Abstract
Corticotropin-releasing factor (CRF) is a neurohormone that mediates stress, anxiety, and affects serotonergic activity. Studies have shown that CRF has dose-dependent opposing effects on serotonergic activity. This effect has been hypothesized to be differentially mediated by CRF1 and CRF2 receptors in the dorsal raphé nucleus. We directly tested this hypothesis by using in vivo microdialysis to determine the effects of CRF and CRF antagonists in the dorsal raphé nucleus on serotonin (5-HT) release in the nucleus accumbens, a brain region implicated in the neuropathology of stress-related psychiatric disorders. Male urethane-anesthetized rats were implanted with a microdialysis probe into the nucleus accumbens, and CRF (0, 100 or 500 ng) was infused into the dorsal raphé. Infusion of CRF into the dorsal raphé nucleus had dose-dependent opposite effects, with 100 ng of CRF significantly decreasing 5-HT levels in the nucleus accumbens and 500 ng CRF significantly increasing accumbal 5-HT levels. In subsequent experiments, the raphé was pre-treated with the CRF1 receptor antagonist antalarmin (0.25 µg) or the CRF2 receptor antagonist antisauvigine-30 (ASV-30; 2 µg) prior to CRF infusion. Antagonism of CRF1 receptors in the dorsal raphé nucleus abolished the decrease in accumbal 5-HT levels elicited by 100 ng CRF, and CRF2 receptor antagonism in the raphé blocked the increase in accumbal 5-HT levels elicited by 500 ng CRF. These results suggest that the opposing effects of dorsal raphé CRF on 5-HT release in the nucleus accumbens are dependent on differential activation of CRF1 and CRF2 receptors in the dorsal raphé nucleus.
Keywords: microdialysis, antalarmin, antisauvigine-30, CRF receptors, stress
1. Introduction
The nucleus accumbens has been the focus of numerous studies on reward and motivation, and exhibits increased dopaminergic activity during both rewarding and aversive conditions. However, these dopaminergic changes in the nucleus accumbens are modulated by other transmitter systems including the serotonergic and corticotropin-releasing factor (CRF) systems (Amato et al., 2006; Bland et al., 2004; Izzo et al., 2005). For instance, CRF in the nucleus accumbens has been shown to be involved in arousal and amplifying motivated behaviors (Holahan et al., 1997; Pecina et al., 2006). Serotonergic innervation of the nucleus accumbens originates from the lateral wings of the dorsal raphé nucleus (Van Bockstaele et al., 1993). Serotonin in the nucleus accumbens is influenced by stress and social interaction (Summers et al., 2003b) and decreased activity of 5-HT in the nucleus accumbens may be associated with impulsivity (Coccaro, 1992; Winstanley et al., 2005). Thus, examining the effects of CRF in the dorsal raphé nucleus on 5-HT release in the nucleus accumbens may help elucidate the roles of CRF and 5-HT in the primary accumbal functions such as reward, motivation, impulsivity, and their pyschopathologies.
Corticotropin-releasing factor plays an important role in integrating multiple components of the stress response. Exposure to a stressor increases central CRF levels (Merali et al., 1998; Merlo et al., 1995), while the behavioral effects of administering CRF to rats resemble those induced by stressors (Dunn and Berridge, 1990a; Dunn and Berridge, 1990b; Harvey and Hennessy, 1995; Koob and Bloom, 1985). The behavioral effects induced by CRF are thought to be mediated, in part, by CRF actions on 5-HT systems within the brain (Forster et al., 2006; Hammack et al., 2002; Kirby et al., 2000), via activation of CRF1 and CRF2 receptors (Radulovic et al., 1998; Radulovic et al., 1999). Experimental evidence suggests that activation of the CRF1 receptor subtype is involved in the initiation of HPA responses to stress (Bale et al., 2002; Risbrough et al., 2003; Smith et al., 1998). Blockade of these receptors results in a reduction of the CRF-potentiated acoustic startle response along with startle elicited by fear-inducing stimuli (Schulz et al., 1996). In addition, CRF2 receptors appear to contribute to some of the anxiogenic functions of CRF, because antagonism of CRF2 receptors decreases conditioned freezing and acoustic startle behaviors (Bakshi et al., 2002; Takahashi et al., 2001). Furthermore, CRF-mediated increases in conditioned freezing appear to be mediated by CRF2 receptors located in the 5-HT cell body region of the dorsal raphé nucleus (Hammack et al., 2003b).
The dorsal and median raphé nuclei are major sources of 5-HT innervation of forebrain and limbic regions (Jacobs and Azmitia, 1992), and receive CRF projections from the amygdala(Gray, 1993). Serotonin is involved in neuronal processes related to conflict behavior, inhibitory control and impulsivity as well as reward-related mechanisms (Evenden and Ryan, 1996; LeMarquand et al., 1994a; LeMarquand et al., 1994b; Roy and Linnoila, 1988; Soubrie, 1986). Changes in limbic 5-HT activity are also associated with stressors, and decreased central 5-HT function is implicated in the symptoms of affective disorders (Carrasco and Van de Kar, 2003; Deakin, 1998; Millan, 2003). Corticotropin-releasing factor in the dorsal raphé nucleus is known to affect serotonergic activity (Forster et al., 2006; Kirby et al., 2000; Price et al., 1998; Price and Lucki, 2001; Valentino et al., 2001). Previous studies have shown that both CRF1 and CRF2 receptors have been detected in the dorsal raphé nucleus (Commons et al., 2003; Funk et al., 2003; Van Pett et al., 2000) and may have opposing roles for 5-HT release (Kirby et al., 2000; Pernar et al., 2004; Valentino et al., 2001). Corticotropin-releasing factor has a higher affinity for CRF1 receptors when compared to CRF2 receptors (Grigoriadis et al., 1996a; Grigoriadis et al., 1996b), and activation of these receptors normally inhibits serotonergic activity in the dorsal raphé (Hammack et al., 2003b; Pernar et al., 2004). In contrast, higher levels of CRF are believed to be required for CRF2 receptor activation, and activation of these receptors normally facilitates serotonergic activity in dorsal raphé (Kirby et al., 2000; Pernar et al., 2004). Combined, these studies suggest that CRF has opposing effects in the dorsal raphé nucleus that depend on CRF1 and CRF2 receptor activation based on the dosage of CRF. If this is the case, then dose-dependent CRF activation of CRF1 and CRF2 receptors in the dorsal raphé nucleus should influence 5-HT release in limbic terminal sites, such as the nucleus accumbens, and thereby influence behavior. This hypothesis has not been directly tested in relation to the nucleus accumbens.
