Abstract
Objective
Because debate continues over the role of combination, platinum-based chemotherapy for platinum sensitive (PS), recurrent ovarian cancer (OC), we compared overall survival (OS), progression-free survival (PFS), confirmed complete response rate and time to treatment failure in this population.
Methods
Patients with recurrent stage III or IV OC, a progression-free and platinum-free interval of 6- 24 months after first-line platinum-based chemotherapy and up to 12 courses of a non-platinum containing consolidation treatment were eligible. Patients were randomized to IV pegylated liposomal doxorubicin (PLD) (30 mg/m2) plus IV carboplatin (AUC=5 mg/mL × min) once every 4 weeks (PLD arm) or IV carboplatin alone (AUC=5mg/mL × min) once every 4 weeks.
Results
The PLD arm enrolled 31 patients and the carboplatin alone arm 30 for a total of 61 patients out of 900 planned. Response rates were 67% for the PLD arm and 32% for the carboplatin only arm (Fisher’s exact p=0.02). The estimated median PFS was 12 and 8 months for PLD versus carboplatin alone. The estimated median OS on the PLD arm was 26 months and 18 months on the carboplatin only arm (p=0.02). Twenty-six percent of the patients on the PLD arm reported grade 4 toxicities, all hematological in nature.
Conclusion
This study was closed early because of slow patient accrual. The response rate, median PFS and OS results are intriguing. These data suggest that there may be an advantage to the PLD plus carboplatin combination treatment in patients with PS, recurrent OC. The regimen should be further tested.
Introduction
The management of advanced, platinum-sensitive (PS) recurrent ovarian cancer continues to be controversial, although the results of two recent phase III trials suggest that either paclitaxel or gemcitabine combined with carboplatin as compared to carboplatin alone improve progression-free survival (PFS), while the former combination improves overall survival [1,2]. Nevertheless, both of these drug combinations carry considerable acute and chronic hematological and non-hematological toxicities and require relatively frequent intervals of dosing.
Beyond the use of platinating agents, pegylated liposomal doxorubicin (PLD)has proven a drug of choice for the treatment of ovarian cancer patients, who have recurred with platinum-sensitive disease following primary platinum-based chemotherapy [3,4]. In fact, PLD prolonged both PFS and overall survival as compared to topotecan in a phase III trial in patients with first disease recurrence [4].
There are phase II clinical data suggesting additivity between PLD and carboplatin in the treatment of advanced ovarian cancer [2,5]. The recommended PLD dose of 30 mg/m2 for combination with carboplatin of every four weeks is virtually free of the usual dose-limiting palmar-plantar erythrodysesthesia (i.e. hand-foot syndrome or PPE). Furthermore, this dose of PLD has extremely low potential for causing either stomatitis or myelosuppression. Thus, in relation to the selection of a non-cross-resistant drug to combine with carboplatin for the treatment of platinum-sensitive recurrent ovarian cancer, PLD may prove to have an even better therapeutic index than either paclitaxel or gemcitabine which is FDA approved for this combination.
Materials and Methods
Between August 2002 and December 2004, Southwest Oncology Group (SWOG) member institutions entered patients with advanced, recurrent epithelial ovarian or peritoneal carcinoma onto SWOG protocol S0200 with the objective to evaluate the addition of PLD to carboplatin with respect to OS, PFS, tumor response, and toxicity.
