Abstract
[11C]Raclopride ([11C]RAC) is a selective dopamine D2/D3 antagonist that is commonly used in positron emission tomography (PET) studies to assess both basal levels of receptor availability and changes in availability caused by alterations in striatal dopamine concentration. When designing [11C]RAC studies, it is important to understand what variables may affect the results. Here, we examined differences in baseline striatal [11C]RAC binding potential (BPND) under two different “rest” conditions. Thirteen subjects received [11C]RAC scans. Eight subjects were aware prior to initiation of scanning that they would receive a “baseline” scan, and that no additional procedures would take place during the scan (“certain rest” group, CER). Five subjects were informed that they might or might not receive an IV alcohol infusion during the scan (“uncertain rest” group, UNC). This group was informed five min after scan start that they would not receive alcohol. Voxel-wise analyses of binding potential (BPND) images generated for both “rest” conditions indicated that receptor availability was higher in UNC than in CER. This result was confirmed by a region-of-interest analysis, which indicated that the average BPND in right and left putamen was statistically higher in UNC. There were no differences in groups with respect to age or raclopride mass dose that could account for the difference in D2/D3 receptor availability. Our findings suggest that even slight differences in cognitive states between groups can have an effect on BPND, presumably mediated by changes in endogenous dopamine concentration.
Keywords: positron emission tomography, raclopride, binding potential, dopamine, baseline, reward
Introduction
[11C]Raclopride ([11C]RAC) is a competitive D2/D3 antagonist (KD ∼ 1-10 nM [6, 9, 10, 12]) that is sensitive to changes in endogenous dopamine (DA) levels [32, 41]. Neuroimagers have taken advantage of the kinetic properties of [11C]RAC to study changes in striatal DA concentration ([DA]) using positron emission tomography [11, 16, 20, 21] (for extensive review, see [16]). Typically, this involves a design requiring two PET scans: one at a “baseline” or “resting” state, and one during which a pharmacological challenge is administered or a cognitive or a motor task is performed (it should be noted that single-scan paradigms have been developed [1, 26]). If the index of receptor availability, the nondisplaceable binding potential (BPND), in the experimental condition is different from the baseline BPND, then the observed changes in BPND are attributed to changes in endogenous [DA] [7, 8, 32, 41]. Decreases in BPND relative to baseline indicate increases in [DA], and increases in BPND relative to the baseline BPND indicate decreases in [DA].
It is assumed that the BPND obtained during the “baseline” or “resting” condition is representative of a subject's striatal D2/D3 receptor state under “normal” conditions. However, it is conceivable that subtle differences in cognitive state may result in changes in [DA], leading to measurable changes in BPND. If baseline BPND is “artificially” altered consistently in one direction by induction of a specific cognitive state, it could affect the reliability of PET measures of stimulus-induced changes in striatal [DA]. To assess whether cognitive states of subjects could affect BPND, we tested for differences in resting BPND in normal subjects who were given different instructions with differing expectancies about what would occur during the [11C]RAC scans.
Materials and Methods
Subjects
All procedures were carried out in accordance with the Declaration of Helsinki, and were approved by the Indiana University Institutional Review Board. The study was carefully explained to the subjects and informed consent was obtained prior to initiation of the study. Subjects were 13 healthy, non-smoking volunteers without histories of significant neurological disturbances or psychiatric diagnoses. None of the subjects were taking medications with central nervous system effects. Subjects received a urine drug screen on the day of scanning and all tested negative for amphetamines, barbiturates, benzodiazepines, cannabis, cocaine, and opiates.
Subjects had previously participated in one of two [11C]RAC PET protocols, each of which assessed the effects of a different dose of IV alcohol infusion on striatal [DA] [39, 40]. These protocols were designed to assess changes in [DA], and thus consisted of two scans, a “baseline” scan, and a scan during which alcohol was administered. The present work is a retrospective analysis of differences in the baseline scans between the two protocols (i.e., only one PET scan from each subject was included in the present comparison).
Each group had 1 female subject, and 1 left-handed subject. Ethnicity of all subjects was non-hispanic/latino. Mean ± SD age were 25.0 ± 5.76 (CER; see below) and 25.2 ± 3.19 (UNC).