Since CRF in the dorsal raphé nucleus is thought to affect 5-HT activity in an opposing manner, we hypothesized that infusion of high and low doses of rat-human CRF into the dorsal raphé nucleus would have contrasting effects on 5-HT release in the nucleus accumbens. Furthermore, we hypothesized that the opposing effects of CRF on accumbal 5-HT release would be a function of opposing effects of CRF1 and CRF2 receptor activation in the dorsal raphé nucleus.
2. Materials and methods
2.1. Animals
Ninety-six male Sprague-Dawley rats (University of South Dakota Laboratory Animal Services, Vermillion, SD, USA) weighing between 250 and 350 g were housed in pairs and maintained at a constant room temperature (22 °C, 60% relative humidity) and a reverse 12h light: 12h dark cycle. Food and water were available ad libitum. The following procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
2.2. Microdialysis and pharmacological experiments
Rats were anesthetized with urethane (1.8 g/kg i.p; Sigma, St. Louis, MO) and placed in a stereotaxic frame (David Kopf Institute, CA, USA) with the incisor bar set at −3.3 mm. Body temperature was maintained at 37 ± 0.5°C with a temperature regulated heating pad (CMA, North Chelmsford, MA). A concentric microdialysis probe (membrane length 2 mm, with MW cut-off 5000; (Hoffman et al., 2002)) was inserted into the nucleus accumbens (AP: +1.2 from bregma; ML: −1.4 from midline; DV: −8.1 from dura; (Paxinos and Watson, 1997)). For CRF alone and control infusions (CRF receptor antagonists and vehicles for each antagonist alone) into the dorsal raphé nucleus, a single stainless-steel infusion guide cannula (26 gauge) was implanted 2 mm above the dorsal raphé nucleus (AP: −7.4 from bregma; ML: +2.8 from midline; DV: −4.6 from dura) at a 26° lateral to medial angle to avoid the cerebral aqueduct (Forster et al., 2006; Paxinos and Watson, 1997). For the CRF antagonist with CRF infusion experiments, two 26 gauge stainless steel guide cannulae were fixed together in such a way that the one cannula was immediately behind the other and these cannulae were implanted into the dorsal raphé nucleus as described for the single guide cannula.
Artificial cerebrospinal fluid (aCSF; Moghaddam and Bunney, 1989) was perfused continuously through the probe at a rate of 0.4 µl/min with a microinfusion pump (CMA) via PE-20 tubing connected to a 1 ml syringe. Three hours after probe insertion, silica cannulae (194 µm od, 2 mm longer than guides; Polymicro Technologies, Phoenix, AZ) were lowered through cannulae. Drug cannulae were fixed to PE-20 tubing connected to a 10 µl Hamilton syringe, and the drug or vehicle was back-loaded into the cannula before implantation as described previously (Forster and Blaha, 2000). Microdialysis sampling began 4 hours after the implantation of the probe (Forster et al., 2006), with perfusates collected at 20 min intervals and analyzed for 5-HT. For the first experiment, following at least 3 stable baseline 5-HT samples, either aCSF (0.5 µl; vehicle for CRF) or rat-human CRF (100 or 500 ng; 0.5 µl; Sigma-Aldrich, St. Louis, MO) was infused through a single cannula directed at the dorsal raphé nucleus. As the natural ligand for rat CRF receptors was used for these experiments, i.e. r/hCRF, from this point we will refer to this form of the neuromodulator as CRF, indicating oCRF for comparison to studies using ovine CRF.
Treatment for the second experiment consisted of (1) infusing antalarmin (0.25 µg/0.5 µl; Sigma-Aldrich), a CRF1 receptor antagonist, into the dorsal raphé nucleus alone or 10 minutes prior to infusion of CRF (100 ng or 500 ng/ 0.5 µl) into the dorsal raphé nucleus; or (2) infusing antisauvagine-30 (2 µg/0.5 µl; Sigma-Aldrich), a CRF2 receptor antagonist, into the dorsal raphé nucleus alone or ten minutes before CRF (100 ng or 500 ng/ 0.5 µl) into the dorsal raphé nucleus. Vehicle treatments for the second experiment consisted of infusing (1) 5% ethanol and 5% camphor in aCSF (0.5 µl; vehicle for antalarmin) into the dorsal raphé nucleus either alone or 10 minutes prior to the infusion of 100 ng/ 0.5 µl CRF into the dorsal raphé nucleus; or (2) 2% ethanol in aCSF (0.5 µl; vehicle for antisauvagine-30) either alone or ten minutes before 500 ng/ 0.5 µl CRF into the dorsal raphé nucleus. All drugs and vehicle solutions were delivered at a rate of 0.5 µl/min using a microinfusion pump (Stoelting, Wood Dale, IL). The doses of CRF were determined from previous studies demonstrating CRF effects on 5-HT activity (Amat et al., 2004; Forster et al., 2006; Kirby et al., 2000; Price et al., 1998). Doses of receptor antagonists were derived from studies demonstrating effective antagonism of CRF receptors as indicated by the drug induced blockade of CRF-dependent effects (Amat et al., 2004; Hammack et al., 2003b; Hammack et al., 2003a; Le et al., 2002). Sampling of 5-HT in perfusates was continued until 5-HT activity returned to pretreatment levels.
2.3. Serotonin analysis
Analysis of 5-HT in dialysates was accomplished by using high-performance liquid chromatography (HPLC) with electrochemical detection (Bradberry et al., 1991; Hoffman et al., 2002). Samples were injected into a chromatographic system with a 5 µl loop. The mobile phase used for 5-HT separation (0.3 g EDTA, 0.432 g of 1-octanesulfonic acid, 4.8 g NaH2PO4, 120 ml acetonitrile, 200 µl triethylamine per 1 L, pH 5.35) was pumped through a UniJet 3 µm C18 silica column (Bioanalytical Systems, West Lafayette, IN) under nitrogen gas pressure (2000 psi). The collection rate of 0.4 µl/ min resulted in approximately 8 µl of dialysate to insure that the loop was overfilled during each sample period. Following separation by the column, 5-HT was detected by a glassy carbon electrode (Bioanalytical Systems) maintained at +0.5 V with respect to the Ag/AgCl2 reference electrode with a LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Serotonin peaks were identified by comparison to a 5-HT standard (7.9 pg/5 µl 5-HT). The 2:1 signal to noise detection limit for 5-HT using this system was 0.08 ± 0.04 pg.
