Abstract
In this work, we describe the isolation of a new cDNA encoding an NADP-dependent isocitrate dehydrogenase (ICDH). The nucleotide sequence in its 5′ region gives a deduced amino acid sequence indicative of a targeting peptide. However, even if this cDNA clearly encodes a noncytosolic ICDH, it is not possible to say from the targeting peptide sequence to which subcellular compartment the protein is addressed. To respond to this question, we have transformed tobacco plants with a construct containing the entire targeting signal-encoding sequence in front of a modified green fluorescent protein (GFP) gene. This construct was placed under the control of the cauliflower mosaic virus 35S promoter, and transgenic tobacco plants were regenerated. At the same time, and as a control, we also have transformed tobacco plants with the same construct but lacking the nucleotide sequence corresponding to the ICDH-targeting peptide, in which the GFP is retained in the cytoplasm. By optical and confocal microscopy of leaf epiderm and Western blot analyses, we show that the putative-targeting sequence encoded by the cDNA addresses the GFP exclusively into the mitochondria of plant cells. Therefore, we conclude that this cDNA encodes a mitochondrial ICDH.
A common feature within both plant and animal kingdoms is the presence of isoenzymes encoded by multi-gene families. Often they are located in different subcellular compartments within the same cell, each isoenzyme being exposed to different chemical environments and each participating in different metabolic pathways. With the development of molecular biology, an ever increasing number of cDNAs encoding different isoforms of a given protein have been described. The establishment of a correspondence between a cDNA and the isoenzyme it encodes is primordial in determining the physiological role(s) of the protein (1, 2). In general, the most used criterium is the suggestion of a putative-targeting peptide (as deduced from the nucleotide sequence) followed by a study of its composition; however, this kind of analysis is often inconclusive (3–5).
The assimilation of ammonia is a good example of a plant metabolic pathway in which differently located isoforms are implicated (6–9). Ammonia produced from nitrogen fixation, nitrate reduction, or photorespiration is incorporated into an organic compound by the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway (10). To be active, this cycle requires a continuous supply of 2-oxoglutarate, which is synthesized by the action of isocitrate dehydrogenase. Two different enzymes catalyzing the oxidative decarboxylation of isocitrate to 2-oxoglutarate can be distinguished by their cofactor specificity: An NAD-dependent form (IDH; E.C.1.1.1.41) is located exclusively in the mitochondria where it plays a role in the Krebs cycle, and several NADP-dependent forms (ICDH; E.C.1.1.1.42) are located in cytosol, mitochondria, chloroplasts, and peroxisomes (see ref. 9 for a review); as yet, their physiological roles have not been elucidated. Although it has been proposed that the cytosolic ICDH isoform is responsible for producing the 2-oxoglutarate necessary for ammonia assimilation (11), proof is still lacking. Up until now, several plant ICDH-encoding cDNAs have been described in the literature (12–14), but they all resemble the tobacco cytosolic isoform (2).
A recent and potentially powerful tool for studying the in vivo subcellular localization of a protein is the green fluorescent protein (GFP). An obvious advantage of this reporter protein is that the fluorescence emission does not require any added cofactor or substrate, and it is not necessary to destroy the tissue to visualize the fluorescence emission. Because the fluorescence emission of wild-type GFP is poorly detectable in a number of plant species including tobacco, several GFP mutants have been created (15–18) to make this protein a suitable marker in plant systems. Major modifications include altering the codon usage to be optimal for humans, maize, and Arabidopsis, and thus eliminating a cryptic intron site and changing the amino acid composition of the chromophore, e.g., 65-SYG–65-TYG. Such modifications have been carried out by J. Sheen and coworkers (15), and the resulting GFP [sGFP(S65T)] gives a 120-fold brighter signal than the original GFP in plant cells. This result has led to several examples of brightly fluorescent transgenic tobacco lines transformed with a modified GFP (16, 18).
In this work, we report the isolation and characterization of the first plant cDNA encoding a noncytosolic ICDH. A modified GFP has been used to study the subcellular localization of this isoform. A chimeric gene encoding the putative-targeting sequence of the ICDH fused to the coding sequence of the sGFP(S65T) mutant has been used to transform tobacco plants. The analysis of the resulting transgenic plants shows that the modified GFP is exclusively located in the mitochondria of plant cells. Therefore, we conclude that the cDNA described in this work encodes a mitochondrial isoform of the plant enzyme.
MATERIALS AND METHODS
Isolation of cDNA Clones.
