Abstract
Cardiovascular disease is associated with chronic low-level inflammation, as evidenced by elevated circulating proinflammatory cytokines. Experimental evidence suggests that inflammation can be suppressed under conditions of high shear stress. We examined the effects of enhanced external counterpulsation (EECP), a non-invasive therapy that increases endothelial shear stress, on circulating levels of inflammatory biomarkers and adhesion molecules in patients with angina pectoris. Twenty-one patients were randomly assigned to either 35 1-hour treatments at cuff pressures of 300 mmHg (EECP; n= 12) or at 75 mmHg (SHAM; n=9). Plasma tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1) were measured before and after 35 1-hour sessions of treatment or SHAM. Patients in the EECP group demonstrated reductions in TNF-α (6.9 ± 2.7 vs. 4.9 ± 2.5 pg/ml, P < 0.01; -29%) and MCP-1 (254.9 ± 55.9 vs. 190.4 ± 47.6 pg/ml, P < 0.01; -19%) following treatment, whereas, there was no change in the SHAM group. Changes in sVCAM-1 were not observed in either group. In conclusion, 35 sessions of EECP decreases circulating levels of proinflammatory biomarkers in patients with symptomatic CAD.
Keywords: enhanced external counterpulsation, inflammation, angina
Patients with CAD demonstrate elevated levels of proinflammatory cytokines and adhesion molecules as compared to levels observed in healthy controls.1,2 Moreover, proinflammatory cytokines appear to be elevated even further in patients with angina.3 Enhanced external counterpulsation (EECP) is a noninvasive treatment for patients with symptomatic CAD and has been shown to decrease refractory angina.4,5 EECP significantly augments diastolic flow and increases shear stress in central and peripheral vascular beds.6 Experimental evidence suggests that high shear stress along the blood vessel wall has a favorable effect on proinflammatory cytokine and adhesion molecule expression and signaling.7,8 Therefore, we hypothesized that the high levels of shear stress produced during EECP would decrease circulating levels of selected proinflammatory markers and adhesion molecules in patients with angina pectoris.
METHODS
This prospective, single-blind, sham controlled study consisted of 21 consecutive patients with angina pectoris who were referred to EECP. All patients were recruited from the Cardiovascular Clinic at Shands Hospital at the University of Florida during a clinical screening procedure performed by a cardiologist that is mandatory for all patients referred for EECP. The study was approved by the University of Florida Health Science Center Institutional Review Board and written informed consent was obtained from all patients. Inclusion criteria were age ≥ 21, symptoms of angina pectoris or angina equivalent present on average of at least twice a week, and angiographic evidence of disease in at least one major epicardial coronary artery. Exclusion criteria included unstable angina, arrhythmia that would interfere with EECP triggering, heart failure and/or left ventricular ejection fraction ≤ 30%, valvular heart disease, severe peripheral vascular disease, or uncontrolled hypertension (>180/100). Patients were randomly assigned to either 35 1-hour sessions of EECP at cuff pressures of 300 mmHg (EECP; n=12) or to a sham-EECP group (SHAM; n=9) at cuff pressures of 75 mmHg.
EECP treatment was performed at Shands Hospital at the University of Florida, Gainesville, FL. Patients were treated 1 hour daily on Monday through Friday for 7 consecutive weeks, resulting in a total of 35 hours of treatment. EECP involved sequential inflation and deflation of compressible cuffs wrapped around the patients’ calves, lower thighs, and upper thighs. Compressed air pressure was applied via the cuffs to the lower extremities in a sequence synchronized with the cardiac cycle via microprocessor-interpreted electrocardiogram signals. The diastolic augmentation pressure was progressively increased by increasing external compression to either 300 mmHg (EECP) or 75 mmHg (SHAM).5 Patients were instructed to continue their usual medications. Canadian Cardiovascular Society angina class was determined before and after completion of the study.
Venous blood samples were collected prior to and following 35-sessions of EECP or SHAM. Plasma levels of tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1) were determined by commercially available enzyme-linked immunosorbent assay (Quantikine, R&D Systems). These specific markers were chosen based on previous experimental evidence.7,8 The intra- and inter assay coefficients of variance were 4.6% and 7.7% for TNF-α, 4.2% and 6.9% for MCP-1, and 3.4% and 6.1% for sVCAM-1, respectively. Levels of serum lipids and glucose were measured in hospital laboratories by standard and validated techniques.