Image Acquisition
[11C]RAC scans were performed as previously described [39, 40] on an EXACT HR+ scanner (CTI, Knoxville, TN) Upon arrival on the study day (typically, 2-3 hours before the baseline scan), subjects were informed in one of two ways about scanning procedures. CER subjects (n = 8) were told that they would receive a baseline scan and that during this scan, no other procedures would be performed while they were in the scanning environment. UNC subjects (n = 5) were informed that they might or might not receive an IV alcohol infusion during the scan, and that the type of scan would be revealed to them immediately after the scan started. UNC subjects were informed 5 min after the start of RAC injection (see below) by study personnel that “This is a No-Alcohol scan.” Both groups were instructed to otherwise lie quietly in the scanner.
Scans were initiated with the IV injection of 14.5 ± 3.09 mCi (CER) or 14.6 ± 8.21 mCi (UNC) of [11C]RAC, and dynamic data were acquired for 60 min. Specific activity at time of injection was 1.12 ± 0.67 Ci/μmol (CER) and 0.42 ± 0.22 Ci/μmol (UNC). Subject weights were 76.5 ± 14.4 kg (CER) and 68.6 ± 12.8 kg (UNC). Injected raclopride mass doses were 0.24 ± 0.15 nmol/kg (CER) and 0.65 ± 0.39 nmol/kg (UNC). Subjects also received a heavily T1-weighted magnetic resonance image (MR; 3D spoiled gradient echo recalled) on a 1.5T GE Echospeed LX scanner (GE Medical Systems, Waukesha, WI).
Image Processing
Image processing procedures using Statistical Parametric Mapping 2 software (SPM2) (http://www.fil.ion.ucl.ac.uk/spm/) have been described in detail elsewhere [39]. For each scan, a summed image was created from the first ten minutes of dynamic [11C]RAC data using the Realign function in SPM2. These summed images contained a mixture of blood flow and specific striatal D2/D3 binding, permitting accurate registration of all time frames to a single image. The summed image was co-registered to the individual subject's MR scan using the SPM2. Motion correction was achieved by coregistering individual PET frames to the coregistered, summed PET image. Each subject's MR was normalized into Montreal Neurological Institute (MNI) stereotactic space using SPM2's default normalization parameters. The transformation matrix obtained from this normalization step was applied to the motion-corrected, coregistered PET data from each subject, placing all dynamic PET data in MNI stereotactic space.
Parametric Binding Potential Images
The binding potential of [11C]RAC (BPND, [14]) is an index of how many DA D2/D3 receptors are available for binding. Parametric BPND images were generated from the spatially normalized dynamic PET data as described previously [39], using a multilinear reformulation of the Logan reference region graphical analysis [13, 17]. The parametric whole brain BPND images were smoothed with an 8 mm kernel [5, 27, 42].
Voxel-Based Analyses
We restricted the search area during the voxel-wise paired t-test analysis, as (1) our sole focus was the striatum, and (2) the striatum has the highest density of D2/D3 receptors in the brain, and is the only brain structure with high enough signal-to-noise ratio to support quantitation of D2/D3 receptor availability using [11C]RAC. Ligands with higher signal-to-noise ratios in extrastriatal areas are required to quantify D2/D3 receptor availability outside of the striatum [4, 22, 23]. A bilateral striatal binary mask smoothed with a 10 mm kernel [39] was applied to the whole brain BPND images to create striatal BPND images that were used for all analyses reported herein. Unidirectional, voxel-wise independent t-tests between the CER and UNC striatal BPND images were conducted in SPM2 as follows: CER BPND > UNC BPND, and UNC BPND > CER BPND. The statistical threshold for the SPM results was p < 0.005 (uncorrected).
Regions of Interest (ROI) Analysis
ROIs were created for the left caudate, right caudate, left putamen, and right putamen from the publically available MarsBaR Automated Anatomic Labeling Region of Interest library (http://marsbar.sourceforge.net/). The anatomic regions are described in Tzourio-Mazoyer et al. [33]. ROIs were smoothed with a 10 mm kernel. Mean BPND values for each ROI were extracted from the smoothed parametric images using the MarsBaR toolbox for SPM2 (http://marsbar.sourceforge.net/). Two-tailed Student's t-tests were used to test for differences in BPND between groups. Statistical significance was set at p < 0.05.