A partial cDNA was isolated from a tobacco cell suspension library constructed in λZAPII as described in ref. 2. A full-length cDNA named pST5 was obtained after rescreening the library with the homologous probe. Labeling with [α-32P]dCTP was realized by using the Nonaprimer labeling kit (Appligene, Strasbourg, France). Positive clones were sequenced by using the T7Sequencing Kit (Pharmacia) and synthetic oligonucleotides as primers. All methods concerning restriction enzyme digestion, ligation, and transformation of bacterial cells were as described elsewhere (19).
Construction of Chimeric Genes.
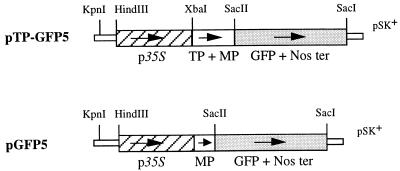
pTP-GFP5 was made as follows: A HindIII/XbaI fragment containing the 800-bp 35S-cauliflower mosaic virus promoter from pBI221 (CLONTECH) was cloned into pBluescript SK+ (pSK+, Stratagene) to give pSK35S. A 414-bp XbaI fragment encoding the targeting peptide and the first 38 amino acids of the mature ICDH protein was obtained from pST5 and cloned into pSK35S to give pSK35S-TP–MP. The correct orientation was shown by DNA sequencing. To clone the GFP in phase with 35S-TP–MP, sGFP (S65T) was PCR-amplified from pSK-sGFP-TYG-nopaline synthetase terminator (15) by using Pfu polymerase, a 5′ primer containing an added SacII restriction site (italic) (ATCCGCGGAATCCCATGGTGA) and a 3′ primer containing a SacI site (AAGAGCTCTTCTCATGTTTGAC). The purified PCR product was blunt-ended, cloned into EcoRV-digested pSK+, and sequenced. Finally, a SacI/SacII restriction fragment containing the GFP-coding sequence was cloned into pSK35S-TP–MP to give the plasmid pTP-GFP5 (Fig. 1).
Figure 1.
Constructs used for transformation of tobacco plants. p35S, 800-bp 35S promoter of the cauliflower mosaic virus recovered from pBI221; TP, sequence from pST5 encoding the ICDH targeting peptide; MP, sequence encoding the first 38 amino acids of mature ICDH protein; GFP, sequence encoding a modified GFP; Nos ter, nopaline synthetase terminator.
pGFP5 was made as follows: A 126-bp fragment was PCR-amplified from pST5 by using a 5′ oligonucleotide containing additional SacI and NcoI restriction sites (TGAGCTCCCATGGCAGCTTCGTC) and a homologous 3′ oligonucleotide containing the internal XbaI site found in pST5 (CTCTAGATAAGGATATAT). The PCR product was cloned into pMOS-blue (Amersham) and sequenced, and the SacI/XbaI fragment was recovered, blunt-ended, and cloned into NotI (blunt-ended) pSK35S. The plasmid containing a correctly orientated 114-bp fragment was called pSK35S-MP. Using the strategy described above, the GFP was cloned in phase with the added methionine (by the NcoI site) to give pGFP5 (Fig. 1).
The two chimeric gene constructs were cloned into pBI121 as follows: pTP-GFP5 and pGFP5 were SacI (blunt-ended) and HindIII digested, and the resulting fragments were cloned into EcoRI (blunt-ended) and HindIII digested pBI121. This strategy gave pBI-TP-GFP5 and pBI-GFP5, which were used in the Agrobacterium-mediated transformation of tobacco plants.
Plant Transformation and Regeneration.
Tobacco (Nicotiana tabacum L.) var. Xanthi plants were grown in a growth chamber as described (20). Leaves of 3-month-old plants were used.
The pBI121 vectors containing the different chimeric genes were mobilized in Agrobacterium tumefaciens strain LBA4404 by the method described by Hofgen and Willmitzer (21). Leaf disks were infected and kanamycin-resistant plantlets regenerated as described (22).
Organelle Isolation and Protein Assays.
Intact chloroplasts and mitochondria were isolated from 40 g of tobacco leaves as described (23, 24). The cytosol-enriched fraction corresponded to the supernatant after the 23,000 × g centrifugation step in which the mitochondria were pelleted. Stroma- or matrix-enriched fractions were obtained by lysis of the intact organella in a small volume of Hepes 10 mM, pH 7.5 and followed by three freeze/thaw cycles.
Protein concentration was determined spectrophotometrically according to M. Bradford (25) with BSA as the standard. Chlorophyll concentration was determined as in ref. 26.
PAGE and Western Blot.
