Summary
The Cdc25A phosphatase positively regulates cell cycle transitions; is degraded by the proteosome throughout interphase and in response to stress; and is overproduced in human cancers. The kinases targeting Cdc25A for proteolysis during early cell cycle phases have not been identified and mechanistic insight into the cause of Cdc25A overproduction in human cancers is lacking. Here, we demonstrate that glycogen synthase kinase-3β (GSK-3β) phosphorylates Cdc25A to promote its proteolysis in early cell cycle phases. Phosphorylation by GSK-3β requires priming of Cdc25A and this can be catalyzed by polo-like kinase 3 (Plk-3). Importantly, a strong correlation between Cdc25A overproduction and GSK-3β inactivation was observed in human tumor tissues indicating that GSK-3β inactivation may account for Cdc25A overproduction in a subset of human tumors.
Significance
Our study identifies two Cdc25A regulators and provides a molecular mechanism to account for Cdc25A overproduction in human cancers. A therapeutic strategy being developed to treat p53-deficient cancers combines DNA damaging agents with drugs, such as UCN-01, to induce Cdc25A accumulation. This results in preferential killing of p53-deficient tumors because all DNA damage checkpoints are eliminated in these tumors. Our study identifies GSK-3β and Plk-3 as potential therapeutic targets whose inhibition may induce checkpoint bypass in tumors by blocking Cdc25A proteolysis. Furthermore, tumors from patients enrolled on clinical trials that depend on Cdc25A stabilization to induce checkpoint bypass, should be tested for the integrity of the GSK-3β/Plk-3/Cdc25A pathway in order to correlate Cdc25A levels with tumor responses.
Introduction
Cancer cells frequently overproduce proteins that positively regulate the cell division cycle in order to maintain their proliferative capacity. The Cdc25A protein phosphatase is an example of a key cell cycle regulator that is overproduced many human cancers (Kristjansdottir and Rudolph, 2004). Cdc25A drives the cell cycle forward by activating cyclin-dependent protein kinases (Cdks). Cells regulate the activity and abundance of Cdc25A through transcriptional activation, reversible phosphorylation, protein-protein interactions, and ubiquitin-mediated proteolysis (Bernardi et al., 2000; Boutros et al., 2006; Busino et al., 2004; Chen et al., 2003). In addition to tightly controlling Cdc25A during a normal cell cycle, cells rapidly shunt Cdc25A for ubiquitin-mediated proteolysis when they are exposed to genotoxic stress (Mailand et al., 2000; Molinari et al., 2000). By eliminating Cdc25A, cells are able to temporarily arrest the cell division cycle to allow time for DNA repair.
Cdc25A ubiquitination is mediated by two distinct E3 ubiquitin ligases (Busino et al., 2004; Busino et al., 2003; Donzelli et al., 2002; Jin et al., 2003; Ray et al., 2005). APC/CCdh1 targets Cdc25A for destruction during mitotic exit and early G1 whereas SCFβTrCP targets Cdc25A during G1, S and G2. The importance of Cdc25A regulation is underscored by the observation that its overproduction leads to accelerated entry of cells into both S-phase (Blomberg and Hoffmann, 1999) and mitosis (Molinari et al., 2000) and failure to regulate Cdc25A during a checkpoint response causes bypass of DNA damage and replication checkpoints, resulting in enhanced DNA damage (Bartek and Lukas, 2001; Falck et al., 2001; Mailand et al., 2000; Molinari et al., 2000; Zhao et al., 2002). Thus, the order and fidelity of cell cycle events in mammals is intimately linked to the Cdc25A-regulatory pathway.
Phosphorylation serves to both promote and protect Cdc25A from ubiquitin-mediated proteolysis. Phosphorylation of Cdc25A on S76 is required for its ubiquitin-mediated proteolysis (Donzelli et al., 2004; Goloudina et al., 2003). In addition, phosphorylation of serines 82 and 88 regulates Cdc25A destruction by facilitating recognition of Cdc25A by SCFβTrCP (Busino et al., 2003; Jin et al., 2003). In contrast, phosphorylation of Cdc25A on serines 18 and 116 prevents proteasome-mediated degradation of Cdc25A during mitosis (Mailand et al., 2002).
Cdc25A is regulated by several protein kinases. Cdk2 activates Cdc25A during the G1/S transition (Hoffmann et al., 1994). Cdk1 phosphorylates Cdc25A during mitosis on serine 18 and 116 to uncouple it from ubiquitin-mediated proteolysis (Mailand et al., 2002). In turn, Cdc25A accumulation enhances Cdk1 activation. The Chk1 protein kinase phosphorylates Cdc25A in S and G2 under normal conditions and in response to checkpoint activation (Zhao et al., 2002). Chk1 phosphorylates Cdc25A on S76 to target it for ubiquitin-mediated proteolysis and on T507 to prevent Cdc25A from activating Cdk1 in S and G2 (Chen et al., 2003; Donzelli et al., 2004; Falck et al., 2001; Goloudina et al., 2003; Hassepass et al., 2003; Sorensen et al., 2003; Zhao et al., 2002). Cdc25A is also targeted for ubiquitin-mediated proteolysis during G1 but the kinase(s) that regulate its ubiquitination in G1 has not been identified.
GSK-3β is a serine/threonine protein kinase that operates in G1 to receive input from several signaling and developmental pathways (Cohen and Frame, 2001; Doble and Woodgett, 2003). GSK-3β phosphorylates cyclin D1 and c-Myc to promote their destruction in G1. Thus, signaling pathways that inactivate GSK-3β such as the PI-3K/AKT and MAPK pathways promote cell cycle entry by stabilizing proteins such as cyclin D1 and c-Myc (Diehl et al., 1998; Doble and Woodgett, 2003). Here we demonstrate that GSK-3β regulates the ubiquitin-mediated proteolysis of Cdc25A during early phases of the cell cycle and overproduction of Cdc25A strongly correlates with GSK-3β inactivation in human tumors.
Results
Identification of GSK-3β as a potential regulator of Cdc25A
Transcriptional activation of Cdc25A begins in G1 and Cdc25A protein levels begin to rise as cells advance towards S-phase. However, Cdc25A accumulation is counterbalanced by its ubiquitin-mediated proteolysis, which occurs throughout the G1-, S- and G2-phases of the cell division cycle (Busino et al., 2004). The Chk1 protein kinase is active during the S- and G2- phases of the cell cycle and contributes to the ubiquitin-mediated proteolysis of Cdc25A during these two phases (Busino et al., 2004; Busino et al., 2003; Jazayeri et al., 2006). However, the protein kinases that regulate the turnover of Cdc25A during mid- to late-G1 have not been identified. In searching for protein kinases that might fulfill this role, we identified kinases that are active in the G1 phase of the cell cycle and we examined Cdc25A to determine if it contained consensus phosphorylation sites for the G1-active kinase(s). GSK-3β met both criteria. GSK-3β activity is highest in quiescent and G1-phase cells and decreases as cells progress through the cell cycle (Cohen and Frame, 2001; Doble and Woodgett, 2003). An examination of the amino acid sequence of Cdc25A revealed that S76, a key regulatory site that is required for the ubiquitin-mediated proteolysis of Cdc25A, conforms to a minimal GSK-3β consensus motif.
The classic GSK-3β consensus motif is (S/T)XXX(S/T)* where X is any amino acid and potential GSK-3β phosphorylation sites are underlined (Cohen and Frame, 2001; Doble and Woodgett, 2003). GSK-3β often requires that its substrates first be phosphorylated at a serine or threonine residue in the +4 position relative to the GSK-3β phosphorylation site. This residue is referred to as the priming site (indicated by an asterisk). Of the residues known to be required for the ubiquitin-mediated proteolysis of Cdc25A (S76, S82 and S88) only sequences surrounding and inclusive of S76 conform to a GSK-3β motif (S76SEST80*).