Eligibility
Patients had to meet the following eligibility criteria: 1. histologically diagnosed Stage III or IV disease consistent with epithelial carcinoma of the ovary, peritoneal carcinoma or mixed mullerian tumors; 2. relapse or progression of disease within 6–24 months of completing frontline platinum-based chemotherapy (either single agent or combination therapy); 3. progressive disease according to RECIST criteria or GCIG CA-125 progression criteria; 4. performance status of 0–1 by Zubrod; 5. consolidation therapy (i.e., up to twelve courses of non-platinum containing, continuing chemotherapy or biological therapy following first-line platinum-based chemotherapy) during the 6–24 month progression-free and platinum-free interval was allowed, provided it was completed at least 28 days prior to registration; 6. surgical debulking for recurrent/progressive disease is allowed with recovery from side effects prior to registration; 7. no prior cumulative anthracycline (e.g., doxorubicin, daunorubicin, epirubicin) dose in excess of 240 mg/m2 and no prior therapy with PLD; 8. no prior abdominopelvic irradiation; 9. free from class 2 or greater cardiac problems as defined by New York Heart Association Criteria; 10. no evidence of active or uncontrolled infection; and 11. no known brain metastases, severe gastrointestinal symptoms or grade 2 or greater sensory neuropathy per CTC 2.0 criteria at the time of registration.
Pre-treatment laboratory data included a serum creatinine less than or equal to 1.9 mg/dL, serum bilirubin less than equal to the institutional upper limit of normal, SGOT, SGPT and alkaline phosphatase less than equal to 2 times the institutional upper limit of normal, and ANC greater than or equal to 1,500/µL, platelet count greater than equal to 100,000/µL, and hemoglobin greater than or equal to 10g/dL. All patients must have had an EKG and a MUGA scan or a 2-d echocardiogram indicating an ejection fraction of >50% within 42 days of registration. All investigations were performed after approval by either a local or central Human Subjects Committee. Written informed consent was obtained from all patients prior to study entry.
Treatment
Patients were treated with one of two regimens. Arm 1 treatment consisted of IV carboplatin (AUC=5mg/mL × min) every 4 weeks administered over a minimum of 15 minutes plus IV PLD (30mg/m2) every 4 weeks administered over 1 hour (PLD Arm). Arm 2 treatment consisted of IV carboplatin (AUC=5mg/mL × min) every 4 weeks administered over a minimum of 15 minutes. Treatment was given until progression, intolerable toxicity or physician/patient desire for removal from study. The maximum cumulative dose allowed for PLD was 600mg/m2.
Dose modifications were allowed based on toxicity to PLD. Any patient with a compromised left ventricular ejection fraction (<45% or decreases by a relative 20% from baseline) was removed from PLD and continued on the carboplatin treatment. For PPE or stomatitis and bilirubin toxicity a dose reduction schedule was created based on grade and previous history in order to minimize this side effect. For all other grade 3 and 4 events, PLD was withheld for up to 4 weeks until the toxicity resolved to less than or equal to a grade 2, after which treatment resumed at a one-level dose reduction (level −1= 25 mg/m2, level −2=20 mg/m2). If treatment was delayed greater than 4 weeks PLD was permanently discontinued.
Carboplatin dose modifications were allowed for gastrointestinal and neurological toxicity. Patients with persistently greater than equal to grade 2 peripheral neuropathy, despite dose reduction, were permanently taken off carboplatin treatment. Dose modifications and treatment delays were also allowed for both decreases and elevations in neutrophil (ANC) and platelet count.
Prophylactic use of G-CSF or GM-CSF was not allowed, but was allowed to treat neutropenia according to ASCO guidelines. G-CSF was administered to patients who developed Grade 3–4 neutropenia following chemotherapy. For patients who experience Grade 3 or 4 neutropenia or developed neutropenic fever between cycles of chemotherapy, G-CSF was then added to all subsequent cycles of chemotherapy, unless there was clinical suspicion that the neutropenia was due to an unrelated medical condition and not due to the chemotherapy. The recommended dosage was 5µg/kg/d subcutaneously beginning 24 hours after completion of chemotherapy and continuing until ANC ≥ to 1,000/mm3 on two successive determinations
Objective response and disease progression were defined according to standard RECIST criteria. The GCIG CA-125 progression criteria were included in defining disease progression [6].
