Synopsis
Complimentary and alternative medicines (CAM) are frequently used for the treatment of sleep disorders, but in many cases, patients do not discuss these therapies directly with their health care provider. There is a growing body of well-designed clinical trials using CAM that have shown the following: 1) Melatonin is an effective agent for the treatment of circadian phase disorders that affect sleep, however, the role of melatonin in the treatment of primary or secondary insomnia is less well established. 2) Valerian has shown a benefit in some, but not all clinical trials. 3) Several other modalities, such as Tai Chi, acupuncture, acupressure, yoga and meditation have improved sleep parameters in a limited number of early trials. Future work examining CAM has the potential to significantly add to our treatment options for sleep disorders in older adults.
Keywords: Complementary and alternative medicine, insomnia, aged, melatonin, valerian
Introduction
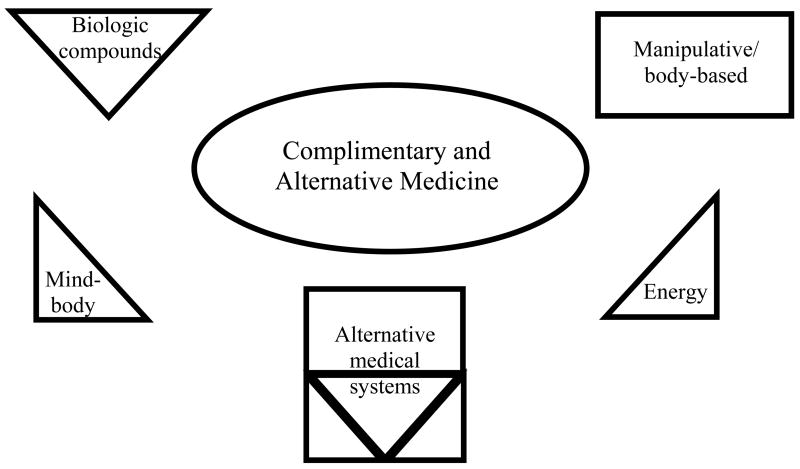
Complimentary and alternative medicines (CAM) have a long history of use for the treatment of sleep disorders. In the 2nd century AD, for example, the prominent ancient Greek physician Galen (Claudius Galenus) prescribed valerian for insomnia.1 CAM therapies are defined by the National Center for Complimentary and Alternative Medicine (NCCAM) as “a group of diverse medical and health care systems, practices, and products that are not presently considered to be part of conventional medicine.” 2 While the terms are often used synonymously, a more accurate statement is that complementary medicines are “used together with conventional medicine”, while alternative medicines are “used in place of conventional medicine (Figure 1).2 Inherent in this definition of CAM is a concept of change: what is considered to be a CAM therapy today may become a conventional form of treatment in the future. An example of this would be the growth of clinical patient support groups, or the use of some forms of cognitive-behavioral therapy as mainstream medical treatments.2
Figure 1.
Diagram of complimentary and alternative medicine subtypes
There exist several hundred different forms of CAM therapy (Figure 1). The classification method used by NCCAM consists of five broad domains: 1) Alternative medical systems that are based on a set of theories and practices that have generally developed apart from conventional medicine. These include acupuncture, ayurveda, or homeopathy. 2) Biologically based practices that consist of compounds often found in nature, such as herbal products. 3) Mind-body medicine which uses systems of thought that can affect bodily functions. This is a broad category that can include meditation, tai chi, yoga and biofeedback. 4) Manipulative and body-based practices which rely on movement of select parts of the body. An example would be massage-based therapies. 5) Energy medicine that emphasizes the use of energy fields including bio-electromagnetic based therapies.2
It is also important to note that many CAM therapies, in particular the biologically based practices and some forms of alternative medical systems, are regulated under the Dietary Supplemental and Education Act of 1994. According to these guidelines, these compounds are not required to undergo purity, safety or efficacy testing. Standardization of compounds across different research studies is thus not always consistent, and some products may not contain the ingredients they advertise. Furthermore, under these guidelines, these products cannot claim treatment effects for specific disease processes in their marketing or advertising.
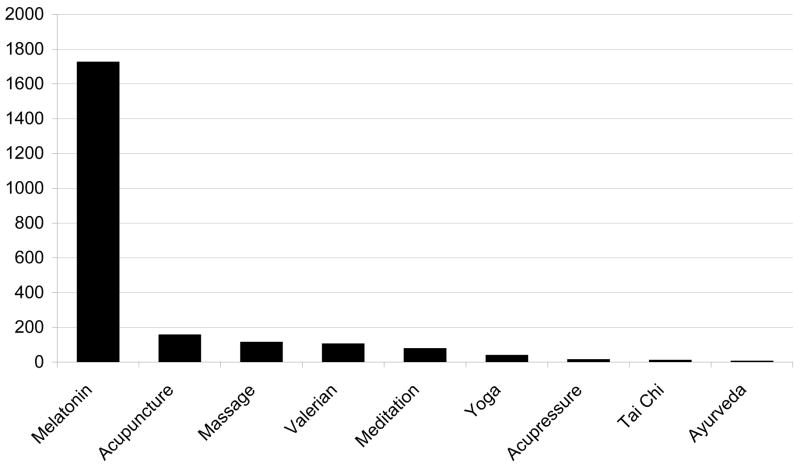
This review will focus on CAM therapies for sleep disorders in older adults. Since there are a large number of CAM therapies, we will limit our discussion to the therapies that are most widely used and for which an adequate body of scientific data exists upon which to base conclusions. Due to the relative paucity of literature for certain modalities, we will also review studies on sleep treatment in younger subjects where appropriate. Several reviews exist of CAM therapy to which the reader is referred for more information about specific modalities.3–14 The major categories that will be discussed in this review include the following (Figure 2): 1) Alternative medical systems—acupuncture, ayurveda; 2) Biological—melatonin, valerian; 3) Mind-body—meditation, yoga, Tai Chi; and 4) Manipulation—massage.
Figure 2.
Number of publications of different types of CAM for sleep based on Medline search.
CAM usage by older adults
A recent telephone survey of 1,559 people over the age of 50 conducted by the American Association of Retired Persons (AARP) and the National Center for Complementary and Alternative Medicine (NCCAM) noted that 54% of persons aged 65 or older had used a CAM therapy or practice.15 The 30% response rate, fairly typical for telephone surveys that do not offer financial compensation, may have overestimated CAM use since users may be more likely to respond than non-users. Nevertheless, the overall prevalence of use is quite high.