Other Statistical Analyses
Two-tailed Student's t-tests were used to test for group differences in subject age and total mass dose. Statistical significance was set at p < 0.05.
Results
There were no differences between groups with respect to age or weight. The specific activity was significantly higher in the CER group (p < 0.05). Total raclopride mass dose per body weight was significantly lower in CER (p < 0.02).
Voxel-Wise Analysis
For the contrast, CER BPND > UNC BPND, no significant voxels were detected, even when the statistical threshold was lowered to p < 0.05. However, for the contrast UNC BPND > CER BPND, significant voxels were detected in the right and left putamen, indicating that the UNC had higher resting D2/D3 receptor availability than CER in these regions (Figure 1).
FIGURE 1.
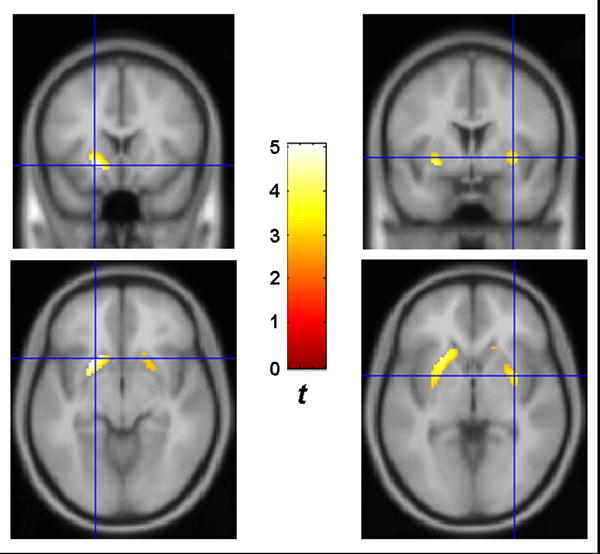
SPM results for contrast between striatal binding potential images; UNC > CER. CER, certain. UNC, uncertain. Statistical threshold: p < 0.005, uncorrected. Display threshold: p < 0.01. Colorbar indicates t-values for each contrast. Crosshairs indicate peak t-values within each cluster. Left: Peak voxel is in the left anterior putamen, at MNI coordinate (-20, 16, -8). Right: Peak voxel is in the right posterior putamen, at MNI coordinate (32, 2, -2).
ROI Analysis
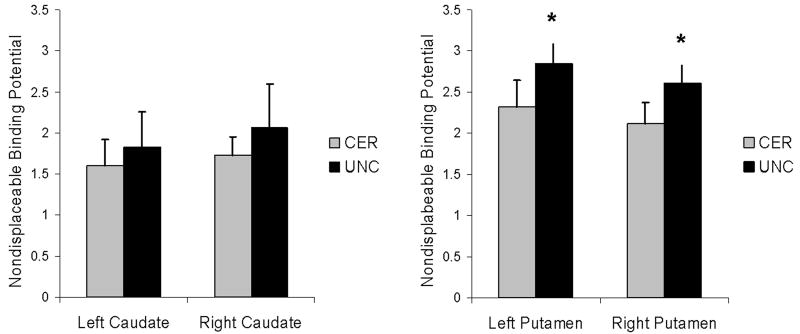
Figure 2 illustrates the mean BPND values for each region, by group. The mean ± SD BPND values (mean ± SD) for CER and UNC, respectively, were: Right caudate, 1.73 ± 0.22; 2.07 ± 0.53. Left caudate: 1.60 ± 0.32; 1.83 ± 0.43. Right putamen: 2.12 ± 0.26; 2.61 ± 0.40. Left putamen: 2.32 ± 0.33; 2.85 ± 0.39. There were no differences between groups in either the right or left caudate. UNC had significantly higher BPND values in both right and left putamen.
FIGURE 2.
Mean [11C]raclopride binding potential from the ROI analysis. CER, certain. UNC, uncertain. A. Mean ± SD for left and right caudate. B. Mean ± SD for left and right putamen. *Statistically different from CER, p < 0.03.