Statistical considerations
The primary goal of this study was to compare the OS in PS, recurrent ovarian or peritoneal cancer patients treated with PLD plus carboplatin versus carboplatin alone. Patients were randomized equally to the two study arms while stratified by disease measurability, number of disease sites and serous histology. Based on previous data, the median OS for the carboplatin arm was estimated to be 18 months [7]. The study sought to detect a 4.5- month increase in median survival to 22.5 months on the PLD arm. Thus, 900 eligible subjects were targeted for accrual over four and a half years. With analysis taking place after an additional two years of follow-up and a true death hazard ratio of 1.25, the power to detect a statistically significant OS increases was 0.85. This calculation assumed uniform patient entry and a one-sided log rank test at a 0.025 level of significance.
Due to extremely slow patient accrual, the study was closed on December 15, 2004 based on the SWOG Data and Safety Monitoring Committee’s recommendation with sixty-one patients enrolled. The results are presented here as those from a randomized phase II study.
Results
A total of 61 patients were registered to the study between August 15, 2002 and December 15, 2004. All 61 patients were eligible by central review, started treatment and were considered evaluable. Thirty-one were randomized to the PLD plus carboplatin arm and 30 to the carboplatin arm.
The median patient age was 66.9 years (range, 43–87 years) for the PLD plus carboplatin arm and 62.5 years (range 31–80) for the carboplatin arm (Table 1). Disease measurability (categorized as elevated CA-125 only, other non-measurable disease and measurable disease), number of disease sites and serous tumor histology were evenly distributed between the two arms.
Table 1.
Patient Characteristics
| Characteristic | PLD +Carboplatin (%) (n=31) | Carboplatin (%) n=30) |
|---|---|---|
| Age | ||
| <50 | 5(16%) | 5(17%) |
| 50–59 | 4(13%) | 5(17%) |
| 60–69 | 9 (29%) | 13(43%) |
| ≥70 | 13(42%) | 7 (23%) |
| Median Age Stage | 66.9 (Range 43–87) | 62.5 (Range 31–80) |
| 3 | 22(71%) | 26(87%) |
| 4 | 9(29%) | 4(13%) |
| Serous Histology | ||
| Yes | 25(81%) | 25(83%) |
| No | 6(19%) | 5(17%) |
| Number of Disease Sites | ||
| ≤2 | 24(77%) | 22(73%) |
| ≥3 | 7(23%) | 8(27%) |
| Disease Measurability | ||
| Elevated CA-125 only | 4(13%) | 2(7%) |
| Other non-measurable disease | 8(26%) | 8(27%) |
| Measurable disease | 19(61%) | 20(67%) |
| Zubrod Performance Status at Study Entry | ||
| 0 | 20(65%) | 16(53%) |
| 1 | 11(35%) | 14(47%) |
| Platinum Free Interval (days) | ||
| Median (range) | 430 (253–774) | 382(192–790) |
| % >365 days | 57% | 50% |
| Chemotherapy Free Interval (days) | ||
| Median (range) | 350(31–774) | 382 (30–685) |
The median platinum–free interval before beginning the study was 430 days in the PLD plus carboplatin arm and 382 days for the carboplatin alone arm. Fifty-seven percent of the PLD plus carboplatin patients and 50% of the carboplatin alone patients were platinum-free for >365 days with an overall total of 53%. The median chemotherapy-free interval prior to starting the S0200 study for the PLD plus carboplatin only arm was 350 days and 382 days for the carboplatin alone arm. Nine patients (5 on the PLD plus carboplatin arm and 4 on the carboplatin alone arm) received consolidation treatment with Taxol (8) or taxotere (1)
All 61 evaluable patients have gone off protocol treatment. The median number of treatment cycles given was 7 (range 1–18) for patients in the PLD plus carboplatin arm and it was 6 (range 2–16) for those in the carboplatin alone arm. There were no major protocol violations reported. Twenty-two (36%) of the total subjects went off protocol treatment for an adverse event or side effect, 15 (48%) in the PLD plus carboplatin arm and 7 (23%) in the carboplatin arm. While all 7 carboplatin only patients went off study due to allergic reactions, the 15 PLD plus carboplatin patients went off study due to different toxicities, 10 of 15 involved hematological toxicities and/or fatigue. The number of treatment courses received by the 15 patients who went off study due to toxicities (median 6, range 1–15) was no different from the number reported by the entire 31 patients on the PLD plus carboplatin arm. For those who went off study due to toxicity in the carboplatin alone arm the median number of cycles was 3 (range 2–6).