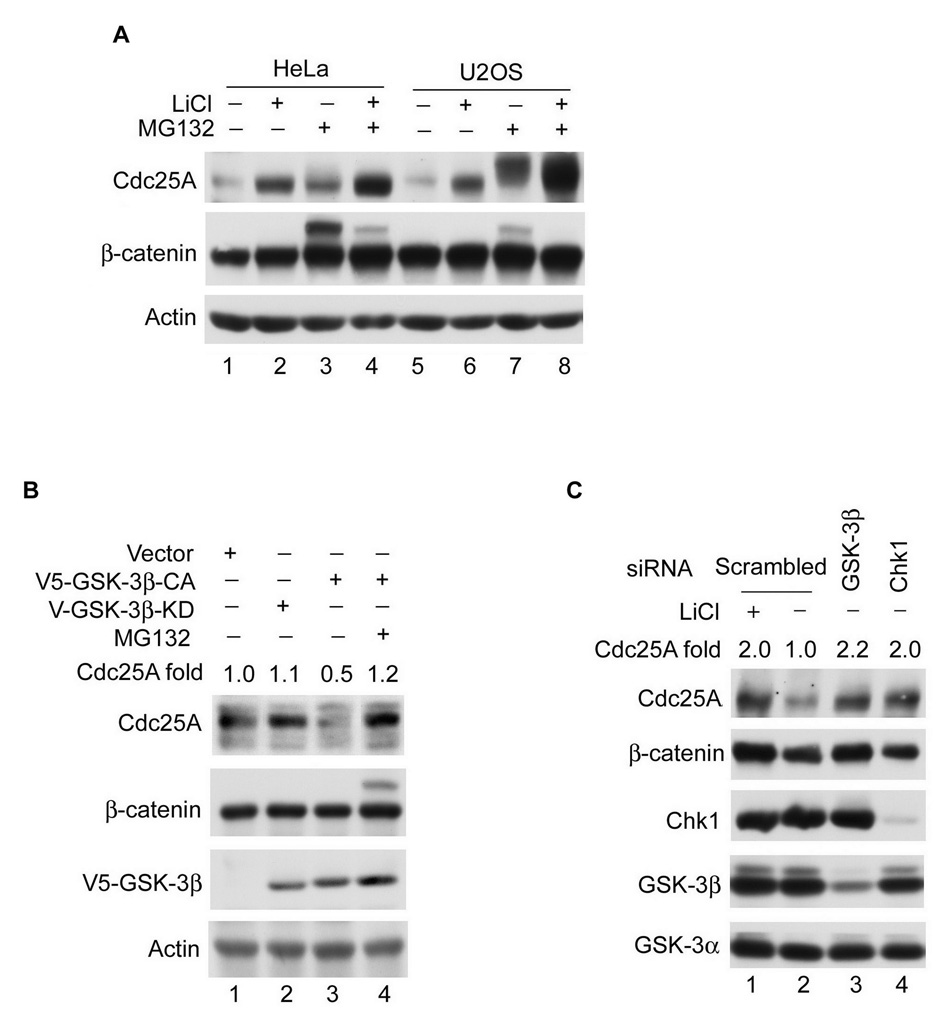
Several experiments were performed to determine if GSK-3β negatively regulates Cdc25A in vivo. First, HeLa and U2OS cells were treated with LiCl, a potent GSK-3β inhibitor (Yin et al., 2006). As seen in Figure 1A, endogenous Cdc25A accumulated in cells treated with either LiCl (lanes 2, 6) or MG132 (lanes 3, 7), an inhibitor of the 26S proteasome. An even greater stabilization of endogenous Cdc25A was observed when cells were treated with both LiCl and MG132 (lanes 4, 8). Note that endogenous β-catenin, a downsteam effector of GSK-3β, was also stabilized by LiCl-treatment (Yin et al., 2006). Endogenous Cdc25A also accumulated in cells treated with other GSK-3β specific inhibitors, including 1-Azakenpaullone, SB216763 and SB415286 (data not shown) (Coghlan et al., 2000; Kunick et al., 2004). Accumulation of Cdc25A was also observed in normal human diploid fibroblasts (IMR90) and several cancer cell lines, including MDA-MB-231, H460, A549, MCF-7, Saos2 and BT549 (Figure S1A and data not shown). Ectopically-produced Cdc25A was also observed to accumulate in cells treated with LiCl, MG132 or both (Figure S1B). Next, endogenous Cdc25A levels were monitored in cells overproducing constitutively-active (CA) or kinase dead (KD) GSK-3β. As seen in Figure 1B, Cdc25A protein levels were reduced in cells expressing GSK-3β-CA (lane 3) relative to cells expressing either vector (lane 1) or kinase-inactive GSK-3β (lane 2), and Cdc25A levels were restored in cells treated with MG132 (lane 4). Experiments were also carried out using GSK-3β- and Chk1-specific siRNAs, with Chk1-siRNAs serving as a positive control (Zhao et al., 2002). As seen in Figure 1C, knockdown of GSK-3β (lane 3) stabilized endogenous Cdc25A, as did knockdown of Chk1 (lane 4). Knockdown of GSK-3β did not affect levels of GSK-3α (lane 3) demonstrating that stabilization of Cdc25A was due to loss of GSK-3β rather than to loss of both GSK-3β and GSK-3α. Cdc25A levels rise as cells advance through the cell division cycle (Busino et al., 2000). FACs analysis was performed to ensure that GSK-3β knockdown did not cause a significant fraction of the cells to move into and remain in late cell cycle phases. If this were the case the observed stabilization of Cdc25A could be due to an indirect effect of cell cycle position rather than a direct effect of GSK-3β loss. Under the time course of the experiment (48 h), knockdown of GSK-3β and Chk1 did not significantly alter cell cycle profiles (Figure S2A–D), demonstrating that Cdc25A stabilization was not an indirect effect of cell cycle alterations. However, GSK-3β inactivation in synchronized populations of G1 cells, led to an accelerated S-phase entry (Figure 6 and S5). Taken together, these results suggest that GSK-3β negatively regulates Cdc25A abundance in vivo. Myc, a downstream target of GSK-3β, has been reported to positively regulate the ubiquitin-mediated proteolysis of Cdc25A (Bernardi et al., 2000). However, GSK-3β inhibition did not change Myc levels under our experimental conditions ruling out the possibility that the stabilization of Cdc25A observed upon GSK-3β inhibition is mediated by increased levels of Myc (Figure S2E).
Figure 1. Cdc25A protein levels are negatively regulated by GSK-3β in vivo.
(A) Asynchronously growing HeLa and U2OS cells were untreated or were incubated with 10 mM LiCl, 10 µM MG132, or both for 4 h (n=3). The indicated proteins were analyzed by Western blotting. (B) H460 cells transfected with plasmids encoding the indicated proteins for 20 h, were untreated or were incubated with 10 µM MG132 for 4 h (n=2), and analyzed by Western blotting. (C) HeLa cells transfected with scrambled siRNA for 44 h were cultured in the absence or presence of 10 mM LiCl for 4 h (n=3). Alternatively cells were transfected with siRNAs specific for GSK-3β or Chk1 for 48 h and the indicated proteins were analyzed by Western blotting.
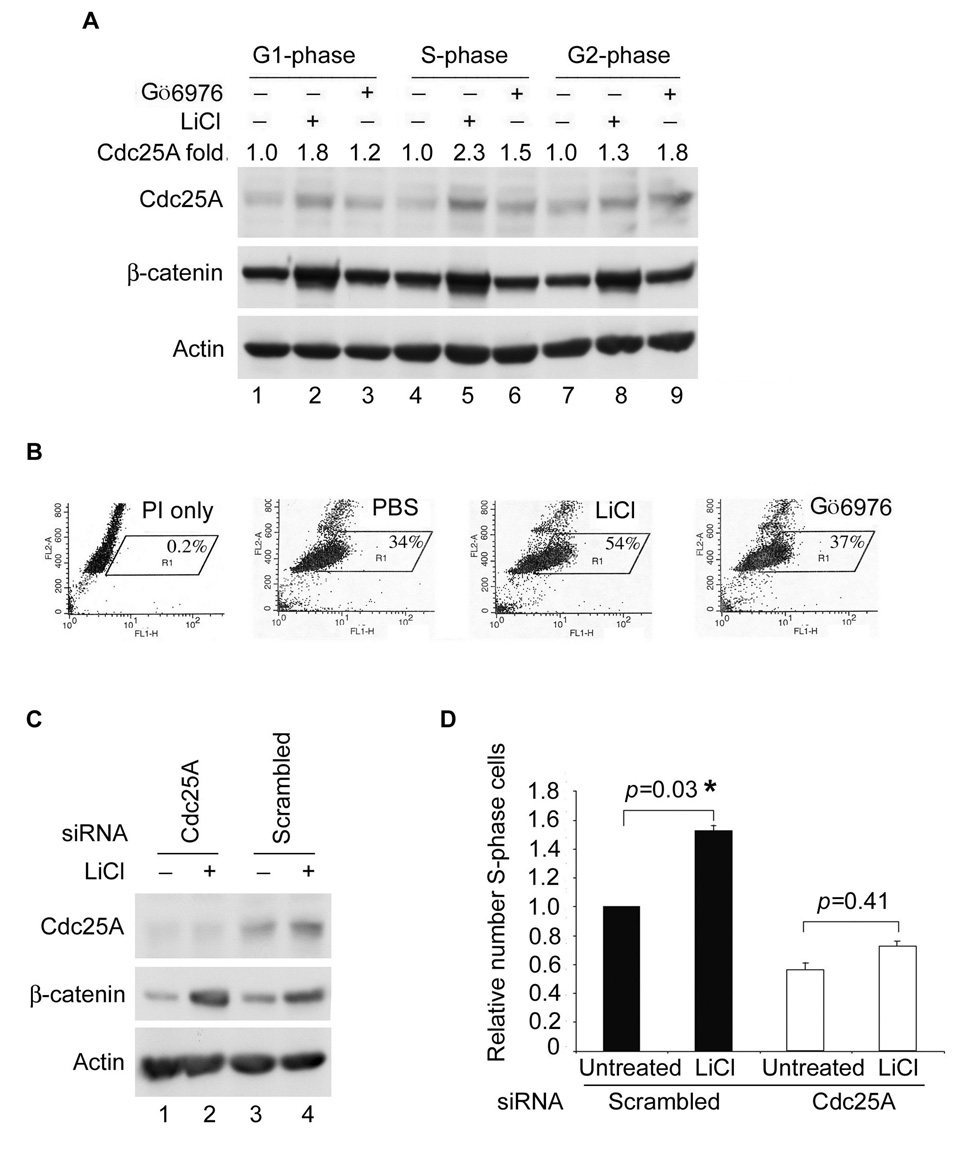
Figure 6. Cell Cycle Regulation of Cdc25A by GSK-3β and Chk1.
(A) HeLa cells synchronized at the G1/S border using a double thymidine block and release protocol were released for 2.5 h (S phase, lanes 4–6), 6 h (G2 phase, lanes 7–9) or 11 h (G1 phase, lanes 1–3). Cells were incubated with either PBS, 20 mM LiCl, or 100 nM Gö6976 for 2 h prior to harvesting (n=2). Cells were analyzed by Western blotting in (A) and by flow cytometry (Figure S5A). Relative levels of Cdc25A were determined from the Western blot using ImageJ program and are indicated above the blot. (B) HeLa cells were synchronized at the G1/S-border by a double-thymidine block and release protocol. Synchronized cells were untreated or were incubated with 20 mM LiCl, or 100 nM Gö6976 immediately after release from the block. One hour later, cells were cultured in the presence of BrdU and harvested 1 h later. Cells were stained with PI and for BrdU. The percentage of BrdU positive cells was determined by flow cytometry, n = 3. (C, D). HeLa cells were transfected with control siRNAs or siRNAs specific for Cdc25A for 24 h. Cells were synchronized as described in (B) and some cells were lysed for Western blotting (C) and the remaining cells were analyzed as described in B (D). A two-tailed t-test was performed for comparisons between groups. Standard error of the mean (SEM) for triplicate samples is shown as error bars along the y axis. The “*” indicates statistically significant.