SDS/PAGE was carried out as described in ref. 20 by using 10% polyacrylamide gels. For Western blot analysis, proteins were electrotransferred to a nitrocellulose membrane. GFP was revealed by using polyclonal anti-GFP antibodies (CLONTECH) and SuperSignal Ultra substrate (Pierce). IDH was revealed as described (20), using antibodies raised against a recombinant tobacco IDH subunit (unpublished results).
In Vitro Transcription/Translation and Import into Chloroplasts.
In vitro-coupled transcription/translation was carried out using the TNT-Coupled Rabbit Reticulocyte Lysate System (Promega), according to the manufacturer’s instructions, in the presence of [35S]methionine (Amersham).
Intact chloroplasts were resuspended in a small volume of import buffer (50 mM Hepes/330 mM sorbitol, pH 8.0). The import assay (250 μl) contained 50 mM Hepes (pH 8.0), 330 mM sorbitol, 2 mM MgCl2, 0.5 mM dithiothreitol, 2 mM ATP, 2 mM methionine, intact chloroplasts (120 μg chlorophyll), and the translation reaction. After a 30-min incubation at 22°C, chloroplasts were washed twice in import buffer and then incubated with 0.1 mg⋅ml−1 trypsin for 15 min at 4°C. The reaction was stopped by addition of 30 mg⋅ml−1 trypsin inhibitor. Intact chloroplasts were recovered by Percoll gradient centrifugation and washed twice in import buffer containing 30 mg⋅ml−1 trypsin inhibitor. Chloroplasts were broken by three freeze/thaw cycles in 10 mM Hepes (pH 7.6) and 30 mg⋅ml−1 trypsin inhibitor, and the stroma was recovered by a 15-min centrifugation at 12,000 g.
Mitochondria Visualization.
Leaf disks were incubated in the presence of 50 mM Hepes (pH 7.0), 330 mM sorbitol, 1 μM Dihydro Rhodamine-123 (Molecular Probes) at room temperature for 1 h. Excess dye was eliminated by washing the disks several times in dye-free buffer. The epidermic tissue was observed by optical microscopy.
Flow Cytometry.
A leaf was taken from the plant and immediately chopped with a razor blade in 500 μl of organelle-stabilizing buffer (50 mM Hepes, pH 7.5/330 mM sorbitol/1 mM MgCl2). After filtration through 48 μm and 10 μm of nylon, chloroplasts were analyzed with an EPICS flow cytometer (Coultonics, France) using 100 mW at 488 nm. Objects were identified by light scatter and with specific filters for green (525 nm ± 20 nm) and for red (685 nm ± 10 nm) fluorescence. In this way, the green fluorescence of 105 individual chloroplasts could be expressed on a logarithmic scale of intensity values within several minutes of taking the leaf.
Microscopy.
The epidermic tissue of tobacco leaves was examined by using Zeiss or Reichert epifluorescence microscopes. For the Zeiss microscope, GFP-specific fluorescence was observed by using Zeiss filters BP480 nm (excitation), FT510 nm (dichroic), and BP515–565 nm (barrier). For the Reichert microscope, fluorescence from either GFP or Dihydro Rhodamine-123 was observed by using filters BP475–495 nm, FT510 nm, and LP520–560 nm. In both, chlorophyll fluorescence was observed with BP450–495-nm, FT510-nm, and LP520-nm filters. Photographs were recorded on Fuji Provia film. Confocal microscopy was carried out by using a Bio-Rad MRC 600 confocal microscope. Cells were imaged by using the standard rhodamine/fluorescein filters. GFP (green channel) and chlorophyll autofluorescence (red channel) were imaged at the same time. Single images were collected by using line-averaging six times. Serial Z-sections (1 μm thick) were collected.
RESULTS AND DISCUSSION
Screening of a tobacco cell suspension cDNA library with a soybean ICDH cDNA (2) allowed us to obtain several ICDH-encoding clones, which formed two distinct classes. We have already shown that the most abundant, strongly hybridizing cDNA class encodes cytosolic ICDH (2). A cDNA 1,728 bp in length corresponding to the rare, weakly hybridizing class, named pST5, was obtained by library rescreening. Whereas the already available plant ICDH protein sequences have a very high identity (87–96%) (2, 12–14), the protein deduced from this ICDH-encoding cDNA showed a sequence identity of only 70–75%. This comparison indicated that the cDNA of pST5 encodes a unique ICDH isoenzyme different from all other known plant ICDH forms.
