Abstract
Helicobacter pylori colonizes the human gastric epithelium and causes diseases such as gastritis, peptic ulcers, and stomach cancer. Undecaprenyl pyrophosphate synthase (UPPS), which catalyzes consecutive condensation reactions of farnesyl pyrophosphate with eight isopentenyl pyrophosphate to form lipid carrier for bacterial peptidoglycan biosynthesis, represents a potential target for developing new antibiotics. In this study, we solved the crystal structure of H. pylori UPPS and performed virtual screening of inhibitors from a library of 58,635 compounds. Two hits were found to exhibit differential activities against Helicobacter pylori and Escherichia coli UPPS, giving the possibility of developing antibiotics specially targeting pathogenic H. pylori without killing the intestinal E. coli.
1. INTRODUCTION
Undecaprenyl pyrophosphate synthase (UPPS) catalyzes consecutive condensation reactions of farnesyl pyrophosphate (FPP) with eight molecules of isopentenyl pyrophosphate (IPP) to form C55 undecaprenyl pyrophosphate (UPP), which acts as a lipid carrier to mediate bacterial peptidoglycan biosynthesis [1, 2]. This enzyme belongs to a group of cis-prenyltransferases which catalyze cis-double bonds during IPP condensation reactions [3, 4]. UPPS was first cloned from Micrococcus luteus and Escherichia coli, and their amino acid sequences were found conserved among the cis-prenyltransferases, but totally different from those of the trans-prenyltransferases [5–7], implying different catalytic mechanism [8, 9].
Helicobacter pylori is a pathogen which causes chronic inflammation in the stomach [10]. The infection may evolve to peptic ulcerations and gastric neoplasias. Due to its unusual ability to survive in stomach under the low pH condition via proton pumps, H. pylori infection becomes wide spreading and accounts for the increased cases of stomach carcinogenesis [11]. Antibiotics, such as proton pump inhibitors (PPI), amoxicillin, and clarithromycin, are used to treat the infected patients. When failed, empirical quadruple therapy (PPI-bismuch-tetracyclin-metronidazole) is then used as the second-line therapy [12]. Since UPPS is essential for bacterial survival, it could possibly serve as a target for new antibiotics. Even though the complex structures of E. coli UPPS with the FPP substrate or with its analogue (farnesyl thiopyrophosphate, FsPP) and IPP have been obtained [9, 13], no UPPS structure-derived inhibitors have been reported so far. As shown in this study, we solved the crystal structures of H. pylori UPPS and performed structure-based inhibitor discovery. Two hits were discovered through computer virtual screening from 58,635 compounds, which exhibited different level of inhibition against E. coli and H. pylori UPPS.
2. MATERIALS AND METHODS
2.1. Overexpression of H. pylori UPPS
The gene encoding UPPS from the H. pylori (ATCC43504) genomic DNA was amplified by using polymerase chain reaction (PCR). The forward primer 5′-GGTATTGAGGGTCGCTTGGATAGCACTCTCAAA-3′ and reverse primer 5′-AGAGGAGAGTTAGAGCCCTAGCATTTTAATTCCCC-3′ were utilized in the PCR. The PCR product was purified from 0.8% agarose gel electrophoresis. The DNA product was ligated with pET-32Xa/LIC vector and transformed into E. coli BL21 (DE3) for protein expression as previously described for expressing E. coli UPPS [14].
The C234A mutant was prepared by using QuikChange Site-Directed Mutagenesis Kit in conjunction with the wild-type gene template in the pET32Xa/Lic vector. The mutagenic forward primer was 5′-CGCAAATTCGGGGAATTAAAA TAGTGAGGCTCTAACTCT-3′. The procedure of mutagenesis utilized a supercoiled double-stranded DNA (dsDNA) vector with an insert of interest and two synthetic forward and backward primers containing the desired mutation. The mutation was confirmed by sequencing the entire UPPS mutant gene of the plasmid obtained from overnight culture. The correct construct was subsequently transformed to E. coli BL21(DE3) for protein expression. The procedure for protein purification followed our reported protocol [15]. Each purified mutant UPPS was verified by mass spectroscopic analysis and its purity (>95%) was checked by SDS-PAGE.
