Abstract
Traumatic brain injury is characterized by neuroinflammatory pathological sequelae which contribute to brain edema and delayed neuronal cell death. Until present, no specific pharmacological compound has been found, which attenuates these pathophysiological events and improves the outcome after head injury. Recent experimental studies suggest that targeting peroxisome proliferator-activated receptors (PPARs) may represent a new anti-inflammatory therapeutic concept for traumatic brain injury. PPARs are “key” transcription factors which inhibit NFκB activity and downstream transcription products, such as proinflammatory and proapoptotic cytokines. The present review outlines our current understanding of PPAR-mediated neuroprotective mechanisms in the injured brain and discusses potential future anti-inflammatory strategies for head-injured patients, with an emphasis on the putative beneficial combination therapy of synthetic cannabinoids (e.g., dexanabinol) with PPARα agonists (e.g., fenofibrate).
1. INTRODUCTION
Research efforts in recent years have provided increasing evidence that the intracerebral inflammatory response is in large part responsible for the devastating neuropathological sequelae and poor outcome of traumatic brain injury [1–3]. The extent of brain damage is determined by primary and secondary injury patterns. While the primary injury results from mechanical forces applied to the skull and brain at the time of impact, secondary brain injury occurs as a delayed consequence of trauma [4–7]. Secondary brain injuries are mediated by endogenous pathophysiological processes which lead to an overwhelming neuroinflammation in the injured brain [6, 8–10]. The main risk factors for developing secondary brain injuries are hypoxemia and systemic hypotension which occur frequently in the trauma patient [11, 12]. These conditions contribute to the ischemic brain damage and perpetuate the intracerebral inflammatory reaction through ischemia/reperfusion-mediated mechanisms [13]. Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of the nuclear receptor superfamily which have recently been shown to exert anti-inflammatory properties in acute neurological disorders. These include cerebrovascular stroke, intracerebral hemorrhage, spinal cord injury, and traumatic brain injury [14–21]. The present paper provides an overview on the so far known anti-inflammatory properties of PPARs in brain injury and discusses potential pharmacological properties of PPAR agonists as future neuroprotective agents.
2. BIOLOGICAL FUNCTIONS OF PPARS
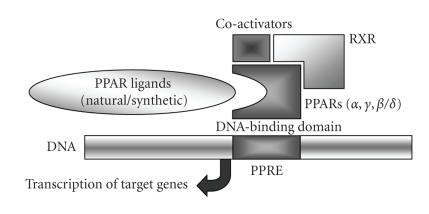
PPARs are nuclear membrane-associated transcription factors belonging to the nuclear receptor family [22]. Three isotypes with a differential tissue distribution have been described: PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3) [23, 24]. While PPARβ/δ has an ubiquitous expression, PPARα and PPARγ are mainly expressed in tissues with high fatty acid catabolism, such as adipose tissue, liver, kidney, and skeletal muscle [25]. Mechanistically, PPARs are activated by heterodimerization with the retinoid-X receptor (RXR) into biologically active transcription factors. PPAR-RXR heterodimers induce the transcription of candidate genes by binding to so-called peroxisome proliferator-response elements (PPRE's) consisting of DNA-specific sequences (see Figure 1).
Figure 1.
Mechanism of gene transcription through ligand binding on peroxisome proliferator-activated receptors (PPARs). In presence of coactivating stimuli, PPARs heterodimerize with retinoid X receptors (RXR) to form active transcription factors. The DNA binding domain on PPAR-RXR heterodimers induces the transcription of target genes by binding to peroxisome proliferator-response elements (PPRE's) which consist of DNA-specific sequences.
PPARs exert a wide variety of physiological functions [24, 26]. They play a central role in the regulation of lipid and lipoprotein metabolism and glucose homeostasis, and have been shown to mediate cellular proliferation and programmed cell death (apoptosis) [27–31]. PPARs have furthermore been involved in bone metabolism and in pathologies of the cardiovascular system and the lung [32–35]. PPARα has been attributed important immunological functions due to its expression on monocytes/macrophages, T cells, and vascular endothelial cells. PPARγ appears to play a crucial role in the regulation of proliferation and differentiation of various cell types. While the biological role of PPARβ/δ has not been defined in detail, recent data imply an antiapoptotic and anti-inflammatory effect after tissue injury, both in vitro and in vivo [29].