GSK-3β promotes the turnover of Cdc25A during an unperturbed cell cycle and in response to ionizing radiation
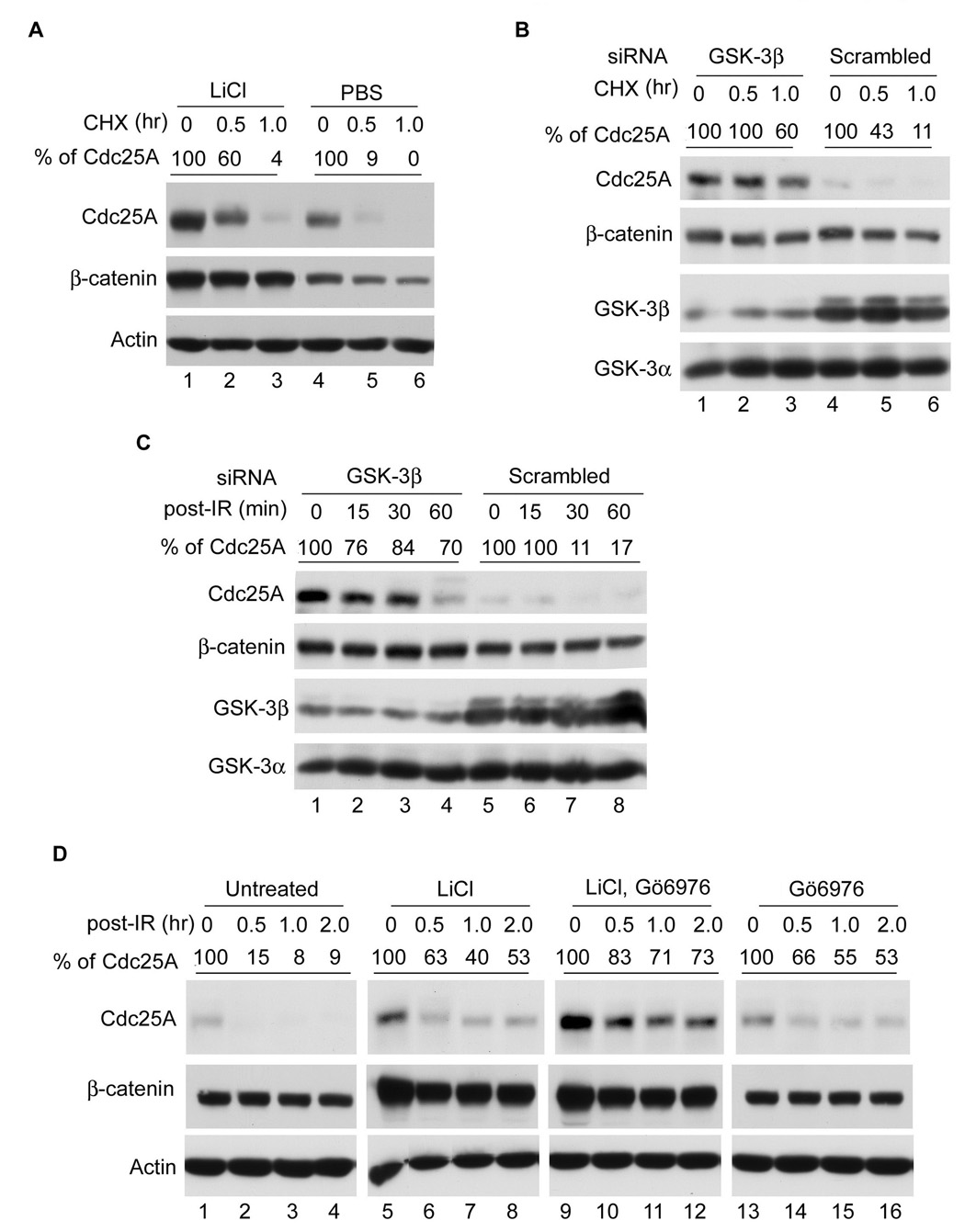
If GSK-3β promotes the turnover of Cdc25A in vivo, then treatment of cells with GSK-3β-specific inhibitors is expected to prolong the half-life of Cdc25A. As seen in Figure 2, both Cdc25A and β-catenin were stabilized in cells treated with either LiCl (panel A) or GSK-3β-specific siRNAs (panel B). Moreover, GSK-3β inhibition also prolonged the half-life of Cdc25A in cells exposed to ionizing radiation (IR) (Figure 2C, D). Thus, GSK-3β contributes to the turnover of Cdc25A both during an unperturbed cell cycle and in response to genotoxic stress. The half-life of Cdc25A was less than 30 min in cells with intact GSK-3β (panel A, lanes 4–6 and panel B, lanes 4–6). In contrast, the half-life of Cdc25A was prolonged in cells where GSK-3β was inhibited pharmacologically (panel A, lanes 1–3) or by knockdown with siRNAs (panel B, lanes 1–3). This was also true in irradiated cells (panels C and D). The contributions made by Chk1 and GSK-3β to Cdc25A degradation was also monitored in cells where each kinase was inhibited singly or simultaneously. LiCl was used to inhibit GSK-3β (lanes 5–8) and Gö6976 (Kohn et al., 2003) was used to inhibit Chk1 (lanes 13–16). As seen in Figure 2D (lanes 9–12), GSK-3β and Chk1 cooperate in regulating Cdc25A proteolysis in vivo both in the presence and absence of genotoxic stress.
Figure 2. GSK-3β promotes Cdc25A turnover during an unperturbed cell cycle and in response to IR.
(A) Asynchronously growing HeLa cells were treated with PBS or 10 mM LiCl for 4 h, and then 10 µg/ml cycloheximide (CHX) was added for the indicated times (n=3). Proteins were analyzed by Western blotting. (B) HeLa cells transfected with either GSK-3β siRNA or scrambled siRNA for 48 h were incubated in the presence of 10 µg/ml CHX for the indicated times (n=3). Proteins were analyzed by Western blotting. (C) HeLa cells were transfected with GSK-3β or scrambled siRNAs for 48 h (n=2), and cells were then mock-irradiated or were treated with 10 Gy IR and harvested at times indicated. Proteins were analyzed by Western blotting. (D) HeLa cells were untreated or were incubated with 10 mM LiCl, 100 nM Gö6976, or both for 4 h. Cells were then mock-irradiated or were treated with 10 Gy IR and harvested at times indicated (n=2). Proteins were analyzed by Western blotting. Relative levels of Cdc25A were determined from Western blots using the ImageJ program and are indicated above the respective blots.
GSK-3β interacts with Cdc25A
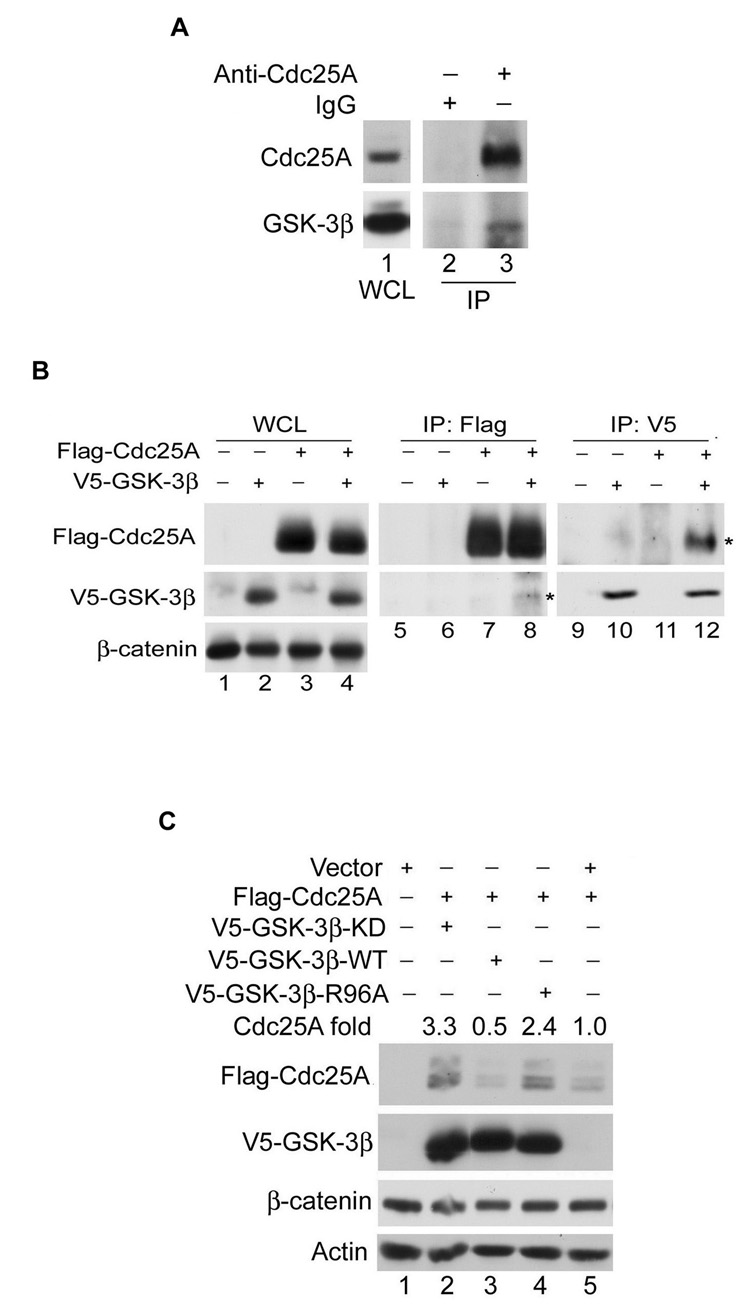
We next asked if complexes between endogenous Cdc25A and GSK-3β could be detected in vivo. As seen in Figure 3A, GSK-3β was present in Cdc25A immunoprecipitates (lane 3) but not in normal rabbit IgG precipitates (lane 2). In addition, interactions between ectopically produced Cdc25A and GSK-3β could also be detected in vivo (Figure 3B). These results demonstrate that Cdc25A and GSK-3β stably associate in vivo. In many cases the association between GSK-3β and its substrates requires that the substrate first be phosphorylated on a priming site (Cohen and Frame, 2001; Doble and Woodgett, 2003). Mutation of R96 to alanine has been shown to disrupt interactions between GSK-3β and those substrates that require priming. Flag-Cdc25A was co-produced with wild-type and mutant forms of GSK-3β. As seen in Figure 3C, Cdc25A levels were lower in cells overproducing wild-type GSK-3β (lane 3) relative to control cells (lane 5) but higher in cells overproducing kinase-inactive GSK-3β (lane 2) or the R96A mutant (lane 4). These results suggest that Cdc25A may be a substrate of GSK-3β and that GSK-3β prefers that Cdc25A first be phosphorylated on a priming site.
Figure 3. Interactions between Cdc25A and GSK-3β.
(A) MDA-MB-231 cells incubated with 10 µM MG132 for 4 h were lysed and either analyzed directly by Western blotting (lane 1) or were first incubated with rabbit anti-Cdc25A antibody (lane 3) or normal rabbit IgG (lane 2). Immunoprecipitates (IP) were analyzed by Western blotting (lanes 2, 3), n=2. WCL: whole cell lysate. (B) Lysates were prepared from HeLa cells transfected with the indicated proteins for 24 h. WCL were resolved directly by SDS-PAGE (lanes 1–4) or were first incubated with antibodies specific for either the Flag epitope (lanes 5–8) or the V5 epitope (lane 9–12). Precipitates were analyzed by Western blotting. Asterisks denote V5-GSK-3β and Flag-Cdc25A in the co-precipitates, n=2. (C) HeLa cells were co-transfected with the indicated plasmids for 24 h (n=2), and proteins were analyzed by Western blotting. Relative levels of Flag-Cdc25A are indicated.