2.2. Crystallization and data collection
H. pylori C234A UPPS mutant was crystallized using the hanging drop method from Hampton Research (Laguna Niguel, Calif, USA) by mixing 2 μL of the UPPS solution (10 mg/mL in 25 mM Tris, 150 mM NaCl, pH 8.0) with 2 μL of the mother liquor (0.15 M KSCN, 15% PEG600, and 2% PEG5KMME), and equilibrating with 500 μL of the mother liquor. Within 4 days, crystals grew to dimensions of about 0.5 × 0.5 × 0.2 mm, and then the crystals were soaked with a cryoprotectant solution of 0.2 M KSCN, 30% PEG600, and 5% PEG5KMME for 1 day. The structure of the C234A H. pylori UPPS in complex with FsPP was obtained by soaking the crystals with cryoprotectant solution of 2.5 mM MgCl2, 2.5 mM IPP, 2.5 mM FsPP, 0.15 M KSCN, 15% PEG600, and 2% PEG5KMME. However, only the pyrophosphate of FsPP was found in the complex structure. The X-ray diffraction datasets for the structures of the C234A UPPS mutant and the complex with FsPP were collected to 1.88 Å and 2.5 Å resolution, respectively. Data for the C234A UPPS crystals were collected at beam line BL17B2 of the National Synchrotron Radiation Research Center (NSRRC, Hsinchu, Taiwan). Data for the C234A UPPS complexed with FsPP were collected in house using a Rigaku MicroMax002 X-ray generator equipped with an R-Axis IV++ image plate detector. The diffraction data were processed using the programs of HKL and HKL2000 [16]. Statistics for the dataset are listed in Table 1. Prior to use in structural refinements, 5% randomly selected reflections were set aside for calculating Rfree as a monitor [17].
Table 1.
Data collection and refinement statistics for the orthorhombic H. pylori UPPS crystals of the apoenyzme and the complex with thiopyrophosphate. C234A mutation was included to prevent intramolecular disulfide bond formation.
| H. pylori UPPS | H. pylori UPPS + PPi | |
|---|---|---|
| Data collection | ||
| Space group | P212121 | |
| Resolution (Å)a | 25 to 1.88 (1.95 to 1.88) | 50 to 2.5 (2.59 to 2.5) |
| Unit cell dimensions | ||
| a, b, c (Å) | 49.63, 58.91, 153.43 | |
| No. of reflections | ||
| Observed | 201171 (18692) | 137910 (12888) |
| Unique | 35917 (3338) | 15618 (1432) |
| Completeness (%) | 95.4 (90.3) | 96.0 (91.2) |
| Rmerge (%) | 5.5 (43.3) | 5.9 (15.9) |
| I/σ(I) | 30.7 (4.1) | 42.3 (5.2) |
| Refinement | ||
| No. of reflectionsb | 34629 (3038) | 15084 (1330) |
| Rwork (%) | 19.34 (22.91) | 21.44 (30.24) |
| Rfree (%) | 24.00 (30.02) | 29.33 (37.43) |
| Geometry deviations | ||
| Bond lengths (Å) | 0.0193 | 0.0061 |
| Bond angles (°) | 1.817 | 1.157 |
| No. of all non-H atoms | 3463 | 3449 |
| No. of water molecules | 581 | 134 |
| Mean B-values (Å2) | 39.54 | 49.49 |
| Ramachandran plot (%) | ||
| Most favored | 92.1 | 92.3 |
| Additionally allowed | 7.9 | 7.7 |
aValues in the parentheses are for the highest resolution shells.
bAll positive reflections are used in the refinements.
2.3. Structure determination and refinement
The crystal structure of C234A UPPS was determined by molecular replacement method using the Crystallography & NMR System (CNS) program [18]. The orthorhombic crystal contained one UPPS dimer in an asymmetric unit. The models of PDB 1V7U (E. coli UPPS structure bound with FPP, chain A) [13] were used as search model to yield a good resolution for the H. pylori UPPS. The space group was determined as P212121. With all solvent and cofactor molecules removed, the model yielded an initial R-value of 0.50 using all positive reflections at 1.88 Å resolution upon rigid-body refinement.
The 2Fo-Fc difference Fourier map showed clear electron densities for most amino acid residues. The residues of catalytic loop of 58–67 in chain A, 56–71 and 150–158 in chain B were disordered. Subsequent refinement with incorporation of 581 water molecules according to 1.0 σ map level yielded R and Rfree values of 0.193 and 0.240, respectively, at 1.88 Å resolution. By employing similar procedures, the C234A H. pylori UPPS and the FsPP-complexed structures were refined with the addition of cofactor and solvent molecules. All manual modifications of the models were performed on an SGI Fuel computer using the program O [19]. Computational refinements, which included maximal likelihood and simulated-annealing protocols, were carried out using CNS. The programs MolScript [20], and Raster3D [21] were used in producing figures.
