic atheroma burdent. Br J Anaesth. 2003;91:656. doi: 10.1093/bja/aeg234. [DOI] [PubMed] [Google Scholar]
- 67.Mathew J, Grocott H, Phillips-Bute B, et al. Extreme hemodilution is associated with increased cognitive dysfunction in the elderly after cardiac surgery. Anesth Analg. 2004;98:SCA21. [Google Scholar]
- 68.Mathew J, Rinder CS, Howe JG, et al. Platelet PLA2 polymorphism enhances the risk of neurocognitive decline after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:663. doi: 10.1016/s0003-4975(00)02335-3. [DOI] [PubMed] [Google Scholar]
- 69.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. J Am Med Assoc. 2004;291:1720. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 70.Mathew JP, Podgoreanu MV, Grocott HP, et al. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 71.McKhann GM, Grega MA, Borowicz LM, Jr, et al. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37:562. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 72.Minamisawa H, Smith ML, BK S. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28:26. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 73.Moody DM, Brown WR, Challa VR, et al. Brain microemboli associated with cardiopulmonary bypass: a histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304. doi: 10.1016/0003-4975(95)00057-r. [DOI] [PubMed] [Google Scholar]
- 74.Moraca R, Lin E, Holmes JH, et al. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131:540. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Morgan TM, Krumholz HM, Lifton RP, et al. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. J Am Med Assoc. 2007;297:1551. doi: 10.1001/jama.297.14.1551. [DOI] [PubMed] [Google Scholar]
- 76.Murkin JM, Farrar JK, Tweed A, et al. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of PaCO2. Anesth Analg. 1987;66:825. [PubMed] [Google Scholar]
- 77.Nathan HJ, Wells GA, Munson JL, et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a randomized trial. Circulation. 2001;104:I–85. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 78.Newman MF, Grocott HP, Mathew JP, et al. Report of the substudy assessing impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874. doi: 10.1161/hs1201.099803. [DOI] [PubMed] [Google Scholar]
- 79.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 80.Novistky D, Boswell BB. Total myocardial revascularization without cardiopulmonary bypass utilizing computer-processed monitoring to assess cerebral perfusion. Heart Surg Forum. 2000;3:198. [PubMed] [Google Scholar]
- 81.Pepe MG. Apolipoprotein E is a biologically active constituent of the normal immunoregulatory lipoprotein, LDL. Int J Immunol. 1986;136:3716. [PubMed] [Google Scholar]
- 82.Pugsley W, Klinger L, Paschalis C, et al. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393. doi: 10.1161/01.str.25.7.1393. [DOI] [PubMed] [Google Scholar]
- 83.Rees K, Beranek-Stanley M, Burke M, et al. (Cochrane Review) Chichester, UK: John Wiley & Sons; 2004. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Vol Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting. Stroke. 2002;33:2909–2915. doi: 10.1161/01.str.0000040408.75704.15. [DOI] [PubMed] [Google Scholar]
- 85.Ribakove GH, Katz ES, Galloway AC, et al. Surgical implications of transesophageal echocardiography to grade the atheromatous aortic arch. Ann Thorac Surg. 1992;53:758. doi: 10.1016/0003-4975(92)91431-8. [DOI] [PubMed] [Google Scholar]
- 86.Roach GW, Kanchuger M, Mora-Mangano C, et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335:1857. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 87.Robson MJ, Alston RP, Andrews PJ, et al. Apolipoprotein E and neurocognitive outcome from coronary artery surgery. J Neurol Neurosurg Psych. 2002;72:675. doi: 10.1136/jnnp.72.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selnes OA, Grega MA, Borowicz LM, et al. Cognitive outcomes three years after coronary artery bypass surgery: a comparison of on-pump coronary artery bypass graft surgery and nonsurgical controls. Ann Thorac Surg. 2005;79:1201. doi: 10.1016/j.athoracsur.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Spiess BD, Royston D, Levy JH, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 90.Stanley TO, Mackensen GB, Grocott HP, et al. The impact of postoperative atrial fibrillation on neurocognitive outcome after coronary artery bypass graft surgery. Anesth Analg. 2002;94:290. doi: 10.1097/00000539-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 91.Steed L, Kong R, Stygall J, et al. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71:823. doi: 10.1016/s0003-4975(00)02511-x. [DOI] [PubMed] [Google Scholar]
- 92.Stump DA, Rogers AT, Hammon JW, et al. Cerebral emboli and cognitive outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:113. doi: 10.1016/s1053-0770(96)80186-8. [DOI] [PubMed] [Google Scholar]
- 93.Swaminathan M, Grocott HP, Mackensen GB, et al. The "sandblasting" effect of aortic cannulation on arch atheroma during cardiopulmonary bypass. Anesth Analg. 2007;104:1350. doi: 10.1213/01.ane.0000264090.24756.08. [DOI] [PubMed] [Google Scholar]
- 94.Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. Ann Thorac Surg. 1997;64:715. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 95.Tunick PA, Perez JL, I K. Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423. doi: 10.7326/0003-4819-115-6-423. [DOI] [PubMed] [Google Scholar]
- 96.Ura M, Sakata R, Nakayama Y, et al. Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol. 2000;35:1303. doi: 10.1016/s0735-1097(00)00548-9. [DOI] [PubMed] [Google Scholar]
- 97.van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 98.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 99.Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery. J Am Med Assoc. 2002;287:1405. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 100.Wang JC, Kwon JM, Shah P, et al. Effect of APOE genotype and promoter polymorphism on risk of Alzheimer’s disease. Neurol. 2000;55:1644. doi: 10.1212/wnl.55.11.1644. [DOI] [PubMed] [Google Scholar]
- 101.Wareing TH, Dávila-Román VG, Barzilia B, et al. Management of the severely atherosclerotic ascending aorta during cardiac operations. J Thorac Cardiovasc Surg. 1992;103:453. [PubMed] [Google Scholar]


