Abstract
We sought to determine whether coherent networks which circumvent lesioned cortex are seen in patients with ideomotor apraxia (IMA) while performing tool use pantomimes. Five normal subjects and five patients with IMA (three patients with corticobasal degeneration and two with left hemisphere stroke) underwent 64-channel EEG recording while performing three tool-use pantomimes with their left hand in a self-paced manner. Beta band (20–22 Hz) coherence indicates that normal subjects have a dominant left hemisphere network responsible for praxis preparation, which was absent in patients. Corticobasal degeneration patients showed significant coherence increase between left parietal - right premotor areas. Left hemisphere stroke patients showed significant coherence increases in a right parietofrontal network. The right hemisphere appears to store useable praxis representations in IMA patients with left hemisphere damage.
Performance of pantomiming tool use and communicative gestures with spatial and temporal errors is called ideomotor apraxia (IMA). There are two common etiologies of IMA, corticobasal degeneration (CBD) and stroke. CBD patients typically have neurodegeneration in frontal cortex and subcortical structures including basal ganglia [24]. Movement disorders commonly encountered in CBD such as tremor and dystonia may interfere with the performance of these tasks. Yet, disorganized motor plans such as making large circular arcs in the air when asked to pantomime using a toothbrush are evident, are not indicative of elementary motor disorders. Stroke patients with left hemisphere lesions suffering from IMA will have the same errors types as those patients with CBD [14].
Using beta band coherence analysis of EEG, we previously showed evidence of left hemisphere networks responsible for right hand praxis in normal subjects [22]. Damage to left hemisphere parietal or premotor cortex or the white matter connecting them may cause IMA [11]. While patients may regain praxis function [1, 21], the neural mechanisms are unclear. The right hemisphere may play a role in assisting in praxis rehabilitation, as it has been shown to be involved with other types of cognitive task rehabilitation (for example [2, 5]). It remains unclear to what extent the right hemisphere actually stores praxis movement representations, as left hemisphere pathology results in the most severe disturbances of praxis [12], even though it is clear that anatomical connectivity between the right hemisphere parietofrontal areas exists, and that the left and right parietal areas are anatomically connected [3, 4, 17]. Knowing this, it is unclear if networks using right hemisphere areas to circumvent lesioned left cortex are functional for praxis. EEG coherence analysis may be useful in determining patterns of brain networks involved in this task. Coherence allows detection of activities in electrode pairs at frequencies that are phase-consistent over many trials. When there is a plausible neuroanatomical basis, one may infer functional connectivity between regions where coherence values are significantly above baseline. Moreover, a loss of coherence may suggest diminished connectivity.
Our aim in this exploratory study is to evaluate whether coherent right hemisphere networks relevant to praxis performance exist in patients with IMA. Based on the findings of beta band coherence in normal subjects on praxis tasks [22], we hypothesize that in normal right-handed subjects using the left arm to perform praxis, we will see a dominant left hemisphere network. In patients, a right (intact) hemisphere network will be seen, suggesting storage in the right hemisphere to facilitate praxis, implying function to known right hemisphere parietofrontal networks.
The Institutional Review Board of the National Institute of Neurological Disorders and Stroke approved this study, and all subjects gave informed consent. The inclusion criteria were right-handed normal subjects and patients with either left hemisphere cortical stroke or primarily left-sided corticobasal degeneration. The normal subjects had both a normal neurologic examination and no history of neurologic disorders. For patients, we excluded those with a second neurologic disorder, previous brain lesions, and cognitively impaired subjects.
