Abstract
While much is known about muscle spindle structure, innervation and function, relatively few factors have been identified that regulate intrafusal fiber differentiation and spindle development. Identification of these factors will be a crucial step in tissue engineering functional muscle systems. In this study, we investigated the role of the growth factor, neuregulin 1-β-1 EGF (Nrg1-β-1), for its ability to influence myotube fate specification in a defined culture system utilizing the non-biological substrate DETA. Based on morphological and immunocytochemical criteria, Nrg 1-β-1 treatment of developing myotubes increases the ratio of nuclear bag fibers to total myotubes from 0.019 to 0.100, approximately a five-fold increase. The myotube cultures were evaluated for expression of the intrafusal fiber specific alpha cardiac-like myosin heavy chain and for the expression of the nonspecific slow myosin heavy chain. Additionally, the expression of ErbB2 receptors on all myotubes was observed, while phosphorylated ErbB2 receptors were only observed in Nrg1-β-1 treated intrafusal fibers. After Nrg1-β-1 treatment, we were able to observe the expression of the intrafusal fiber specific transcription factor Egr3 only in fibers exhibiting the nuclear bag phenotype. Finally, nuclear bag fibers were characterized electrophysiologically for the first time in vitro. This data shows conclusively, in a serum-free system, that Nrg 1-β-1 is necessary to drive specification of forming myotubes to the nuclear bag phenotype.
Introduction
Investigations into primary myocyte growth and differentiation have significant clinical applications ranging from functional prosthetic device development to muscular dystrophy. While much research has focused on the existence of extrafusal muscle fibers, much less effort has been applied to understanding spindle development in vitro and the intrafusal fibers that comprise the spindle. Research on spindle development would not only have interest from a basic research prospective, but identification of the factors regulating muscle spindle development would also enhance scientists' understanding of proprioception/kinesthesia. Interspersed in most muscles, muscle spindles act as mechanoreceptors, providing proprioceptive sensory information to the brain and form the feedback circuit of the stretch reflex arc [1, 2]. Structurally, each muscle spindle contains several intrafusal myotubes; identified morphologically as either nuclear bag fibers with nuclei clustered at the center of the myotube or nuclear chain fibers with nuclei aligned to form a chain-like appearance [3, 4]. Furthermore, individual intrafusal fibers, either nuclear bag or nuclear chain, are also characterized by distinct patterns of myosin heavy chain expression profiles [5]. A key unanswered question in developmental muscle biology is the cellular origin of the intrafusal fibers that compose the muscle spindle, as well as the factors responsible for their differentiation.
Currently, two theories on the origin of intrafusal fibers dominate the field: one suggests a single bipotential population of myocytes that develop into both fiber types while the other advocates the presence of two distinct population of cells, one for extrafusal fibers and the other for intrafusal fibers [6, 7]. To resolve this debate, investigations focused on identifying proteins and cell types necessary for intrafusal fiber differentiation are ongoing. For example, the Ia sensory neuron's impact on spindle morphogenesis through ErbB2 signaling has been clearly established, in vivo [8, 9]. Additionally, the transcription factor Egr3 has been shown to play a role in protein expression profiles necessary for muscle spindle development [10]. Finally, the extracellular matrix molecule type IV collagen, observed using scanning electron microscopy, has been proposed as a molecule important in intrafusal fiber differentiation [11, 12]. While significant advances have been made using in vivo techniques, a clear definition of all the factors required for intrafusal fiber differentiation has not been documented [13-17]. Therefore, one approach to gain additional insight into intrafusal fiber myogenesis is to utilize a defined in vitro [18]. Such a system would permit the analysis of specific factors, in a defined environment, on the differentiation of skeletal muscle without variable interference from undefined sources such as serum or adsorbed biological substrates.