Discussion
We examined nondisplaceable [11C]raclopride binding potential in two groups that received different instructions regarding their scan condition. CER (“certain”) was instructed that they would undergo a “baseline” scan, during which no procedures would occur. UNC (“uncertain”) was told that they may or may not receive an IV alcohol infusion during the scan, and were not informed that it was a “No-Alcohol” scan until 5 min after scan start. Both voxel-wise and ROI-based analyses demonstrated that UNC had significantly higher BPND values in putamen bilaterally than CER. Although this suggests that differences in cognitive state contributed to this result, that is not the only potential source of bias in BPND. We also tested for other group differences that could have had direct impact on D2/D3 receptor availability, namely, age and mass dose of raclopride.
The age-related decline of striatal D2/D3 receptors is well-documented [3, 19, 28, 34-36, 38], which raised the possibility that age differences between CER and UNC could possibly have accounted for the results. However, subjects in our studies were not statistically different in age by group. The tight distribution around the mean of 25 years of age also makes it very unlikely that age-related correlations with BPND would be detectable, as the rates of receptor loss are slow (e.g., 0.6% per year [3], 7.9% per decade [35]) and are even slower with proper correction for partial volume effects [19]). Therefore, we are confident that age of the subjects did not play a role in the differential BPND values.
In addition, mass effects were not responsible for the differences in striatal BPND between groups. A high mass dose per body weight given during tracer administration could cause underestimation of BPND. This happens when the amount of cold ligand administered is not truly a “trace” amount (i.e., “non-tracer” conditions). Although mass dose is traditionally a concern limited to the realm of small animal neuroligand imaging, it is theoretically possible for mass dose effects to occur in human studies. With [11C]RAC, non-tracer conditions cause two significant sources of confound: (1) Mass effect: Occupation of receptors by cold raclopride could become significant, reducing the number of receptors available for binding by the hot ligand, and causing underestimation of BPND. (2) Drug effect: Raclopride itself could induce DA release via antagonism of pre-synaptic D2 autoreceptors [2, 30, 31, 37]. The DA release caused by cold raclopride could also contribute to reduction of [11C]RAC signal, and contribute to the underestimation of BPND. However, it is highly unlikely that our scans were conducted under non-tracer conditions, especially if the “safe” mass dose range determined in small animals may be extrapolated to human studies. The highest dose given was 1.1 nmol/kg, which is still under the cutoff for a mass dose effect in rats (1.5 nmol/kg, [15, 24]). Furthermore, the data do not support evidence for a mass dose effect causing differences in BPND between groups: the mass doses given per subject were significantly different, but UNC had a significantly higher mass dose than CER (this was driven by two outliers in the UNC group at 1.09 and 1.06 nmol/kg; all other subjects – in both groups- were under 0.45 nmol/kg). This is inconsistent with the concept of a mass dose effect. For mass dose to have played a role in the differences in BPND, UNC (which had a higher BPND than CER) would have to have had a significantly lower mass dose compared to CER. Therefore, we can safely rule out mass dose as the cause of higher BPND in UNC.
A more plausible and theoretically meaningful explanation for the differences in BPND between CER and UNC could lie in the expectations the subjects had for the scan condition. While somewhat speculative in the absence of quantitative data on subjects' expectations, it nevertheless seems reasonable to assume that subjects in CER had no ambivalence about the scan condition; they were told definitively they would be undergoing a resting scan. UNC, however, had several hours to contemplate the possibility of receiving alcohol, and perhaps cultivate an anticipation or hope of alcohol administration during the first scan (alcohol administration was highlighted during recruitment). It is also possible that when the UNC subjects were told they were not going to receive alcohol, this constituted a negative prediction error (e.g., an anticipated reward was not delivered), which can result in a decrease in the firing rates of dopamine neurons [18, 25, 29], and presumably a concomitant decrease in striatal [DA].
Our present retrospective analysis has limitations: most notably, small sample size and the lack of a quantitative assessment of subjective state regarding anticipation, expectation, and disappointment before, during, and after the “baseline” scan. Future studies are needed to assess the effects of various cognitive states on “resting” measures of binding potential for [11C]raclopride and other tracers.
In summary, striatal D2/D3 receptor availability was significantly different in two subject groups provided with different instructions about what to expect during a “baseline” scan. Our results suggest that alterations in DA level induced by cognitive state may affect measurements of binding potential. When designing studies that assess changes in dopamine concentration via changes in BPND relative to a baseline scan, investigators should carefully consider the conditions under which “baseline” scans are conducted.