Three right-handed CBD and two right-handed stroke patients were recruited from the Human Motor Control Section, Stroke Clinic, and Cognitive Neuroscience Section, both of NINDS, NIH (Bethesda, MD) and National Rehabilitation Hospital (Washington, DC). Table 1 lists the area of lesion for the patients as assessed by MRI. All patients had IMA, based on the Florida Apraxia Battery [20]. CBD and stroke patients underwent a standard neurological evaluation to ensure that movement disorders or cognitive deficiency would not interfere with normal praxis function or be confused with apraxia. Patients practiced three tool-use pantomimes (use of hammer, scissors and screwdriver) over a one-half day training session to achieve performance similar to that of the normal subjects. Tool use pantomime was specifically studied as it was deficient in all patients, and is common in IMA [13]. Practice sessions were necessary for several reasons. One, the apraxia patients each had different types of errors, which would not allow for valid comparisons. Two, accurate description of these errors would be challenging. Three, any comparison of normal movements with severely disordered movements would provide data that would be difficult to interpret. Four, we needed to ensure that all patients had a level of effort when executing the tasks similar to that of the normal subjects. Each practice session consisted of verbal communication by the investigator (LW) to facilitate proper pantomimes. All movements were made with the left hand since the right hand was paretic in each patient. Once the movement had a consistent normal appearance (as deemed by observation of the investigators), the EEG study began.
Table 1.
Study population
| Diagnosis | Patient | Age | Sites of brain lesion estimated from MRI | Other motor deficits | Duration |
|---|---|---|---|---|---|
| CBD | 1 | 60 | Mild left frontal cerebral atrophy, scattered lesions of left frontal, periventricular and pontine white matter | Mild disturbances in walking, right hand hemiparesis | 2 years |
| 2 | 68 | Left hemisphere cerebral atrophy, mostly involving left frontal lobe and mild atrophy of left temporal lobe | Right hand hemiparesis | 5 years | |
| 3 | 63 | Mild left frontal atrophy | Right arm hemiparesis | 4 years | |
| Stroke | 1 | 55 | Ischemic stroke in left MCA distribution, involving left frontal lobe, inferior left parietal and temporal cortices, mildly affecting left insula and basal ganglia | Right arm hemiparesis | 5 years |
| 2 | 35 | Hemorrhagic stroke in left MCA distribution, involving frontal ventral white matter, temporal and parietal cortices, as well as basal ganglia | Right arm hemiparesis, shuffling gait, mild oral apraxia | 3 months |
Clinical profiles of the patients studied. Duration is the time interval from the clinical onset. MCA, middle cerebral artery.
Because we studied non-dominant hand activation in the patient group, we also aimed to identify the activations seen with movements of the non-dominant hand in normal subjects. Five right-handed normal subjects age-matched to the patient population were studied for this portion of the experiment. A short training session was used to ensure proper task performance. Normal subjects required little or no training because their movements were unimpaired. The left hand was tested in the normal subjects to match the constraint of the patient studies.
All subjects were instructed to briskly perform the practiced tool-use pantomime praxis movements (use of hammer, scissors and screwdriver) with the left hand with two repetitions (e.g., pretend to hit a nail with a hammer twice) with the distal arm in the same manner as previous studies [23] (self-paced repetitions, 10–15s between movements). Each type of praxis movement was performed for two 7-min blocks, for a total of 6 blocks. All participants were monitored by 2 of the investigators (LW and SV) during the entire 7-min block to ensure proper task performance (Table 2). Praxis movements were averaged together to characterize tool use movements.
Table 2.
Apraxia scale during the study
| Hammer | Scissors | Screwdriver | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Training | Block 1 | Mid | Block 2 | Before Training | Block 1 | Mid | Block 2 | Before Training | Block 1 | Mid | Block 2 | |
| CBD | ||||||||||||
| 1 | 1 | 2 | 3 | 4 | 0 | 3 | 3 | 3 | 1 | 3 | 4 | 3 |
| 2 | 0 | 1 | 3 | 3 | 1 | 2 | 3 | 2 | 1 | 2 | 3 | 3 |
| 3 | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 2 | 3 | 3 | 3 |
| Stroke | ||||||||||||
| 1 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 3 |
| 2 | 1 | 2 | 3 | 3 | 1 | 3 | 2 | 3 | 2 | 3 | 3 | 4 |
| Control | ||||||||||||
| 1 | 4 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 3 | 4 | 4 |
| 2 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 4 | 4 |
| 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
Results of the rating scale for praxis performance evaluated before the training session, during the first block of repetition of the movement during the EEG study which took place after training (block1), between the first and second blocks (mid), and during the last block (block 2). The scale is defined as follows: 0, no recognizable movement; 1, movement present, but hard to decipher, prolonged with pauses; 2, movement with moderately severe temporal and spatial errors; 3, movement fair, but with any of the following errors: temporal or spatial errors, context errors, slightly prolonged movement sequences; 4, movement error-free.