2.4. Histology
At the conclusion of these experiments, rats were administered a lethal dose of Fatal-plus (Vortech, Dearborn, MI, 0.5 ml, ip.). Brains were removed and fixed in 10% formalin. Sections (60 µm) were cut using a Leica Jung CM1800 cryostat (Wetzlar, Germany) at −12 °C, and analyzed under a light microscope to confirm appropriate placements of microdialysis probes and drug infusion cannulae. Only data from rats with correct probe and cannulae placements were included in the data analyses (n=4-9 per group).
2.5. Data Analysis
Serotonin levels are expressed as a percentage change (±SEM) from baseline levels where the baseline level is determined by averaging the 3 baseline measurements. Significance levels for all statistical tests were set at P<0.05 (SigmaStat v2.03). The effects of CRF or CRF receptor antagonists in the dorsal raphé nucleus on NAc 5-HT concentrations were analyzed using a two-way ANOVA (treatment × time) with repeated measures where the repeated factor was time. In analyses where a significant treatment × time interaction was present, effects of treatment were analyzed further using one-way repeated measures ANOVA. Comparisons between drug and control treatments at a given time were analyzed using Student Newman-Keuls posthoc analysis. The effects of treatments across time were analyzed using one-way repeated measures ANOVA in analyses where a significant treatment × time interaction was present. The effects of treatment over time were then compared using the Dunnett’s test where the sample immediately preceding treatment was used as the control 5-HT value.
3. Results
3.1. Probe and injection placement
The placement of microdialysis probes ensured that the 2 mm length of dialysis membrane sampled 5-HT from both the nucleus accumbens shell and core (Figs. 1A, 1C, and 1E). Drug infusion cannulae were located in the mid to posterior aspect of the dorsal raphé nucleus (Figs. 1B, 1D, and 1E). The area of the dorsal raphé nucleus infused has been shown to provide 5-HT innervation to the nucleus accumbens (Van Bockstaele et al., 1993). Although infusions into the dorsal raphé nucleus were close to the cerebral aqueduct, cannulae were placed on a lateral to medial angle to minimize the possibility of diffusion into the cerebral aqueduct (Forster et al., 2006). Probe placements outside of the dorsal raphé nucleus were used for anatomical control (Figs. 1B).
Figure 1. Probe and Cannula Placements in the Nucleus Accumbens and dorsal Raphé.
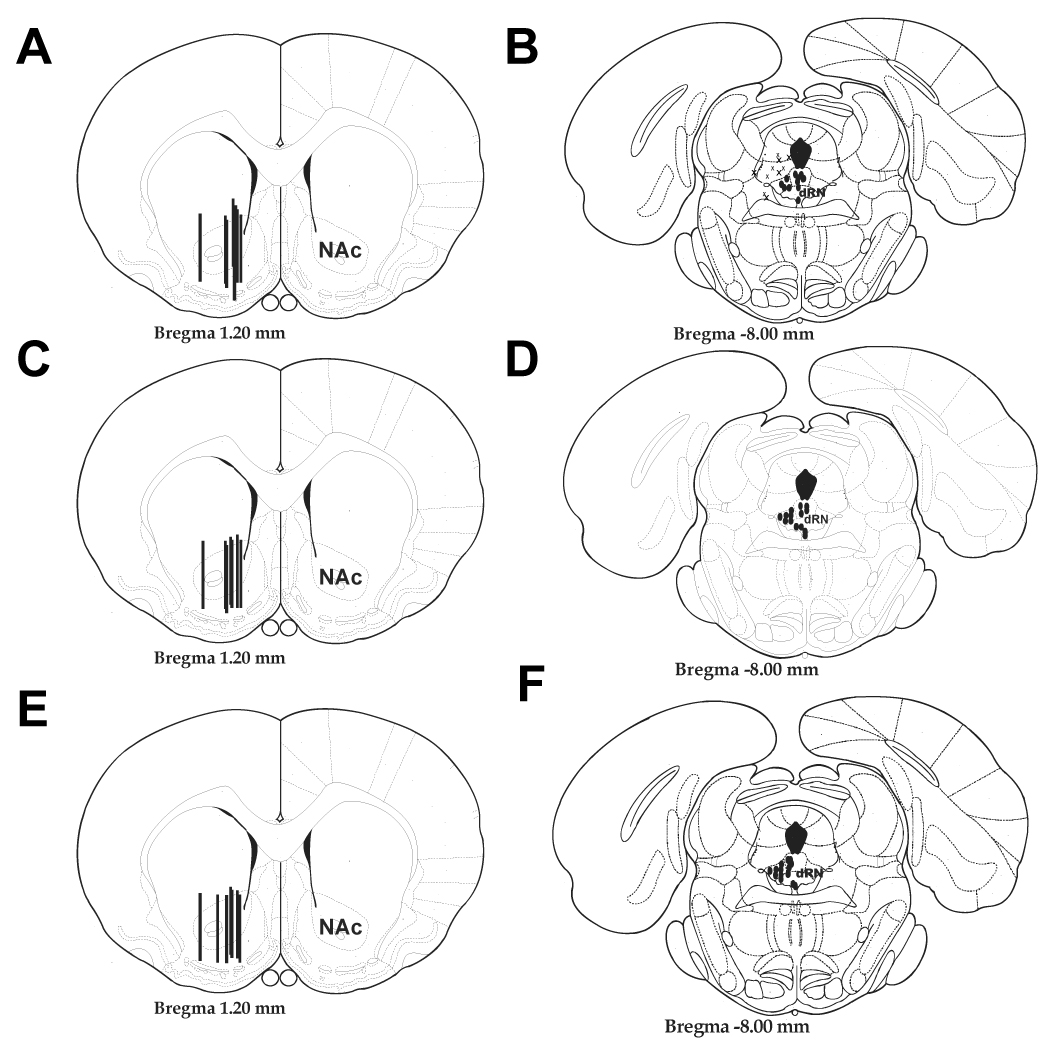
Schematic representations of microdialysis probe (A,C,E) and dorsal raphé nucleus drug infusion cannulae (B, D, F) for CRF dose-response experiments (A and B), CRF1 receptor antagonist experiments (C and D), and CRF2 receptor antagonist experiments (E and F). Microdialysis probes (black bars which represent more than one placement) were placed in the nucleus accumbens (NAc) with a 2 mm membrane to record from both the shell and the core (A, C, E; Figures adapted from Paxinos and Watson (1997); AP: +1.2 from bregma; ML: −1.4 from midline; DV: 8.1 from dura). Drug cannulae (black circles) were placed in regions encompassing areas of the dorsal raphé nucleus (AP: −7.4 from bregma; ML: +2.8 from midline; DV: −4.6 from dura) that project to the nucleus accumbens (B, D, and F). Figure B also illustrates cannula placements outside the dorsal raphé nucleus (crosses).