Acknowledgments
This work was supported by the Whitaker Foundation (RG-02-0126), the NIAAA-sponsored Alcohol Research Center at Indiana University (P60 AA07611-17), and the General Clinical Research Center at the Indiana University School of Medicine (MO1 RR000750).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alpert NM, Badgaiyan RD, Livni E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. Neuroimage. 2003;19:1049–60. doi: 10.1016/s1053-8119(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 2.Andersson JL, Marcus M, Nomikos GG, Svensson TH. Prazosin modulates the changes in firing pattern and transmitter release induced by raclopride in the mesolimbic, but not in the nigrostriatal dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:236–43. doi: 10.1007/BF00169289. [DOI] [PubMed] [Google Scholar]
- 3.Antonini A, Leenders KL, Reist H, Thomann R, Beer HF, Locher J. Effect of age on D2 dopamine receptors in normal human brain measured by positron emission tomography and 11C-raclopride. Arch Neurol. 1993;50:474–80. doi: 10.1001/archneur.1993.00540050026010. [DOI] [PubMed] [Google Scholar]
- 4.Christian BT, Narayanan T, Shi B, Morris ED, Mantil J, Mukherjee J. Measuring the in vivo binding parameters of [18F]-fallypride in monkeys using a PET multiple-injection protocol. J Cereb Blood Flow Metab. 2004;24:309–22. doi: 10.1097/01.WCB.0000105020.93708.DD. [DOI] [PubMed] [Google Scholar]
- 5.Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46:1980–9. [PubMed] [Google Scholar]
- 6.Dewar KM, Montreuil B, Grondin L, Reader TA. Dopamine D2 receptors labeled with [3H]raclopride in rat and rabbit brains. Equilibrium binding, kinetics, distribution and selectivity. J Pharmacol Exp Ther. 1989;250:696–706. [PubMed] [Google Scholar]
- 7.Dewey S, Smith G, Logan J, Brodie J, Fowler J, Wolf A. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- 8.Dewey S, Smith G, Logan J, Brodie J, Yu D, Ferrieri R, King P, MacGregor R, Martin T, Wolf A, Volkow N, Fowler J, Meller E. GABAergic inhibition of endogenous dopamine release measure in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET--a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989;9:696–708. doi: 10.1038/jcbfm.1989.98. [DOI] [PubMed] [Google Scholar]
- 10.Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986;231:258–61. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RE, Morris ED, Alpert NM, Fischman AJ. In vivo imaging of neuromodulatory synaptic transmission using PET: A review of relevant physiology. Human Brain Mapping. 1995;3:24–34. [Google Scholar]
- 12.Hall H, Kohler C, Gawell L, Farde L, Sedvall G. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:559–68. doi: 10.1016/0278-5846(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–81. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 14.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Maguire RP, Mintun M, Morris ED, Parsey R, Slifstein M, Sossi V, Suhara T, Votaw J, Wong DF, Carson RE. Consensus nomeclature for binding potential and related terms used in radioligand imaging. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600493. in press. [DOI] [PubMed] [Google Scholar]
- 15.Kung MP, Kung HF. Mass effect of injected dose in small rodent imaging by SPECT and PET. Nucl Med Biol. 2005;32:673–8. doi: 10.1016/j.nucmedbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–7. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 19.Morris ED, Chefer SI, Lane MA, Muzic RF, Jr, Wong DF, Dannals RF, Matochik JA, Bonab AA, Villemagne VL, Grant SJ, Ingram DK, Roth GS, London ED. Loss of D2 receptor binding with age in rhesus monkeys: importance of correction for differences in striatal size. J Cereb Blood Flow Metab. 1999;19:218–29. doi: 10.1097/00004647-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Morris ED, Fisher RE, Alpert NM, Rauch SL, Fischman AJ. In vivo imaging of neuromodulation using positron emission tomography: optimal ligand characteristics and task length for detection of activation. Human Brain Mapping. 1995;3:35–55. [Google Scholar]