In vitro primary myocyte culture has attracted the interest of multi-disciplinary scientific endeavors such as cell patterning and tissue engineering for the development of biosensors and bio-robotic systems [19-21). Also, much effort has been towards identifying growth factors, hormones and culture conditions that yield robust primary myocyte growth, as well as subsequent fusion into contractile myotubes in vitro [22-24]. For example, in vitro studies have identified neuregulin as a growth factor that induces expression of the transcription factor Egr3 which is critical for intrafusal fiber development [25-27]. These studies have provided some insight into the conditions required for myocyte growth and differentiation. However, variability of adsorbed biological growth substrates, and the undefined factors present in serum containing media formulations introduce unknown variability into these in vitro systems.
Definition of the minimal components required to create a functional system and subsequent control of those conditions are paramount for truly answering mechanistic questions using an in vitro system. We have developed a serum-free medium composition facilitating myocyte growth and fusion into functional myotubes on the non-biological substrate N-1[3-(trimethoxysilyl)propyl]-diethylenetriamine (DETA) [28, 29]. The nonbiological substrate, DETA, is ideally suited for in vitro studies due to its ability to form self-assembling monolayers on glass coverslips, effectively eliminating inconsistencies in growth surfaces [29]. Furthermore, the use of a defined, serum-free media formulation eliminates the unwanted variability of serum containing media. These two modifications facilitate direct correlation of the tested variable to the observed outcome. In this study we have utilized such a system to understand the role of neuregulin 1-β-1 on the development of nuclear bag fibers.
Materials and Methods
DETA Surface Preparation and Characterization
Glass coverslips (Thomas Scientific 6661F52) were cleaned using an O2 plasma cleaner (Harrick PDC-32G) for 20 minutes at 100 mTorr. The N-1[3 (trimethoxysilyl) propyl] diethylenetriamine (DETA) (United Chemical Technologies Inc. T2910KG) self assembling monolayer film was formed by reacting the cleaned coverslip with a 0.1% (v/v) mixture of the DETA organosilane in freshly distilled toluene (Fisher T2904). The DETA coated coverslips were heated in toluene to just below boiling for 30 minutes, rinsed with fresh toluene, reheated to just below boiling again for 30 minutes and then oven dried overnight. The DETA surfaces were characterized by contact angle measurements using an optical contact angle goniometer (KSV Instruments, Cam 200) and by N 1s peak monitoring using XPS (Kratos Axis 165) as previously described [29-32]. The values are reported as the mean ± SEM.
Collagen Surface Preparation
The collagen surfaces were prepared by adsorbing type IV collagen [100μg/mL] on to previously prepared DETA surfaces for one hour at room temperature. These surfaces will be evaluated for their ability to influence intrafusal fiber formation relative to the DETA surfaces.
Animals
Dated pregnant Sprague-Dawley rats were housed in an animal facility at the University of Central Florida. All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines. Pregnant rats were anesthetized and sacrificed at embryonic day 18, embryos were removed by caesarean section and fetuses dissected under a stereomicroscope (Carl Zeiss, Stemi 2000).
Primary Culture of Rattus norvegicus Myocytes
Skeletal muscle was removed from the hind limb of E18 rat fetuses, collected in a 15ml tube in cold Hibernate E + GlutaMAX™ + antibiotic-antimycotic + B27 (dissection media) and incubated in 0.05% trypsin-EDTA for 1 hour in a 37°C water bath. Following incubation, the trypsin-EDTA was removed and the cells suspended in dissection media + 10% FBS and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 800g for 10 minutes at 4°C to pellet the cells. Following centrifugation, the supernatant was aspirated and the cells resuspended in dissection media + 10% FBS. Fibroblasts were removed by panning the cell suspension in a 100 mm cell culture dish containing dissection media + 10% FBS for 20 min [33]. After panning, the myocytes were aspirated off the panning dish and then pelleted by centrifugation at 800g for 10 minutes at 4°C [34]. Finally, the supernatant was removed and the myocytes were suspended in the serum-free culture medium and a cell count was conducted using the trypan blue method. Myocytes were then plated on DETA coverslips at a density of 600-700 cells/mm2.
Neuregulin 1-β-1 EGF Domain Treatment
Myocytes were initially grown for 3 days in the serum-free culture medium. On the third day the medium was changed and Nrg1-β-1 EGF was added to the culture wells at a concentration of 100 ng/ml. At each subsequent medium change Nrg1-β-1 EGF was supplemented at the same concentration as the original treatment.