When considering factors that influenced CAM use, individuals with a higher income or more years of college education were more likely to use CAM.15 Much of the information obtained by older adults regarding CAM therapies came from family or friends (22%), publications (14%) or radio/TV/internet (20%). Only 31% of CAM users had discussed CAM with their physician15 and only 12% obtained information about CAM from their physicians.15 This highlights the need for physicians to inquire about CAM and help educate their patients regarding CAM. Furthermore, asking about CAM use can help a physician to minimize the risk of polypharmacy: 75% of those who had taken an herbal or dietary product during their lifetime were also taking one or more prescription medications.15
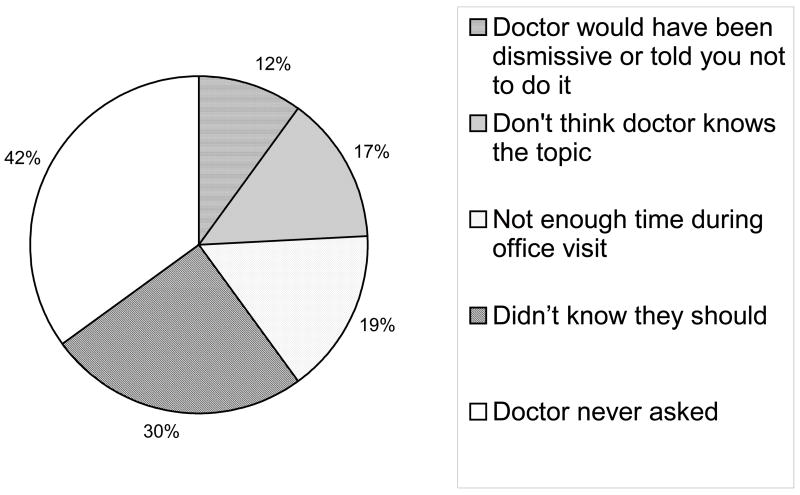
The majority of patients with insomnia also have comorbidities: only 4.1% of patients with insomnia did not have a comorbidity.16 In older adults, many of whom are taking multiple medications, the potential interactions between CAM products and conventional medicine are important to consider. Unfortunately, there is a relative paucity of literature dealing with this topic (see Figure 3).
Figure 3.
Reasons cited for not having discussed CAM with their physician. Adapted from American Association of Retired Persons/National Center for Complimentary and Alternative Medicine, “Complementary and Alternative Medicine: What People 50 and Over Are Using and Discussing with Their Physicians”, 2007
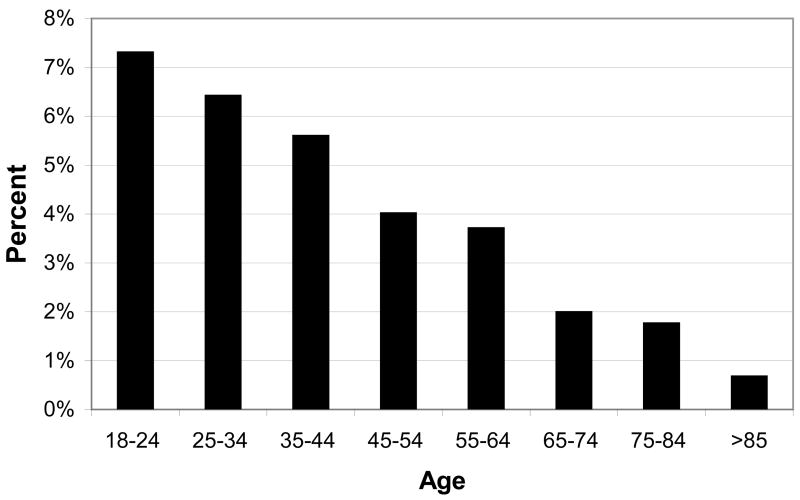
When considering the use of CAM for insomnia in particular, a recent analysis of the National Health Interview Survey (NHIS) dataset (74.3% response rate) by Pearson et al. revealed that 4.5% adults (over age 18, noninstitutionalized) used some form of CAM for their insomnia or trouble sleeping in the past year.16 This extrapolates to 1,615,699 adults over age 18 in the United States, with 113,000 adults over the age of 65 using CAM. Other sedative prescription drugs, by comparison, are used by approximately 5–10% of adults (age over 16–18 years) with insomnia.17, 18 Another interesting observation was that the prevalence of CAM use in those with insomnia tended to decrease with age (Figure 4). The most commonly used CAM modalities in adults were biologically based therapies (64.8% of adult CAM users) and mind-body therapies (39.1% of adult CAM users); those with comorbidities tended to prefer biologically based therapies 16.
Figure 4.
Prevalence of CAM use by age group. Adapted from Pearson et al., “Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data”, Arch Inter Med, 2006.
Melatonin
Melatonin is one of the most thoroughly studied CAM biologic compounds used for sleep. It is a hormone produced by the pineal gland that is postulated to play a significant role in regulating the sleep-wake cycle 19. It has low daytime circulating levels and elevated nocturnal levels that coincide with the sleep phase 20. Melatonin has the ability to influence the timing of the circadian sleep-wake cycle, as has been demonstrated by work in subjects with a free-running circadian rhythm 21. It may also have sedative effects possibly via direct inhibition of the suprachiasmatic nucleus via a feedback loop 22, 23. Melatonin injected into other brain regions, such as the medial preoptic area, can induce sleep as well 24.
Numerous studies have shown decreased melatonin levels in the elderly relative to subjects under age 30 25–27. This may in part be due to declines in the number of pinealocytes 28, or neuronal degeneration of the SCN and resultant circadian desynchrony 29. The presence of insomnia itself is also independently associated with serum melatonin deficiency in some studies 30, 31 but not in others 29, 32–34. Melatonin deficiency is due to three potential factors: medications, age-related changes, and melatonin suppression from comorbid medical condition. Melatonin is profoundly decreased by a variety of medications commonly used by the elderly, including beta-blockers 35 and non-steroidal anti-inflammatory drugs (NSAIDs)36 and the extent of melatonin suppression may be more profound in the elderly than in younger subjects 37. In addition to medications, a variety of primary conditions, such as chronic pain 38, myocardial infarction 39, and ischemic stroke 40 are strongly associated with decreased melatonin levels. This melatonin deficiency is particularly problematic in the elderly as animal studies have shown decreased levels of the Mel1a receptor with aging 41.
These findings have led to a strong interest in using exogenous melatonin to treat chronic primary insomnia in older adults. Doses have ranged from 0.1–0.3 mg (which results in physiologic melatonin levels) to 5–10 mg (pharmacologic melatonin levels). Initial studies showed evidence of a benefit 42 including reduced sleep latency in patients with cognitive impairment 43, however these studies relied on wrist-activity assessment of sleep-wake. Other wrist-activity studies have been negative, however 44. In the largest study to date that uses objective measures, Singer et al. studied 157 older adults with Alzheimer’s Disease and randomized them into three groups: placebo, melatonin 2.5 mg sustained release, and melatonin 10 mg 45. There was no statistically significant benefit on objective parameters (wrist-activity monitoring) with melatonin therapy; this was the primary study measure. Caregivers did note improvement in sleep (as assessed using a sleep diary) for patients using the 2.5 mg sustained-release formulation relative to placebo or the 10 mg immediate release formulation; however there was no significant difference in the subjective Sleep Disturbance Index scores amongst the study arms.
More rigorous studies relying on polysomnography (which is often required by the FDA-for sedative-hypnotics to show efficacy) have also been conducted. Some studies have shown a benefit 46, 47, while others did not demonstrate significant improvements in sleep patterns 48, 49.
Recently, a large meta-analysis was conducted of melatonin therapy for sleep 50. The meta-analysis suggested that melatonin had a clinically insignificant benefit for primary insomnia in older adults: there was a 7.8 min improvement in sleep latency, but no overall improvement in sleep efficiency. Patients with delayed sleep phase syndrome did show a larger, clinically significant benefit of 38.8 min. No benefit was noted for secondary insomnia.