- 21.Morris ED, Yoder KK. Positron emission tomography displacement sensitivity: predicting binding potential change for positron emission tomography tracers based on their kinetic characteristics. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600359. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee J, Christian BT, Narayanan TK, Shi B, Collins D. Measurement of d-amphetamine-induced effects on the binding of dopamine D-2/D-3 receptor radioligand, 18F-fallypride in extrastriatal brain regions in non-human primates using PET. Brain Res. 2005;1032:77–84. doi: 10.1016/j.brainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee J, Yang ZY, Lew R, Brown T, Kronmal S, Cooper MD, Seiden LS. Evaluation of d-amphetamine effects on the binding of dopamine D-2 receptor radioligand, 18F-fallypride in nonhuman primates using positron emission tomography. Synapse. 1997;27:1–13. doi: 10.1002/(SICI)1098-2396(199709)27:1<1::AID-SYN1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Opacka-Juffry J, Ashworth S, Ahier RG, Hume SP. Modulatory effects of L-DOPA on D2 dopamine receptors in rat striatum, measured using in vivo microdialysis and PET. J Neural Transm. 1998;105:349–64. doi: 10.1007/s007020050063. [DOI] [PubMed] [Google Scholar]
- 25.Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–42. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappata S, Dehaene S, Poline J, Gregoire M, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A. In vivo detection of striatal dopamine release during reward: a PET study with [11C]raclopride and a single dynamic scan approach. Neuroimage. 2002;16:1015–1027. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- 27.Picard F, Bruel D, Servent D, Saba W, Fruchart-Gaillard C, Schollhorn-Peyronneau MA, Roumenov D, Brodtkorb E, Zuberi S, Gambardella A, Steinborn B, Hufnagel A, Valette H, Bottlaender M. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129:2047–60. doi: 10.1093/brain/awl156. [DOI] [PubMed] [Google Scholar]
- 28.Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, Lehikoinen P, Oikonen V, Syvalahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–4. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- 29.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 30.See RE. Striatal dopamine metabolism increases during long-term haloperidol administration in rats but shows tolerance in response to acute challenge with raclopride. Neurosci Lett. 1991;129:265–8. doi: 10.1016/0304-3940(91)90477-b. [DOI] [PubMed] [Google Scholar]
- 31.See RE, Murray CE. Changes in striatal dopamine release and metabolism during and after subchronic haloperidol administration in rats. Neurosci Lett. 1992;142:100–4. doi: 10.1016/0304-3940(92)90629-l. [DOI] [PubMed] [Google Scholar]
- 32.Seeman P, Guan HC, Niznik HB. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: implications for positron emission tomography of the human brain. Synapse. 1989;3:96–7. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–9. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–6. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang GJ, Volkow ND, Logan J, Fowler JS, Schlyer D, MacGregor RR, Hitzemann RJ, Gur RC, Wolf AP. Evaluation of age-related changes in serotonin 5-HT2 and dopamine D2 receptor availability in healthy human subjects. Life Sci. 1995;56:PL249–53. doi: 10.1016/0024-3205(95)00066-f. [DOI] [PubMed] [Google Scholar]
- 37.Westerink BH, Kawahara Y, De Boer P, Geels C, De Vries JB, Wikstrom HV, Van Kalkeren A, Van Vliet B, Kruse CG, Long SK. Antipsychotic drugs classified by their effects on the release of dopamine and noradrenaline in the prefrontal cortex and striatum. Eur J Pharmacol. 2001;412:127–38. doi: 10.1016/s0014-2999(00)00935-3. [DOI] [PubMed] [Google Scholar]
- 38.Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226:1393–6. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- 39.Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–73. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–70. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- 41.Young LT, Wong DF, Goldman S, Minkin E, Chen C, Matsumura K, Scheffel U, Wagner HN., Jr Effects of endogenous dopamine on kinetics of [3H]N-methylspiperone and [3H]raclopride binding in the rat brain. Synapse. 1991;9:188–94. doi: 10.1002/syn.890090305. [DOI] [PubMed] [Google Scholar]
- 42.Ziolko SK, Weissfeld LA, Klunk WE, Mathis CA, Hoge JA, Lopresti BJ, DeKosky ST, Price JC. Evaluation of voxel-based methods for the statistical analysis of PIB PET amyloid imaging studies in Alzheimer's disease. Neuroimage. 2006;33:94–102. doi: 10.1016/j.neuroimage.2006.05.063. [DOI] [PubMed] [Google Scholar]