As shown in Table 2, eight patients (26%) on the PLD plus carboplatin arm reported grade 4 hematological adverse events; neutropenia and thrombocytopenia occurred in 19% and 10% of the patients, respectively. These events did not result in either sepsis or bleeding. There were no reports of other grade 4 adverse events on the PLD arm. The most common Grade 3 toxicities on the PLD arm were also of hematological nature (neutropenia 29%, thrombocytopenia 29%, leucopenia 26% and anemia 16%). There were no allergic reactions in the patients treated on the PLD arm, but there was a 30% allergic reaction rate on the carboplatin only arm: 2 (7%) grade 1, 2 (7%) grade 2, 4 (13%) grade 3 and 1 (3%) grade 4, the most common grade 3 toxicities were allergy (13%) and thrombocytopenia (10%).
Table 2.
Proportions of Patients with Adverse Events
| PLD + Carboplatin (n=31) | Carboplatin (n=30) | ||||||
|---|---|---|---|---|---|---|---|
| Grade | Grade | ||||||
| ADVERSE EVENT | ≤2 | 3 | 4 | ≤2 | 3 | 4 | |
| Abdominal pain/cramping | 97% | 3% | 0% | 100% | 0% | 0% | |
| Allergy/hypersensitivity | 100% | 0% | 0% | 83% | 13% | 3% | |
| Anemia | 84% | 16% | 0% | 100% | 0% | 0% | |
| Catheter related infection | 97% | 3% | 0% | 100% | 0% | 0% | |
| Constipation/bowel obstruction | 94% | 6% | 0% | 97% | 3% | 0% | |
| Depression | 100% | 0% | 0% | 97% | 3% | 0% | |
| Dyspnea | 94% | 3% | 0% | 93% | 3% | 3% | |
| Fatigue/malaise/lethargy | 90% | 10% | 0% | 93% | 7% | 0% | |
| Febrile neutropenia | 90% | 10% | 0% | 100% | 0% | 0% | |
| Hand-foot skin reaction | 97% | 3% | 0% | 100% | 0% | 0% | |
| Hypomagnesemia | 97% | 3% | 0% | 100% | 0% | 0% | |
| Hyponatremia | 97% | 3% | 0% | 100% | 0% | 0% | |
| Hypotension | 100% | 0% | 0% | 97% | 3% | 0% | |
| Infection with 3-4 neutropenia | 94% | 6% | 0% | 100% | 0% | 0% | |
| Leukopenia | 71% | 26% | 3% | 100% | 0% | 0% | |
| Myalgia | 100% | 0% | 0% | 97% | 3% | 0% | |
| Nausea | 94% | 6% | 0% | 100% | 0% | 0% | |
| Neutropenia/granulocytopenia | 52% | 29% | 19% | 97% | 3% | 0% | |
| PRBC transfusion | 90% | 10% | 0% | 100% | 0% | 0% | |
| Platelet transfusion | 94% | 6% | 0% | 100% | 0% | 0% | |
| Respiratory infect w/o neutropenia | 97% | 3% | 0% | 100% | 0% | 0% | |
| Thrombocytopenia | 61% | 29% | 10% | 90% | 10% | 0% | |
| Vomiting | 97% | 3% | 0% | 100% | 0% | 0% | |
| MAXIMUM GRADE ANY ADVERSE EVENT | 29% | 45% | 26% | 60% | 37% | 3% | |
Four patients on the PLD plus carboplatin arm and two on the carboplatin arm had CA-125 elevations as the only disease at study entry; these 6 were not eligible for objective response evaluation. Twenty-seven patients on the PLD plus carboplatin arm and 28 patients on the carboplatin arm were evaluable for response. The confirmed response rates (CR +PR) were 52% (14/27) on the PLD plus carboplatin arm and 29% (8/28) on the carboplatin only arm (Fishers’ exact p=0.10). The confirmed plus unconfirmed response rates were 67% (18/27) for the PLD plus carboplatin arm and 32% (9/28) on the carboplatin only arm (Fisher’s exact p=0.02).