Regulation of Cdc25A by GSK-3β and Plk-3 in vivo and in vitro
Sequences surrounding and inclusive of S76 (S76XXXT80*) conform to the minimal motif (S/TxxxS/T*) for the priming-required substrates of GSK-3β (Cohen and Frame, 2001; Doble and Woodgett, 2003). Interestingly, phosphorylation of Cdc25A on S76 but not S123 was reduced in cells treated with LiCl (Figure 4A, lane 3) and co-production of Cdc25A with constitutively active GSK-3β enhanced S76 phosphorylation (Figure 4B, lane 2). These data are consistent with the hypothesis that GSK-3β regulates S76 phosphorylation. Next, experiments were performed to determine if T80 served a priming function to enable phosphorylation of S76. An antibody that specifically recognizes Cdc25A when it is phosphorylated on T80 demonstrated that Cdc25A is phosphorylated on T80 in vivo (Figure 4C) and substitution of alanine for threonine at position 80 (T80A) severely curtailed phosphorylation of Cdc25A on S76 in vivo (Figure 4D, lane 3). In addition, although S76 phosphorylation was enhanced when Cdc25A was co-expressed with constitutively active GSK-3β in murine embryonic fibroblasts (MEFs) null for GSK-3β (Figure 4E, lane 2), enhanced phosphorylation of Cdc25A on S76 was not observed when the T80A mutant of Cdc25A was co-expressed with constitutively active GSK-3β in null MEFs (Figure 4E, lane 4). As expected, T80A bound less GSK-3β (Figure 4E, lane 4) than did WT Cdc25A (lane 2). The S76A and T80A mutants were relatively insensitive to co-expression with GSK-3β (Figure S3A) and to LiCl-treatment (Figure S3B). We conclude that S76 phosphorylation by GSK-3β is dependent upon prior phosphorylation of Cdc25A on T80. It is worth noting that S76 phosphorylation was still detected, albeit at a much lower level, when either wild-type Cdc25A (Figure 4E, lane 1) or the T80A mutant (Figure 4E, lane 3) were expressed in GSK-3β null MEFs. Importantly, S76 phosphorylation was observed to decrease when GSK-3β null MEFs were treated with the Chk1 inhibitor, Gö6976 (Kohn et al., 2003), suggesting that Chk1 contributes to S76 phosphorylation in cells null for GSK-3β (Figure S4A).
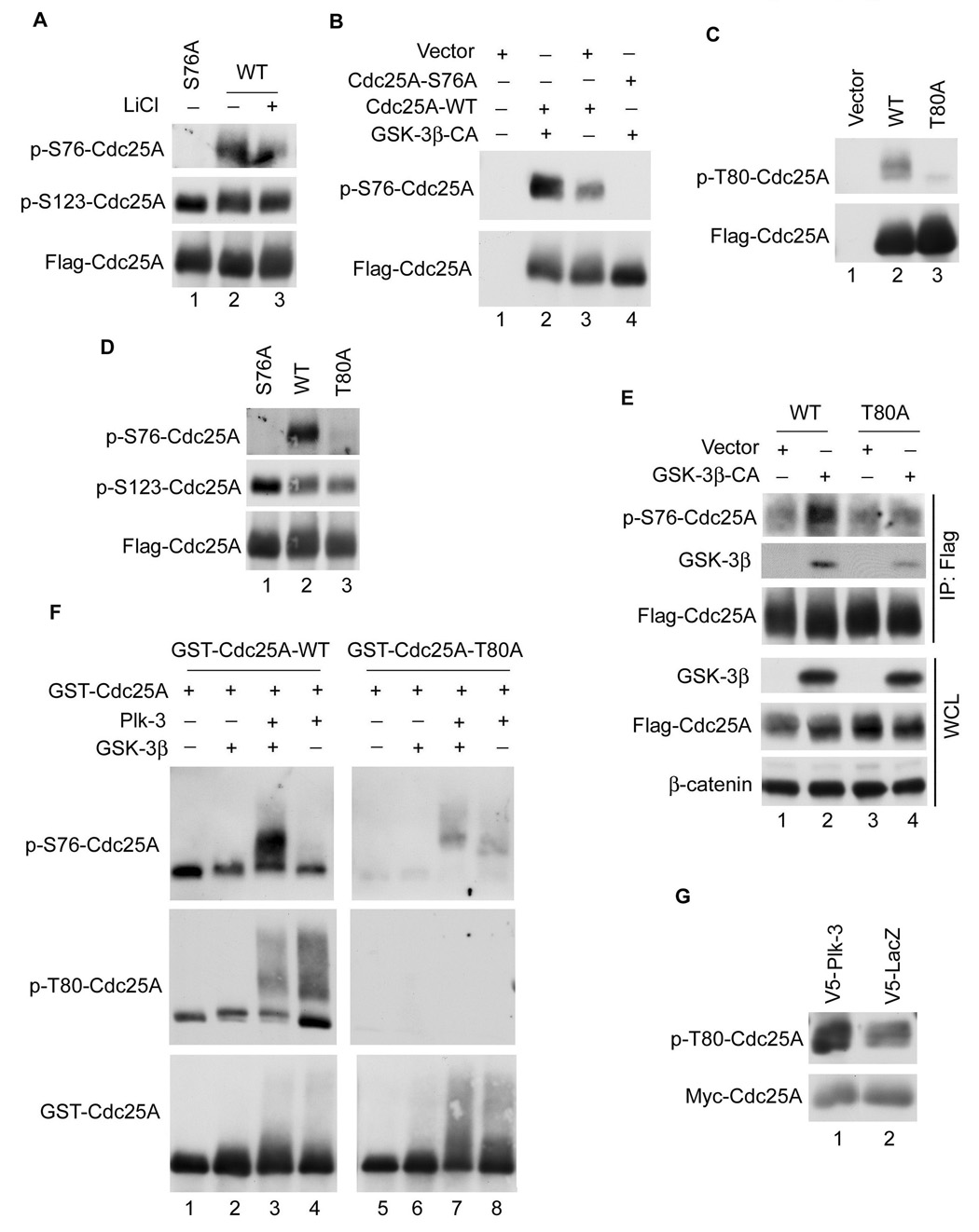
Figure 4. Regulation of Cdc25A by GSK-3β and Plk-3.
(A) U2OS cells transfected with Flag-tagged wild-type Cdc25A (WT) or S76A for 20 h were cultured in the absence or presence of 10 mM LiCl for 4 h. Tagged proteins were precipitated and analyzed by Western blotting using anti-p-S76-Cdc25A antibody (top panel), anti-p-S123-Cdc25A antibody (middle panel), or anti-Cdc25A antibody (bottom panel), n =2. (B) U2OS cells transfected with the indicated plasmids for 24 h were lysed and Cdc25A was precipitated with antibodies specific to the Flag tag. Precipitates were analyzed by Western blotting, n = 2. (C) HEK293 cells were transfected with the indicated plasmids for 24 h followed by Western blotting, n = 2. (D) U2OS cells were transfected with the indicated plasmids for 24 h and analyzed as described in (A), n = 2. (E) Immortalized GSK-3β null MEFs were transfected with plasmids encoding Flag-Cdc25A together with either empty vector or vector encoding HA-tagged constitutively active (CA) GSK-3β for 20 h. Cells were treated with MG132 for 4 h, lysed and analyzed directly by Western blotting (bottom 3 panels) or were incubated with Flag agarose prior to Western blotting (top 3 panels), n = 2. (F) Recombinant GST-Cdc25A was purified from bacteria and kinase reactions were performed in vitro in the absence or presence of purified Plk-3, GSK-3β, or both, as described in Experimental Procedures. Reaction products were analyzed by Western blotting with the indicated antibodies, n = 4. (G) HEK293 cells were co-transfected with plasmids encoding the indicated proteins for 24 h followed by Western blotting, n = 2.
Next, kinase reactions were carried out in vitro to determine if GSK-3β directly phosphorylates Cdc25A on S76. Cdc25A was purified as a GST fusion protein from bacteria and S76 phosphorylation was monitored with a phosphospecific antibody that recognizes Cdc25A when it is phosphorylated on S76. As seen in Figure 4F, incubation of GST-Cdc25A with purified GSK-3β did not increase the reactivity of Cdc25A with the pS76-specific antibody above background (compare lanes 1 and 2). Given that the recombinant Cdc25A used in the assay was purified from bacteria it was unlikely to be phosphorylated on T80, the priming site. Therefore, we searched for kinases that would phosphorylate Cdc25A on T80 in vitro. Sequences surrounding and inclusive of T80 (ExT80) conform to a Polo-like kinase (Plk) phosphorylation site (D/ExS/T). The Plk family consists of four family members Plk (1–4). Plk-2 and -3 are active in the G1 phase of the cell cycle when GSK-3β is expected to regulate Cdc25A whereas Plk-1 and -4 function in late G2 and mitosis (Eckerdt et al., 2005; Winkles and Alberts, 2005; Zimmerman and Erikson, 2007). Plk-3 was tested for its ability to prime Cdc25A for subsequent phosphorylation by GSK-3β. As seen in Figure 4F, incubation of GST-Cdc25A with purified Plk-3 did not increase the reactivity of Cdc25A with the pS76-specific antibody above background (top panel, lanes 1 and 4). However, prior phosphorylation of Cdc25A by Plk-3 significantly enhanced the ability of GSK-3β to phosphorylate Cdc25A on S76 in vitro (top panel, lane 3). Importantly, Plk-3 directly phosphorylated Cdc25A on T80 in vitro (middle panel, lanes 3, 4) and incubation of the T80A mutant with Plk-3 did not facilitate S76 phosphorylation by GSK-3β (lane 7). The altered electrophoretic mobility of wild-type Cdc25A and the T80A mutant indicated that sites other than T80 were also phosphorylated by Plk-3 in vitro.
To further investigate the contribution made by Plk family members to Cdc25A regulation in vivo, Cdc25A was co-produced with Plk-3 in vivo. As seen in Figure 4G, this resulted in enhanced phosphorylation of Cdc25A on T80 and siRNA knockdown of Plk-3 but not other family members resulted in Cdc25A stabilization in vivo (data not shown). These results are consistent with Plk-3 serving a priming function for Cdc25A in vivo.