2.4. Computer screening to identify the inhibitors
The X-ray structure of H. pylori UPPS reported here and the complex structure of E. coli UPPS (PDB code 1V7U) were chosen as the templates in the virtual screening. The program GOLD V2.1 was used to screen Maybridge database, a commercially available compound database obtained from Maybridge Chemical Company (Tintagel, Cornwall, England). The binding pocket for the docking study was defined as a 15 Å radius sphere centered on the active site Asp13 of H. pylori UPPS or Asp26 of E. coli UPPS. The scoring function, GoldScore, implemented in GOLD was used to rank the docking positions of the compounds. 26 compounds with the highest score ranked by GoldScore were selected for inhibition assays.
2.5. IC50 determination
The IC50 values of the two hits were measured in a buffer of 100 mM Hepes (pH 7.5), 50 mM KCl, 0.5 mM MgCl2, and 0.1% Triton X-100, containing 0.05 μM of E. coli or H. pylori UPPS. The concentrations of inhibitors used were ranged from 0 to 500 μM. To obtain the IC50, the does-response curves were fitted with the equation, A(I) = A(0) × {1 −[I/(I+IC50)]}, where A(I) is the enzyme activity with inhibitor concentration I, A(0) is enzyme activity without inhibitor, and I is the inhibitor concentration.
3. RESULTS
3.1. 3D structures of H. pylori UPPS
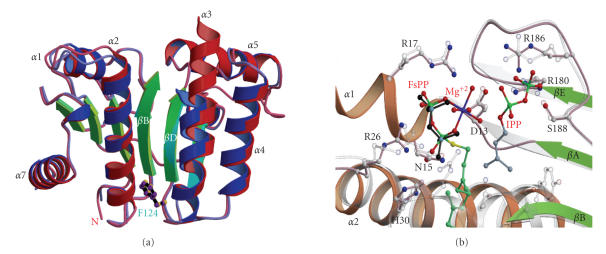
To develop structure-based inhibitors, the crystal structures of H. pylori UPPS were solved in this study. One is the structure of H. pylori UPPS containing C234A mutation to prevent intra-molecular disulfide bond formed during the long period of crystallization process (Figure 1(a)), and the other is the structure of C234A complexed with FsPP, but only the pyrophosphate portion is visible (Figure 1(b)). The C234A mutant has unchanged kinetic property compared with the wild type (kcat, FPP Km and IPP Km of C234A were 0.20 ± 0.08 s−1, 0.15 ± 0.04 μM and 9.6 ± 0.2 μM, almost equal to 0.22 ± 0.05 s−1, 0.11 ± 0.02 μM and 9.2 ± 0.1 μM for the wild type, resp.). The overall structure of H. pylori UPPS was similar to that of E. coli UPPS [22]. The protein is a dimer and each subunit contains a catalytic domain and a pairing domain. Two subunits are tightly associated through the central β-sheet and a pair of long α-helices (α5 and α6). However, H. pylori UPPS has a 1.5-turn shorter α5 helix in the dimer interface. This may weaken the dimer formation for H. pylori UPPS. The catalytic domain is composed of six β-strands and four β-helices and the central tunnel-shaped active site is surrounded by 2 α-helices (α2 and α3) and 4 β-strands (βA-βB-βD-βC) (Figure 1(a)).
Figure 1.
Crystal structures of H. pylori UPPS. (a) Two subunits of the apoenzyme are superimposed. The most obvious disposition occurs in α3 helix which adopts an open form and a closed form in subunit A and B, respectively. At the top of the tunnel-shaped crevice surrounded by 2α-helices and 4β-strands is the substrate-binding site. Phe124 located at the bottom of the H. pylori UPPS tunnel adopts a similar position to that of Leu137 in E. coli UPPS, essential for determining product chain length. (b) Superimposition of active site structures of H. pylori UPPS with FsPP and E. coli UPPS with FsPP, Mg2+, and IPP [9]. The active site residues in H. pylori UPPS are shown in pink and those in E. coli UPPS in white for carbon-carbon bonds in ball-and-stick model. The thiopyrophosphate (visible in crystal structure) is shown in black, the nitrogen atoms and Mg2+ ion are shown in blue, and oxygen atoms are shown in red. Asp13 in H. pylori UPPS occupies a similar position to that of Asp26 in E. coli UPPS to coordinate with an Mg2+ for binding with the pyrophosphate leaving group of FPP.