64-channel EEG (DC-100 Hz, 1 kHz sampling rate, linked ear reference) was recorded using Synamps1 and NeuroScan 4.2 Acquisition Software (Compumedics, El Paso, TX). Surface electromyography (EMG) was recorded (5–200 Hz) from the left abductor pollicis brevis (APB) and flexor carpi ulnaris (FCU) muscles. EEG signal was bandpass filtered offline to DC-50 Hz and marked to indicate EMG onset. Epochs were made from −3.5 s (before movement onset) to 0 s (at movement onset). Upon visual inspection, trials with blinks or other artifacts were removed. Approximately 150 artifact-free trials were acquired for each participant and analyzed for coherence using in-house functions in Matlab (Mathworks, Natick, MA). Beta band (20 – 22 Hz) coherence was chosen for this study based on findings of the preliminary channel pair coherence analysis which revealed consistent changes in coherence within this bandwidth across subjects. Analysis was made for coherence using methodology previously described [22]. Briefly, coherence is a measure of the linear dependency of two signals at a specific frequency. If zi (ω) and zj (ω) are the respective (complex valued) Fourier transforms of channels i and j, then the cross-spectrum Bij (ω) is defined as
| (1) |
where * denotes complex conjugation and 〈 〉 denotes ‘expected value’, i.e. the hypothetical average over an infinite number of samples. The complex valued ‘Coherency’ Cij (ω) is now simply the normalized cross-spectrum
| (2) |
In the case of event-related coherency, as done here, each epoch is divided into 512 ms, with a Hanning window characterized by the time t of its center. The absolute value of coherency (coherence) is always between 0 (no coherence) and 1 (perfect coherence). If a baseline is subtracted, coherence has values from −1 to +1, with increases (relative to baseline) in coherence being positive and decreases (relative to baseline) in coherence being negative.
Coherence values are relative to a subtracted baseline of the first 512 ms of the epoch. All electrodes were used to make topographic maps of coherence. Based on our hypothesis, six coherence pathways were assessed: intrahemispheric parietofrontal (left parietal – left premotor, right parietal – right premotor), interhemispheric parietofrontal (left parietal – right premotor, right parietal – left premotor), and interhemispheric homologous (left premotor – right premotor, left parietal – right parietal). Electrodes used were as follows: left parietal, P3; right parietal, P4; left premotor, F3; right premotor, F4. Analysis was done on the pre-movement time periods only because kinematic differences in the movements may exist that could not be accounted for which could affect the results. Stroke and CBD patients were analyzed separately, as their lesion profiles varied extensively. Because subjects were few and differed from each other, we only examined increases in significance of each group separately. Thus, the results are intended to be exploratory. Only values significantly (p = 0.05) above the 512 ms baseline, as computed by the two tailed t-statistic, are displayed in head plots to avoid consideration of spurious interactions. A significance threshold for a significant change in coherence was assessed as ± 2 S.D. from the zero baseline period (normal subjects = 0.09; CBD 1 = 0.12, CBD2 = 0.11, CBD3 = 0.13; stroke 1 = 0.13; stroke 2 = 0.16). Values exceeding this limit were considered significant increases in coherence. Because of the descriptive nature of the study, no corrections for multiple comparisons were made.
Behavioral data (Table 2) illustrate an increase in ability to perform the praxis movements after practice in each patient. Normal subjects could perform the movements accurately before practice, which continued for the duration of the study.