Regulation of Cdc25A stability by S76 and T80 phosphorylation
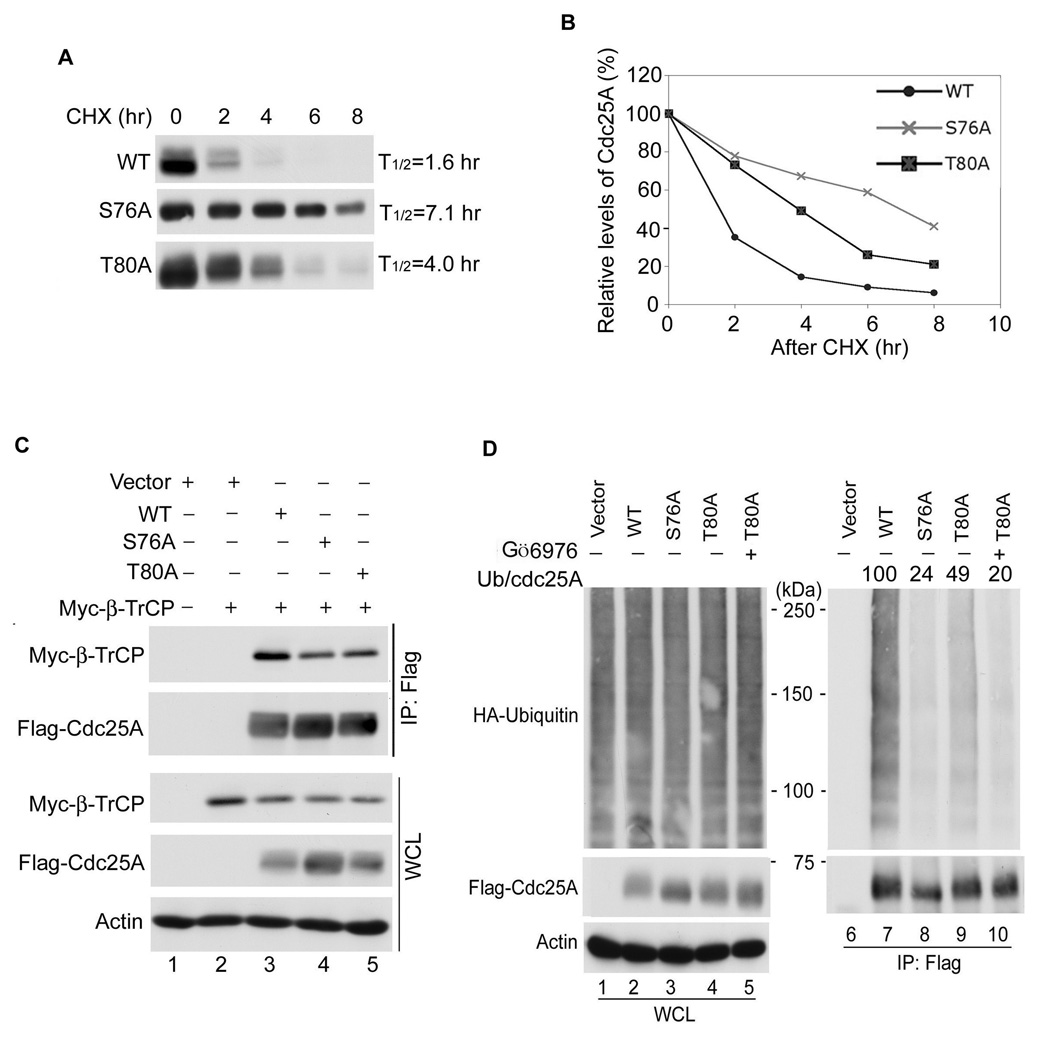
Given that S76 phosphorylation by GSK-3β is dependent on T80 phosphorylation and that the ubiquitin-mediated proteolysis of Cdc25A is dependent on S76 phosphorylation, it is predicted that mutants of Cdc25A that cannot be phosphorylated on T80 would have a longer half-life than wild-type Cdc25A. This was indeed the case as shown in Figure 5A, B. The half-life of the T80A mutant was ~2.5 times longer than that of wild-type Cdc25A. Consistent with its longer half-life, the T80A mutant bound less SCFβTrCP, a component of SCF E3 ligase (Figure 5C, lane 5) and was less ubiquitinated than wild-type Cdc25A (Figure 5D, compare lanes 7 and 9). Given that Chk1 also regulates S76 and Chk1 does not require a priming phosphate, it is expected that the properties of T80A mutant, which should still be a Chk1 substrate, would be less severe than that of the S76A mutant. The half-life of the S76A mutant was longer than T80A (Figure 5A, B) and it was less ubiquitinated than the T80A mutant (Figure 5D, compare lanes 8 and 9). Furthermore, Chk1 inhibition decreased T80A ubiquitination to the level observed for S76A (Figure 5D, compare lanes 8 and 10) and inhibition of Chk1, but not GSK-3β extended the half-life of T80A (Figure S4B). These results demonstrate that although T80A is insensitive to GSK-3β inhibition in vivo, it retains sensitivity to Chk1 inhibition.
Figure 5. Phosphorylation of Cdc25A by GSK-3β promotes β-TrCP binding and ubiquitin-mediated proteolysis.
(A), (B) U2OS cells transfected with Flag-tagged versions of Cdc25A for 16 h were incubated with 10 µg/ml CHX for the indicated times (n=3). Cdc25A protein levels were quantified from Western blots using chemiluminescence on a STORM imager (A) and the data is illustrated graphically (B). (C) U2OS were co-transfected with plasmids encoding Myc-β-TrCP and the indicated forms of Flag-Cdc25A for 24 h. Lysates were analyzed directly by Western blotting (bottom three panels) or lysates were first incubated with Flag agarose and Cdc25A precipitates were analyzed by Western blotting (top two panels). The relative ratio of β–TrCP to Cdc25A was determined from three independent experiments using chemiluminescence on a STORM imager or the ImageJ program. A representative Western blot is shown. The binding of β-TrCP to WT Cdc25A was set at 1. The relative binding of β-TrCP to S76A and T80A was calculated to be 0.55+/− 0.09 and 0.63 +/− 0.09, respectively. The data is presented as mean +/− SEM, n = 3. (D) U2OS co-transfected with plasmids encoding HA-ubiquitin and the indicated forms of Flag-Cdc25A for 20 h were untreated (lanes 1–4, 6–9) or were incubated with 100 nM Gö6976 for 4 h (lanes 5, 10) (n=3). Lysates were analyzed directly by Western blotting (lane 1–5) or lysates were first incubated with Flag agarose and Cdc25A precipitates were analyzed for ubiquitin by Western blotting (lanes 6–10). A representative Western blot is shown. The ratio of ubiquitination to Flag-Cdc25A in each precipitate was determined and normalized to wild-type Cdc25A which was set at 100.
Regulation of Cdc25A by GSK-3β and Chk1 as a function of the cell cycle
To monitor the regulation of Cdc25A by GSK-3β and Chk1 throughout the cell division cycle, synchronized cells were treated with LiCl to inhibit GSK-3β or with Gö6976 to inhibit Chk1 and Cdc25A levels were examined by Western blotting (Figure 6A) and DNA content was determined by flow cytometry (Figure S5A). LiCl-treatment was most effective at stabilizing Cdc25A during the G1- and S- phases of the cell cycle (Figure 6A, lanes 2, 5) whereas Gö6976-treatment was most effective in the S- and G2-phases of the cell cycle (lanes 6, 9). These results suggest that GSK-3β regulates Cdc25A at early times in the cell division cycle and Chk1 takes over at later times. Furthermore, an elevation in BrdU incorporation was measured when cells synchronized at the G1/S-border were released from the block in the presence of the GSK-3β inhibitor LiCl but not the Chk1 inhibitor Gö6976 (Figure 6B). To determine if the enhanced BrdU-incorporation in LiCl-treated cells was due to Cdc25A stabilization, the experiment was repeated in control cells and cells knocked-down for Cdc25A (Figure 6C, D). An increase in the number of BrdU-labeled cells was observed when control cells were treated with LiCl and this increase was statistically significant (p = 0.03). In contrast, a significant increase in the number of BrdU-positive cells was not observed when cells knocked-down for Cdc25A were treated with LiCl (p = 0.41). These results suggest that the enhanced BrdU incorporation observed upon GSK-3β inhibition by LiCl was due to stabilization of Cdc25A. To determine if the effects of LiCl were due to GSK-3β inhibition, the experiments were repeated in cells knocked down for GSK-3β Figure S5B, C). Cdc25A accumulated in cells deficient in GSK-3β and enhanced BrdU incorporation was also observed (p = 0.02). LiCl-treatment did not significantly increase Cdc25A or levels of BrdU incorporation (p = 0.09) in cells knocked-down for GSK-3β (Figure S5B, C). A rise in Cdc2A levels and a significant increase in BrdU incorporation were also observed when cells were released from an M-phase arrest and then treated with LiCl (p = 0.03; Figure S5D, E). These results suggest that Cdc25A stabilization by GSK-3β inhibition causes accelerated S-phase entry. The relative contribution of APC(Cdh1) and SCF to the degradation of Cdc25A in G1 was investigated through siRNA knockdown experiments. As seen in Figure S6, stabilization of Cdc25A in G1 was observed in cells deficient for either Cdh1 or GSK-3β and even further stabilization was observed in cells deficient for both proteins. These results demonstrate that Cdc25A is regulated by APC(Cdh1) and SCF(β-TrCP) in G1.
Correlation between GSK-3β-inactivation and Cdc25A overproduction in multiple human tumor tissues
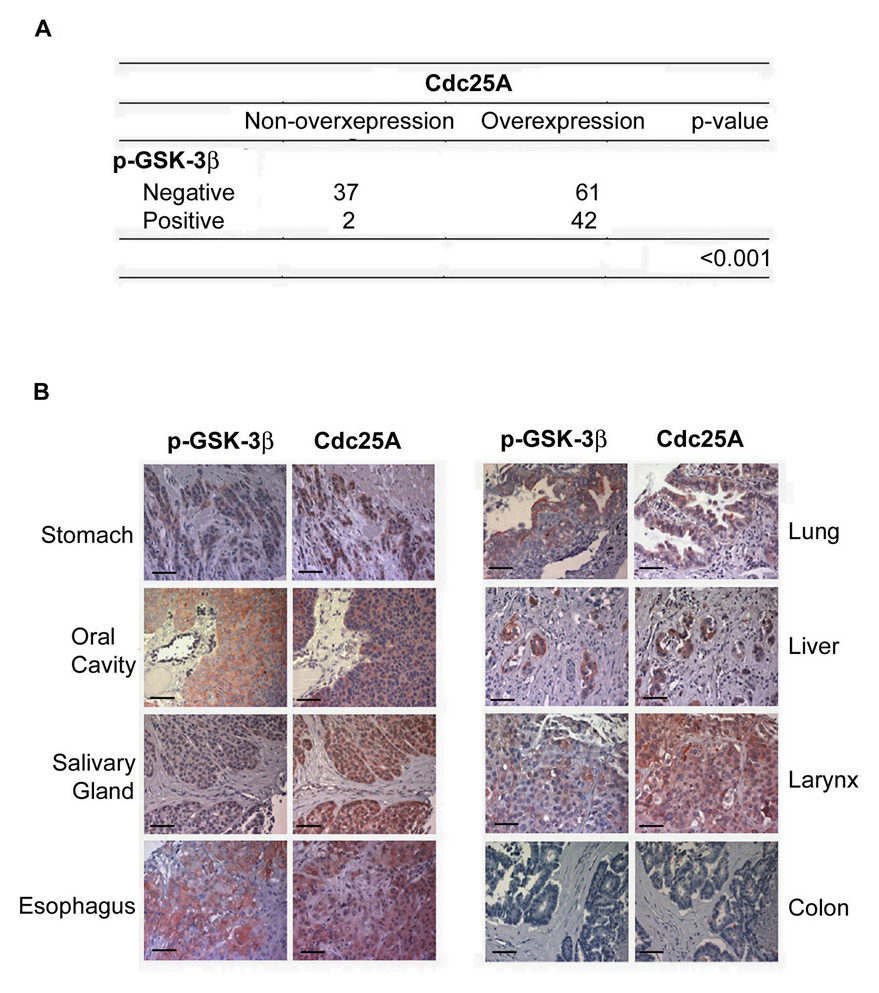
Cdc25A is overproduced in a wide variety of human cancers and its overproduction correlates with poor clinical outcome (Kristjansdottir and Rudolph, 2004; Loffler et al., 2003). Mechanistic insight into the cause of Cdc25A overproduction in these cancers is lacking. Given that GSK-3β is frequently inactivated in a variety of tumors (Cohen and Frame, 2001; Doble and Woodgett, 2003; Zhou et al., 2004), we tested whether there was a correlation between Cdc25A overproduction and GSK-3β inactivation using human tumor tissue microarrays derived from different cancer types (Figure 7 and Table S1). Immunohistochemistry was performed with a Cdc25A-specific antibody and an antibody that recognizes GSK-3β when it is phosphorylated on serine 9 (p-GSK-3β), a modification that inactivates GSK-3β (Cohen and Frame, 2001; Doble and Woodgett, 2003). The specificity of each antibody is shown in Figure S7. As shown in Figure 7, 44 out of 142 tumor tissues stained positive for p-GSK-3β, demonstrating that GSK-3β was inactivated in more than 30% of the tumors. Importantly, Cdc25A was overproduced in 42 out of the 44 tumors containing inactive GSK-3β (p < 0.001 using χ2 tests). These findings provide a strong correlation between GSK-3β inactivation and Cdc25A overproduction in human cancers. Interestingly, there were 61 tumors that contained high levels of Cdc25A but stained negative for p-GSK-3β, demonstrating that pathways, in addition to GSK-3β inactivation, may be involved in Cdc25A overproduction in human tumors. There was no correlation between Cdc25A levels with Chk1 or Cdh1 levels in these tumors (Tables S2 and S3).