The estimated median PFS on the PLD plus carboplatin and carboplatin arms were 12 months and 8 months, respectively. The unadjusted PLD plus carboplatin versus carboplatin only PFS survival hazard ratio was 0.54 (p=0.03, 95% CI 0.32–0.93). Adjusting for randomization stratification factors, the Cox model hazard ratio was 0.59 (p=0.06 95% CI 0.34–1.02).
The estimated median OS on the PLD plus carboplatin and carboplatin arms were 26 months and 18 months respectively. The unadjusted PLD plus carboplatin versus carboplatin death hazard ratio was 0.46 (p=0.03, 95% CI 0.22–0.95). Adjusting for randomization stratification factors, the Cox model death hazard ratio was 0.42 (p=0.02, 95% CI 0.20–0.89). Inclusion of platinum-free interval in the Cox model did not affect the treatment p value for either the PFS or the OS.
Discussion
This phase III trial was closed early by the SWOG Data and Safety Monitoring Committee, because of extremely slow patient accrual with 61 evaluable patients. With a median follow-up of approximately 2 years (22.4 months), the PLD plus carboplatin arm appeared to have outperformed carboplatin used as a single agent in relation to objective response rates and PFS and OS durations. Obviously, because of the small sample size (61 evaluable patients) in this trial, these data cannot be considered definitive and are only hypothesis generating concerning the activity of the combination of PLD plus carboplatin.
Several factors, beyond the control of the coordinators of this phase III trial mitigated against adequate patient accrual, including: 1. The SWOG Gynecologic Cancer Committee was dissolved after this trial was initiated, depriving the trial of a stable group of support institutions and investigators; 2. The initial results of ICON 4 were reported, showing survival superiority of the combination of paclitaxel plus carboplatin over carboplatin alone in a PS, recurrent disease population [1]; and 3. GOG-0182 was initiated, comparing 4 different 3-drug combinations, including a combination of PLD added to a paclitaxel plus carboplatin combination as up front ovarian cancer chemotherapy, and seemingly abrogating the need for the present SWOG study [8]. These factors together contributed to the lack of patient accrual to SWOG-0200.
With positive PFS results reported for the AGO/MRC phase III trial comparing gemcitabine plus carboplatin to carboplatin in PS, recurrent disease, the use of gemcitabine or paclitaxel plus carboplatin have become standard of care in this patient population [1]. Although our results concerning the activity of PLD plus carboplatin in this same patient population are far from being definitive, we believe that this combination deserves additional consideration for the following reasons: 1. The combination is more convenient to administer with the dosing interval being every 4 weeks, instead of the every 3 weeks of the paclitaxel/carboplatin and gemcitabine /carboplatin combinations; 2. The PLD plus carboplatin combination was well tolerated with respect to the expected spectrum of hematologic and non-hematologic toxicities; there were no cases of sepsis, bleeding, stomatitis or significant neurologic or hand-foot syndrome problems; and 3. PFS and OS durations appeared prolonged in this limited randomized study.
In the carboplatin alone arm 30% of the patients reported an allergic reaction, however there were no allergic reactions reported on the PLD plus carboplatin arm. It is possible that PLD protects against carboplatin allergy; however this conjecture must be studied prospectively. If this proves to be true then the PLD plus carboplatin combination may have an additional benefit over the combinations of paclitaxel plus carboplatin or gemcitabine plus carboplatin since allergic reactions to carboplatin during treatment of platinum sensitive, recurrent disease have become dose-limiting.
Figure 1.