3.2. Effects of CRF infusions into the dorsal raphé on 5-HT levels in nucleus accumbens
Infusion of CRF into the dorsal raphé nucleus had opposing effects depending on dose on extracellular 5-HT levels in the nucleus accumbens (Fig. 2A). There was a significant effect of CRF treatment (F2, 165 = 3.53, P < 0.05) and a significant treatment × time interaction (F16, 165 = 8.85, P < 0.001). Infusion of 100 ng of CRF produced a significant effect on accumbal 5-HT over time (F8, 55 = 6.605, P < 0.001). Serotonin decreased in the nucleus accumbens in the first twenty minutes following 100 ng CRF infusion into the dorsal raphé nucleus when compared to pretreatment 5-HT levels (Dunnett’s q 55, 9 = 5.86, P < 0.05). There was also a significant effect on 5-HT in the nucleus accumbens in response to the infusion of 500 ng CRF into the dorsal raphé nucleus (F8, 55 = 7.378, P < 0.001). In the first sample collected from the nucleus accumbens following the infusion of 500 ng CRF into the dorsal raphé nucleus, 5-HT was increased approximately 55% over baseline values (Dunnett’s q 55, 9 = 5.78, P < 0.05). When CRF treatments were compared within each time point, a significant effect was found at 20 min post treatment (F2, 21 = 21.45, P < 0.001). The decrease in accumbal 5-HT levels observed after the infusion of 100 ng into the dorsal raphé nucleus was significant when compared to both aCSF (SNK P = 0.02) and 500 ng (SNK P < 0.001) CRF infusions. In contrast, when 500 ng CRF was infused into the dorsal raphé, a significant increase was evident at 20 min when compared to aCSF (SNK P = 0.001).
Figure 2. Effects of CRF Infusion into the dorsal Raphé nucleus on Accumbal 5-HT levels.
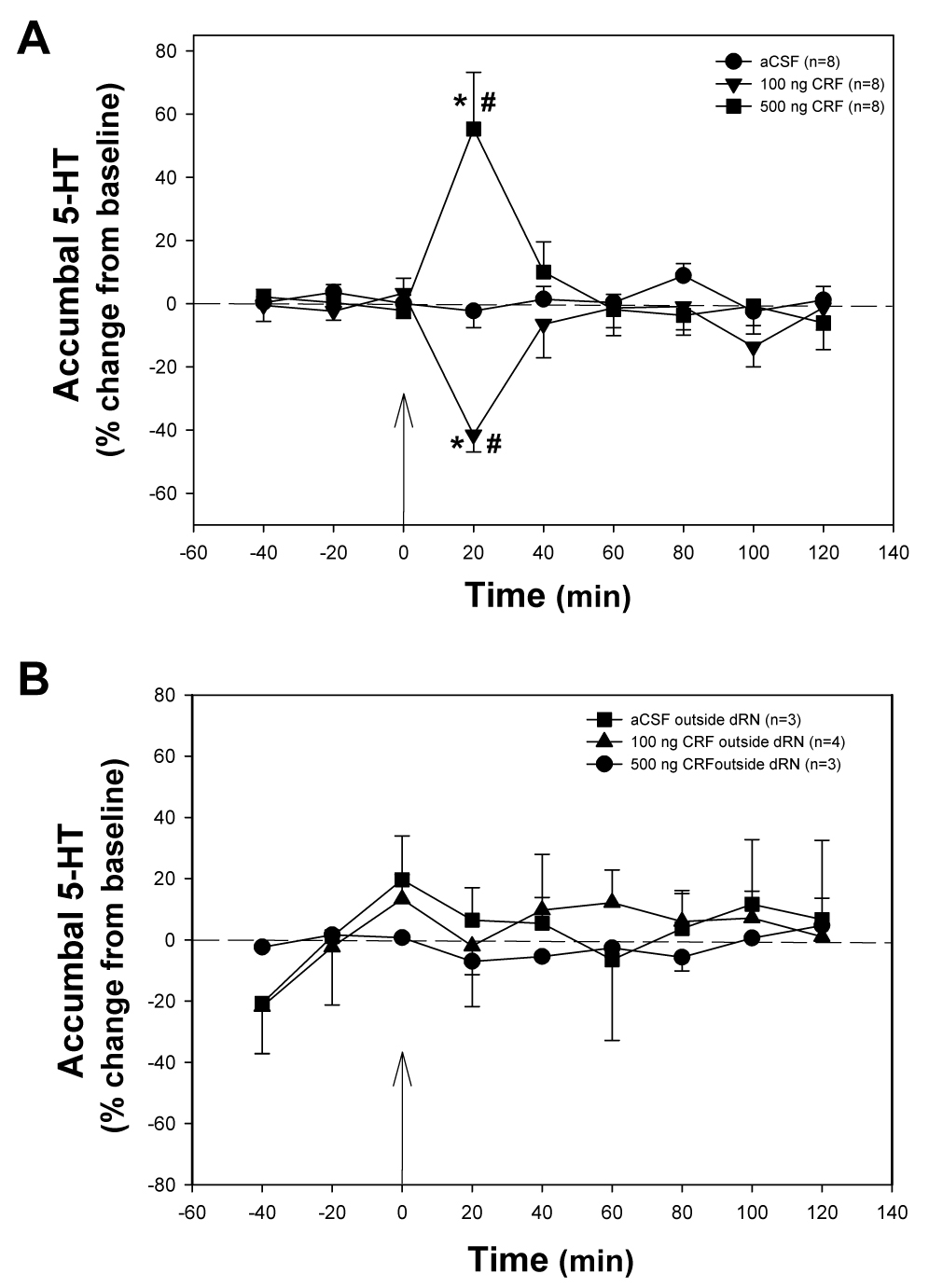
Infusion of 100 ng CRF into the dorsal raphé nucleus (A) resulted in a significant decrease in accumbal 5-HT when compared to CSF (A; *significant difference in accumbal 5-HT levels as compared to aCSF infusion, P<0.05), and compared to pre-infusion baseline 5-HT overflow (# significant difference over time in accumbal 5-HT levels, P<0.05). Arrow indicates time of intra-dorsal raphé injection. In contrast, infusion of 500 ng into the dorsal raphé nucleus (A) resulted in a significant increase in accumbal 5-HT levels when compared to controls (A). When cannulae placements were outside the dorsal raphé nucleus (B), no change in 5-HT overflow was recorded, regardless of the treatment.