From an immunological viewpoint, PPARs have been identified as important regulators of inflammatory gene expression [36–40]. PPARs have also been shown to attenuate adaptive immune responses by inhibiting helper T cell functions and by mediating apoptosis of B cells [41, 42]. PPARs are activated by naturally ocurring fatty acid derivatives, eicosanoides, and by synthetic pharmacological agents, such as fibrates (PPARα) and glitazones (PPARγ) [18, 22, 43]. PPAR ligands have been shown to exert anti-inflammatory activities in various cell types by inhibiting the gene expression for proinflammatory cytokines, metalloproteinases, and hepatic acute-phase proteins.
3. PPARS: “KEY” REGULATORS OF NEUROINFLAMMATION
Mechanistically, the activation of PPARα has been shown to inhibit proinflammatory gene transcription by repressing the central inflammatory transcription factor, nuclear factor-κB (NF-κB) [43–45]. Along with suppression of NF-κB, PPARα acts by inhibition of signal transduction through activator protein-1 (AP-1) signaling [43]. It appears that the inhibitory effect of PPARα on these crucial inflammatory transcription factors creates a negative feedback loop for controlling acute posttraumatic inflammation [44–46]. First in vivo data on the involvement of PPARs in the regulation of inflammation were reported from studies in PPARα knockout mice [47]. Cuzzocrea et al. showed that the targeted deletion of the PPARα gene leads to a significantly increased inflammatory response in different experimental models of acute inflammation outside the central nervous system (CNS) [47]. Within the CNS, the constitutive expression of PPARs has been described for some time [48, 49]. Interestingly, PPAR gene expression was detected not only on vascular endothelial cells in the brain and spinal cord, but also on resident cells in the CNS, such as neurons and glial cells [49].
4. ROLE OF PPARS IN CNS INJURY
In recent years, experimental studies in models of cerebral ischemia/reperfusion injury, ischemic stroke, intracerebral hemorrhage, and spinal cord injury have revealed a crucial role of PPARs in attenuating neuroinflammation and neuronal cell death in the injured CNS (see Table 1) [14–16, 19, 20, 50–52]. PPARα gene-deficient mice (PPARα−/−) were shown to have a significantly worsened neurological outcome, associated with an increased neuroinflammatory response to experimental spinal cord injury, as compared to wild-type littermates [16]. The postulated neuroprotective effects of natural PPARα ligands include the attenuation of polymorphonuclear leukocyte (PMNL) recruitment and associated neurotoxicity, as determined by a significantly reduced expression of myeloperoxidase in the injured spinal cord of PPARα−/− mice [16]. In addition, tumor necrosis factor (TNF), a “key” mediator of neuroinflammation and neurotoxicity, was shown to be upregulated and associated with neuronal apoptosis in the injured spinal cord of PPARα−/− mice [16]. In traumatic brain injury, experimental studies in the past decade have shown that TNF is upregulated in the intracranial compartment within a few hours after trauma, and contributes to secondary neuronal injury [53–55]. The deleterious neurotoxic effects were shown to be abrogated by pharmacological inhibition of TNF [56]. Since PPARs inhibit proinflammatory gene transcription by attenuating NF-κB signaling [43–45], the potent PPAR-mediated neuroprotective effects may be dependent on inhibition of NF-κB-dependent proinflammatory cytokines released in the injured brain, such as TNF, interleukin (IL)-1, IL-8, IL-12, and IL-18 [57–61]. The central role of NF-κB signaling in inflammation and oxidative stress explains why PPARs have been considered possible targets for neuroprotection in inflammatory CNS diseases, including traumatic brain injury [14, 20, 62, 63].
Table 1.