Normal subjects showed a pattern of coherence only within the left hemisphere parietofrontal network (Figure 1) similar to what we reported previously for the right hand [22]. Significant coherence increases to a magnitude 0.2 occurred at −1.8 s and continued for the pre-movement period. Figure 2A (top) shows the spatial pattern for the coherence for the left premotor area.
Figure 1.
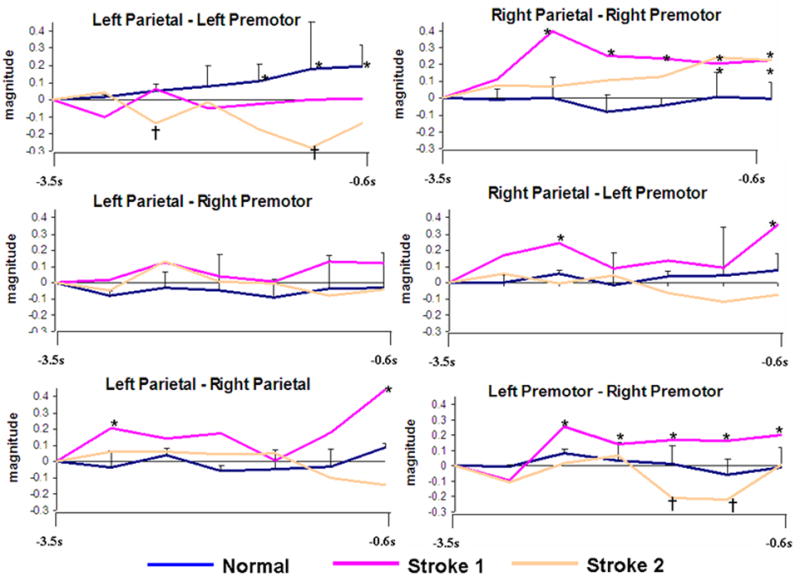
Time-magnitude plots of individual (stroke) and mean coherence (normal subjects) divided into each network of interest relative to movement onset (s). Standard error bars represent the distribution of normal subject coherence. Asterisks (*) indicate significant increases, † denotes significant decreases.
Figure 2.
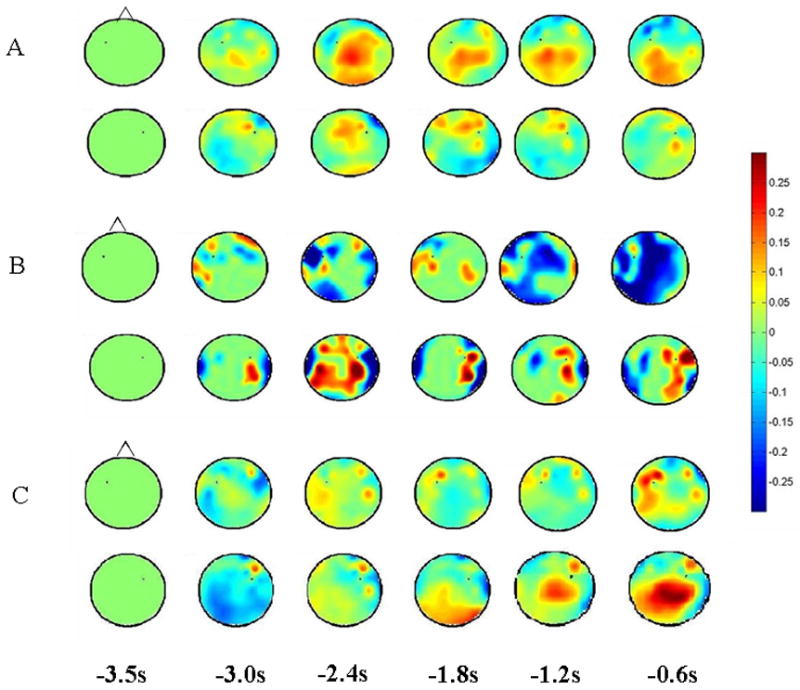
Head plots of coherence relative to left and right premotor cortex in normal subjects (A), stroke patient #2 (B) and CBD patient #3 (C). A, B, and C (top) shows coherence relative to the electrode over the left premotor cortex. A, B, and C (bottom) shows coherence relative to the electrode over the right premotor cortex. Time (s) is relative to EMG onset. Coherence increases are shown in red; decreases in blue.