A few cannulae for infusion of CRF did miss the dorsal raphé nucleus, resulting in CRF delivery to regions outside, but close to, the serotonergic cells of interest (Figs. 1B). Infusion of CRF outside the dorsal raphé nucleus did not affect accumbal 5-HT release (Fig. 2B). This suggests that although the CRF delivered to the dorsal raphé nucleus is contained in a large volume (0.05 µl), the effects are specific to the dorsal raphé as no influence of CRF on 5-HT overflow were observed when CRF was infused outside the dorsal raphé nucleus.
3.3. Effects of CRF receptor antagonists in dorsal raphé on accumbal 5-HT
Prior infusion of the CRF1 receptor antagonist, antalarmin, into the dorsal raphé nucleus blocked the 100 ng CRF-induced decrease in accumbal 5-HT levels (Fig. 3). There was a significant effect of time (F9, 216 = 3.85, P < 0.001) and treatment × time interaction (F45, 216 = 2.61, P < 0.001). Consistent with the results from experiment one, infusion of 100 ng CRF in the dorsal raphé following infusion of the vehicle, resulted in a significant decrease in accumbal 5-HT levels at 20 min (F9,45 = 5.76, P < 0.001; Dunnett’s q 45, 10 = 5.97, P < 0.05). Similarly, infusion of 100 ng CRF into the dorsal raphé nucleus following infusion of the CRF2 receptor antagonist resulted in a significant decrease in accumbal 5-HT levels at 20 min (F9,36 = 36.24, P < 0.001; Dunnett’s q 36, 10 = 13.11, P < 0.05). In the group treated with the CRF1 receptor antagonist antalarmin, there was a trend towards an effect across time (F9, 45 = 5.76, P = 0.07). Furthermore, the comparison of the CRF response elicited with antalarmin compared to vehicle at 20 min post infusion showed antalarmin pretreatment effectively blocked the 100 ng CRF effect on 5-HT in the nucleus accumbens (P = 0.004). When comparing between groups within each time point, there were significant differences between treatment groups at post 20 (F5, 23 = 7.034, P < 0.001), post 100 (F5, 23 = 3.753, P = 0.012), and post 120 (F5, 23 = 5.398, P = 0.002). At 20 min post infusion, antalarmin alone (SNK=0.007), vehicle for antalarmin alone (SNK = 0.012), aCSF (SNK= 0.008), and infusion of antalarmin 10 min prior to the infusion of 100 ng CRF (SNK= 0.005) were all significantly different from the infusion of either vehicle or ASV-30 prior to the infusion of 100 ng CRF. There was a gradual, unexplained decline in baseline after the infusion of antalarmin 10 min prior to the infusion of 100 ng CRF into the dorsal raphé, resulting in significant differences at post 100 and post 120 from all other treatment groups (SNK P < 0.05). No effect on accumbal 5-HT levels over time were observed after the infusion of antalarmin (F8, 24 = 1.57, p = 0.187) or the infusion of vehicle for antalarmin (F8, 32 = 1.013, p = 0.447) without the presence of CRF into the dorsal raphé nucleus.
Figure 3. Infusion of a CRF1 Antagonist into the dorsal Raphé nucleus Blocks the Effects of 100 ng CRF Infusion into the dorsal Raphé.
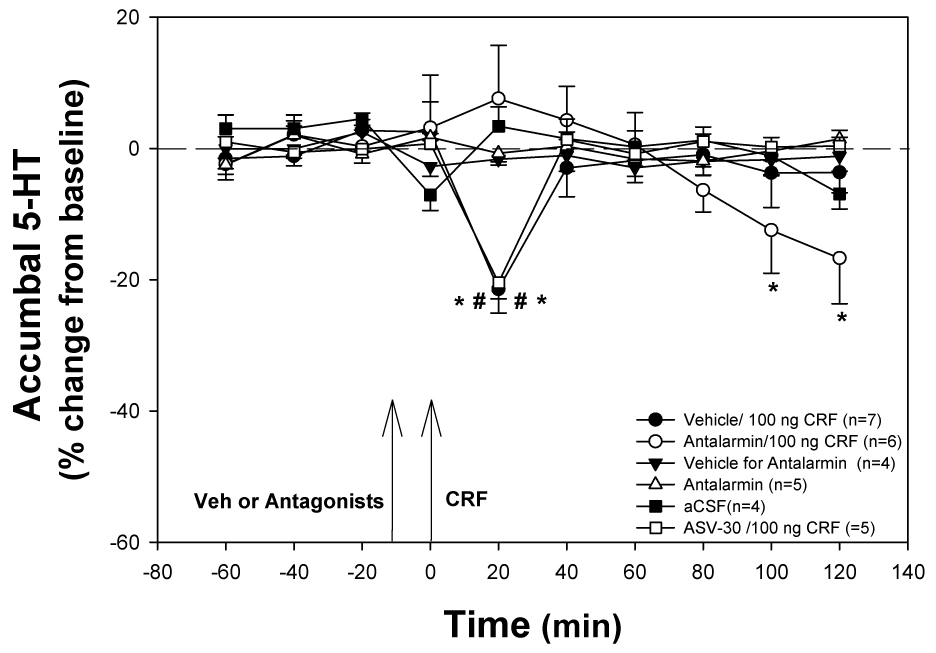
Infusion of 100 ng CRF into the dorsal raphé nucleus (in the absence of the CRF1 antagonist antalarmin) resulted in a significant 5-HT decrease in the nucleus accumbens (* significant compared to control groups; # significant difference over time; P<0.05). The effect of 100 ng CRF on accumbal 5-HT levels was not affected by infusion of the CRF2 antagonist (ASV-30) into the dorsal raphé nucleus 10 min prior to the infusion of 100 ng CRF. In contrast, infusion of antalarmin, a CRF1 receptor antagonist, into the dorsal raphé nucleus completely blocked the 100 ng CRF effect. No effects on accumbal 5-HT levels were observed after infusion of vehicle or antalarmin alone in the absence of CRF. Arrows indicate time of intra-dorsal raphé injection: first arrow represents infusion of receptor antagonist or vehicle, and the second arrow represents infusion of CRF.