The N-terminal region of the protein deduced from pST5 was compared with those of available plant ICDH proteins (Fig. 2). The sequence deduced from pST5 showed an N-terminal extension, which was absent from all the other known plant ICDH proteins. It appeared to have the characteristics of a targeting sequence (27). Because it was enriched in basic residues at the N-terminal extremity of the targeting peptide, which are normally absent from chloroplastic transit peptides (27–28), it appeared to be more closely related to mitochondrial presequences. However, it is not possible to assign unequivocally the subcellular localization of the mature protein on the basis of only this kind of analysis. For instance, the transit peptide of the chloroplastic triose-phosphate translocator, an inner membrane protein, has the N-terminal characteristics of a mitochondrial presequence. Recently, this transit peptide has been shown to be capable of in vitro targeting the chloramphenicol acetyl transferase into both mitochondria and chloroplasts (5). Furthermore, it is now known that certain isoenzymes are encoded by a single gene and that their targeting sequence is capable of addressing the same protein into two different compartments (3, 4).
Figure 2.
Comparison between the N-terminal sequences deduced from different plant ICDH-encoding cDNAs. pST5 (this work, X96728), tobacco (3), potato (15), alfalfa (13), soybean (14), and Eucalyptus (U80912). With respect to the pST5 sequence, identical residues are replaced by hyphens although different amino acids are shown. ⇓ indicates the amino acid changed to the “start” methionine in pGFP5. The 38 amino acids of the putative mature protein situated between ▿ and ⇓ were placed in front of the GFP.
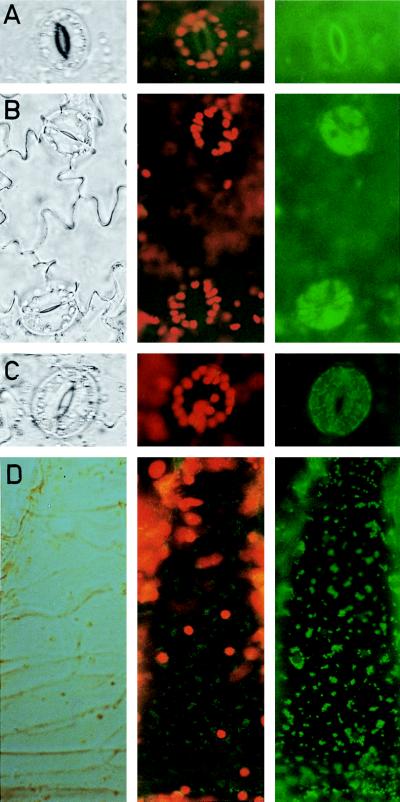
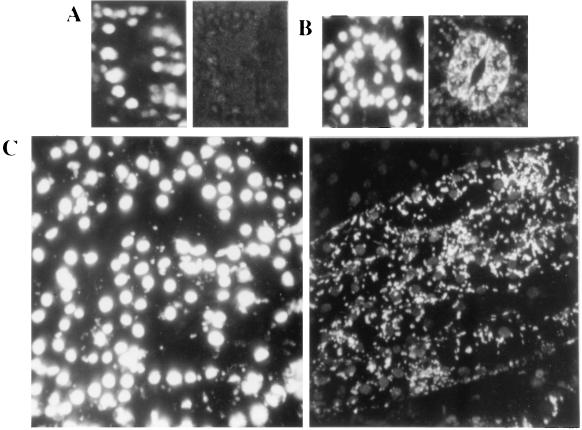
We have taken advantage of a modified GFP, sGFP(S65T) (kindly provided by J. Sheen, Harvard, Boston), to assess the subcellular localization of the protein encoded by the cDNA of pST5. Two constructs were made (Fig. 1) in which the pST5 sequence encoding either the whole targeting sequence plus 38 amino acids of the mature ICDH protein (pTP-GFP5) or only the 38 residues of the mature protein (pGFP5) were placed in phase with the GFP-coding sequence with expression being under the control of the cauliflower mosaic virus 35S promoter. The 38 residues of the mature ICDH protein were included in these constructs because it has been shown that the N-terminal region can play a role in targeting, such is the case for a chlorophyll a/b-binding protein (29). After A. tumefaciens-mediated transformation of tobacco plants with the chimeric genes placed in pBI-TP-GFP5 and pBI-GFP5, the presence of an active GFP in kanamycin-resistant primary transformants was followed by fluorescence microscopy. No significant green fluorescence was observed in untransformed, control tobacco plants (Fig. 3A, Right). The red chlorophyll autofluorescence (Fig. 3A, Center) makes it possible to identify the chloroplasts that are present in the stomatal cells (Fig. 3A, Left). In stomatal cells of pBI-GFP5-transformed plants (Fig. 3B, Left), green fluorescence was observed in the cytoplasm located around the nucleus whereas labeling was absent in cellular organella (Fig. 3B, Right). This distribution has been described (18) when using a GFP having no addressing sequence. In contrast, plants transformed with pBI-TP-GFP5 showed a very strong, visible green fluorescence in 1- to 3-μm long intracellular compartments in the guard (Fig. 3C, Right) and hair cells (Fig. 3D, Right), often situated very close to the chloroplasts.