Attempts have also been made to target specific subgroups of patients by their melatonin profile. For example, one study by Hughes et al. found no overall benefit, but noted that the most prominent improvement occurred in patients who had a short duration of endogenous melatonin secretion 48. Of note, this study had an unusual treatment regimen in which all subjects were awoken 4.5 hours after the initial melatonin dose to take a middle-of-the-night dose. One actigraphy based study in patients with low melatonin levels noted improved sleep 51, however only the first six hours of sleep were analyzed in this study. Another self-report study found evidence of benefit 52 however the interpretation of these results is limited by the study design which consisted of a non-randomized placebo/melatonin paradigm. Alternative forms of melatonin have also been tested, including transbuccal melatonin, with no significant benefit in elderly subjects with insomnia 49.
In general, melatonin is well tolerated in the dose range of 0.1 mg to 10 mg with few reported adverse events 53–55. One particular concern in the elderly, however, is that of daytime sleepiness 56. No clinical trials of melatonin in the elderly have measured objective markers of daytime sleepiness. However, one study in younger subjects using objective measures of performance suggests that the sedative effects of melatonin persist even up to seven hours after ingestion 57 and another study noted “tiredness at rising” in four (out of eleven) subjects treated with melatonin 58. Thus while melatonin has the potential to improve sleep efficiency and thereby result in decreased daytime sleepiness, its use may directly lead to daytime sleepiness. It is also possible that melatonin doses of 3–5 mg may lead to increased sleep disruption as has been noted in 4 of 16 younger subjects given melatonin in a temporal isolation study 59. Despite these concerns, Jean-Louis et al., in their previously mentioned study of melatonin 6 mg in cognitively-impaired subjects, noted improved memory recall and concentration, and no increased subjective fatigue 43. In addition, melatonin is less likely to lead to dependence and abuse as can occur with other sedative-hypnotics as it does not cause euphoria 60.
A large body of work has been done examining melatonin’s effects on other organ systems in regards to its safety profile. These findings are summarized below.
Cardiovascular Effects
Human studies using doses such as melatonin 5 mg have observed that melatonin may impair the antihypertensive efficacy of calcium channel blockers to a mild degree. In subjects on nifedipine who were treated with melatonin, there was a mean increase in systolic blood pressure of 6.5 mmHg and in diastolic blood pressure of 4.9 mmHg 61. Human studies show mild reduction in blood pressure with physiologic doses of melatonin 62.
Immune System Effects
Melatonin may have immunomodulatory effects, although no clear consensus exists in this regard as to overall safety 63. Some studies have suggested a pro-inflammatory role in conditions such as autoimmune arthritis 64, 65, while others have found melatonin to be protective against the development of autoimmune disorders such as Type I Diabetes Mellitus 66 and experimental models of inflammation such as carrageenan 67. There has also been one case report regarding the development of auto-immune hepatitis in a patient taking melatonin 68. Of note, the melatonin formulation taken by the patient may have contained unknown additives 69.
Other Effects
The use of melatonin 10 mg for three months has been associated with suppression of endogenous melatonin and the development of an unentrained (free-running) sleep-wake cycle after melatonin withdrawal in two of five subjects in one study of bipolar disorder 70; research in non-bipolar disorder subjects has shown no suppression of endogenous melatonin, however (A. Lewy, personal communication). Melatonin may increase the severity of sleep apnea as noted in one study which documented mild-moderate increases in sleep apnea in some subjects taking melatonin 71. Melatonin may affect follicle stimulating hormone (FSH), luteotropic hormone (LH) and thyroid hormones, however this has not been found to have significant clinical effects in older adults 72, 73. Other side effects noted with melatonin include headache and pruritis in less than 10% of subjects 74.
To study these concerns, several studies have evaluated the safety of melatonin. One study using 10.0 mg over a one month period documented no evidence of adverse events in younger subjects 54. An open-label study of 22 older patients (mean age 60.1 years) with 3 mg of melatonin for six months found no significant changes in endocrine and routine chemistry/liver function analysis, including FSH, LH and thyroid stimulating hormone 53. Another study administered 1.0 mg for 2 months in elderly subjects with no reported side effects 51. In an extended open-label study of 2 mg of melatonin nightly for six months, elderly subjects had decreased estradiol levels, and increased IGD-I and DHEAS levels, but no adverse clinical events occurred 75.
There have also been two large reviews of melatonin that have been published recently (2004). The first review was conducted by the Institute of Medicine/National Academies (Dietary Supplements: A Framework for Evaluation Safety, Prototype Monograph on Melatonin)76. This 70 page review observed that the only cardiovascular adverse events noted with melatonin from approximately 60 clinical studies (including both young, middle aged and elderly subjects) were an increase in blood pressure of approximately 6.5 mmHg in hypertensive patients on calcium channel blockers as mentioned earlier. A second large review of melatonin was recently published by the Agency for Healthcare Research and Quality (AHRQ), titled: Evidence Report/Technology Assessment: Number 108, Melatonin for Treatment of Sleep Disorders.50. The review concluded that adverse “effects were not significant compared to placebo”. They also concluded that melatonin is a “relatively safe substance” in the short term.
Overall, the current evidence suggests that melatonin has a clear role in the management of circadian sleep disorders, such as delayed sleep phase syndrome. The evidence is more equivocal for primary or secondary insomnia, or for the treatment of insomnia in patients with cognitive impairment. It may be that in certain subgroups, such as patients with low melatonin levels or abnormal timing of their melatonin cycle, exogenous melatonin may have a beneficial role; however more work is needed in this area. Fortunately, melatonin appears to have a relatively benign side effect profile.
Valerian
The plant species Valeriana, in particular Valeriana officinalis and to a lesser extent Valeriana edulis, is the source of the ingredients in valerian. These ingredients can be divided into the following categories: valepotriates, sesquiterpenes (volatile oil components which account for valerian’s unpleasant odor), and amino acids (such as GABA and glutamine) 77. Putative sites of action of valerian include the GABA receptor 78, binding at A(1) adenosine receptors 79 or, as more recently noted, the 5-HT-5a receptor 80.
The extraction and preparation method can influence the relative concentrations of each of the valerian components in a given formulation, thus one issue with clinical studies of valerian compounds is while each study may use “valerian”, the specific components may differ. As noted earlier, standardization and purity of biologically based compounds can be a concern, and this applies also to valerian compounds. A recent report by ConsumerLab.com noted that 4 of 17 valerian products had no detectable valerian content, 4 had half the amount listed, two had lead contamination, and one had cadmium contamination 81.