Similarly, prior infusion of the CRF2 receptor antagonist, ASV-30, into the dorsal raphé nucleus blocked the 500 ng CRF-induced increase in accumbal 5-HT levels (F9, 72 = 3.579; Fig. 4). There was a significant effect of treatment (F5, 252 = 3.047, P = 0.026), time (F9, 252 = 4.892, P < 0.001), and treatment × time interaction (F45, 252 = 2.984, P < 0.001). As in the first experiment, infusion of 500 ng CRF preceded by infusion of the vehicle for the CRF2 receptor antagonist, stimulated a significant increase at 20 min (F9, 54 = 4.38, P < 0.001; Dunnett’s q 54, 10 = 4.23, P < 0.05). Similarly, infusion of 500 ng CRF in the dorsal raphé nucleus following infusion of the CRF1 receptor antagonist (antalarmin) resulted in a significant increase in accumbal 5-HT levels at 20 min (F9, 45 = 26.514, P < 0.001; Dunnett’s q 45, 10 = 12.158, P < 0.05). There was however, a gradual but significant (F9, 72 = 3.579, P < 0.001) decline of baseline at the final sampling time points (Post 100 min Dunnett’s q 72, 10 = 3.107, P < 0.05; Post 120 min Dunnett’s q 72, 10 = 3.169, P < 0.05) in the ASV-30 with CRF group, which were not significantly different from the same time points when compared to the ASV-30 alone or vehicle treated groups. Due to the gradual decline in baseline observed in the ASV-30 prior to the infusion of 500 ng group, a significant difference was observed at time points post 100 (SNK P=0.041) and post 120 (SNK P=0.022) when compared to prior infusion of antalarmin into the dorsal raphé nucleus. No significant effects on accumbal 5-HT levels across time were observed when vehicle (SNK P = 0.061) or ASV-30 (SNK P = 0.589) were infused alone into the dorsal raphé nucleus in the absence of CRF.
Figure 4. Infusion of a CRF2 Antagonist into the dorsal Raphé nucleus Blocks the Effects of 500 ng CRF Infused into the dorsal Raphé.
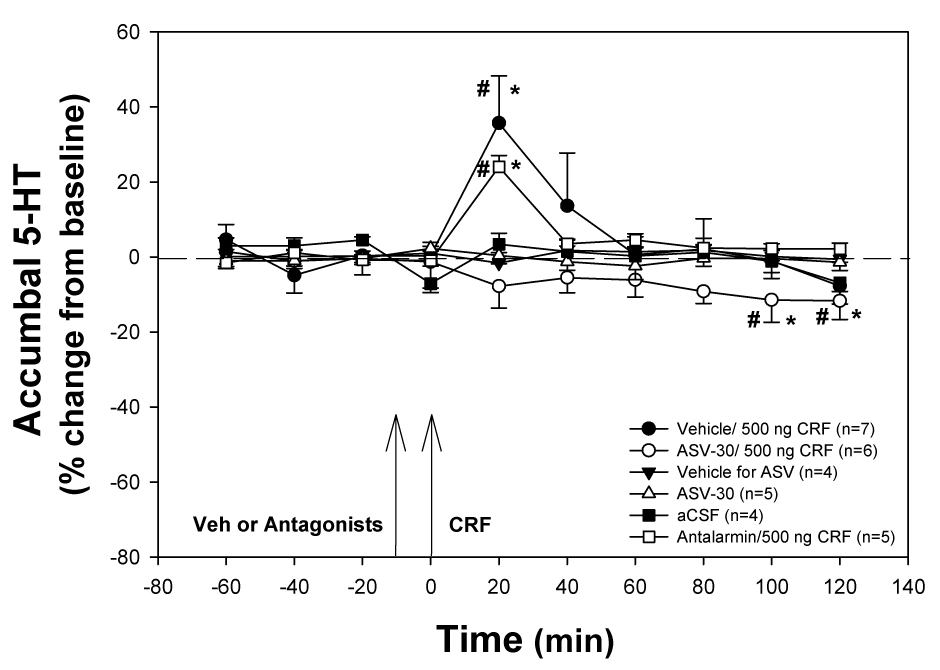
Infusion of 500 ng CRF into the dorsal raphé nucleus (in the absence of the CRF2 antagonist ASV-30) resulted in a significant increase in accumbal 5-HT (* significant compared to control groups; # significant difference over time; P<0.05). The effect of 500 ng CRF on accumbal 5-HT levels was not affected by infusion of the CRF1 antagonist (antalarmin) into the dorsal raphé nucleus 10 min prior to the infusion of 500 ng CRF. In contrast, infusion of ASV-30, blocked the effects of 500 ng CRF into the dorsal raphé nucleus. No effects on accumbal 5-HT levels were observed after infusion of vehicle or ASV-30 alone in the absence of CRF. Arrows indicate time of intra-dorsal raphé injection: first arrow represents infusion of receptor antagonist or vehicle, and the second arrow represents infusion of CRF.
4. Discussion
Our results demonstrate for the first time that CRF infused into the dorsal raphé nucleus alters 5-HT release in the nucleus accumbens. Infusion of 100 ng CRF into the dorsal raphé decreases extracellular 5-HT concentrations in the nucleus accumbens (Fig. 2). In contrast, a higher dose of 500 ng CRF infused into the dorsal raphé resulted in an increase in accumbal 5-HT. Furthermore, the results suggest that the decrease in accumbal 5-HT release induced by 100 ng CRF is dependent on CRF1 receptors in the dorsal raphé nucleus while the increase in 5-HT release in the nucleus accumbens following 500 ng CRF infusions into the dorsal raphé appears to be mediated by CRF2 activation.