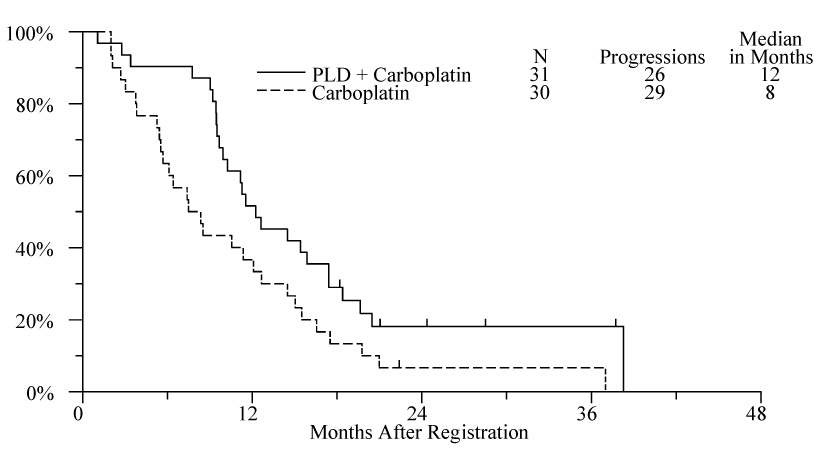
Progression free survival by treatment arm
Figure 2.
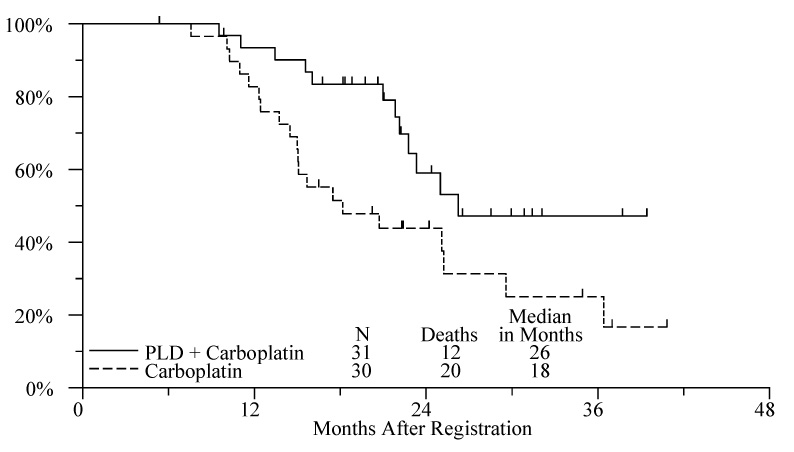
Overall Survival by Treatment Arm
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA35178, CA45807, CA67575, CA45560, CA46441, CA35176, CA13612, CA04919, CA63844, CA27057, CA35119, CA35431, and supported in part by Ortho Biotech.
Footnotes
Article Précis There may be an advantage to the PLD plus carboplatin combination treatment in patients with PS, recurrent OC and the regimen should be further tested.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 2.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGOOVAR, the NCIC CTG, and the EORTC CGC. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 3.Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, et al. Phase II study of liposomal doxorubicin in platinum-and paclitaxel refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AN, Tonda M, Sun S, Rackoff W. Doxil Study 30–49 Investigators. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero JM, Weber B, Geay JF, Lepille D, Orfeuvre H, Combe M, et al. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: a GINECO phase II trial. Ann Oncol. 2007;18:263–268. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Rustin GJ, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MK, et al. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer] J Natl Cancer Inst. 2000;92:1534–1535. doi: 10.1093/jnci/92.18.1534. [DOI] [PubMed] [Google Scholar]
- 7.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian-carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 8.Bookman MA. Gynecologic Cancer InterGroup. GOG 0182-ICON5: 5-arm phase III randomized trial of paclitaxel and carboplatin vs combinations with gemcitabine, PEG-liposomal doxorubicin, or topotecan in patients with advanced-stage epithelial ovarian or primary peritoneal carcinoma. J Clin Oncol, ASCO Annual Meeting Proceedings. 2006;18S:5002. [Google Scholar]