Figure 7. Inactivation of GSK-3β correlates with overexpression of Cdc25A in multiple cancerous tissues.
(A), (B) Immunohistochemical staining of p-GSK-3β (Ser9) and Cdc25A were performed using multiple cancerous tissue microarrays. Representative examples of p-GSK-3β and Cdc25A staining in the same tumor are shown in (B), and a summary of the results is shown in (A). The scale bar is 50 µm.
Discussion
In this study, we identify GSK-3β as a key kinase that regulates the ubiquitin-mediated proteolysis of Cdc25A in early phases of the cell division cycle. We demonstrate that GSK-3β phosphorylates Cdc25A on S76 to facilitate the binding of the E3 ubiquitin ligase, SCFβ-TrCP. In addition, we demonstrate that Cdc25A phosphorylation by GSK-3β requires prior phosphorylation of Cdc25A on T80 and this can be catalyzed by Plk-3. Importantly, we report a strong correlation between GSK-3β inactivation and Cdc25A overproduction in several human tumors of diverse origins suggesting that GSK-3β inactivation may account for the prevalence of Cdc25A overproduction in many human cancers.
The periodic fluctuations observed in Cdc25A levels throughout the cell cycle can be attributed to the action of two distinct ubiquitin ligase complexes, APCCdh1 and SCFβ-TrCP(Busino et al., 2004; Donzelli et al., 2002; Jin et al., 2003). At mitotic exit and in G1, Cdc25A ubiquitination and degradation is regulated by APCCdh1 whereas SCFβ-TrCP regulates Cdc25A destruction in G1 as well as in the S- and G2-phases of the cell cycle (Busino et al., 2004; Busino et al., 2003; Jin et al., 2003). Chk1 phosphorylates Cdc25A during the S- and G2-phases of the cell cycle to regulate its ubiquitin-mediated proteolysis (Hassepass et al., 2003; Jazayeri et al., 2006; Jin et al., 2003). Consistent with this, we observed Cdc25A levels rise when S-and G2- but not when G1-phase cells were treated with the Chk1 inhibitor Gö6976. Until now, the identity of the kinase(s) that regulate recognition of Cdc25A by SCFβ-TrCP in late G1 have not been identified. We observed elevated levels of Cdc25A in G1 cells that were treated with the GSK-3β inhibitor, LiCl, and we observed that inhibition of GSK-3β, but not Chk1, accelerated the rate of S-phase entry. These results support the conclusion that Cdc25A levels are regulated by GSK-3β in G1. Interestingly, the activity of GSK-3β is the highest in G1 and gradually decreases as cells progress toward mitosis (Cohen and Frame, 2001; Doble and Woodgett, 2003).
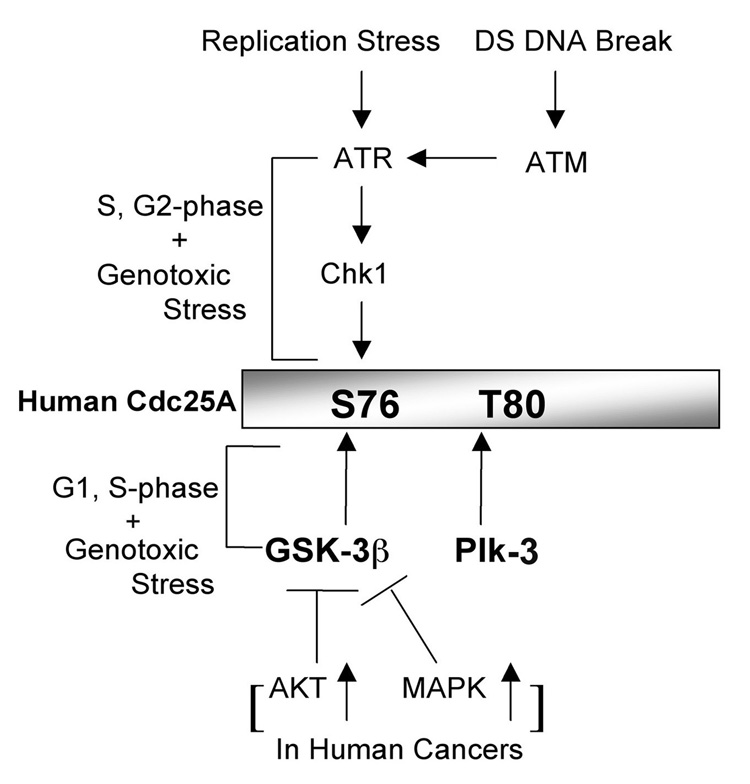
Elevated levels of Cdc25A were observed when GSK-3β was inhibited both pharmacologically and with siRNA-treatment. In addition, GSK-3β bound to and phosphorylated Cdc25A on a key regulatory site, S76. However, efficient phosphorylation of Cdc25A by GSK-3β required that Cdc25A first be phosphorylated on a priming site, T80. We found that Plk-3 phosphorylated Cdc25A on T80 and thereby primed Cdc25A for subsequent phosphorylation by GSK-3β. In addition, ectopic expression of Plk-3 increased levels of T80-phosphorylated Cdc25A in vivo. This suggests that Plk-3 functions to negatively regulate Cdc25A by serving as a priming kinase for GSK-3β, consistent with the literature showing that Plk-3 is active in the G1 phase of the cell cycle when GSK-3β is expected to regulate Cdc25A (Myer et al., 2005). Given that S76 phosphorylation is a prerequisite for subsequent phosphorylation of S82, which is critical for β-TrCP binding, and that Chk1 also phosphorylates S76 (Bartek and Lukas, 2003; Busino et al., 2003; Donzelli et al., 2004; Goloudina et al., 2003; Hassepass et al., 2003; Jin et al., 2003; Ray et al., 2005; Sorensen et al., 2003), we propose that the coordinated activities of GSK-3β and Chk1 tightly regulate Cdc25A levels throughout the cell cycle (Figure 8). The PI-3K/AKT pathway negatively regulates both GSK-3β and Chk1 and this in turn promotes cell cycle advancement by elevating Cdc25A levels (Cohen and Frame, 2001; Puc et al., 2005; Shtivelman et al., 2002). Recently, Ray et al. (2005) reported that transforming growth factor β (TGF-β) signaling induces ubiquitin-mediated proteolysis of Cdc25A through SCFβTrCP-mediated binding and this occurs in a Chk1-independent manner. It will be interesting to determine if GSK-3β plays a role in the destruction of Cdc25A by TGF-β.
Figure 8. Model of Cdc25A regulation.
A proposed model to illustrate how the GSK-3β pathway coordinates with the Chk1 pathway to modulate Cdc25A levels during an unperturbed cell cycle, in response to DNA damage and in certain cancer cells.
Cdc25A is rapidly targeted for ubiquitin-mediated proteolysis in cells exposed to genotoxic stress (Falck et al., 2001; Falck et al., 2002; Mailand et al., 2000; Zhao et al., 2002) and Chk1 plays a key role in regulating Cdc25A levels following exposure of cells to replicative stress and to DNA damage (Zhao et al., 2002). Here we demonstrate that the ability of cells to efficiently degrade Cdc25A in response to genotoxic stress also requires GSK-3β activity, and that GSK-3β cooperates with Chk1 to target Cdc25A for degradation in response to IR. Although it is known that Chk1 acts downstream of ATR in the replicative stress pathway and downstream of ATM/ATR in the DNA double strand break pathway (Jazayeri et al., 2006; Liu et al., 2000; Zhao and Piwnica-Worms, 2001), it is not known how GSK-3β activity is regulated by various forms of genotoxic stress.
Cdc25A is able to transform primary mouse embryo fibroblasts in cooperation with activated Ras or loss of RB and Cdc25A is frequently overproduced in human cancers both at the RNA and protein levels (Broggini et al., 2000; Cangi et al., 2000; Galaktionov et al., 1995; Gasparotto et al., 1997; Kristjansdottir and Rudolph, 2004; Loffler et al., 2003; Nadal et al., 2000). Until now, pathways leading to Cdc25A accumulation in human cancers have not been identified. Importantly, GSK-3β inactivation is a common occurrence in human cancers and we observed a strong correlation between GSK-3β inactivation and Cdc25A overproduction in several human tumors of diverse origins. Thus, inactivation of GSK-3β signaling pathways correlates with Cdc25A overproduction in a subset of human cancers.