The observation that CRF has receptor-dependent opposite effects on 5-HT release may explain seemingly inconsistent findings from other studies that infused CRF into the dorsal raphé nucleus and measured 5-HT neuronal activity (Kirby et al., 2000; Lowry et al., 2000) or 5-HT release and activity in terminal sites (Kirby et al., 2000; Kirby et al., 2003; Pernar et al., 2004; Price et al., 1998; Price and Lucki, 2001; Thomas et al., 2003). Previous studies suggest that lower doses of CRF (intra-dorsal raphé 3–10 ng; icv 0.1–1 µg; Kirby et al., 2000; Price et al., 1998) inhibit 5-HT release in the lateral septum and striatum, whereas higher doses were shown to either increase (intra-dorsal raphé 1µg CRF; Price and Lucki, 2001) or have no effect on 5-HT concentrations. Our results combined with those of previous studies suggest that the opposing effects of CRF on 5-HT terminal release are probably due to variable concentrations of CRF acting differentially on CRF1 and CRF2 receptors to inhibit or excite 5-HT neuronal cell firing.
Opposing actions by varying doses of CRF suggest mediation by different CRF receptors in the dorsal raphé nucleus (Hammack et al., 2003b; Hammack et al., 2003a; Kirby et al., 2000; Pernar et al., 2004). The dorsal raphé nucleus contains an unusually high density of CRF1 and CRF2 receptors. Decreased accumbal 5-HT release elicited by a lower CRF dose was dependent upon CRF1 receptors, demonstrated by the CRF1 receptor antagonist (antalarmin) blocking the effect of 100 ng CRF delivered to the dorsal raphé, whereas the CRF2 receptor antagonist had no effect (Fig. 3). Similarly, dorsal raphé firing rates inhibited by CRF, were reversed by a selective CRF1 receptor antagonist (Kirby et al., 2000). Furthermore, a higher dose of CRF in the dorsal raphé nucleus stimulated increased 5-HT release in the nucleus accumbens as a result of CRF2 activation (Fig. 4). That is, the stimulatory effect of 500 ng CRF infused into the dorsal raphé nucleus on accumbal 5-HT release was inhibited by a CRF2 receptor antagonist (ASV-30) but was not affected by a CRF1 receptor antagonist.
It is possible that the divergent effects of CRF we observed are mediated by neuroanatomical heterogeneity of CRF1 and CRF2 receptor subtypes associated with regional specificity of function of the raphé (Lowry et al., 2000; Lowry, 2002; Summers et al., 2003a; Valentino et al., 2001). Regional specificity in raphé may yield direct control of terminal 5-HT output, such as from the lateral wings of the dorsal raphé nucleus to the nucleus accumbens (Van Bockstaele et al., 1993). Of course, in our model, presumably CRF binds to both CRF1 and CRF2 receptors regardless of the quantity released or the dosage delivered, and the effect is then modulated by the affinity of the receptor type, which is greater for CRF binding to the type 1 receptor (Grigoriadis et al., 1996a; Grigoriadis et al., 1996b). At lower concentrations, primarily CRF1 receptors are activated by CRF due to their higher affinity for CRF. Higher concentrations of CRF are required to bind and stimulate CRF2 receptor activity, and activation of CRF2 receptors may mask CRF1 receptor function by some unidentified mechanism. The possible mechanisms could include inhibitory receptor-to-receptor interactions or perhaps more likely inhibitory effects from inhibition by other neurotransmitter systems within the raphé. The raphé includes numerous GABAergic interneurons, particularly in the lateral wings, and also catecholaminergic input (Day et al., 2004). These inhibitory systems may have reduced accumbal 5-HT release observed in the slightly declining baseline of receptor antagonist treated preparations. However, only animals treated with receptor antagonist followed by CRF showed this decline suggesting an inhibitory feedback mechanism. In addition, the raphé also produces CRF binding proteins (Peto et al., 1999; Potter et al., 1992). These binding proteins may be involved in modulating cellular events that occur following CRF receptor binding, such as processing and degradation of CRF and/or ligand-receptor complexes (Peto et al., 1999). Therefore, direct or indirect mechanisms may be operational when concentration differences of CRF influence the output of 5-HT.
A decrease in serotonergic activity in the nucleus accumbens has been associated with increased aggression (Giacalone et al., 1968; Haney et al., 1990; Welch and Welch, 1968) and impulsivity (Cardinal et al., 2001; Tobin and Logue, 1994). In contrast, an increase in serotonergic activity in the nucleus accumbens is associated with stressful conditions (Petty et al., 1994; Rueter and Jacobs, 1996; Wilkinson et al., 1996), such as in the forced swim test (Amat et al., 1998a; Amat et al., 1998b; Kirby et al., 1997). In addition behavioral states or processes mediated by increased accumbal dopamine include salient events such as reward, memory, motivation, and hedonia (Ikemoto and Panksepp, 1999; Kalivas, 1993; Pennartz et al., 1994; Robinson and Berridge, 1993; Zangen et al., 2001), that may also be influenced by 5-HT. The nucleus accumbens has been shown to regulate impulsive choice through interactions between dopamine and 5-HT (Winstanley et al., 2005). Therefore, CRF effects on the raphé serotonergic output to nucleus accumbens has the potential for modifying a suite of behaviors that involve anticipation, impulsivity, motivation, reward, aggression and/or depression.
The results of our experiments suggest that different concentrations of CRF yield differential CRF receptor binding in the dorsal raphé nucleus. This implies that CRF may act with a dual potential to elicit functional modulation of 5-HT in nucleus accumbens, thereby modifying behavior. Similarities between the effects of centrally administered CRF and the behavioral and physiological symptoms of human affective disorders, suggest an important role for CRF (Nemeroff, 1996; Nemeroff, 1998). Depressed individuals show elevated CRF levels and abnormal CRF functioning (Dunn and Berridge, 1990a; Dunn and Berridge, 1990b; Gold and Chrousos, 1999; Nemeroff, 1996; Nemeroff, 1998; Plotsky et al., 1998; Wong et al., 2000), as well as altered serotonergic functioning in the nucleus accumbens (Avgustinovich et al., 2004; Dremencov et al., 2005) which normalize in CSF after antidepressant treatment (Arborelius et al., 1999). The production of anxiety appears to be influenced by CRF2 receptors in the dorsal raphé via 5-HT projections from the dorsal raphé nucleus (Graeff et al., 1996) during anxiety-arousing circumstances such as exposure to uncontrollable stressors (Bakshi et al., 2002; Hammack et al., 2003b; Ho et al., 2001; Takahashi et al., 2001). The importance of our results is to suggest that CRF1 and CRF2 receptors can be differentially activated simply by controlling the level of CRF neuronal output. If this is the case, then neurotransmitter activity modified by CRF, such as 5-HT in terminal regions like the nucleus accumbens, can be up- or down-regulated by modulation of the dorsal raphé nucleus through changes in the activity of CRF projections from cell body regions such as the central amygdala to affect or fine tune behavior.