Figure 3.
Detection of GFP in tobacco leaf epidermic tissue. Transmission (Left column) and epifluorescence microscopy to visualize either the red chlorophyll fluorescence and the green GFP fluorescence (Center column) or only the GFP fluorescence (Right column). Stomatal guard cells from control, untransformed tobacco plant (A), plant transformed with pBI-GFP5 (B), lacking the targeting sequence, and plant transformed with pBI-TP-GFP5 (C), containing the targeting peptide. (D) A hair cell of a plant as described in C. (10 μm = 3.2 mm).
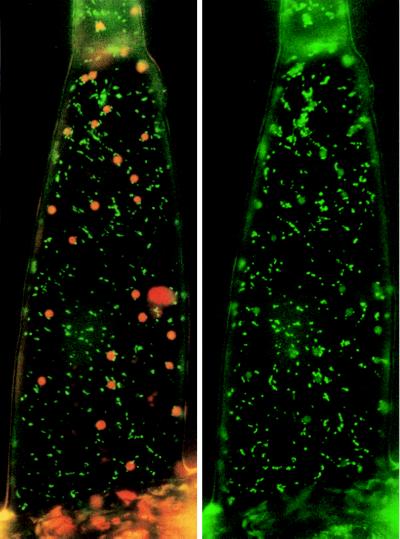
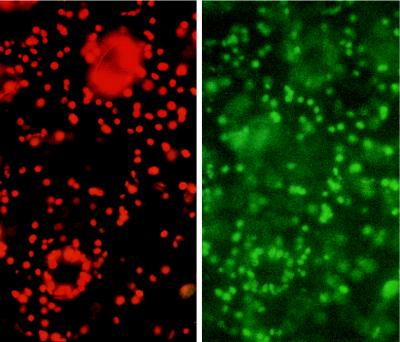
To identify the subcellular compartment that was labeled with the GFP in these plants, we incubated leaf discs of untransformed tobacco in the presence of Dihydro Rhodamine-123, a permeant form of the mitochondrial-specific dye Rhodamine-123 (Fig. 4). Because the GFP and the dye share similar spectral properties, their subcellular localization was followed by using the same filter set. The labeling using this compound was very similar to that seen in tobacco plants transformed with pBI-TP-GFP5 (Fig. 3 C and D), suggesting that the GFP-labeled compartments are mitochondria. Taken together, these results indicate that the ICDH encoded by our cDNA is addressed to the mitochondria by its targeting peptide. However, the chloroplasts (Fig. 3D, Center) also showed a weak green fluorescence signal (Fig. 3D, Right), indicating a possible chloroplastic cotargeting of the GFP by the ICDH-signaling peptide. Recently (30), it has been shown that chloroplast-located GFP gives rise to only a weak green fluorescent signal; these researchers concluded that this could be due to energy transfer from the GFP to chloroplast pigments. During this work, we have observed that under laboratory conditions, chloroplasts of tobacco leaf epidermic tissue can routinely give rise to an intense green fluorescence emission even in nontransformed material when observed under the optical microscope for >20–30 min (Fig. 5). This rise often coincided with a change in the chlorophyll fluorescence emission from red to orange. The principal endogenous compounds contributing green autofluorescence in leaf tissues are flavins and flavoproteins in their oxidized state (blue excited, emission ≈530 nm). The metabolic redox balance of an excised leaf is expected to shift toward the oxidized state: Amongst other processes, photoreduction of accessory pigments is impaired and the terminal flavins remain oxidized (31). In the absence of photosynthesis, the stromal pH would fall and the quantum yield of flavoproteins would increase. Therefore, we have tried to discriminate between the intrinsic unspecific chloroplast green fluorescence and that of the GFP.
Figure 4.
Green fluorescence of hair cells from untransformed tobacco after incubation with the mitochondrial-specific dye Dihydro Rhodamine 123. Illumination with blue light plus a specific filter set to visualize either red chlorophyll and green dye fluorescence (Left) or only green dye fluorescence (Right). (10 μm = 3.2 mm).
Figure 5.