Selected publications on the role of PPARs in CNS injury and inflammation.
| Models of CNS injury and neuroinflammation | PPAR isotype | Main findings | Reference no. |
|---|---|---|---|
| Different models of CNS injury | PPARγ | Review on the mechanisms of neuroprotection by PPARγ agonists | Kapadia et al. [20] |
| Different models of CNS injury | PPARα, PPARγ | Review on pharmacological neuroprotection by PPARs | Bordet et al. [14] |
| Brain inflammation | PPARγ | Review on regulation of microglial activation by PPARγ agonists | Bernardo and Minghetti [63] |
| Spinal cord injury | All isotypes | Review on the role of PPAR signal transduction in spinal cord injury | Van Neerven and Mey [15] |
| Spinal cord injury | PPARα | Experimental model of spinal cord injury in PPARα gene knockout mice. Lack of PPARα leads to worse outcome and increased neuroinflammation. | Genovese et al. [16] |
| Cerebral ischemia/reperfusion injury | PPARγ | The PPARγ agonists rosiglitazone and pioglitazone exert neuroprotective effects in a rat model of cerebral ischemia/reperfusion injury by reducing neuroinflammation and oxidative stress. | Collino et al. [50] |
| Intracerebral hemorrhage | PPARγ | PPARγ expressed by microglia and macrophages promotes the resolution of intracerebral hemorrhage and attenuates the neuroinflammatory response. | Zhao et al. [19] |
| Traumatic brain injury | PPARα | The PPARα agonist fenofibrate reduces brain edema and improves the neurological outcome after experimental fluid percussion brain injury in male Sprague-Dawley rats. | Besson et al. [21] |
| Traumatic brain injury | PPARα | The PPARα agonist fenofibrate promotes neurological recovery by reducing inflammation and oxidative stress in rat brains after experimental fluid percussion brain injury. | Chen et al. [17] |
| Neuroinflammation | All isotypes | Review on the interaction between cannabinoids and PPARs as inhibitors of neuroinflammation | Sun and Bennett [83] |
5. PHARMACOLOGY OF HEAD INJURY: ARE PPAR-AGONISTS AND CANNABINOIDS THE LONG SOUGHT “GOLDEN BULLET”?
A wide variety of natural and synthetic PPARγ agonists have been described in recent years as regulators of microglial activation and cerebral inflammation [63]. For example, the thiazolinedione pioglitazone has been shown to reduce the extent of neuroinflammation and the severity of disease in experimental autoimmune encephalomyelitis (EAE), the animal model for multiple sclerosis (MS) [64, 65]. A recent case report described the impressive clinical improvement of a patient with chronic progressive MS, after a 3-year period of treatment with pioglitazone [66]. This unexpected clinical recovery implies that PPARγ agonists may represent a promising new strategy for attenuating neuroinflammation in patients with CNS autoimmune diseases [62, 63, 67].
In cerebrovascular stroke, the combination therapy of a PPARγ agonist (rosiglitazone) with an antiexcitotoxic glutamate receptor antagonist (MK-801) led to an improved neurological recovery in rats undergoing middle cerebral artery occlusion [18]. A study by another group assessed the therapeutic efficacy of two different PPARγ agonists, rosiglitazone and pioglitazone, in a rat model of cerebral ischemia/reperfusion injury [50]. The authors showed that the pretreatment with either compound led to a significant attenuation of inflammation and oxidative stress in injured rat brains [50].
Pharmacological ligands to PPARα, such as fenofibrate, have also been shown to exert neuroprotective effects in inflammatory CNS conditions. Deplanque et al. demonstrated a significant neuroprotective effect of fenofibrate administration in C57BL/6 mice with cerebrovascular stroke [68]. The authors suggested that PPARα may represent a new pharmacological target to reduce the neuroinflammatory and neuropathological sequelae of cerebrovascular stroke [68].