Acknowledgements
This work was supported by NIH grants NIDA R01 DA019921, NIMH R03 MH068303 and NIH COBRE P20 RR15567, but is solely the responsibility of the authors and does not necessarily represent the official views of NIH. We would also like to thank Michael Watt, Pat Ronan and Nicholas Mouw for their valuable contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Amato JL, Bankson MG, Yamamoto BK. Prior Exposure to Chronic Stress and MDMA Potentiates Mesoaccumbens Dopamine Release Mediated by the 5-HT(1B) Receptor. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301174. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Alekseenko OV, Bakshtanovskaia IV, Koriakina LA, Lipina TV, Tenditnik MV, Bondar' NP, Kovalenko IL, Kudriavtseva NN. Dynamic changes of brain serotonergic and dopaminergic activities during development of anxious depression: experimental study. Usp. Fiziol. Nauk. 2004;35:19–40. [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin- releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J. Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Twining C, Schmid MJ, Der-Avakian A, Watkins LR, Maier SF. Stress potentiation of morphine-induced dopamine efflux in the nucleus accumbens shell is dependent upon stressor uncontrollability and is mediated by the dorsal raphe nucleus. Neuroscience. 2004;126:705–715. doi: 10.1016/j.neuroscience.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Sprouse JS, Aghajanian GK, Roth RH. Sub-picogram determination of serotonin using HPLC with electrochemical detection for microdialysis studies of serotonin release. Adv. Exp. Med. Biol. 1991;294:81–89. doi: 10.1007/978-1-4684-5952-4_7. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. Int. Clin. Psychopharmacol. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J. Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF. The role of serotonin in panic, anxiety and depression. Int. Clin. Psychopharmacol. 1998;13 Suppl 4:S1–S5. doi: 10.1097/00004850-199804004-00001. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, Yadid G. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology. 2005;48:34–42. doi: 10.1016/j.neuropharm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann. N. Y. Acad. Sci. 1990a;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990b;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur. J. Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Giacalone E, Tansella M, Valzelli L, Garattini S. Brain serotonin metabolism in isolated aggressive mice. Biochem. Pharmacol. 1968;17:1315–1327. doi: 10.1016/0006-2952(68)90069-5. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences; Proc. Assoc. Am. Physicians; 1999. pp. 22–34. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann. N. Y. Acad. Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol. Pharmacol. 1996a;50:679–686. [PubMed] [Google Scholar]
- Grigoriadis DE, Lovenberg TW, Chalmers DT, Liaw C, De Souze EB. Characterization of corticotropin-releasing factor receptor subtypes. Ann. N. Y. Acad. Sci. 1996b;780:60–80. doi: 10.1111/j.1749-6632.1996.tb15112.x. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav. Brain Res. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J. Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J. Neurosci. 2003b;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Noda Y, Kream R, Miczek K. Regional serotonin and dopamine activity; sensitivity to amphetamine and aggressive behavior in mice. Aggressive Behav. 1990;16:259–270. [Google Scholar]
- Harvey AT, Hennessy MB. Corticotropin-releasing factor modulation of the ultrasonic vocalization rate of isolated rat pups. Brain Res. Dev. Brain Res. 1995;87:125–134. doi: 10.1016/0165-3806(95)00064-k. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res. Mol. Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Westin TM, Miner HM, Johnson PL, Summers CH, Renner KJ. GABAergic drugs alter hypothalamic serotonin release and lordosis in estrogen-primed rats. Brain Res. 2002;946:96–103. doi: 10.1016/s0006-8993(02)02867-6. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–196. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacol. Biochem. Behav. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res. Brain Res. Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin- containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed. Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994a;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994b;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J. Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J. Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo PE, Lorang M, Yeganeh M, Rodriguez dF, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Ionic composition of microdialysis perfusing solution alters the pharmacological responsiveness and basal outflow of striatal dopamine. J. Neurochem. 1989;53:652–654. doi: 10.1111/j.1471-4159.1989.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol. Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Nemeroff CB. The neurobiology of depression. Sci. Am. 1998;278:42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd ed. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC. Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J. Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto CA, Arias C, Vale WW, Sawchenko PE. Ultrastructural localization of the corticotropin-releasing factor-binding protein in rat brain and pituitary. J. Comp Neurol. 1999;413:241–254. [PubMed] [Google Scholar]
- Petty F, Kramer G, Wilson L, Jordan S. In vivo serotonin release and learned helplessness. Psychiatry Res. 1994;52:285–293. doi: 10.1016/0165-1781(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)- binding protein predicts multiple sites and modes of interaction with CRF. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J. Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci. Res. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roy A, Linnoila M. Suicidal behavior, impulsiveness and serotonin. Acta Psychiatr. Scand. 1988;78:529–535. doi: 10.1111/j.1600-0447.1988.tb06380.x. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, III, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Soubrie P. Serotonergic neurons and behavior. J Pharmacol. 1986;17:107–112. [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. J. Neuroendocrinol. 2003a;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Summers CH, Summers TR, Moore MC, Korzan WJ, Woodley SK, Ronan PJ, Höglund E, Watt MJ, Greenberg N. Temporal patterns of limbic monoamine and plasma corticosterone response during social stress. Neuroscience. 2003b;116:553–563. doi: 10.1016/s0306-4522(02)00708-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain Res. 2003;960:201–208. doi: 10.1016/s0006-8993(02)03882-9. [DOI] [PubMed] [Google Scholar]
- Tobin H, Logue AW. Self-control across species (Columba livia, Homo sapiens, and Rattus norvegicus) J Comp Psychol. 1994;108:126–133. doi: 10.1037/0735-7036.108.2.126. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J. Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Welch AS, Welch BL. Effect of stress and para-chlorophenylalanine upon brain serotonin, 5-hydroxyindoleacetic acid and catecholamines in grouped and isolated mice. Biochem. Pharmacol. 1968;17:699–708. doi: 10.1016/0006-2952(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Humby T, Killcross S, Robbins TW, Everitt BJ. Dissociations in hippocampal 5-hydroxytryptamine release in the rat following Pavlovian aversive conditioning to discrete and contextual stimuli. Eur. J Neurosci. 1996;8:1479–1487. doi: 10.1111/j.1460-9568.1996.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. U. S. A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]