Green fluorescence of untransformed tobacco leaf epidermic tissue. After 20–30 min on the microscope slide, the chloroplasts became green-fluorescent. Illumination with blue light plus a specific filter set to visualize either the red chlorophyll and green fluorescence (Left) or only the green fluorescence (Right). (10 μm = 2.7 mm).
To determine whether the GFP has been targeted to the chloroplasts, we used confocal laser microscopy. Stomatal cells of control tissues showed only a trace fluorescence in a 45-μm thick projection (Fig. 6A). However, plants transformed with pBI-TP-GFP5 gave a very strong green signal in the mitochondria (Fig. 6B and C) but also a weak emission in the chloroplasts when several serial sections were projected (Fig. 6C). Again, under laboratory conditions even the control tissues gave rise to a green fluorescence emission from the chloroplasts.
Figure 6.
Confocal images of tobacco leaf epidermic tissues. Projection of 43- to 58-serial sections taken at 1-μm intervals; Left column, images obtained in the red channel (chlorophyll fluorescence); Right column, images visualized in the green channel (GFP fluorescence). Stomatal guard cells of a control, untransformed tobacco plant (A), stomate (B), and epidermic tissue (C) of a tobacco plant transformed with pBI-PT-GFP5, containing the targeting peptide. Mitochondria are observed in the green channel. (10 μm = 4 mm).
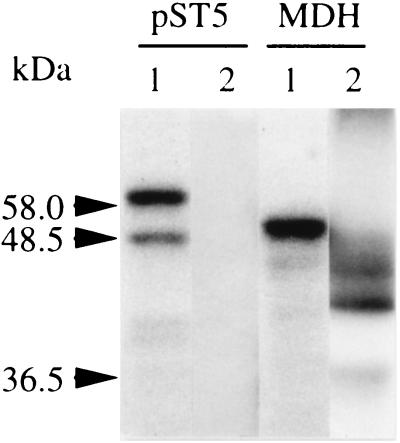
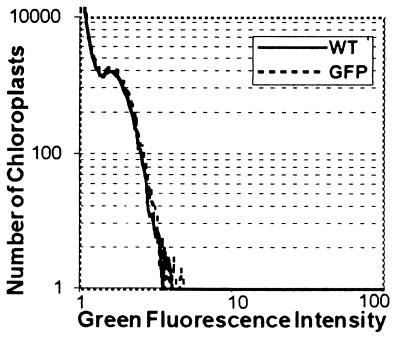
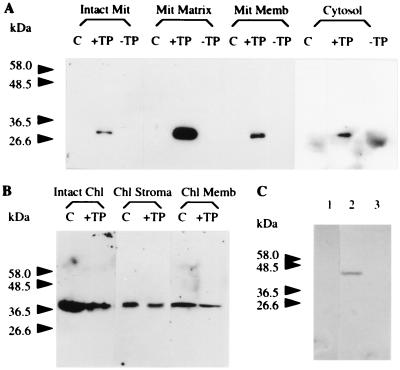
However, several observations suggest that ICDH is not targeted to the chloroplasts. Firstly, in vitro import studies were carried out (Fig. 7). Although the chloroplastic NADP-dependent malate dehydrogenase (32) was efficiently addressed into isolated tobacco chloroplasts, the protein translated from pST5 was not imported. Secondly, an analysis by flow cytometry of the fluorescence properties of the chloroplast population present in pBI-PT-GFP5 transformed plants compared with untransformed plants showed no significant differences (Fig. 8). To confirm the unique subcellular targeting of the GFP to mitochondria by the ICDH presequence, we have used commercially available anti-GFP antibodies in Western blot experiments. Chloroplastic, mitochondrial, and cytosolic fractions were isolated from control and transformed tobacco plants. A single protein band having the expected molecular weight was recognized in the mitochondrial fraction of tobacco transformed with pBI-TP-GFP5, being absent in both control plants and pBI-GFP5-transformed plants (Fig. 9A). The intensity of the signal was most intense in the matrix fraction, suggesting that the targeting peptide had all the information needed to introduce the GFP into the mitochondrium. GFP also was present in the cytosolic fraction of transgenic plants transformed with either pBI-TP-GFP5 or pBI-GFP5 but undetectable in control plants (Fig. 9A). The presence of GFP in this fraction of pBI-TP-GFP5-transformed plants seems to be due to newly synthesized GFP before translocation into the mitochondria because the detected protein band migrated more slowly than the cytosolic GFP in which the peptide was lacking. Surprisingly, in isolated chloroplasts, the polyclonal anti-GFP antibodies crossreacted with a protein band having a molecular mass ≈10 kDa higher than that of the GFP containing the first 38 residues of the mature ICDH (Fig. 9B). This protein band was detected in untransformed, control plants as well as transformed ones. Therefore, any future immunological analysis of chloroplasts using these antibodies should keep this observation in mind. Antibodies raised against a typical mitochondrial protein, the IDH, were used as a mitochondrial marker. This protein was exclusively present in the mitochondrial-enriched fraction (Fig. 9C).