GSK-3β is present in the nucleus and the cytoplasm of cells and its activity is modulated by several signaling and development pathways, including the Wnt, PI-3K/AKT and Ras/MAPK pathways (Cohen and Frame, 2001; Ding et al., 2005; Doble and Woodgett, 2003; Zhou et al., 2004). GSK-3β inactivation is a common occurrence in human cancers and this is due, in part, to the tendency of cancer cells to constitutively activate the PI-3K/AKT and Ras/MAPK pathways. Indeed, 44 out of 142 cancer tissues examined contained inactive GSK-3β Importantly, 42 out of the 44 tissues containing inactive GSK-3β also contained high levels of Cdc25A supporting the conclusion that derailment of GSK-3β signaling correlates with the overproduction of Cdc25A in a subset of human cancers. Cdc25A overproduction may contribute to the genomic instability observed in these cancers. We also found 61 tumors that contained active GSK-3β and high levels of Cdc25A demonstrating that pathways, in addition to GSK-3β inactivation, may be involved in Cdc25A overproduction. We demonstrated that Plk-3 can phosphorylate and thereby prime Cdc25A for subsequent phosphorylation by GSK-3β and that Cdc25A accumulates in cells knocked down for Plk-3. This suggests that Plk-3 may function to negatively regulate Cdc25A by serving as a priming kinase for GSK-3β. Interestingly, Plk-3 has been shown to be inactivated in multiple tumor samples (Takai et al., 2005). Tumors that inactivate Plk-3 are predicted to overproduce Cdc25A by reducing phosphorylation of S76 by GSK-3β. Thus, Plk-3 inactivation may account for Cdc25A overproduction in a subset of human tumors as well.
A therapeutic strategy that is currently being developed to treat p53-deficient cancers is to combine DNA damaging agents with drugs, such as UCN-01, that cause Cdc25A levels to rise. This induces preferential killing of p53-deficient tumor cells because it eliminates all DNA damage checkpoints in p53-deficient tumors. Phase I and II clinical trials combining UCN-01 with DNA damaging agents are currently underway to treat various cancer types (Bunch and Eastman, 1996; Busby et al., 2000; Dees et al., 2005; Fuse, 1998; Graves et al., 2000; Hotte et al., 2006; Kohn et al., 2003; Kortmansky et al., 2005; Levesque et al., 2005; Perez et al., 2006; Sampath et al., 2006; Sausville et al., 2001; Wang et al., 1996; Yu et al., 1998). Our study identifies GSK-3β and Plk-3 as potential therapeutic targets whose inhibition may induce checkpoint bypass in tumors by blocking Cdc25A proteolysis. Furthermore, tumors from patients enrolled on clinical trials that depend on Cdc25A stabilization to induce checkpoint bypass, should be tested for the integrity of the GSK-3β/Plk-3/Cdc25A pathway in order to correlate Cdc25A levels with tumor responses.
Experimental Procedures
Cell lines
Cells (HeLa, U2OS, MCF-7, HEK293, Saos2, BT549, MDA-MB-231, A549, H460 and IMR90) were cultured according to the instructions from ATCC, and immortalized MEFs (wild-type or null for GSK-3β) were grown in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% bovine growth serum (BGS, Hyclone), 1 mM glutamine and 100 U/ml each of penicillin and streptomycin.
Plasmids
Plasmids encoding human Cdc25A have been described (Chen et al., 2003). V5-tagged Wild-type (WT), constitutively active (CA) and kinase-inactive (KD) GSK-3β were obtained using the Gateway System (Invitrogen) with myc-tagged plasmids as templates. Plasmids encoding HA-GSK-3β-CA (Wei et al., 2005), Myc-GSK-3β, Myc-β-TrCP and HA-Ubiquitin (Zhou et al., 2004) have been described. Mutations were introduced using the Quick-Change site directed mutagenesis kit (Stratagene) and all mutations were verified by DNA sequencing.
Antibodies
Human Cdc25A was detected with mouse monoclonal antibody Ab-3 (Neomarkers). Antibodies specific for Cdc25A phosphorylated on S76 have been described (Goloudina et al., 2003). GSK-3β antibodies and antibodies specific for GSK-3β phosphorylated on serine 9 (p-Ser9) were purchased from Cell Signaling Technology. Other primary antibodies used for Western blotting were anti-Flag (Sigma Chemical Co.), anti-V5 (Invitrogen), anti-β-catenin (BD Transduction), anti-actin (Sigma Chemical Co.), anti-GSK-3α (Santa Cruz) which also reacts with GSK-3β, anti-Chk1(Santa Cruz), anti-Myc (A-14, Santa Cruz) for tagged protein, anti-Myc (N-262, Santa Cruz) for endogenous Myc, anti-HA (Santa Cruz), anti-Plk-2 (Santa Cruz) and anti-Plk-3 (BL1697, BETHYL Laboratories, Inc). Antibodies specific for Cdc25A phosphorylated on T80 and S123 were generated by immunizing rabbits with the coupled phosphopeptides C-GSSES-pT-DSGFC and C-LKRSH-pS-DSLD, respectively. Bound primary antibodies were detected with either horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (Jackson) or HRP-goat anti-rabbit antibody (Zymed) and proteins were visualized by chemiluminescence.
Transfection Experiments
Asynchronously growing cells seeded at 2.5 × 105 cells per well of a 6-well tissue culture dish or at 1 × 106 cells per 100 mm tissue culture dish were transfected with 2 µg or 10 µg plasmid DNA, respectively, using Lipofectamie™ 2000 (Invitrogen).
Treatment of cells with chemical inhibitors
Asynchronously growing cells were incubated in media containing either 100 nM Gö6976, 10 mM LiCl, 10 µM MG132, or a combination of LiCl and MG132 or Gö6976 at the indicated concentrations for 4 h. To obtain mitotic cells, cells were incubated with 50ng/ml of nocodazole for 16–18 h. Cells were lysed in mammalian cell lysis buffer (MCLB: 50 mM Tris-HCl pH 8.0, 2 mM DTT, 5 mM EDTA, 0.5% Nonidet P-40, 100 mM NaCl, 1 µM microcystin, 1 mM sodium orthovanadate, 2 µM PMSF, protease (Sigma Chemical Co.) and phosphatase inhibitor cocktail (Calbiochem). LiCl was purchased from Sigma Chemical Co. and MG132 and Gö6976 were purchased from Calbiochem.
RNAi treatment
Knockdown of GSK-3β, Chk1, Cdc25A, Cdh1, Plk-1, Plk-2, Plk-3 and Plk-4 was accomplished using Smartpool siRNA (Dharmacon). Approximately 2 × 105 HeLa cells were seeded per well of a 6-well tissue culture dish the day before transfection. Transfection was performed according to the manufacturer’s instructions using Dharmafect 3 transfection reagent (Dharmacon) and 100 nM siRNA. Forty-eight hours post-transfection, cells were incubated in the presence or absence of 20 µg/ml cycloheximide for the indicated times and harvested in MCLB. Alternatively cells were exposed to 10 Gy IR and harvested in MCLB. Smartpool siRNA reagents (Dharmacon) were: scrambled control (D00121002), luciferase GL3 (D00140001), GSK-3β (M00301003), Chk1 (M00892700), Cdc25A (Zhao et al., 2002), Cdh1 (M01537701), Plk-3 (L00325700), Plk1 (L00329000), Plk2 (L00332500), and Plk4 (L00503600).
Western blotting and immunoprecipitations
Cells were lysed in MCLB. Clarified lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting using either ECL detection reagents (Amersham Biosciences), Millipore Immobilon detection reagents (Millipore Co.) or SuperSignal reagents (Pierce). Alternatively, clarified supernatants were first incubated with anti-Flag- or anti-V5-agarose (Sigma Chemical Co.) for 1 to 2 h at 4°C, and precipitates were washed 4 times with MCLB. In the case of endogenous Cdc25A, rabbit anti-Cdc25A (sc-97, Santa Cruz) and protein A plus G beads (Santa Cruz) were used. In some cases proteins were visualized and quantified using the ECL plus reagent (Amersham), which contains a chemifluorescence component for quantification on a Molecular Dynamics Storm Imager (Molecular Biosystems, Piscataway, NJ).
Kinase assays
GST-Cdc25A was produced in bacteria and purified as described previously. GST-Cdc25A was eluted from GSH agarose in buffer consisting of 50 mM Tris (pH 7.4), 20 mM glutathione, 2 mM PMSF, 10 µg/ml of aprotinin and 20 µM leupeptin at 4°C. Plk-3 was purified from Sf9 cells infected with recombinant baculovirus encoding His6-tagged-Plk-3. Active GST-GSK-3β was purchased from Cell Signaling. Kinase reactions were carried out in a reaction buffer consisting of 50 mM Tris-HCl, pH 7.4, 1 mM DTT, 10 mM MgCl2, 500 µM ATP, 2 µg of soluble GST-Cdc25A, 100 ng of either GSK-3β or Plk3 or a combination of 100 ng each of GSK-3β and Plk-3. Reactions were incubated at 30°C for 1 to 2 h and then resolved by SDS-PAGE followed by Western blotting.
Synchronization of HeLa cells
HeLa cells, synchronized at the G1/S-border using a double thymidine block and release protocol (Chen et al., 2003), were released into culture media for 2.5 h (S-phase cells), 6 h (G2-phase cells) or 11 h (G1-phase cells). Synchronized cells were either not treated or were incubated with 20 mM LiCl or 100 nM Gö6976 for 2 h prior to collection for analyzing by flow cytometry and Western blotting. Alternatively, HeLa cells were cultured in the presence of 50 ng/ml nocodazole for 18 h and mitotic cells were isolated by mitotic shake off. Cells were washed twice with PBS and cultured for 8 h (G1-phase cells), 12 h (S-phase) or 15 h (G2-phase). Synchronized cells were either not treated or were treated with 20 mM LiCl or 100 nM Gö6976 for 2 h. Cell were collected and analyzed by flow cytometry and Western blotting.
Flow cytometry
Cells were harvested by trypsinization and collected by centrifugation. Cells were washed once with PBS and fixed in 5 ml of 70% ethanol at 4°C. Cells were washed once with PBS/ 1% BSA and then incubated with 1 ml of PBS/1% BSA containing 30 µg/ml propidium iodide (PI) and 0.25 mg/ml RNase A for 1 h at room temperature. Cells were analyzed for DNA content by flow cytometry using a FACS Calibur (BD Biosciences). The data were analyzed using CellQuest Analysis software (BD Biosciences).
BrdU labeling
HeLa cells, synchronized in mitosis with nocodazole were washed twice with PBS and cultured for 9 h. Alternatively, HeLa cells were synchronized at the G1/S-border using a double thymidine block and release protocol (Chen et al., 2003). Cells were treated with vehicle (PBS), 20 mM LiCl or 100 nM Gö6976 for 1h. Cells were then incubated with 20 mM bromodeoxyuridine (BrdU) for 1h and then processed for flow cytometry as described (Ferguson et al., 2005). In some cases, HeLa cells were first transfected with siRNAs for 24 h. Statistical analysis was performed using a two-tailed t-test.