In traumatic brain injury, the PPARα agonist fenofibrate appears to represent a highly promising new anti-inflammatory compound. Besson et al. assessed the pharmacological role of fenofibrate in a model of experimental fluid-percussion injury in adult male Sprague-Dawley rats [21]. The authors revealed that the administration of fenofibrate during a clinically relevant therapeutic “time window of opportunity” at 1 hour after trauma mediated a significant posttraumatic neuroprotection. This was demonstrated by improved neurological scores in the fenofibrate group at 24 hours and 7 days after trauma, compared to vehicle-treated animals [21]. Morphologically, fenofibrate treatment resulted in significantly decreased extent of brain edema at 24 hours after head injury, compared to the placebo group. The authors furthermore described a marked reduction in intercellular adhesion molecule (ICAM)-1 expression at the protein level by immunohistochemistry in injured rat brain sections after fenofibrate administration [21]. This finding implies a reduced extent of intracerebral immunoactivation and neuroinflammation in rats treated by the PPARα agonist, compared to vehicle controls.
A more recent follow-up study by the same research group assessed the role of PPARα in modulating the oxidative stress in the injured rat brain [17]. Oxidative stress and ischemia/reperfusion-mediated injuries contribute significantly to the extent of posttraumatic intracerebral inflammation and delayed secondary brain damage after head injury [13, 69, 70]. Pathophysiologically, contused brain areas are surrounded by a penumbra zone which is hypoperfused due to traumatic vascular damage, loss of cerebrovascular autoregulation, and systemic hypotension. After resuscitation, the hypoperfused, ischemic brain areas in the penumbra zone are reperfused, which leads to activation of the complement cascade and of reactive oxygen intermediates by activation of the xanthine oxidase [71, 72]. Oxygen-derived free radicals such as hydroxyl ions, hydrogen peroxide, and superoxide anion induce lipid peroxidation, cell membrane disintegration, and delayed neuronal cell death (see Figure 2). Lipid peroxidation is facilitated in the brain due to its genuine vulnerability to oxidative stress based on specific morphological characteristics, such as a high ratio of “membrane to cytoplasm” and high levels of polyunsaturated fatty acids in the CNS [70]. In addition to reactive oxygen intermediates, the generation of nitric oxide (NO) by inducible NO synthase (iNOS) up-regulation also occurs after head injury and adds to the extent of secondary brain damage [73]. Metabolites emerging from the interaction between superoxide anion and NO, such as the highly reactive oxidant peroxynitrite, have been shown to mediate neurotoxicity and delayed neuronal cell death after traumatic brain injury [74].
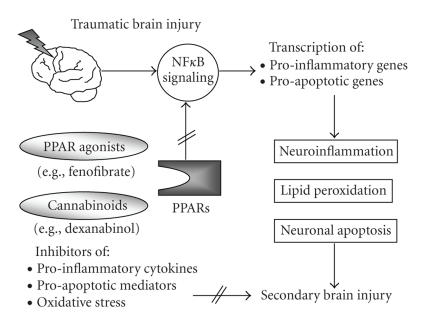
Figure 2.
Working hypothesis of PPAR-mediated mechanisms of neuroprotection after traumatic brain injury. The neuropathological sequelae of head injury include the posttraumatic activation of NFκB-dependent inflammatory genes. The transcription of neuroinflammatory mediators in the injured brain induces and perpetuates the intracranial inflammatory response and leads to formation of brain edema and adverse outcome. Activation of PPARs by binding of synthetic ligands, such as the PPARα agonist fenofibrate, leads to inhibition of NFκB and of downstream transcribed proinflammatory and proapoptotic mediators. In addition, cannabinoids have a dual neuroprotective function, (1) by acting as ligands to PPARs and (2) by inhibiting “key” mediators of neuroinflammation and apoptosis, such as tumor necrosis factor (TNF). The combination therapy of synthetic PPAR agonists and cannabinoids may represent the long sought pharmacological “golden bullet” for the treatment of traumatic brain injury in the future.
The pharmacological administration of the PPARα agonist fenofibrate after experimental fluid-percussion injury resulted in a significant decrease of intracerebral iNOS expression [17]. This was associated with a decreased neuroinflammation in the injured brain and an improved neurological recovery after trauma [17]. These important findings imply that the attenuation of oxidative stress may represent a “key” mechanistic aspect of PPAR-mediated neuroprotection after head injury. The pleiotropic beneficial effects of PPARs in the injured brain, however, are far from being elucidated in detail until present. For example, in contrast to PPARα, no studies have yet been performed to analyze the effect of PPARγ in experimental models of traumatic brain injury (see Table 1).