Figure 1 illustrates the coherence increases for the stroke patients individually.
In the first stroke patient (left frontal and left inferior parietal lesion), an early increase in coherence was present in a right parietofrontal network at 2.4 s before movement (magnitude = 0.39), and remained above baseline for the duration of preparation (Figure 1). However, the magnitude fell during this period to 0.21 at −0.6 s. In a bilateral parietal network, brief significance was at −3.2 s (magnitude = 0.21), and again became significant at −1.2 s (magnitude = 0.18) until the end of preparation (magnitude = 0.45). In the bilateral premotor network, the first stroke patient showed increased coherence beginning at −2.4 s, which was maintained for the duration of the preparation phase. There was no significant increase within the left parietofrontal network.
For the second stroke patient (left frontal, temporal and parietal stroke), the left parietofrontal network showed a significant decrease in coherence which began at −2.5 s (magnitude = −0.16), then returned to baseline levels, reached a significant decrease again at −1.2 s (magnitude = −0.17; Figure 2B, top). A right parietofrontal network was seen which showed significant coherence increases at −1.2 s (magnitude = 0.13), and remained throughout preparation (Fig 1 and 3, bottom). In the bilateral premotor network, significant decreases in coherence were seen from −1.6 – −1.2 s before movement.
Figure 3.
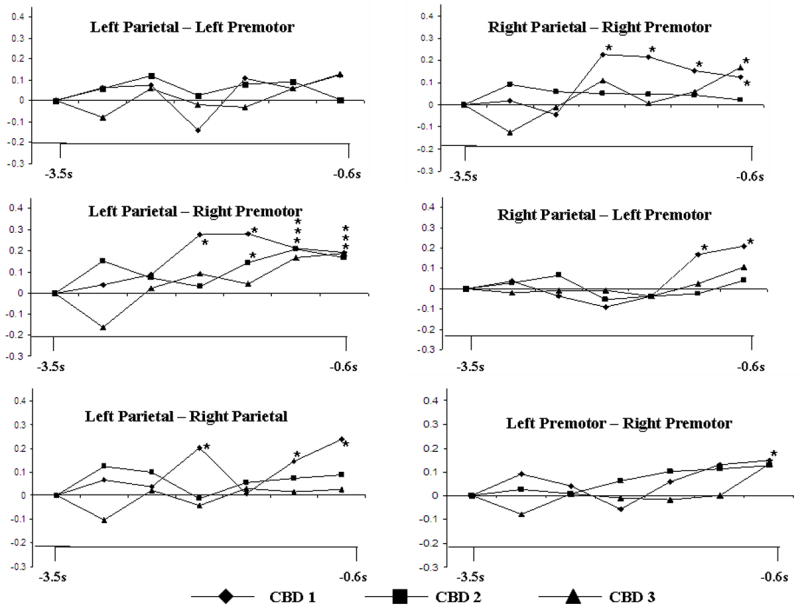
Time-magnitude plot of individual coherence values for patients with CBD for each network of interest for all participants relative to movement onset (s). Asterisks (*) indicate significant increases.
Figure 3 illustrates the network changes for each of the CBD patients. CBD 1 showed significant coherence increases in a left parietal-right premotor network beginning at −2.0 s at a magnitude of 0.28. This significant increase continued for the duration of the pre-movement time period. There was a coherence increase within the right parietofrontal network beginning at −2.0 s (magnitude = 0.25), which remained significant. An increase in coherence was seen between the bilateral parietal hemispheres at −2.0 s, which decreased, then regained significance at −1.2 s. Significant bilateral premotor coherence was noted only just before movement onset (magnitude = 0.15). There was no significant increase in the left parietofrontal network.