Myotube Fusion Index and Bag Fiber Differentiation Index
The myotube fusion index was calculated as the number of nuclei incorporated into myotubes relative to the total number of nuclei per unit area [35]. For each culture, a minimum of 10 random fields were counted and the fusion index expressed as the mean fused myocytes: total myocytes ± SEM. The intrafusal fiber differentiation index (bag fibers: total myotubes) of myocytes on untreated control coverslips and on Nrg1-β-1 EGF treated coverslips was assessed at day 12. The differentiation index was determined as the ratio of bag fiber myotubes to the total number of myotubes (greater than 4 nuclei). The index was calculated as the mean from three coverslips ± SEM for each experiment (treated and controls) and the experiments were conducted over 10 different cultures. A differentiation index was also calculated for experiments conducted on collagen adsorbed surfaces in order to address its potential enhancement of intrafusal fiber formation.
Preparation BA-G5 alpha cardiac myosin heavy chain
The mouse B lymphocyte hybridoma cell line HB-276 was cultured according to ATCC guidelines [36]. Briefly, cells were grown in DMEM (Gibco 10313-021) + 10% FBS (Gibco 16000-077) or Hybridoma-SFM (Gibco 12045-076) in 75 mm2 tissue culture flasks and placed in an incubator at 37°C and 5% CO2 at a concentration of between 1×105 and 1×106 cells/ml. The medium was changed twice weekly. The BA-G5 antibody is constantly secreted by the cells in culture and was harvested by removing 12ml of conditioned culture medium, transferring it to a 15 ml tube, followed by centrifugation at 4000g for 15 minutes at 4°C. The antibody containing supernatant was then removed and the concentration quantified using the microBCA method (Pierce 23235). The antibody concentration ranged from 9-12 μg/ml (data not shown) and was used 4 to 1 in blocking solution (see below).
Myotube Assessment: Immunocytochemistry & Laser Scanning Confocal Microscopy
The myocyte cultures were fixed in fresh 4% paraformaldehyde in PBS for 5 minutes and then rinsed twice with phosphate buffered saline (PBS). Next, cells were permeabilized with a solution of 0.05% saponin in PBS + 5% bovine serum albumin (BSA) for 5 minutes, rinsed once with PBS and then blocked with permeabilization solution + 5% donkey serum [37]. The cells were then incubated with primary antibodies (ATCC: HB-276) diluted in blocking solution (4:1) overnight at 4°C. The primary antibodies used include: mouse anti-alpha cardiac-like myosin heavy chain (see above), mouse anti-slow myosin heavy chain (A4.840 Developmental Studies Hybridoma Bank), rabbit anti-ErbB2 (06-562 Upstate), rabbit anti-phospho ErbB2 (06-229 Upstate), rabbit anti-Egr3 (sc-22801 Santa Cruz Biotechnology, Inc). The next day primary antibody solutions were aspirated and the cells rinsed three times with PBS. Then, AlexaFluor® 488nm secondary antibodies (Molecular Probes A21200) diluted 1:200 in blocking solution were added to the cells and incubated for 2 hours at room temperature in the dark. The secondary antibody solution was then aspirated and the coverslips rinsed three times in PBS. Finally, coverslips were mounted on glass slides using VectaShield mounting medium with DAPI (Vector Labs, H-1200) and fixed using clear nail polish.