Despite increasing insights into the pathophysiological mechanisms of posttraumatic neuroinflammation and neurodegeneration, clinical neuroprotection trials have failed to provide a benefit of anti-inflammatory pharmacological strategies with regard to the outcome after head injury [75, 76]. Cannabinoids have recently evolved as a promising new therapeutic avenue for neuroprotection after head injury [77–79]. This group of compounds consists of natural (endocannabinoids) and synthetic ligands, such as dexanabinol (HU-211). The endocannabinoid 2-arachidonoyl glycerol (2-AG) has received increased attention in recent years due to its strong neuroprotective effect after head injury, by inhibition of proinflammatory cytokines, reactive oxygen intermediates, and excitotoxic aminoacids, such as glutamate [80, 81]. The pharmacological agent dexanabinol was shown to mediate neuroprotection by inhibition of TNF production in injured rodent brains [77, 82] and was recently proposed as an effective neuroprotective strategy to reduce the extent of secondary brain injury in humans (see Figure 2) [78, 79]. Dexanabinol (HU-211) is a nonpsychotropic, synthetic cannabinoid which exerts beneficial effects by cytokine inhibition and radical scavenging associated with reduction of brain edema [77–79, 82]. Cannabinoids were attributed a new role as neuroprotective agents by agonistic action to PPARs [83]. The functional interaction between cannabinoids and PPARs was first described based on the finding of oleylethanolamide (OEA), a lipid derivate structurally related to anandamide, as a regulator of feeding behavior in rats through activation of PPARα [68, 84]. Aside from OEA, which is a low-affinity agonist to cannabinoid receptors, other cannabinoids were recently described as PPAR ligands [83]. As such, Δ9-tetrahydrocannabinol (THC) was found to activate PPARγ in human cell lines [85]. Of particular interest for neuroprotection in traumatic brain injury is the finding that the potent endocannabinoid 2-AG [80, 81] has been found to suppress the proinflammatory cytokine IL-2 through PPARγ signaling, independent of 2-AG binding to cannabinoid receptors [86]. Future studies will have to determine whether cannabinoids represent the long sought “golden bullet” for reduction of secondary brain damage after head injury. It seems reasonable to suggest that a combination of neuroprotective cannabinoids, such as dexanabinol, with other potent anti-inflammatory therapeutic agents, such as synthetic PPAR ligands, may represent a promising new therapeutic avenue for improving the outcome of traumatic brain injury.
References
- 1.McMillan TM, Teasdale GM. Death rate is increased for at least 7 years after head injury: a prospective study. Brain. 2007;130(10):2520–2527. doi: 10.1093/brain/awm185. [DOI] [PubMed] [Google Scholar]
- 2.Holmes JF, Hendey GW, Oman JA, et al. Epidemiology of blunt head injury victims undergoing ED cranial computed tomographic scanning. American Journal of Emergency Medicine. 2006;24(2):167–173. doi: 10.1016/j.ajem.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Doppenberg EMR, Choi SC, Bullock R. Clinical trials in traumatic brain injury: lessons for the future. Journal of Neurosurgical Anesthesiology. 2004;16(1):87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Chesnut RM. Care of central nervous system injuries. Surgical Clinics of North America. 2007;87(1):119–156. doi: 10.1016/j.suc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Bayir H, Kochanek PM, Clark RSB. Traumatic brain injury in infants and children mechanisms of secondary damage and treatment in the intensive care unit. Critical Care Clinics. 2003;19(3):529–549. doi: 10.1016/s0749-0704(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 6.Fritz HG, Bauer R. Secondary injuries in brain trauma: effects of hypothermia. Journal of Neurosurgical Anesthesiology. 2004;16(1):43–52. doi: 10.1097/00008506-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kochanek PM, Clark RSB, Ruppel RA, et al. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: lessons learned from the bedside. Pediatric Critical Care Medicine. 2000;1(1):4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury: an inflammatory disease? Brain Research Reviews. 2005;48(2):388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Stahel PF, Smith WR, Moore EE. Role of biological modifiers regulating the immune response after trauma. Injury. 2007;38(12):1409–1422. doi: 10.1016/j.injury.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Kelley BJ, Lifshitz J, Povlishock JT. Neuroinflammatory responses after experimental diffuse traumatic brain injury. Journal of Neuropathology and Experimental Neurology. 2007;66(11):989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- 11.Stahel PF, Smith WR, Moore EE. Hypoxia and hypotension, the “lethal duo” in traumatic brain injury: implications for prehospital care. Intensive Care Medicine. 2008;34(3):402–404. doi: 10.1007/s00134-007-0889-3. [DOI] [PubMed] [Google Scholar]