Analysis of human tumor tissue
Human tumor tissue microarrays were purchased from IMGENEX (cat# IMH-365). This tissue microarray is a high density slide (146 samples in which 142 were available for analysis in this study) containing multiple cancerous tissues. Rabbit polyclonal anti-human Cdc25A antibody (SC-97, Santa Cruz) was used at 1:500. Rabbit polyclonal anti-human phospho-GSK3β (Ser9) (#9336, Cell Signaling Technology) was used at 1:30. Rabbit polyclonal anti-human Cdh1 antibody (Zymed, #34-2000) was used at 1:20 and monoclonal anti-human Chk1 antibody (Sigma, #C9358) was used at 1:3,000. Tissue slides were deparaffinized in xylene and rehydrated in a series of graded alcohols and the antigen was retrieved in 0.01 M sodium citrate buffer (pH 6.0) using a microwave oven. The sections were then treated with 1% hydrogen peroxide in methanol for 30 minutes to exhaust endogenous peroxidase activity. After a 1h pre-incubation in 10% normal goat serum to prevent nonspecific staining, the samples were incubated with primary antibody at 4°C overnight. The sections were then treated with biotinylated goat-anti rabbit immunoglobulin followed by incubation with avidin-biotin peroxidase complex solution for 1h at room temperature. The peroxidase reaction was visualized by incubating the sections with 3-amino-9-ethylcarbazole solution. The counterstaining was carried out using Mayer’s hematoxylin. Cdc25A expression was classified as positive in cases with more than 50% positive-staining cells, with other cases classified as negative. In the case of GSK-3β, cases with more than 10% positive-stained cells were classified as positive and others as negative. Statistical analysis was done using SPSS, version 10. Association between the phospho-GSK-3β and Cdc25A abundance was assessed using χ2 tests. The Institutional Review Board of the University of Texas MD Anderson Cancer Center has determined that the use of the human tumor tissue microarray is exempt under Category Number 4.
Peptide Competition
Peptides were mixed with primary antibodies prior to incubating with the sequential breast cancer tissue sections. Peptides included Cdc25A blocking peptide (SC-97P, Santa Cruz) diluted 1:125, phospho-GSK3β (Ser 9) blocking peptide (# 900, Cell Signaling Technology) diluted 1:7.5. Control peptides were unrelated to the antibodies used and were diluted as indicated above.
Supplementary Material
Acknowledgments
We thank Janis L. Watkins for preparing recombinant GST-Cdc25A and Chris Ryan for editing the manuscript. We thank Dr. James R. Woodgett for providing immortalized wild-type and GSK-3β null MEFs. Dr. Wenyi Wei for providing plasmid encoding HA-GSK-3β, Dr. Binhua P. Zhou for providing plasmids encoding HA-Ub, Myc-GSK-3β and Myc-β-TrCP and Dr. Ray Erikson for providing recombinant baculovirus encoding His6-Plk3. Members of the laboratory are thanked for their helpful comments on the manuscript. This work was supported by a grant from the National Institutes of Health and the Siteman Cancer Center. T. K. is an Associate and H.P.-W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Lieberman DA, Hoffman B. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene. 2000;19:2447–2454. doi: 10.1038/sj.onc.1203564. [DOI] [PubMed] [Google Scholar]
- Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Broggini M, Buraggi G, Brenna A, Riva L, Codegoni A, Torri V, Lissoni A, Mangioni C, D'Incalci M. Cell cycle-related phosphatases Cdc25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 2000;20:4835–4840. [PubMed] [Google Scholar]
- Bunch RT, Eastman A. Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (UCN-01), a new G2 checkpoint inhibitor. Clin Cancer Res. 1996;2:791–797. [PubMed] [Google Scholar]
- Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Research. 2000;60:2108–2212. [PubMed] [Google Scholar]
- Busino L, Chiesa M, Draetta GF, Donzelli M. Cdc25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene. 2004;23:2050–2056. doi: 10.1038/sj.onc.1207394. [DOI] [PubMed] [Google Scholar]
- Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M, Draetta GF. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- Cangi MG, Cukor B, Soung P, Signoretti S, Moreira G, Jr, Ranashinge M, Cady B, Pagano M, Loda M. Role of the Cdc25A phosphatase in human breast cancer. J Clin Invest. 2000;106:753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-S, Ryan CE, Piwnica-Worms H. Chk1 Kinase Negatively Regulates Mitotic Function of Cdc25A Phosphatase through 14-3-3 Binding. Mol Cell Biol. 2003;23:7488–7497. doi: 10.1128/MCB.23.21.7488-7497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Dees EC, Baker SD, O'Reilly S, Rudek MA, Davidson SB, Aylesworth C, Elza-Brown K, Carducci MA, Donehower RC. A phase I and pharmacokinetic study of short infusions of UCN-01 in patients with refractory solid tumors. Clin Cancer Res. 2005;11:664–671. [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli M, Busino L, Chiesa M, Ganoth D, Hershko A, Draetta GF. Hierarchical order of phosphorylation events commits Cdc25A to betaTrCP-dependent degradation. Cell Cycle. 2004;3:469–471. [PubMed] [Google Scholar]
- Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M, Draetta GF. Dual mode of degradation of Cdc25 A phosphatase. Embo J. 2002;21:4875–4884. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30:290–294. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol. 2005;25:2853–2860. doi: 10.1128/MCB.25.7.2853-2860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse E, Tanii H, Kurata N, Kobayashi H, Shimada Y, Tamura T, Sasaki Y, Tanigawara Y, Lush RD, Headlee D, Figg WD, Arbuck SG, Senderowicz AM, Sausville EA, Akinaga S, Kuwabara T, Kobayashi S. Unpredicted clinical pharmacology of UCN-01 caused by specific binding to human alphal-acid glycoprotein. Cancer Research. 1998;58:3248–3253. [PubMed] [Google Scholar]
- Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366–2368. [PubMed] [Google Scholar]
- Goloudina A, Yamaguchi H, Chervyakova DB, Appella E, Fornace AJ, Jr, Bulavin DV. Regulation of human Cdc25A stability by serine 75 phosphorylation is not sufficient to activate a S-phase checkpoint. Cell Cycle. 2003;2:473–478. [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O'Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathway are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- Hassepass I, Voit R, Hoffmann I. Phosphorylation at serine-75 is required for UV-mediated degradation of human Cdc25A phosphatase at the S-phase checkpoint. J Biol Chem. 2003;278:29824–29829. doi: 10.1074/jbc.M302704200. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13:4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotte SJ, Oza A, Winquist EW, Moore M, Chen EX, Brown S, Pond GR, Dancey JE, Hirte HW. Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II Consortium study. Ann Oncol. 2006;17:334–340. doi: 10.1093/annonc/mdj076. [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–3074. doi: 10.1101/gad.1157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn EA, Yoo CJ, Eastman A. The protein kinase C inhibitor Go6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res. 2003;63:31–35. [PubMed] [Google Scholar]
- Kortmansky J, Shah MA, Kaubisch A, Weyerbacher A, Yi S, Tong W, Sowers R, Gonen M, O'Reilly E, Kemeny N, et al. Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol. 2005;23:1875–1884. doi: 10.1200/JCO.2005.03.116. [DOI] [PubMed] [Google Scholar]
- Kristjansdottir K, Rudolph J. Cdc25 phosphatases and cancer. Chem Biol. 2004;11:1043–1051. doi: 10.1016/j.chembiol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Kunick C, Lauenroth K, Leost M, Meijer L, Lemcke T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 beta. Bioorg Med Chem Lett. 2004;14:413–416. doi: 10.1016/j.bmcl.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Levesque AA, Kohn EA, Bresnick E, Eastman A. Distinct roles for p53 transactivation and repression in preventing UCN-01-mediated abrogation of DNA damage-induced arrest at S and G2 cell cycle checkpoints. Oncogene. 2005;24:3786–3796. doi: 10.1038/sj.onc.1208451. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Loffler H, Syljuasen RG, Bartkova J, Worm J, Lukas J, Bartek J. Distinct modes of deregulation of the proto-oncogenic Cdc25A phosphatase in human breast cancer cell lines. Oncogene. 2003;22:8063–8071. doi: 10.1038/sj.onc.1206976. [DOI] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J, Lukas J. Regulation of G2/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21:5911–5920. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Mercurio C, Dominguez J, Goubin F, Draetta GF. Human Cdc25A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO Reports. 2000;1:71–79. doi: 10.1093/embo-reports/kvd018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DL, Bahassi el M, Stambrook PJ. The Plk3-Cdc25 circuit. Oncogene. 2005;24:299–305. doi: 10.1038/sj.onc.1208278. [DOI] [PubMed] [Google Scholar]
- Nadal A, Ferrer A, Fernandez PL, Montserrat E, Cardesa A, Campo E. Cdc25A and the splicing variant cdc25b2, but not cdc25B1, -B3 or -C, are over-expressed in aggressive human non Hodgkin's lymphomas. International Journal of Cancer. 2000;89:148–152. [PubMed] [Google Scholar]
- Perez RP, Lewis LD, Beelen AP, Olszanski AJ, Johnston N, Rhodes CH, Beaulieu B, Ernstoff MS, Eastman A. A. Modulation of cell cycle progression in human tumors: a pharmacokinetic and tumor molecular pharmacodynamic study of cisplatin plus the Chk1 inhibitor UCN-01 (NSC 638850) Clin Cancer Res. 2006;12:7079–7085. doi: 10.1158/1078-0432.CCR-06-0197. [DOI] [PubMed] [Google Scholar]
- Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ray D, Terao Y, Nimbalkar D, Chu LH, Donzelli M, Tsutsui T, Zou X, Ghosh AK, Varga J, Draetta GF, Kiyokawa H. Transforming growth factor beta facilitates beta-TrCP-mediated degradation of Cdc25A in a Smad3-dependent manner. Mol Cell Biol. 2005;25:3338–3347. doi: 10.1128/MCB.25.8.3338-3347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, Gandhi V, Plunkett W. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107:2517–2524. doi: 10.1182/blood-2005-08-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville EA, Arbuck SG, Messmann R, Headlee D, Bauer KS, Lush RM, Murgo A, Figg WD, Lahusen T, Jaken S, et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol. 2001;19:2319–2333. doi: 10.1200/JCO.2001.19.8.2319. [DOI] [PubMed] [Google Scholar]
- Shtivelman E, Sussman J, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr Biol. 2002;12:919–924. doi: 10.1016/s0960-9822(02)00843-6. [DOI] [PubMed] [Google Scholar]
- Sorensen CS, Syluasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou B-B, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- Wang O, Fan S, Eastman A, Worland PJ, Sausville EA, O'Conner PM. UCN-01: a Potent Abrogator of G2 Checkpoint Function in Cancer Cells With Disrupted p53. Journal of National Cancer Institute. 1996;88(No14):956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Winkles JA, Alberts GF. Differential regulation of polo-like kinase 1, 2, 3, and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260–266. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yu L, Orlandi L, Wang P, Orr MS, Senderowicz AM, Sausville EA, Silvestrini R, Watanabe N, Piwnica-Worms H, O'Connor PM. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J Biol Chem. 1998;273:33455–33464. doi: 10.1074/jbc.273.50.33455. [DOI] [PubMed] [Google Scholar]
- Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci USA. 2002;99:14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci U S A. 2007;104:1847–1852. doi: 10.1073/pnas.0610856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.