Valerian has been studied in several randomized, placebo-controlled studies, in doses ranging from 400–900 mg 5, 6 One study using both subjective and objective (polysomnography and actigraphy) measures in 18 subjects without sleep problems noted that while subjective measures improved, objective measures showed small, clinical and statistically insignificant improvements 82. Another actigraphy-based study observed a statistically significant improvement in sleep latency (from 15.8 +/− 5.8 min to 9.0 +/− 3.9 min, p<0.01) in patients with insomnia, however, higher doses of 900 mg were associated with morning sleepiness 83. Polysomnography-based research has also shown that a prolonged (2 week) course of treatment was associated with reductions in sleep latency, but no overall change in sleep efficiency 84. Leathwood et al conducted a study in which subgroup analysis included older adult poor sleepers 85. They noted that 63% reported subjective improvement with valerian; however, 43% also noted improvement with placebo and there was thus no statistically significant difference. Valerian has also been used as a tool to assist with weaning patients from benzodiazepines with some limited success. Sleep quality improved and wakefulness after sleep onset decreased, however, there was an increased sleep latency 86. Comparisons with benzodiazepines, such as oxazepam have also been performed and have found that valerian was as effective as oxazepam in improving sleep; however, it was not a placebo controlled study and relied on subjective self-report 87. Valerian has also been used in combination with other agents, such as hops. This valerian-hops combination resulted in a reduced self-reported sleep latency of 5.6 min (p=0.079) relative to placebo, and all other subjective and polysomnographic measures were similar.
Studies specifically targeting older adults with insomnia have also been conducted using objective measures. A randomized, placebo-controlled study of 14 subjects noted that total sleep time and slow-wave sleep improved with valerian 88. The valerian group, however, had significantly worse baseline sleep parameters, thus making it difficult to determine if these results represent a true effect of valerian or a regression towards the mean phenomenon. A later study using a one night dosing paradigm found no significant benefit of valerian relative to placebo 89.
In general, valerian has been found to be safe with minimal side effects in the published literature. Rare side effects that have been reported include gastrointestinal upset, contact allergies, headache, restless sleep, and mydriasis 90. In comparison to placebo, adverse events occurred at a similar rate, and there was no rebound insomnia or withdrawal effects with valerian 91. Valerian has been considered as a possible treatment for sleep disruption in comorbid medical states, such as cancer 92 and rheumatoid arthritis 93, however due to uncertainty regarding polypharmacy and metabolism, it is not recommended for use in critically ill patients 94. Valepotriates may also alkylate DNA, and can thus have theoretical cytotoxic and carcinogenic potential 77. Since valerian may act on GABA receptors, valerian may potentiate the sedative effects of other central nervous system depressants 95. Research examining the daytime “hangover” cognitive effects found no difference between placebo and valerian 91, 96. There is also a case report of valerian withdrawal symptoms which were characterized by delirium in a patient who had been using one-half to two grams per dose up to five times daily for several years; symptoms improved with benzodiazepine therapy 97.
In summary, valerian has been studied in several randomized, placebo-controlled trials, including several studies in older adults. There appears to be evidence of a mild subjective improvement in sleep with valerian, especially when used for two weeks or more. However, the objective testing has had less consistent results with little or no improvement noted. Some, but not all, studies have observed increased slow wave sleep, which could be an important finding if validated in additional work. Methodological limitations, non-standardized formulations and the small sample size of the existing literature (which creates a higher likelihood of type 2 errors), suggests that future larger studies are needed once a well-characterized and standardized form of valerian is developed.
Manipulative and Body-Based Practices
The manipulative and body-based practices encompass a broad range of therapies that involve hands-on interventions. The majority of the published literature on massage therapy are for sleep disorders in infants and children. Studies in adult populations are limited and tend to focus on patients with comorbid medical conditions. One study examined the use of aromatherapy massage for hospice patients using self-report measures of sleep. This randomized, placebo-controlled study noted no benefits in quality of life or pain control; however, there were statistically significant improvements in sleep and depression 98. Another randomized study comparing therapeutic massage and relaxation tapes in the management of stress noted that while patients expressed a preference for massage, both modalities showed improvements in sleep, with no significant benefit of one over the other 99. Combination therapies using massage have also suggested a benefit, although these often have not included a placebo arm 100. Polysomnography studies of massage have also been conducted: Richards et al. conducted a randomized trial of a massage intervention compared to placebo and observed a one hour increase in sleep time for the massage therapy group 101. Massage therapy has also been used for fibromyalgia, where it has been found to increase sleep time and reduce pain levels 102, 103.
Another form of manipulative therapy is acupressure, which is a non-invasive technique that involves stimulation of meridian or acupoints on the body using finger pressing movements. It can be administered by nursing staff, or by family members of a patient. Acupressure has been studied in a randomized design in institutionalized older adults 104. This study noted statistically significant improvements in both the Pittsburgh Sleep Quality Index (primary outcome measure) and number of nocturnal awakenings in the acupressure group relative to the two placebo arms (sham acupressure and conversation). This group has also studied acupressure in end-stage renal patients using a similar design, and also observed evidence of statistically significant improvements in self-reported sleep quality, sleep latency and sleep efficiency 105. Another study has replicated these findings 106. Agitated behavior in patients with dementia has also been treated with acupressure, with one study noting reductions in the Cohen-Mansfield Agitation Inventory score, and other metrics of agitation in patients during the acupressure arm relative to the control arm 107.
Another form of acupressure is auricular therapy, which involves applying pressure to acupoints either via the fingertips, medicinal seeds, or magnets 108, 109. Suen et al. conducted a 3 week randomized, single-blind placebo-controlled study using wrist-activity monitoring to provide an objective assessment of sleep parameters between magnetic auricular acupressure and two controls 109. They observed statistically significant improvements in sleep latency, and sleep efficiency, with an overall increase of approximately 35 min in the total sleep time. Adverse effects were not discussed in their manuscript; however, auricular therapy is generally considered safe. A six month follow-up of this cohort was also conducted and found that insomnia symptoms remained ameliorated in the treatment group relative to the control groups 108.
The results of these studies are very intriguing. However, this work needs to be replicated in additional studies amongst more diverse cohorts before it can be routinely recommended for the management of insomnia.
Acupuncture
Acupuncture, which is considered in the category of alternative medical systems, also acts on meridian points to influence health 9. The majority of studies utilizing acupuncture have relied on subjective measures or have not had a placebo control, thus making interpretation of study findings difficult 9. Few studies have examined the effects of acupuncture in insomnia using polysomnography 110, 111. While both demonstrated evidence of improved sleep, one was a pilot study that was not placebo-controlled 110. This study also demonstrated increases in nocturnal melatonin secretion and reductions in stress/anxiety scores compared to pre-treatment levels.
Acupuncture has also been examined as a treatment modality for sleep disruption due to other conditions, including insomnia post stroke 112 and post menopausal symptoms 113, 114. Other sleep disorders that have been treated with acupuncture include fibromyalgia 115, 116 and sleep apnea 117, 118.
Meditation
While there are several forms of meditation, one of the most commonly studied for insomnia is mindfulness meditation. Stress reduction may be one of the mechanisms by which meditation can exert a beneficial effect on sleep and most of the studies that have demonstrated improved sleep during meditation therapy have been conducted as stress reduction studies. In this regard, it can be used as part of a cognitive therapy approach. In addition to stress reduction, there may also be differences in slow-wave sleep as a result of meditation 119. Meditation therapy has also been used in cancer patients and found to help improve sleep in two studies 120, 121.