- 12.Eastridge BJ, Salinas J, McManus JG, et al. Hypotension begins at 110 mm Hg: redefining “hypotension” with data. Journal of Trauma-Injury Infection & Critical Care. 2007;63(2):291–297. doi: 10.1097/TA.0b013e31809ed924. discussion 297–299. [DOI] [PubMed] [Google Scholar]
- 13.Leker RR, Shohami E. Cerebral ischemia and trauma—different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Research Reviews. 2002;39(1):55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- 14.Bordet R, Ouk T, Petrault O, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochemical Society Transactions. 2006;34(6):1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 15.van Neerven S, Mey J. RAR/RXR and PPAR/RXR signaling in spinal cord injury. PPAR Research. 2007;2007:14. doi: 10.1155/2007/29275. Article ID 29275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genovese T, Mazzon E, Di Paola R, et al. Role of endogenous ligands for the peroxisome proliferators activated receptors α in the secondary damage in experimental spinal cord trauma. Experimental Neurology. 2005;194(1):267–278. doi: 10.1016/j.expneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR α agonist, in traumatic brain injury. Journal of Neurotrauma. 2007;24(7):1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- 18.Allahtavakoli M, Shabanzadeh A, Roohbakhsh A, Pourshanazari A. Combination therapy of rosiglitazone, a peroxisome proliferator-activated receptor-γ ligand, and NMDA receptor antagonist (MK-801) on experimental embolic stroke in rats. Basic & Clinical Pharmacology & Toxicology. 2007;101(5):309–314. doi: 10.1111/j.1742-7843.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Annals of Neurology. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia R, Yi J-H, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-γ agonists. Frontiers in Bioscience. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor α agonist, exerts neuroprotective effects in traumatic brain injury. Neuroscience Letters. 2005;388(1):7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 23.Michalik L, Wahli W. Peroxisome proliferator-activated receptors: three isotypes for a multitude of functions. Current Opinion in Biotechnology. 1999;10(6):564–570. doi: 10.1016/s0958-1669(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 24.Kliewer SA, Xu HE, Lambert MH, Willson TM. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Progress in Hormone Research. 2001;56:239–263. doi: 10.1210/rp.56.1.239. [DOI] [PubMed] [Google Scholar]
- 25.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO Journal. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 26.Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 28.Brun RP, Tontonoz P, Forman BM, et al. Differential activation of adipogenesis by multiple PPAR isoforms. Genes and Development. 1996;10(8):974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 29.Tan NS, Michalik L, Noy N, et al. Critical roles of PPAR β/δ in keratinocyte response to inflammation. Genes & Development. 2001;15(24):3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersten S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Research. 2008;2008:11. doi: 10.1155/2008/132960. Article ID 132960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinasso G, Oraldi M, Trombetta A, et al. Involvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell lines. PPAR Research. 2007;2007:9. doi: 10.1155/2007/93416. Article ID 93416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecka-Czernik B. PPARs and bone metabolism. PPAR Research. 2006;2006:1 page. doi: 10.1155/PPAR/2006/18089. Article ID 18089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan SZ, Ivashchenko CY, Usher MG, Mortensen RM. PPAR-γ in the cardiovascular system. PPAR Research. 2008;2008:10. doi: 10.1155/2008/745804. Article ID 745804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Paola R, Cuzzocrea S. Peroxisome proliferator-activated receptors and acute lung injury. PPAR Research. 2007;2007:8. doi: 10.1155/2007/63745. Article ID 63745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang TH-W, Razmovski-Naumovski V, Kota BP, Lin DS-H, Roufogalis BD. The pathophysiological function of peroxisome proliferator-activated receptor-γ in lung-related diseases. Respiratory Research. 2005;6:9. doi: 10.1186/1465-9921-6-102. Article ID 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. Journal of Endocrinology. 2001;169(3):453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 37.Cabrero A, Laguna JC, Vazquez M. Peroxisome proliferator-activated receptors and the control of inflammation. Current Drug Targets—Inflammation & Allergy. 2002;1(3):243–248. doi: 10.2174/1568010023344616. [DOI] [PubMed] [Google Scholar]