At the bottom of the tunnel, a large amino acid F124 occupies a similar position to that of L137 at the bottom of E. coli UPPS tunnel, which is a key residue to shield the final product and determine its chain length [22]. At the top of this tunnel, several amino acids including D13, R17, R26, H30, F57, S58, R180, and E184 are located in the substrate binding site (Figure 1(b)). The position of the pyrophosphate (shown in black sticks in Figure 1(b)) of FsPP in the complex is almost identical to that of the FPP pyrophosphate in the E. coli UPPS active site [13].
The positions of the α3 helix in the two subunits of H. pylori UPPS are slightly different (Figure 1(a)), resembling the open and closed forms of E. coli UPPS [22]. H. pylori UPPS A-chain strongly resembles the Triton-bound open form of E. coli UPPS [23], with root mean square deviation (r.m.s.d) of 0.78 Å for 200 match pairs of α-carbon atoms. Compare to the closed-form structure of E. coli UPPS with FsPP and IPP bound [9], the H. pylori UPPS B-chain is with the r.m.s.d. of 1.08 Å for 191 match pairs of α-carbon atoms. This suggests a conformational change in the H. pylori UPPS reaction.
3.2. Virtual screening of the H. pylori UPPS inhibitors
Based on the structures, computer virtual screening was carried out to search for selective inhibitors of E. coli and H. pylori UPPS. The screening procedure is summarized in Figure 2. The crystal structure of E. coli UPPS bound with FPP (1V7U) was used as a template first for the virtual screening since the electron density of a small loop responsible for conformational change near the active site is not visible in H. pylori UPPS, which might confound the virtual screening result. A compound database containing 58,635 compounds available from Maybridge Chemical Company were screened using the program GOLD V2.1. Each compound in the database was docked into the active site of E. coli UPPS, defined as 15 Å radius sphere around Asp26, an essential residue responsible to coordinate with the catalytic Mg2+. The docked molecules were then ranked by the GoldScore fitness function, according to the sum of H-bond energy, van der Waals energy, internal ligand van der Waals and internal torsional strain energy. The top 26 compounds ranked by GoldScore were then purchased and experimentally evaluated for their ability to inhibit H. pylori and E. coli UPPS.
Figure 2.
The flow chart for computer screening of H. pylori UPPS inhibitors. The active zone for screening was focused on Asp13, an important amino acid residue for coordinating with catalytic Mg2+. In parentheses are the numbers of compounds. BTB06061 and HTS04781 are the final hits.
3.3. Inhibition against E. coli and H. pylori UPPS
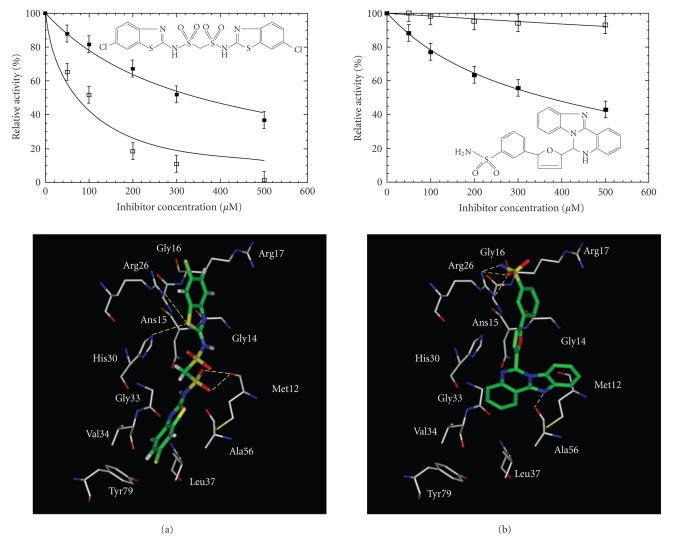
Of these 26 compounds, 2 compounds numbered BTB06061 and HTS04781, were found inhibitory to H. pylori UPPS almost equally with IC50 values of 350 μM and 362 μM, respectively (Figure 3). The IC50 values of these two compounds against the C234A and wild-type enzyme were almost equal. As revealed by the predicted models shown in Figures 3(a) and 3(b), two inhibitors are likely bound to H. pylori UPPS with a similar orientation to that of the substrate FPP. The sulfur atom in the thiazole ring of BTB06061 may form H-bonds with Asn15 and His30 while the SO2 group is hydrogen bound with Met12. In addition, the aromatic rings of BTB06061 form hydrophobic interactions with the surrounding hydrophobic residues, including Val34, Leu37, Ala56 and Tyr79. As shown in the predicted model of HTS04781 with H. pylori UPPS, the sulfonamide group forms H-bonds with Gly16 and Arg26 and the N atom in the tetracyclic ring is hydrogen bound to the main chain of Met12. Extensive hydrophobic interactions were found between the tetracyclic ring with the surrounding residues including Met12, His30, Gly33 and Val34.