Electrophysiology
Whole-cell patch clamp recordings were performed in a recording chamber located on the stage of a Zeiss Axioscope 2FS Plus upright microscope. The chamber was continuously perfused (2 ml/min) with the extracellular solution (culture medium @ 35 °C). Patch pipettes were prepared from borosilicate glass (BF150-86-10; Sutter, Novato, CA) with a Sutter P97 pipette puller and filled with intracellular solution (in mM: K-gluconate 140, EGTA 1, MgCl2 2, Na2ATP 2, Phosphocreatine 5, Phosphocreatine kinase 2.4, Hepes 10; pH = 7.2). The resistance of the electrode was 6–8 MΩ. Voltage clamp and current clamp experiments were performed with a Multiclamp 700A amplifier (Axon, Union City, CA). Signals were filtered at 2 kHz and digitized at 20 kHz with an Axon Digidata 1322A interface. Data recording and analysis were performed with pClamp 9.2 software (Axon). Membrane potentials were corrected by subtraction of a 15 mV tip potential, which was calculated using Axon's pClamp 9.2 program. Membrane resistance and capacitance were calculated using 50 ms voltage steps from −85 to −95 mV without any whole-cell or series resistance compensation. Sodium and potassium currents were measured in voltage clamp mode using voltage steps from a −85 mV holding potential. Action potentials were evoked with 1 s depolarizing current injections from a −85 mV holding potential [28, 30, 38]. For these experiments, five Nrg1-β-1 EGF treated myotubes meeting the morphological requirements of nuclear bag fibers were selected and patched from three different experiments (n=15).
Results
DETA surface modification
The aminosilane, trimethoxy-silylpropyl-diethylenetriamine (DETA), functions efficiently as a non-biological substrate due to its self-assembling monolayer properties and the multiple amines contained in the terminal group. This group confers hydrophilic properties to the surface, and that combined with the partial positive charge on the amines at physiological pH make it an ideal surface for cellular attachment and survival. The system is similar to poly-D-lysine, but has been found to be more robust and consistent [29]. X-ray photon spectroscopic (XPS) measurements of the DETA coated coverslips indicated a complete monolayer formed during the self-assembly process. The normalized area values of N 1s (401 and 399 eV) to the Si 2p3/2 peaks were stable throughout the study at 1500 ± 200 and were similar to previously published results [39, 40]. Static contact angle measurements of 40.13 ± 1.35° validated the hydrophilicity of the DETA surfaces. Stable XPS readings and contact angles across coverslips throughout the study indicate uniformity and reproducibility of the self-assembly of the DETA monolayer.
Serum-free medium and defined system formulation
The basic serum-free medium composition developed in our laboratory for the co-culture of embryonic motoneurons and myocytes was used as a starting point for these experiments [41]. The additional critical component added to the previous formulations was nerve growth factor (NGF) at a concentration of 10 ng/ml (Table 1). Using media supplemented with NGF, myotube formation progressed at the usual rate, but the longevity of the culture increased. NGF was added to the medium to assist in myocyte recovery and to more accurately represent the plausible environment that exists inside a muscle spindle in vivo due to secretion of the factor by schwann cells and myocytes and to also serve as a starting point for future sensory neuron / muscle co-culture experiments [42, 43] (Table 1).
Table 1.
Serum-free medium composition in Neurobasal used for myocyte growth and intrafusal fiber differentiation.
| Component | Concentration | Company | Catalog Number |
|---|---|---|---|
| B27 | 20μl/ml | Gibco | 17504-044 |
| Glutamax | 10μl/ml | Invitrogen | 35050-061 |
| Antibiotic/Antimycotic | 10μl/ml | Invitrogen | 15240-062 |
| aFGF | 20ng/ml | Invitrogen | 13241-013 |
| VEGF 165 | 20ng/ml | Invitrogen | P2654 |
| G5 (10×) | 2μl/ml | Invitrogen | 17503-012 |
| h BDNF | 20ng/ml | Cell Sciences | CRB 600B |
| h GDNF | 20ng/ml | Cell Sciences | CRG 400B |
| r CNTF | 50ng/ml | Cell Sciences | CRC 401B |
| h CT-1 | 20ng/ml | Cell Sciences | CRC 700B |
| NT-3 | 20ng/ml | Cell Sciences | CRN 500B |
| NT-4 | 20ng/ml | Cell Sciences | CRN 501B |
| De-N-sulphated | |||
| N-acetylated heparan sulfate | 80ng/ml | Sigma | D9809 |
| Vitronectin | 100ng/ml | Sigma | V0132 |
| h β-NGF | 10ng/ml | R&D Systems | 256-GF |
| h Nrg 1-β-1* | 100ng/ml | R&D Systems | 396-HB |
Supplemental component added only as indicated.