CBD 2 showed significance coherence increase between the left parietal – right premotor areas. Significance was first seen at −1.6 s (magnitude = 0.15, which persisted for the duration of the pre-movement period. No other networks of interest showed significant increases in coherence.
CBD 3 showed significant increases in coherence between the left parietal – right premotor area. Significance was first seen at −1.2 s (magnitude = 0.18), and persisted for the duration of the pre-movement period (figure 2C). As well, significant coherence increase was also seen in the right parietofrontal network, but only just before movement onset (−0.6 s, magnitude = 0.19). No other networks of interest showed significant increases in coherence.
Several combinations of connectivity may exist to contribute to praxis planning. The left parietal cortex has connections with right parietal and bilateral premotor cortices in primates [3, 4] and anatomical connections link the bilateral premotor areas [17]. Normal subjects only showed coherence increases within the left hemisphere parietofrontal network (Figure 2A) similar to that seen with right (dominant) hand movements [22]. None of the patients showed any increases in this network. Patient groups showed coherence increases involving the non-lesioned homologous cortex, suggesting that the undamaged right hemisphere parietal and/or frontal areas assist in planning praxis in these pathologies.
Our aim was to evaluate whether patients use the intact, non-dominant right hemisphere networks in cognitive motor control. Structures such as the basal ganglia and cerebellum could be involved [7], but could not be evaluated using EEG. It is possible that non-apraxic stroke patients with similar lesion localizations may have use of right hemisphere pathways. It remains unclear whether this is the case, as the notion of right hemisphere parietofrontal representations of praxis is unclear [11, 12]. Our aim was not directed at lasting praxis rehabilitation, thus the effect seen may be a result of short term task training which requires evaluation in larger longitudinal studies. Knowledge that right hemisphere networks are used suggests that praxis representations may not be completely lost with left hemisphere damage.
Pioneers of apraxia research, Liepmann and Geschwind, hypothesized retrieval of praxis function from storage in the right hemisphere [10, 15]. Rapcsak and colleagues indicated that “the right hemisphere has an impoverished store of visuokinesthetic engrams, or that intrahemispheric connections between the engrams and motor areas are less well developed in the right hemisphere” [19]. This concept has been shown for aphasia [5] and acalculia [2]. Neuroimaging work also supports this hypothesis in patients with left premotor lesions, which establish right premotor activity [16]. If the same principles apply in this study, a patient may have access to “engrams” useful for not only intransitive gestures and actual tool use [19], but also tool-use pantomime.
Another possibility is perilesional change, which may be a tenable hypothesis for improvement of function [8]. If so, we may see increases in significance to a region of the injured brain which may be spatially incongruent (but consistent within the general cortical area) with what is seen in normal subjects. This was not apparent. For example, if a left hemisphere parietofrontal network utilizing parietal perilesional areas existed in stroke patient #2 (Figure 2B, top), we would expect to see a pattern of increased coherence from the remaining parietal cortex to the intact frontal cortex. This was not seen. Unfortunately, determining which electrodes record from the brain adjacent to the lesion is unclear. Considering the nature of EEG and coherence, volume conduction of an active source should be detected in nearby electrodes [18]. Because we saw no spatial evidence of stable increases, we suspect that this network is no longer functioning.
The right parietal cortex either stores the representations of these movements or acquires them from its contralateral homologue. Aside from the fluctuating values in the first stroke patient, there was no clear and consistently increased bilateral parietal coherence which may indicate right hemisphere praxis representations before the stroke. The right hemisphere may contain praxis representations useful in recovery, but this needs better evaluation in longitudinal studies aimed at recovery.