Figure 7.
In vitro import into intact chloroplasts. 35S-labeled proteins were synthesized from plasmids containing either the entire ICDH cDNA (pST5) or the NADP-dependent malate dehydrogenase (MDH) by coupled transcription/translation. Lane 1, total products of the coupled reaction (1/25th); 2, stroma-enriched fraction obtained from intact chloroplasts after import
Figure 8.
Overlain flow cytometry histograms (log/log) of green fluorescence intensities (arbitrary units) measured in 105 chloroplasts of untransformed (—) or pTP-GFP5 transformed (- - -) plants in organelle-stabilizing buffer.
Figure 9.
Protein blots showing GFP compartmentalization in tobacco. The mitochondrial and cytosolic fractions (A) and the chloroplastic fractions (B) of control, untransformed plants (lanes C), tobacco transformed with pBI-TP-GFP5 (lanes +TP) or pBI-GFP5 (lanes −TP) were probed with GFP antibodies. Ten micrograms of protein from crude mitochondrial (Intact Mit) or chloroplastic (Intact Chl) fractions; 25 μg of soluble proteins from purified mitochondria (Mit Matrix) or chloroplasts (Chl Stroma); 20 μg of insoluble membrane fraction of purified mitochondria (Mit Memb) or chloroplasts (Chl Memb); and 100 μg of soluble proteins from the cytosolic fraction (Cytosol) were loaded per lane. (C) Immunodetection of the mitochondrial IDH in untransformed tobacco. Lane 1, cytosol (100 μg); 2, mitochondria (25 μg); and 3, chloroplasts (25 μg).
Up until now, the only reports about the existence of a mitochondrial ICDH isoenzyme in plants were based on the measure of an ICDH activity in isolated mitochondria. The presence of an ICDH isoenzyme in mitochondria raises the question of its role in mitochondrial metabolism. Several hypotheses have been suggested (9), perhaps the most attractive being that the mitochondrial ICDH could be implicated in producing the NADPH needed for glutathione reduction (33). Verification of its role(s) in plant metabolism has been until now impossible because (i) the protein has never been purified to homogeneity from a plant material, (ii) antibodies raised against this isoform are not available, and (iii) it is necessary to isolate mitochondria without any cytosolic contamination before measuring its activity. For these reasons, the establishment of a correspondence between the ICDH-encoding cDNA described here and the subcellular localization of its product will be primordial to advance our understanding of the physiological role(s) of this ICDH isoenzyme. Antibodies may now be raised against the recombinant protein thereby allowing expression studies to be carried out at both the protein and mRNA levels with respect to nitrogen nutrition and anoxic stress.
Acknowledgments
We are grateful to J. Sheen (Harvard Medical School, Boston) for the modified GFP-containing plasmids. We thank A. Forchioni (Université de Paris Sud, France) for her assistance with confocal microscopy and R. Boyer for his photographic skills. This work and S.G. were supported by the European communities’ BIOTECH Program, as part of the Project of Technological Priority 1993–1996.
ABBREVIATIONS
- ICDH
NADP-dependent isocitrate dehydrogenase
- GFP
green fluorescent protein
- pSK+
pBluescript SK+
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. X96728).