Yoga
Yoga is a multicomponent practice that consists of physical activity associated with specific postures, breathing exercises, and a specific philosophical attitude towards life. It has been shown to reduce anxiety levels and physiologic arousal. A randomized, parallel group study conducted over a six month treatment period compared yoga (60 minute session six days a week, with a 15 minute evening session), Ayurvedic therapy, and wait-list control in 69 older adults 122. Self-reported sleep measures were assessed and demonstrated a one-hour increase in total sleep time relative to pre-treatment that was significantly higher than changes in the wait-list or Ayurveda groups. When studied in other populations, such as lymphoma patients, yoga was found to improve subjective sleep parameters when compared to a wait-list control group 123, 124.
Tai Chi
Tai Chi is a low- to moderate-intensity Chinese exercise that includes a meditational component. A study of the effects of Tai Chi (consisting of three 60 minutes sessions for 24 weeks) in 118 older adults in comparison to low-impact exercise noted that Tai Chi improved self-reported sleep duration by 48 min 125. General health-related quality of life and daytime sleepiness levels also improved. No injuries were reported in either group. Of note, 33% of subjects withdrew from the study (no significant difference between the Tai Chi and exercise groups). These findings are very interesting and if replicated by additional research using objective measures, could add to the CAM treatment options for insomnia.
Conclusion
This review presents the main CAM therapies for sleep disorders in older adults on which adequate published evidence exists. By far, the largest body of work has been done with melatonin, which has been found to have a benefit in the treatment of circadian sleep disorders, with more equivocal results for primary or secondary insomnia. Valerian has also been found to improve sleep in some studies, but variability in extraction and formulation remains an issue. Other therapies that have shown promise in a limited number of studies include acupuncture, acupressure, yoga, meditation and Tai Chi. The findings from these studies need to be replicated in additional work at different sites that would help to validate the early findings in more diverse patient groups. Most of the CAM therapies discussed have benign side effect profiles from the limited body of data currently available. Thus, they hold the potential to significantly benefit sleep in older adults, a population most at risk for polypharmacy and altered drug metabolism. Increased emphasis on objective measures, more rigorous study design (parallel arm placebo-controlled designs) and larger study sample sizes are crucial next steps for the field.
Acknowledgments
Funding Support: NCCAM R01 AT001521, NIA K23 AG01021, UL1 RR024134 (University of Pennsylvania CTSA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Natural Standard Research Collaboration. [Accessed May 20, 2007];Valerian (Valeriana officinalis L) [internet]. Oct 1, 2006; http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-valerian.html.
- 2.National Center for Complimentary and Alternative Medicine. What Is CAM? NCCAM Publication No. D347. [internet]. February 2007; http://nccam.nih.gov/health/whatiscam/
- 3.Meoli AL, Rosen CL, Kristo D, et al. Non-Pharmacologic Treatments for Insomnia. J Clin Sleep Med. 2005;1:173–187. [PubMed] [Google Scholar]
- 4.Shimazaki M, Martin JL. Do herbal agents have a place in the treatment of sleep problems in long-term care? J Am Med Dir Assoc. 2007 May;8(4):248–252. doi: 10.1016/j.jamda.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bent S, Padula A, Moore D, Patterson M, Mehling W. Valerian for sleep: a systematic review and meta-analysis. Am J Med. 2006 Dec;119(12):1005–1012. doi: 10.1016/j.amjmed.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevinson C, Ernst E. Valerian for insomnia: a systematic review of randomized clinical trials. Sleep Med. 2000 Apr 1;1(2):91–99. doi: 10.1016/s1389-9457(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley D. Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J Psychopharmacol. 2005 Jul;19(4):414–421. doi: 10.1177/0269881105053309. [DOI] [PubMed] [Google Scholar]
- 8.Management of insomnia: a place for traditional herbal remedies. Prescrire Int. 2005 Jun;14(77):104–107. [PubMed] [Google Scholar]
- 9.Sok SR, Erlen JA, Kim KB. Effects of acupuncture therapy on insomnia. J Adv Nurs. 2003 Nov;44(4):375–384. doi: 10.1046/j.0309-2402.2003.02816.x. [DOI] [PubMed] [Google Scholar]
- 10.Larzelere MM, Wiseman P. Anxiety, depression, and insomnia. Prim Care. 2002 Jun;29(2):339–360. vii. doi: 10.1016/s0095-4543(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Crofford LJ, Appleton BE. Complementary and alternative therapies for fibromyalgia. Curr Rheumatol Rep. 2001 Apr;3(2):147–156. doi: 10.1007/s11926-001-0010-9. [DOI] [PubMed] [Google Scholar]
- 12.Gyllenhaal C, Merritt SL, Peterson SD, Block KI, Gochenour T. Efficacy and safety of herbal stimulants and sedatives in sleep disorders. Sleep Med Rev. 2000 Jun;4(3):229–251. doi: 10.1053/smrv.1999.0093. [DOI] [PubMed] [Google Scholar]
- 13.Vallbona C, Richards T. Evolution of magnetic therapy from alternative to traditional medicine. Phys Med Rehabil Clin N Am. 1999 Aug;10(3):729–754. [PubMed] [Google Scholar]
- 14.Lin Y. Acupuncture treatment for insomnia and acupuncture analgesia. Psychiatry Clin Neurosci. 1995 May;49(2):119–120. doi: 10.1111/j.1440-1819.1995.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Retired Persons, National Center for Complimentary and Alternative Medicine. Complementary and Alternative Medicine: What People 50 and Over Are Using and Discussing with Their Physicians. Washington, D.C.: AARP; 2007. [Google Scholar]
- 16.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006 Sep 18;166(16):1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum HT, Kania CM, Kong SX, Wong JM, Mendelson WB. Prevalence of insomnia: a survey of the enrollees at five managed care organizations. Am J Manag Care. 1998 Jan;4(1):79–86. [PubMed] [Google Scholar]