Analysis of variance was used to analyze baseline group differences between the EECP and SHAM groups. Changes in the continuous dependent variables were analyzed by repeated measures analysis of variance measures before and after 35 hrs of EECP or SHAM. When a significant group-by-time interaction was observed, within-group comparisons between time points and between-group comparisons at each time point were performed using Tukey’s post hoc analysis. All statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL). All data are reported as mean ± standard deviation (SD). An alpha level of P < 0.05 was required for statistical significance.
RESULTS
Baseline characteristics are shown in Table 1. There were no differences between the two groups at study entry with respect to blood pressure, drug therapy, prior cardiovascular history and/or procedures, or cardiovascular risk factors. Following the intervention, patients that received EECP demonstrated an improvement in CCS angina class (3.1 ± 0.5 vs. 1.2 ± 0.4, P < 0.01) and reductions in anginal episodes (1.6 ± 1.4 vs. 0.4 ± 0.6, P < 0.05) and nitroglycerine usage per day (0.5 ± 0.7 vs. 0.1 ± 0.2, P < 0.05). There were no changes in any measure of symptom improvement in the SHAM group.
Table 1.
Baseline Patient Characteristics
| Variable | EECP (n=12) | SHAM (n=9) |
|---|---|---|
| Age (years) | 63 ± 11 | 62 ± 10 |
| Male/Female | 8 / 4 | 7 / 2 |
| Body mass index (kg/m2) | 29.8 ± 3.4 | 33.0 ± 4.2 |
| Total cholesterol (mg/dl) | 138 ± 41 | 142 ± 25 |
| Low-density lipoprotein (mg/dl) | 68 ± 37 | 72 ± 22 |
| High-density lipoprotein (mg/dl) | 45 ± 15 | 33 ± 7 |
| Triglycerides (mg/dl) | 123 ± 10 | 165 ± 76 |
| Glucose (mg/dl) | 116 ± 22 | 107 ± 16 |
| Prior myocardial infarction | 4 | 3 |