References
- 1.Edwards J W, Walker E L, Coruzzi G M. Proc Nat Acad Sci USA. 1990;87:3456–3463. doi: 10.1073/pnas.87.9.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gálvez S, Hodges M, Decottignies P, Bismuth E, Lancien M, Sangwan R S, Dubois F, LeMarechal P, Cretin C, Gadal P. Plant Mol Biol. 1996;30:307–320. doi: 10.1007/BF00020116. [DOI] [PubMed] [Google Scholar]
- 3.Creissen G, Reynolds H, Xue Y, Mullineaux P. Plant J. 1995;8:167–175. doi: 10.1046/j.1365-313x.1995.08020167.x. [DOI] [PubMed] [Google Scholar]
- 4.Mireau H, Lancelin D, Small I. Plant Cell. 1996;8:1027–1039. doi: 10.1105/tpc.8.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva-Filho M de C, Wieèrs M-C, Flügge U-I, Chaumont F, Boutry M. J Biol Chem. 1997;272:15264–15269. doi: 10.1074/jbc.272.24.15264. [DOI] [PubMed] [Google Scholar]
- 6.McNally S F, Hirel B, Gadal P, Mann A F, Stewart Plant Physiol. 1983;72:22–25. doi: 10.1104/pp.72.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath R B, Coruzzi G M. Plant J. 1991;1:275–280. doi: 10.1046/j.1365-313x.1991.00999.x. [DOI] [PubMed] [Google Scholar]
- 8.Schultz C J, Coruzzi G M. Plant J. 1995;7:61–75. doi: 10.1046/j.1365-313x.1995.07010061.x. [DOI] [PubMed] [Google Scholar]
- 9.Gálvez S, Gadal P. Plant Sci. 1995;105:1–14. [Google Scholar]
- 10.Miflin B J, Wallsgrove R M, Lea P J. In: Current Topics in Cellular Regulation. Horecker B L, Stadtman E R, editors. Vol. 20. New York: Academic; 1981. pp. 1–43. [DOI] [PubMed] [Google Scholar]
- 11.Chen R D, Gadal P. Plant Physiol Biochem. 1990;28:141–145. [Google Scholar]
- 12.Sorrosh B S, Dixon R A. Plant Mol Biol. 1992;20:801–807. doi: 10.1007/BF00027151. [DOI] [PubMed] [Google Scholar]
- 13.Udvardi M K, McDermott T R, Kahn M L. Plant Mol Biol. 1993;21:739–752. doi: 10.1007/BF00027108. [DOI] [PubMed] [Google Scholar]
- 14.Fieuw S, Müller-Röber B, Gálvez S, Willmitzer L. Plant Physiol. 1995;107:905–913. doi: 10.1104/pp.107.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu W-L, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Curr Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- 16.Reichel C, Mathur J, Eckes P, Langenkemper K, Koncz C, Schell J, Reiss B, Maas C. Proc Natl Acad Sci USA. 1996;93:5888–5893. doi: 10.1073/pnas.93.12.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haseloff J, Siemering K R, Prasher D C, Hodge S. Proc Natl Acad Sci USA. 1997;94:2122–2127. doi: 10.1073/pnas.94.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler R H, Zipfel W R, Webb W W, Hanson M R. Plant J. 1997;11:613–621. doi: 10.1046/j.1365-313x.1997.11030613.x. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. In: Molecular Cloning: A Laboratory Manual. 2nd Ed. Nolan C, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Gálvez S, Bismuth E, Sarda C, Gadal P. Plant Physiol. 1994;105:593–600. doi: 10.1104/pp.105.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Höfgen R, Willmitzer L. Nucleic Acids Res. 1988;17:9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagu D, Crétin C, Bergounioux C, Lepiniec L, Gadal P. Plant Cell Rep. 1991;9:688–690. doi: 10.1007/BF00235358. [DOI] [PubMed] [Google Scholar]
- 23.Chen R D, Bismuth E, Champigny M L, Gadal P. Planta. 1989;178:157–163. doi: 10.1007/BF00393190. [DOI] [PubMed] [Google Scholar]
- 24.Boutry M, Thomas D, Chaumont F. Plant Mol. Biol. Manual. J 2. 1994. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Arnon D. Plant Physiol. 1949;24:1–3. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heijne G, Steppuhn J, Herrmann R G. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- 28.Whelan J, Glaser E. Plant Mol Biol. 1997;33:771–789. doi: 10.1023/a:1005755505738. [DOI] [PubMed] [Google Scholar]
- 29.Silva Filho M C, Chaumont F, Leterme S, Boutry M. Plant Mol Biol. 1996;30:769–780. doi: 10.1007/BF00019010. [DOI] [PubMed] [Google Scholar]
- 30.Schulte W, Töpfer R, Stracke R, Schell J, Martini N. Proc Natl Acad Sci USA. 1997;94:3465–3470. doi: 10.1073/pnas.94.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widell S, Sundqvist C. Physiol Plant. 1987;70:27–34. [Google Scholar]
- 32.Crétin C, Luchetta P, Joly C, Decottignies P, Lepiniec L, Gadal P, Sallantin M, Huet J-C, Pernollet J-C. Eur J Biochem. 1990;192:299–303. doi: 10.1111/j.1432-1033.1990.tb19227.x. [DOI] [PubMed] [Google Scholar]
- 33.Rasmusson A, Möller I M. Plant Physiol. 1990;94:1012–1018. doi: 10.1104/pp.94.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]