- 38.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Research. 2000;49(10):497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 39.Clark RSB. The role of PPARs in inflammation and immunity. Journal of Leukocyte Biology. 2002;71(3):388–400. [PubMed] [Google Scholar]
- 40.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nature Reviews Immunology. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 41.Clark RSB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR γ and immunoregulation: PPAR γ mediates inhibition of helper T cell responses. Journal of Immunology. 2000;164(3):1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 42.Ray DM, Akbiyik F, Bernstein SH, Phipps RP. CD40 engagement prevents peroxisome proliferator-activated receptor γ agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-κB-dependent mechanism. Journal of Immunology. 2005;174:4060–4069. doi: 10.4049/jimmunol.174.7.4060. [DOI] [PubMed] [Google Scholar]
- 43.Staels B, Fruchart J-C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54(8):2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 44.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. Journal of Biological Chemistry. 1998;273(49):32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 45.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 46.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. Journal of Clinical Investigation. 2006;116(3):598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuzzocrea S, Mazzon E, Di Paola R, et al. The role of the peroxisome proliferator-activated receptor-α (PPAR-α) in the regulation of acute inflammation. Journal of Leukocyte Biology. 2006;79(5):999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 48.Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor α, β, and γ and the retinoid X receptor α, β, and γ in rat central nervous system. Journal of Neurochemistry. 1998;70(4):1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- 49.Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123(1):131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 50.Collino M, Aragno M, Mastrocola R, et al. Modulation of the oxidative stress and inflammatory response by PPAR-γ agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. European Journal of Pharmacology. 2006;530(1-2):70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 51.Chen S-D, Wu H-Y, Yang D-I, et al. Effects of rosiglitazone on global ischemia-induced hippocampal injury and expression of mitochondrial uncoupling protein 2. Biochemical and Biophysical Research Communications. 2006;351(1):198–203. doi: 10.1016/j.bbrc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Yin W, Signore AP, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Journal of Neurochemistry. 2006;97(2):435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 53.Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF-α and IL-6 by brain tissue. Journal of Cerebral Blood Flow & Metabolism. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- 54.Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Molecular Brain Research. 1996;36(2):287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 55.Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-α after experimental brain injury contributes to neurological impairment. Journal of Neuroimmunology. 1999;95(1-2):115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- 56.Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor α (TNFα) activity in rat brain is associated with cerebroprotection after closed head injury. Journal of Cerebral Blood Flow & Metabolism. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor α in brain injury. Cytokine and Growth Factor Reviews. 1999;10(2):119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 58.Gopcevic A, Mazul-Sunko B, Marout J, et al. Plasma interleukin-8 as a potential predictor of mortality in adult patients with severe traumatic brain injury. Tohoku Journal of Experimental Medicine. 2007;211(4):387–393. doi: 10.1620/tjem.211.387. [DOI] [PubMed] [Google Scholar]
- 59.Stahel PF, Kossmann T, Joller H, Trentz O, Morganti-Kossmann MC. Increased interleukin-12 levels in human cerebrospinal fluid following severe head trauma. Neuroscience Letters. 1998;249(2-3):123–126. doi: 10.1016/s0304-3940(98)00410-8. [DOI] [PubMed] [Google Scholar]