| Multivessel coronary artery disease | 10 | 8 |
| Prior percutaneous coronary intervention | 8 | 7 |
| Prior coronary artery bypass graft | 7 | 5 |
| Diabetes | 6 | 4 |
| Hypertension | 9 | 6 |
| Hyperlipidemia | 10 | 8 |
Values are mean ± SD.
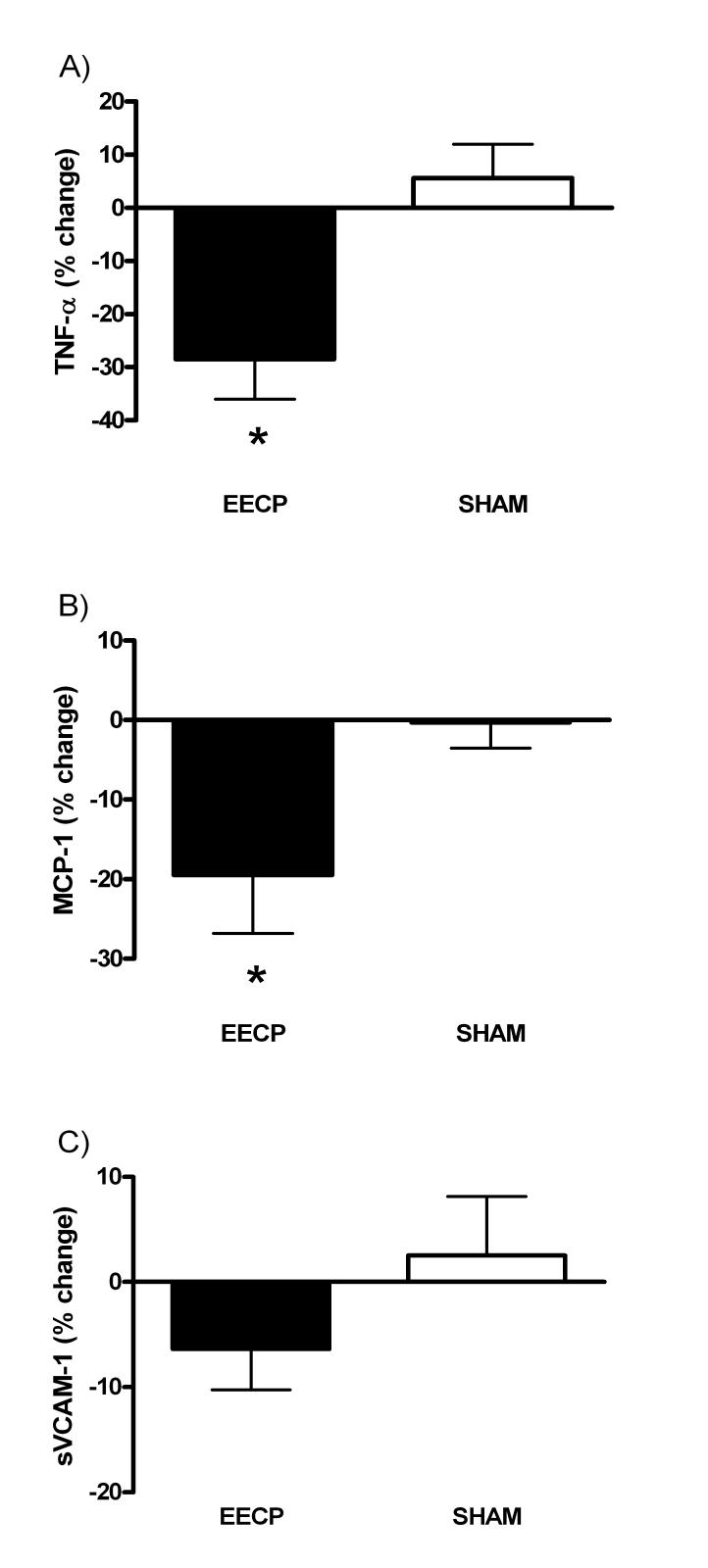
Following 35 hours of treatment, circulating levels of TNF-α (6.9 ± 2.7 vs. 4.9 ± 2.5 pg/ml, P < 0.01) and MCP-1 (255 ± 56 vs. 190 ± 48 pg/ml, P < 0.01) were decreased in the EECP group, but did not change in the SHAM group (6.4 ± 1.9 vs. 6.7 ± 1.9 pg/ml, P = 0.54 and 270 ± 82 vs. 264 ± 66 pg/ml, P = 0.51, respectively). There was no change for sVCAM-1 in either the EECP group (776 ± 280 vs. 726 ± 278 ng/ml, P = 0.14) or the SHAM group (847 ± 177 vs. 859 ± 160 ng/ml, P = 0.81) (Figure 1A-C).
Figure 1.
A) % change in TNF-α % after 35-sessions; B) % change in MCP-1 after 35-sessions; C) % change in sVCAM-1 after 35-sessions; * P < 0.05 versus pre treatment values; values expressed as mean ± S.E.M.
DISCUSSION
This is the first randomized, controlled study examining the effect of EECP on inflammatory and adhesion molecules in CAD patients with refractory angina pectoris. Our results indicate that EECP has an anti-inflammatory effect in patients with angina pectoris. The percent reduction in TNF-α (-29%) observed in the present study following EECP is similar to what has been previously reported with interventions such as exercise in patients with cardiovascular disease.9 EECP was also effective in reducing plasma levels of MCP-1. Increased plasma levels of TNF-α and MCP-1 have been shown to predict future coronary events.10,11 Therefore, the reductions observed in the present study may have clinical significance in regards to reducing the risk for future cardiovascular events in this patient population.