- 18.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006 Nov 1;29(11):1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 19.Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Med Rev. 2005 Feb;9(1):51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Brzezinksi A. Melatonin in Humans. N Engl J Med. 1997 Jan;336(3):186. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 21.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343(15):1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 22.Dubocovitch M. Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997 Jul;19(1):91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson WB. Melatonin microinjection into the medial preoptic area increases sleep in the rat. Life Sci. 2002 Sep 13;71(17):2067–2070. doi: 10.1016/s0024-3205(02)01991-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhou JN, Liu RY, Van Heerikhuize J, Hofman MA, Swaab DF. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J Pineal Res. 2003 Jan;34(1):11–16. doi: 10.1034/j.1600-079x.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 26.Touitou Y, Fevre M, Lagoguey M, et al. Age- and mental health-related circadian rhythms of plasma levels of melatonin, prolactin, luteinizing hormone and follicle-stimulating hormone in man. J Endocrinol. 1981;91(3):467–475. doi: 10.1677/joe.0.0910467. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M, Palacios-Bois J, Schwartz G, et al. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25(3):305–319. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- 28.Humbert W, Pevet P. Calcium content and concretions of pineal glands of young and old rats. A scanning and X-ray microanalytical study. Cell Tissue Res. 1991 Mar;263(3):593–596. doi: 10.1007/BF00327294. [DOI] [PubMed] [Google Scholar]
- 29.Kripke DF, Elliot JA, Youngstedt SD, Smith JS. Melatonin: marvel or marker? Ann Med. 1998;30(1):81–87. doi: 10.3109/07853899808999388. [DOI] [PubMed] [Google Scholar]
- 30.Riemann D, Klein T, Rodenbeck A, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002 Dec 15;113(1–2):17–27. doi: 10.1016/s0165-1781(02)00249-4. [DOI] [PubMed] [Google Scholar]
- 31.Hajak G, Rodenbeck A, Staedt J, Bandelow B, Huether G, Ruther E. Nocturnal plasma melatonin levels in patients suffering from chronic primary insomnia. J Pineal Res. 1995 Oct;19(3):116–122. doi: 10.1111/j.1600-079x.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 32.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001 Oct;31(3):264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 33.Lushington K, Dawson D, Kennaway DJ, Lack L. The relationship between 6-sulphatoxymelatonin and polysomnographic sleep in good sleeping controls and wake maintenance insomniacs, aged 55–80 years. J Sleep Res. 1999 Mar;8(1):57–64. doi: 10.1046/j.1365-2869.1999.00130.x. [DOI] [PubMed] [Google Scholar]
- 34.Baskett JJ, Wood PC, Broad JB, Duncan JR, English J, Arendt J. Melatonin in older people with age-related sleep maintenance problems: a comparison with age matched normal sleepers. Sleep. 2001;24(4):418–424. doi: 10.1093/sleep/24.4.418. [DOI] [PubMed] [Google Scholar]
- 35.Van Den Heuvel CJ, Reid KJ, Dawson D. Effect of atenolol on nocturnal sleep and temperature in young men: reversal by pharmacological doses of melatonin. Physiol Behav. 1997 Jun;61(6):795–802. doi: 10.1016/s0031-9384(96)00534-3. [DOI] [PubMed] [Google Scholar]
- 36.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59(1):133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 37.Surrall K, Smith JA, Bird H, Okala B, Othman H, Padwick DJ. Effect of ibuprofen and indomethacin on human plasma melatonin. J Pharm Pharmacol. 1987;39(10):840–843. doi: 10.1111/j.2042-7158.1987.tb05129.x. [DOI] [PubMed] [Google Scholar]
- 38.Almay BG, von Knorring L, Wetterberg L. Melatonin in serum and urine in patients with idiopathic pain syndromes. Psychiatry Res. 1987;22(3):179–191. doi: 10.1016/0165-1781(87)90033-3. [DOI] [PubMed] [Google Scholar]
- 39.Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995 Jun;345(8962):1408. doi: 10.1016/s0140-6736(95)92600-3. [DOI] [PubMed] [Google Scholar]
- 40.Fiorina P, Lattuada G, Silvestrini C, Ponari O, Dall’Aglio P. Disruption of nocturnal melatonin rhythm and immunological involvement in ischaemic stroke patients. Scand J Immunol. 1999 Aug;50(2):228–231. doi: 10.1046/j.1365-3083.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 41.Richardson G, Tate B. Hormonal and pharmacological manipulation of the circadian clock: recent developments and future strategies. Sleep. 2000;23 (Suppl 3):S77–85. [PubMed] [Google Scholar]
- 42.Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995 Aug 26;346(8974):541–544. doi: 10.1016/s0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- 43.Jean-Louis G, von Gizycki H, Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J Pineal Res. 1998;25(3):177–183. doi: 10.1111/j.1600-079x.1998.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 44.Baskett JJ, Broad JB, Wood PC, et al. Does melatonin improve sleep in older people? A randomised crossover trial. Age Ageing. 2003 Mar;32(2):164–170. doi: 10.1093/ageing/32.2.164. [DOI] [PubMed] [Google Scholar]
- 45.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003 Nov 1;26(7):893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 2001 Oct;86(10):4727–4730. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- 47.Monti JM, Alvarino D, Cardinali D, Savio I, Pintos A. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Archives of Gerontology and Geriatrics. 1999;28:85–98. doi: 10.1016/s0167-4943(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 48.Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep. 1998;21(1):52–68. [PubMed] [Google Scholar]
- 49.Dawson D, Rogers NL, van den Heuvel CJ, Kennaway DJ, Lushington K. Effect of sustained nocturnal transbuccal melatonin administration on sleep and temperature in elderly insomniacs. J Biol Rhythms. 1998;13(6):532–538. doi: 10.1177/074873098129000354. [DOI] [PubMed] [Google Scholar]
- 50.Buscemi N, Vandermeer B, Pandya R, et al. Melatonin for Treatment of Sleep Disorders, Evidence Report/Technology Assessment No. 108. Rockville, MD: Agency for Healthcare Research and Quality; Prepared by the University of Alberta Evidence-based Practice Center, under Contract No. 290–02–0023; November 2004 2004. AHRQ Publication No. 05–E002–2. [Google Scholar]
- 51.Haimov I, Lavie P, Laudon M, Herer P, Vigder C, Zisapel N. Melatonin replacement therapy of elderly insomniacs. Sleep. 1995 Sep;18(7):598–603. doi: 10.1093/sleep/18.7.598. [DOI] [PubMed] [Google Scholar]
- 52.Leger D, Laudon M, Zisapel N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am J Med. 2004 Jan 15;116(2):91–95. doi: 10.1016/j.amjmed.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist C, Benedetti C, Orlando A, et al. Lack of changes in serum prolactin, FSH, TSH, and estradiol after melatonin treatment in doses that improve sleep and reduce benzodiazepine consumption in sleep-disturbed, middle-aged, and elderly patients. J Pineal Res. 2001;30(1):34–42. doi: 10.1034/j.1600-079x.2001.300105.x. [DOI] [PubMed] [Google Scholar]
- 54.Seabra ML, Bignotto M, Pinto LR, Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000 Nov;29(4):193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 55.Buscemi N, Vandermeer B, Hooton N, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med. 2005 Dec;20(12):1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendelson WB. A critical evaluation of the hypnotic efficacy of melatonin. Sleep. 1997;20(10):916–919. doi: 10.1093/sleep/20.10.916. [DOI] [PubMed] [Google Scholar]