Figure 3.
Computer virtual screening of the H. pylori UPPS inhibitors. Two compounds, BTB06061 shown in (a) and HTS04781 in (b), were identified from the computer fitting of the Maybridge compounds into the active site of E. coli and H. pylori UPPS. The data of enzyme activities in the presence of different concentrations of the inhibitors were used to determine the IC50 values of the inhibitors. The compounds displayed IC50 of 350 and 363 μM, respectively, in inhibiting H. pylori UPPS activity. However, the IC50 of BTB06061 became 71 μM in inhibiting E. coli UPPS and HTS04781 was almost inactive against the enzyme. The modeled structures of the inhibitor bound in the active site of H. pylori UPPS are shown at the bottom.
Surprisingly, BTB06061 showed 5-fold smaller IC50 (71 μM) against E. coli UPPS and HTS04781 almost did not inhibit E. coli UPPS, although two compounds inhibited H. pylori equally. From the modeling (not shown), the smaller entrance in E. coli UPPS compared to H. pylori UPPS at the top of the tunnel due to the partial blockage by the amino acids such as Trp75 from the flexible loop might restrict, or at least partially limit the access of bulky compound HTS04781 that contains four rigid aromatic rings to the active site, thereby leading to the loss of inhibitory activity when competing with the substrate for binding.
4. DISCUSSION
In this paper we describe the crystal structures of UPPS from H. pyroli, a wide-spreading and life-threatening pathogen, and the first structure-derived inhibitors from computer virtual screening. Although a high-throughput screening has been performed for UPPS by a pharmaceutical company [24], none of the inhibitors have been reported. So far, a series of IPP analogues with a dicarboxylate moiety in place of the diphosphate were synthesized and the E-pentenylbutanedioic acid showed inhibition of UPPS with an IC50 of 135 μM [25]. Based on the known structure of UPPS (9), two carboxylate groups may coordinate with the catalytic Mg2+ ion which was bound with the pyrophosphate group of the substrates. Recently, we reported some bisphosphonates, which inhibited trans-type FPPs, which could also inhibit cis-type UPPS with sub-μM IC50 when containing suitable hydrophobic side-chains [26]. The crystal structures show that four molecules of inhibitors are bound in the active site and one of them occupies the FPP site with a phosphoate group chelating with the Mg2+. Here, we report the first two novel inhibitors identified from a randomized compound library through virtual screening. These two inhibitors likely occupy the FPP site of H. pylori UPPS based on computer modeling. Two inhibitors displayed similar inhibition against H. pylori UPPS, but very different inhibition on E. coli UPPS. The one with bulky skeleton did not inhibit E. coli UPPS, likely owing to the partially blocked opening at the top of tunnel by the flexible loop in the E. coli UPPS active site. Our results shed light on the possibility of developing antibiotics specially targeting pathogenic H. pylori without killing the intestinal E. coli.