The mechanism responsible for the anti-inflammatory action of EECP is likely related to the intermittent bouts of shear stress created with each inflation/deflation cycle of the cuffs. Shear stress is a potent stimulus for the synthesis and release of endothelial-derived nitric oxide (NO).7 In addition to being a potent vasodilator, NO also serves an anti-inflammatory and anti-atherosclerotic role by inhibiting expression of MCP-1 and reducing VCAM-1 expression.12 Moreover, in arterial regions of low shear stress there is a decrease in NO bioavailability and an upregulation of proinflammatory biomarkers.13 Although NO production was not assessed in the present study, EECP has previously been shown to increase plasma nitrite/nitrate levels, a marker of NO production.14,15 Decreased levels in TNF-α following EECP may have contributed to the decrease in MCP-1 levels. Chiu et al.,16 showed that endothelial cells exposed to a high level of shear stress have attenuated TNF-α induced MCP-1 expression. Although we did not observe a significant change in sVCAM-1 levels following EECP using a plasma enzyme-linked immunosorbent assay, it is possible that membrane bound VCAM-1 levels may change in response to EECP. Unfortunately, the design of the present study did not permit the assessment of membrane bound VCAM-1.
In conclusion, the results from the present study indicate that EECP is an effective intervention in reducing plasma levels of TNF-α and MCP-1 and these changes are paralleled by decreases in anginal symptoms. Together, these results suggest that an anti-inflammatory mechanism may help explain the symptomatic benefits of EECP. Studies involving a larger sample size and other biomarkers of inflammation are necessary to confirm our findings.
Acknowledgments
This study was supported by NIH R01 HL077571-01 to Dr. Braith
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100:793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 2.Mizia-Stec K, Mandecki T, Zahorska-Markiewicz B, Janowska J, Szulc A, Jastrzebska-Maj E, Szymanski L, Majewski T. Selected cytokines and soluble forms of cytokine receptors in coronary artery disease. Eur J Intern Med. 2002;13:115–122. doi: 10.1016/s0953-6205(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, Brunsvig A, Muller F, Forfang K, Froland SS, Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J Am Coll Cardiol. 2001;37:485–491. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW, Estrada JC, Braith RW, Owens K, Conti CR. Enhanced external counterpulsation treatment improves arterial wall properties and wave reflection characteristics in patients with refractory angina. J Am Coll Cardiol. 2006;48:1208–1214. doi: 10.1016/j.jacc.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 5.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 6.Werner D, Schneider M, Weise M, Nonnast-Daniel B, Daniel WG. Pneumatic external counterpulsation: a new noninvasive method to improve organ perfusion. Am J Cardiol. 1999;84:950–952. A7–8. doi: 10.1016/s0002-9149(99)00477-4. [DOI] [PubMed] [Google Scholar]
- 7.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 8.Lerner-Marmarosh N, Yoshizumi M, Che W, Surapisitchat J, Kawakatsu H, Akaike M, Ding B, Huang Q, Yan C, Berk BC, Abe J. Inhibition of tumor necrosis factor-[alpha]-induced SHP-2 phosphatase activity by shear stress: a mechanism to reduce endothelial inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1775–1781. doi: 10.1161/01.ATV.0000094432.98445.36. [DOI] [PubMed] [Google Scholar]
- 9.Adamopoulos S, Parissis J, Karatzas D, Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou K, Coats AJ, Kremastinos DT. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 10.Kervinen H, Manttari M, Kaartinen M, Makynen H, Palosuo T, Pulkki K, Kovanen PT. Prognostic usefulness of plasma monocyte/macrophage and T-lymphocyte activation markers in patients with acute coronary syndromes. Am J Cardiol. 2004;94:993–996. doi: 10.1016/j.amjcard.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 13.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Masuda D, Nohara R, Hirai T, Kataoka K, Chen LG, Hosokawa R, Inubushi M, Tadamura E, Fujita M, Sasayama S. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by(13)N-ammonia positron emission tomography. Eur Heart J. 2001;22:1451–1458. doi: 10.1053/euhj.2000.2545. [DOI] [PubMed] [Google Scholar]
- 15.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98:28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 16.Chiu JJ, Lee PL, Lee CI, Chen LJ, Chen CN, Ko YC, Lien SC. Shear stress attenuates tumor necrosis factor-alpha-induced monocyte chemotactic protein-1 expressions in endothelial cells. Chin J Physiol. 2002;45:169–176. [PubMed] [Google Scholar]