- 57.Rogers NL, Phan O, Kennaway DJ, Dawson D. Effect of daytime oral melatonin administration on neurobehavioral performance in humans. J Pineal Res. 1998 Aug;25(1):47–53. doi: 10.1111/j.1600-079x.1998.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 58.Okawa M, Uchiyama M, Ozaki S, et al. Melatonin treatment for circadian rhythm sleep disorders. Psychiatry Clin Neurosci. 1998;52(2):259–260. doi: 10.1111/j.1440-1819.1998.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 59.Middleton BA, Stone BM, Arendt J. Melatonin and fragmented sleep patterns. Lancet. 1996;348(9026):551–552. doi: 10.1016/S0140-6736(05)64715-0. [DOI] [PubMed] [Google Scholar]
- 60.Dawson D, van den Heuvel CJ. Integrating the actions of melatonin on human physiology. Ann Med. 1998 Feb;30(1):95–102. doi: 10.3109/07853899808999390. [DOI] [PubMed] [Google Scholar]
- 61.Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br J Clin Pharmacol. 2000;49(5):423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sewerynek E. Melatonin and the cardiovascular system. Neuroendocrinol Lett. 2002 Apr;23 (Suppl 1):79–83. [PubMed] [Google Scholar]
- 63.Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001 Mar;10(3):467–476. doi: 10.1517/13543784.10.3.467. [DOI] [PubMed] [Google Scholar]
- 64.Hansson I, Holmdahl R, Mattsson R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J Neuroimmunol. 1990 Apr;27(1):79–84. doi: 10.1016/0165-5728(90)90139-e. [DOI] [PubMed] [Google Scholar]
- 65.Maestroni GJ, Sulli A, Pizzorni C, Villaggio B, Cutolo M. Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci. 2002 Jun;966:271–275. doi: 10.1111/j.1749-6632.2002.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 66.Conti A, Maestroni GJ. Melatonin rhythms in mice: role in autoimmune and lymphoproliferative diseases. Ann N Y Acad Sci. 1998 May 1;840:395–410. doi: 10.1007/978-3-642-59512-7_21. [DOI] [PubMed] [Google Scholar]
- 67.Bilici D, Akpinar E, Kiziltunc A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol Res. 2002 Aug;46(2):133–139. doi: 10.1016/s1043-6618(02)00089-0. [DOI] [PubMed] [Google Scholar]
- 68.Hong YG, Riegler JL. Is melatonin associated with the development of autoimmune hepatitis? J Clin Gastroenterol. 1997;25(1):376–378. doi: 10.1097/00004836-199707000-00020. [DOI] [PubMed] [Google Scholar]
- 69.Medical Letter. Melatonin. Med Lett Drugs Ther. 1995;37(962):111–112. [PubMed] [Google Scholar]
- 70.Leibenluft E, Feldman-Naim S, Turner EH, Wehr TA, Rosenthal NE. Effects of exogenous melatonin administration and withdrawal in five patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997 Sep;58(9):383–388. doi: 10.4088/jcp.v58n0902. [DOI] [PubMed] [Google Scholar]
- 71.Maksoud A, Moore CA, Harshkowitz M. The effect of melatonin administration on patients with sleep apnea. Sleep Res. 1997;26:114. [Google Scholar]
- 72.Olde Rikkert MG, Rigaud AS. Melatonin in elderly patients with insomnia. A systematic review. Z Gerontol Geriatr. 2001 Dec;34(6):491–497. doi: 10.1007/s003910170025. [DOI] [PubMed] [Google Scholar]
- 73.Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E. Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp Gerontol. 2001 Feb;36(2):297–310. doi: 10.1016/s0531-5565(00)00217-5. [DOI] [PubMed] [Google Scholar]
- 74.Attele AS, Xie JT, Yuan CS. Treatment of insomnia: an alternative approach. Altern Med Rev. 2000;5(3):249–259. [PubMed] [Google Scholar]
- 75.Pawlikowski M, Kolomecka M, Wojtczak A, Karasek M. Effects of six months melatonin treatment on sleep quality and serum concentrations of estradiol, cortisol, dehydroepiandrosterone sulfate, and somatomedin C in elderly women. Neuroendocrinol Lett. 2002 Apr;23 (Suppl 1):17–19. [PubMed] [Google Scholar]
- 76.National Academies--Committee on the Framework for Evaluating the Safety of Dietary Supplements. Prototype Monograph on Melatonin. Dietary Supplements: A Framework for Evaluation Safety. Washington, D.C.: The National Academies Press; 2004. pp. D1–D71. [Google Scholar]
- 77.Houghton PJ. The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol. 1999 May;51(5):505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 78.Santos MS, Ferreira F, Cunha AP, Carvalho AP, Ribeiro CF, Macedo T. Synaptosomal GABA release as influenced by valerian root extract--involvement of the GABA carrier. Arch Int Pharmacodyn Ther Mar-Apr. 1994;327(2):220–231. [PubMed] [Google Scholar]
- 79.Muller CE, Schumacher B, Brattstrom A, Abourashed EA, Koetter U. Interactions of valerian extracts and a fixed valerian-hop extract combination with adenosine receptors. Life Sci. 2002 Sep 6;71(16):1939–1949. doi: 10.1016/s0024-3205(02)01964-1. [DOI] [PubMed] [Google Scholar]
- 80.Dietz BM, Mahady GB, Pauli GF, Farnsworth NR. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005 Aug 18;138(2):191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ConsumerLab.com. [Accessed May 20, 2007];Valerian: product review. internet. www.consumerlab.com.
- 82.Balderer G, Borbely AA. Effect of valerian on human sleep. Psychopharmacology. 1985;87(4):406–409. doi: 10.1007/BF00432503. [DOI] [PubMed] [Google Scholar]
- 83.Leathwood PD, Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med. 1985 Apr;(2):144–148. doi: 10.1055/s-2007-969430. [DOI] [PubMed] [Google Scholar]
- 84.Donath F, Quispe S, Diefenbach K, Maurer A, Fietze I, Roots I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry. 2000;33(2):47–53. doi: 10.1055/s-2000-7972. [DOI] [PubMed] [Google Scholar]
- 85.Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982 Jul;17(1):65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 86.Poyares DR, Guilleminault C, Ohayon MM, Tufik S. Can valerian improve the sleep of insomniacs after benzodiazepine withdrawal? Prog Neuropsychopharmacol Biol Psychiatry. 2002 Apr;26(3):539–545. doi: 10.1016/s0278-5846(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 87.Ziegler G, Ploch M, Miettinen-Baumann A, Collet W. Efficacy and tolerability of valerian extract LI 156 compared with oxazepam in the treatment of non-organic insomnia--a randomized, double-blind, comparative clinical study. Eur J Med Res. 2002 Nov 25;7(11):480–486. [PubMed] [Google Scholar]
- 88.Schulz H, Stolz C, Muller J. The effect of valerian extract on sleep polygraphy in poor sleepers: a pilot study. Pharmacopsychiatry. 1994;27(4):147–151. doi: 10.1055/s-2007-1014295. [DOI] [PubMed] [Google Scholar]
- 89.Diaper A, Hindmarch I. A double-blind, placebo-controlled investigation of the effects of two doses of a valerian preparation on the sleep, cognitive and psychomotor function of sleep-disturbed older adults. Phytother Res. 2004 Oct;18(10):831–836. doi: 10.1002/ptr.1574. [DOI] [PubMed] [Google Scholar]
- 90.PDR for Herbal Remedies. Montvale, NJ: Medical Economics; 1998. [Google Scholar]
- 91.Morin CM, Koetter U, Bastien C, Ware JC, Wooten V. Valerian-hops combination and diphenhydramine for treating insomnia: a randomized placebo-controlled clinical trial. Sleep. 2005 Nov 1;28(11):1465–1471. doi: 10.1093/sleep/28.11.1465. [DOI] [PubMed] [Google Scholar]