References
- 1.Allen CM. Purification and characterization of undecaprenyl pyrophosphate synthetase. Methods in Enzymology. 1985;110:281–299. doi: 10.1016/s0076-6879(85)10085-6. [DOI] [PubMed] [Google Scholar]
- 2.Robyt J. Essential of Carbohydrate Chemistry. New York, NY, USA: Springer; 1998. chapter 10. [Google Scholar]
- 3.Ogura K, Koyama T. Enzymatic aspects of isoprenoid chain elongation. Chemical Reviews. 1998;98(4):1263–1276. doi: 10.1021/cr9600464. [DOI] [PubMed] [Google Scholar]
- 4.Liang P-H, Ko T-P, Wang AH-J. Structure, mechanism and function of prenyltransferases. European Journal of Biochemistry. 2002;269(14):3339–3354. doi: 10.1046/j.1432-1033.2002.03014.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen A, Kroon PA, Poulter CD. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Science. 1994;3(4):600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu N, Koyama T, Ogura K. Molecular cloning, expression, and purification of undecaprenyl diphosphate synthase. No sequence similarity between E- and Z-prenyl diphosphate synthases. Journal of Biological Chemistry. 1998;273(31):19476–19481. doi: 10.1074/jbc.273.31.19476. [DOI] [PubMed] [Google Scholar]
- 7.Apfel CM, Takács B, Fountoulakis M, Stieger M, Keck W. Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential UPPS gene. Journal of Bacteriology. 1999;181(2):483–492. doi: 10.1128/jb.181.2.483-492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii H, Koyama T, Ogura K. Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochimica et Biophysica Acta. 1982;712(3):716–718. [PubMed] [Google Scholar]
- 9.Guo R-T, Ko T-P, Chen AP-C, Kuo C-J, Wang AH-J, Liang P-H. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate: roles of the metal ion and conserved residues in catalysis. Journal of Biological Chemistry. 2005;280(21):20762–20774. doi: 10.1074/jbc.M502121200. [DOI] [PubMed] [Google Scholar]
- 10.Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GJ, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Annals of Internal Medicine. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Mauch F, Bode G, Malfertheiner P. Identification and characterization of an ATPase system of Helicobacter pylori and the effect of proton pump inhibitors. American Journal of Gastroenterology. 1993;88(10):1801–1802. [PubMed] [Google Scholar]
- 12.Bytzer P, O'Morain C. Treatment of Helicobacter pylori . Helicobacter. 2005;10(S1):40–46. doi: 10.1111/j.1523-5378.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang S-Y, Ko T-P, Chen AP-C, Wang AH-J, Liang P-H. Substrate binding mode and reaction mechanism of undecaprenyl pyrophosphate synthase deduced from crystallographic studies. Protein Science. 2004;13(4):971–978. doi: 10.1110/ps.03519904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan J-J, Yang L-W, Liang P-H. Effect of site-directed mutagenesis of the conserved aspartate and glutamate on E. coli undecaprenyl pyrophosphate synthase catalysis. Biochemistry. 2000;39(45):13856–13861. doi: 10.1021/bi001226h. [DOI] [PubMed] [Google Scholar]
- 15.Chen AP-C, Chang S-Y, Lin Y-C, et al. Substrate and product specificities of cis-type undecaprenyl pyrophosphate synthase. Biochemical Journal. 2005;386(1):169–176. doi: 10.1042/BJ20040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 17.Brunger AT. Assessment of phase accuracy by cross validation: the free R value. Methods and applications. Acta Crystallographica Section D. 1998;49(1):24–36. doi: 10.1107/S0907444992007352. [DOI] [PubMed] [Google Scholar]
- 18.Brünger AT, Adams PD, Clore GM, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallographica Section D. 1998;54(5):905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 19.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallographica Section A. 1991;47(2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 20.Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. Journal of Applied Crystallography. 1991;24(5):947–950. [Google Scholar]
- 21.Merritt EA, Murphy MEP. Raster3D version 2.0 A program for photorealistic molecular graphics. Acta Crystallographica Section D. 1994;50(6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 22.Ko T-P, Chen Y-K, Robinson H, et al. Mechanism of product chain length determination and the role of a flexible loop in Escherichia coli undecaprenyl-pyrophosphate synthase catalysis. Journal of Biological Chemistry. 2001;276(50):47474–47482. doi: 10.1074/jbc.M106747200. [DOI] [PubMed] [Google Scholar]
- 23.Chang S-Y, Ko T-P, Liang P-H, Wang AH-J. Catalytic mechanism revealed by the crystal structure of undecaprenyl pyrophosphate synthase in complex with sulfate, magnesium, and triton. Journal of Biological Chemistry. 2003;278(31):29298–29307. doi: 10.1074/jbc.M302687200. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Huang J, Jiang X, Seefeld M, McQueney M, Macarron R. The effect of triton concentration on the activity of undecaprenyl pyrophosphate synthase inhibitors. Journal of Biomolecular Screening. 2003;8(6):712–715. doi: 10.1177/1087057103258185. [DOI] [PubMed] [Google Scholar]
- 25.Scholte AA, Eubanks LM, Poulter CD, Vederas JC. Synthesis and biological activity of isopentenyl diphosphate analogues. Bioorganic and Medicinal Chemistry. 2004;12(4):763–770. doi: 10.1016/j.bmc.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Guo R-T, Cao R, Liang P-H, et al. Bisphosphonates target multiple sites in both cis- and trans- prenyltransferases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10022–10027. doi: 10.1073/pnas.0702254104. [DOI] [PMC free article] [PubMed] [Google Scholar]