The present result indicates that there is potential for coherent networks, including the non-dominant right hemisphere in patients performing praxis. Whether this potential neuronal mechanism is permanent is unclear, as the right hemisphere may not be optimal for lasting recovery [6]. Further analyses of other patients with IMA, perhaps longitudinal studies of recovery, are worthwhile to assess the duration of network changes.
Acknowledgments
This research was supported by Intramural Research Program, NINDS, NIH. Thanks to Drs. Edward Healton and Brendan Conroy, National Rehabilitation Hospital, Washington D.C., for assistance with patient accrual. Special thanks to Devera Schoenberg, M.Sc., for editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basso A, Capitani E, Della Sala S, Laiacona M, Spinnler H. Ideomotor apraxia: a study of initial severity. Acta Neurol Scand. 1987;76:142–6. doi: 10.1111/j.1600-0404.1987.tb03557.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernal B, Ardila A, Altman N. Acalculia: an fMRI study with implications with respect to brain plasticity. Int J Neurosci. 2003;113:1505–23. doi: 10.1080/00207450390231545. [DOI] [PubMed] [Google Scholar]
- 3.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 4.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–45. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 5.Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, Fabrizio KS, Peck KK, Soltysik D, Milsted C, Briggs RW, Conway TW, Gonzalez Rothi LJ. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17:392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- 6.Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–7. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 7.Fregni F, Pascual-Leone A. Hand motor recovery after stroke: tuning the orchestra to improve hand motor function. Cogn Behav Neurol. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–58. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 9.Fridman EA, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Wheaton L, Wu T, Hallett M. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–28. doi: 10.1016/j.neuroimage.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 11.Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–13. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- 12.Halsband U, Schmitt J, Weyers M, Binkofski F, Grutzner G, Freund HJ. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: a perspective on apraxia. Neuropsychologia. 2001;39:200–16. doi: 10.1016/s0028-3932(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 13.Hanna-Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, Foundas AL. Praxis lateralization: errors in right and left hemisphere stroke. Cortex. 2001;37:219–30. doi: 10.1016/s0010-9452(08)70569-0. [DOI] [PubMed] [Google Scholar]
- 14.Hanna-Pladdy B, Heilman KM, Foundas AL. Cortical and subcortical contributions to ideomotor apraxia: analysis of task demands and error types. Brain. 2001;124:2513–27. doi: 10.1093/brain/124.12.2513. [DOI] [PubMed] [Google Scholar]
- 15.Liepmann H. Die linke Hemisphare und das Handeln. Munchener Medizinische Wochenschrift. 1905;49:2322–2326. 2365–2378. [Google Scholar]
- 16.Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage. 2004;21:924–35. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 17.McGuire PK, Bates JF, Goldman-Rakic PS. Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb Cortex. 1991;1:390–407. doi: 10.1093/cercor/1.5.390. [DOI] [PubMed] [Google Scholar]
- 18.Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ. EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol. 1997;103:499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 19.Rapcsak SZ, Ochipa C, Beeson PM, Rubens AB. Praxis and the right hemisphere. Brain Cogn. 1993;23:181–202. doi: 10.1006/brcg.1993.1054. [DOI] [PubMed] [Google Scholar]
- 20.Rothi LG, Raymer AM, Ochipa C, Maher L, Greenwald M, Heilman KM. Florida Apraxia Battery, experimental edition. 1992 [Google Scholar]
- 21.Sunderland A, Sluman SM. Ideomotor apraxia, visuomotor control and the explicit representation of posture. Neuropsychologia. 2000;38:923–34. doi: 10.1016/s0028-3932(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 22.Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallett M. Synchronization of parietal and premotor areas during preparation and execution of praxis hand movements. Clin Neurophysiol. 2005;116:1382–90. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Wheaton LA, Shibasaki H, Hallett M. Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol. 2005;116:1201–1212. doi: 10.1016/j.clinph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Zadikoff C, Lang AE. Apraxia in movement disorders. Brain. 2005;128:1480–97. doi: 10.1093/brain/awh560. [DOI] [PubMed] [Google Scholar]