- 92.Block KI, Gyllenhaal C, Mead MN. Safety and efficacy of herbal sedatives in cancer care. Integr Cancer Ther. 2004 Jun;3(2):128–148. doi: 10.1177/1534735404265003. [DOI] [PubMed] [Google Scholar]
- 93.Taibi DM, Bourguignon C, Taylor AG. Valerian use for sleep disturbances related to rheumatoid arthritis. Holist Nurs Pract May-Jun. 2004;18(3):120–126. doi: 10.1097/00004650-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 94.Richards K, Nagel C, Markie M, Elwell J, Barone C. Use of complementary and alternative therapies to promote sleep in critically ill patients. Crit Care Nurs Clin North Am. 2003 Sep;15(3):329–340. doi: 10.1016/s0899-5885(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 95.Plushner SL. Valerian: Valeriana officinalis. Am J Health Syst Pharm. 2000;57(4):328–333. doi: 10.1093/ajhp/57.4.328. [DOI] [PubMed] [Google Scholar]
- 96.Kuhlmann J, Berger W, Podzuweit H, Schmidt U. The influence of valerian treatment on “reaction time, alertness and concentration” in volunteers. Pharmacopsychiatry. 1999 Nov;32(6):235–241. doi: 10.1055/s-2007-991100. [DOI] [PubMed] [Google Scholar]
- 97.Garges HP, Varia I, Doraiswamy PM. Cardiac complications and delirium associated with valerian root withdrawal [letter] Jama. 1998;280(18):1566–1567. doi: 10.1001/jama.280.18.1566-a. [DOI] [PubMed] [Google Scholar]
- 98.Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med. 2004 Mar;18(2):87–92. doi: 10.1191/0269216304pm874oa. [DOI] [PubMed] [Google Scholar]
- 99.Hanley J, Stirling P, Brown C. Randomised controlled trial of therapeutic massage in the management of stress. Br J Gen Pract. 2003 Jan;53(486):20–25. [PMC free article] [PubMed] [Google Scholar]
- 100.McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705. doi: 10.1111/j.1532-5415.1998.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 101.Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. Am J Crit Care. 1998 Jul;7(4):288–299. [PubMed] [Google Scholar]
- 102.Field T, Diego M, Cullen C, Hernandez-Reif M, Sunshine W, Douglas S. Fibromyalgia Pain and Substance P Decrease and Sleep Improves After Massage Therapy. J Clin Rheumatol. 2002 Apr;8(2):72–76. doi: 10.1097/00124743-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 103.Sarac AJ, Gur A. Complementary and alternative medical therapies in fibromyalgia. Curr Pharm Des. 2006;12(1):47–57. [PubMed] [Google Scholar]
- 104.Chen ML, Lin LC, Wu SC, Lin JG. The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. J Gerontol A Biol Sci Med Sci. 1999;54(8):M389–394. doi: 10.1093/gerona/54.8.m389. [DOI] [PubMed] [Google Scholar]
- 105.Tsay SL, Chen ML. Acupressure and quality of sleep in patients with end-stage renal disease--a randomized controlled trial. Int J Nurs Stud. 2003 Jan;40(1):1–7. doi: 10.1016/s0020-7489(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 106.Tsay SL, Cho YC, Chen ML. Acupressure and Transcutaneous Electrical Acupoint Stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. Am J Chin Med. 2004;32(3):407–416. doi: 10.1142/S0192415X04002065. [DOI] [PubMed] [Google Scholar]
- 107.Yang MH, Wu SC, Lin JG, Lin LC. The efficacy of acupressure for decreasing agitated behaviour in dementia: a pilot study. J Clin Nurs. 2007 Feb;16(2):308–315. doi: 10.1111/j.1365-2702.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 108.Suen LK, Wong TK, Leung AW, Ip WC. The long-term effects of auricular therapy using magnetic pearls on elderly with insomnia. Complement Ther Med. 2003 Jun;11(2):85–92. doi: 10.1016/s0965-2299(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 109.Suen LK, Wong TK, Leung AW. Effectiveness of auricular therapy on sleep promotion in the elderly. Am J Chin Med. 2002;30(4):429–449. doi: 10.1142/S0192415X0200051X. [DOI] [PubMed] [Google Scholar]
- 110.Spence DW, Kayumov L, Chen A, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatry Clin Neurosci. 2004 Winter;16(1):19–28. doi: 10.1176/jnp.16.1.19. [DOI] [PubMed] [Google Scholar]
- 111.Montakab H. [Acupuncture and insomnia] Forsch Komplementarmed. 1999 Feb;6 (Suppl 1):29–31. doi: 10.1159/000057127. [DOI] [PubMed] [Google Scholar]
- 112.Kim YS, Lee SH, Jung WS, et al. Intradermal acupuncture on shen-men and neikuan acupoints in patients with insomnia after stroke. Am J Chin Med. 2004;32(5):771–778. doi: 10.1142/S0192415X04002399. [DOI] [PubMed] [Google Scholar]
- 113.Huang MI, Nir Y, Chen B, Schnyer R, Manber R. A randomized controlled pilot study of acupuncture for postmenopausal hot flashes: effect on nocturnal hot flashes and sleep quality. Fertil Steril. 2006 Sep;86(3):700–710. doi: 10.1016/j.fertnstert.2006.02.100. [DOI] [PubMed] [Google Scholar]
- 114.Carpenter JS, Neal JG. Other complementary and alternative medicine modalities: acupuncture, magnets, reflexology, and homeopathy. Am J Med. 2005 Dec 19;118(Suppl 12B):109–117. doi: 10.1016/j.amjmed.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 115.Rooks DS. Fibromyalgia treatment update. Curr Opin Rheumatol. 2007 Mar;19(2):111–117. doi: 10.1097/BOR.0b013e328040bffa. [DOI] [PubMed] [Google Scholar]
- 116.Holdcraft LC, Assefi N, Buchwald D. Complementary and alternative medicine in fibromyalgia and related syndromes. Best Pract Res Clin Rheumatol. 2003 Aug;17(4):667–683. doi: 10.1016/s1521-6942(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 117.Freire AO, Sugai GC, Chrispin FS, et al. Treatment of moderate obstructive sleep apnea syndrome with acupuncture: a randomised, placebo-controlled pilot trial. Sleep Med. 2007 Jan;8(1):43–50. doi: 10.1016/j.sleep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Wang XH, Yuan YD, Wang BF. [Clinical observation on effect of auricular acupoint pressing in treating sleep apnea syndrome] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003 Oct;23(10):747–749. [PubMed] [Google Scholar]
- 119.Mason LI, Alexander CN, Travis FT, et al. Electrophysiological correlates of higher states of consciousness during sleep in long-term practitioners of the Transcendental Meditation program. Sleep. 1997 Feb;20(2):102–110. doi: 10.1093/sleep/20.2.102. [DOI] [PubMed] [Google Scholar]
- 120.Smith JE, Richardson J, Hoffman C, Pilkington K. Mindfulness-Based Stress Reduction as supportive therapy in cancer care: systematic review. J Adv Nurs. 2005 Nov;52(3):315–327. doi: 10.1111/j.1365-2648.2005.03592.x. [DOI] [PubMed] [Google Scholar]
- 121.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12(4):278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 122.Manjunath NK, Telles S. Influence of Yoga and Ayurveda on self-rated sleep in a geriatric population. Indian J Med Res. 2005 May;121(5):683–690. [PubMed] [Google Scholar]
- 123.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004 May 15;100(10):2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 124.Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005 Jul;12(3):165–171. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- 125.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004 Jun;52(6):892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]