- 60.Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF. IL-18: a key player in neuroinflammation and neurodegeneration? Trends in Neurosciences. 2005;28(9):487–493. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Lucas S-M, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. British Journal of Pharmacology. 2006;147, supplement 1:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bordet R, Gelé P, Duriez P, Fruchart J-C. PPARs: a new target for neuroprotection. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(3):285–286. doi: 10.1136/jnnp.2005.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernando A, Minghetti L. PPAR-γ agonists as regulators of microglial activation and brain inflammation. Current Pharmaceutical Design. 2006;12(1):93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- 64.Niino M, Iwabuchi K, Kikuchi S, et al. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by an agonist of peroxisome proliferator-activated receptor-γ . Journal of Neuroimmunology. 2001;116(1):40–48. doi: 10.1016/s0165-5728(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 65.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-γ agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes & Immunity. 2002;3(2):59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 66.Pershadsingh HA, Heneka MT, Saini R. (pershadh@kernmedctr.com), Amin NM, Broeske DJ, Feinstein DL. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. Journal of Neuroinflammation. 2004;1, article 3:1–4. doi: 10.1186/1742-2094-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mrak RE, Landreth GE. PPARγ, neuroinflammation, and disease. Journal of Neuroinflammation. 2004;1, article 5:1–3. doi: 10.1186/1742-2094-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deplanque D, Gelé P, Pétrault O, et al. Peroxisome proliferator-activated receptor-α activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. Journal of Neuroscience. 2003;23(15):6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt OI, Infanger M, Heyde CE, Ertel W, Stahel PF. The role of neuroinflammation in traumatic brain injury. European Journal of Trauma. 2004;30(3):135–149. [Google Scholar]
- 70.Evans PH. Free radicals in brain metabolism and pathology. British Medical Bulletin. 1993;49(3):577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 71.Stahel PF, Morganti-Kossmann MC, Kossmann T. The role of the complement system in traumatic brain injury. Brain Research Reviews. 1998;27(3):243–256. doi: 10.1016/s0165-0173(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 72.D'Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Molecular Medicine. 2001;7(6):367–382. [PMC free article] [PubMed] [Google Scholar]
- 73.Cherian L, Goodman JC, Robertson CS. Brain nitric oxide changes after controlled cortical impact injury in rats. Journal of Neurophysiology. 2000;83(4):2171–2178. doi: 10.1152/jn.2000.83.4.2171. [DOI] [PubMed] [Google Scholar]
- 74.Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Annals of the New York Academy of Sciences. 1999;890(1):462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- 75.Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. Journal of Neurotrauma. 2002;19(5):503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeGraba TJ, Pettigrew LC. Why do neuroprotective drugs work in animals but not humans? Neurologic Clinics. 2000;18(2):475–493. doi: 10.1016/s0733-8619(05)70203-6. [DOI] [PubMed] [Google Scholar]
- 77.Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-α inhibitor and an effective neuroprotectant. Journal of Neuroimmunology. 1997;72(2):169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 78.Mechoulam R, Panikashvili D, Shohami E. Cannabinoids and brain injury: therapeutic implications. Trends in Molecular Medicine. 2002;8(2):58–61. doi: 10.1016/s1471-4914(02)02276-1. [DOI] [PubMed] [Google Scholar]
- 79.Bayir H, Clark RSB, Kochanek PM. Promising strategies to minimize secondary brain injury after head trauma. Critical Care Medicine. 2003;31, supplement 1:S112–S117. doi: 10.1097/00003246-200301001-00016. [DOI] [PubMed] [Google Scholar]
- 80.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 81.Mechoulam R, Shohami E. Endocannabinoids and traumatic brain injury. Molecular Neurobiology. 2007;36(1):68–74. doi: 10.1007/s12035-007-8008-6. [DOI] [PubMed] [Google Scholar]
- 82.Shohami E, Novikov M, Mechoulam R. A nonpsychotropic cannabinoid, HU-211, has cerebroprotective effects after closed head injury in the rat. Journal of Neurotrauma. 1993;10(2):109–119. doi: 10.1089/neu.1993.10.109. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Research. 2007;2007:7. doi: 10.1155/2007/23513. Article ID 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-α agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48(8):1147–1153. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 85.O'Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor γ . Biochemical and Biophysical Research Communications. 2005;337(3):824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 86.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Molecular Pharmacology. 2006;70(1):101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]