Immunocytochemical evaluation of nuclear bag and chain fibers
Initially, myotube cultures were grown on DETA and fusion indexes were determined to be 0.59 ± 0.02 for untreated cultures. While evaluating these cultures, we observed a distinct morphology that appeared to be nuclear bag fibers (Figure 1). The monoclonal antibody BA-G5 was used to evaluate alpha cardiac-like myosin heavy chain (MHC) (Figure 1A,B) expression in the morphologically apparent nuclear bag fibers. This antibody has been previously shown to be specific for intrafusal fibers in vivo, and was therefore the choice for evaluating their presence in vitro [7, 16, 44]. The presence of myotubes expressing intrafusal fiber specific, alpha cardiac-like MHC, was clearly observed in all cultures (Figure 2A, B). All of the myotubes stained displayed medially clustered nuclei characteristic of nuclear bag fibers in vivo. This immunocytochemical data shown in Figure 2 confirmed the morphological data illustrated in Figure 1, and verified the formation of nuclear bag fibers. Although their presence was not quantified, myotubes that were consistent with the morphology of nuclear chain fibers were also positive for BA-G5 MHC antibody staining in all cultures (Figure 2C, D), while other fibers exhibiting similar morphologies, assumed to be extrafusal fibers, did not. In certain cases, myocytes in proximity to bag fibers were positive for alpha cardiac MHC (Table 3) (Figure 2D). Additionally, all myotubes were evaluated for their expression of slow MHC. In both treated and untreated cultures, myotubes as well as some myocytes were positive for slow myosin heavy chain expression (Figure 2 E, F). This expression pattern is consistent with in vivo data where slow myosin heavy chain expression is present in both intrafusal and extrafusal fiber subtypes. In our system, this likely represents an incomplete development of extrafusal fibers due to lack of the necessary cell-cell interactions or trophic factors required for their complete differentiation and is an area of further investigation in our laboratory.
Figure 1.
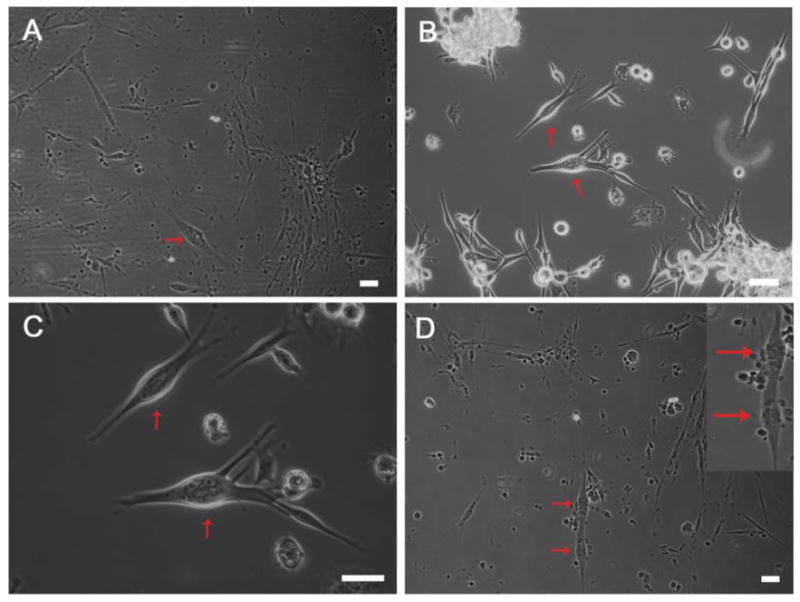
Nrg 1-β-1 induces the formation of nuclear bag fibers in vitro. (A-C) Representative images used to tabulate the number of bag fibers present in treated and untreated cultures and total myotubes (red arrows indicate counted bag fibers), (D) continuous double bag fibers were observed sporadically in treated cultures (red arrows) and represent a physiologically relevant phenomenon. Scale bars = 100μm.
Figure 2.
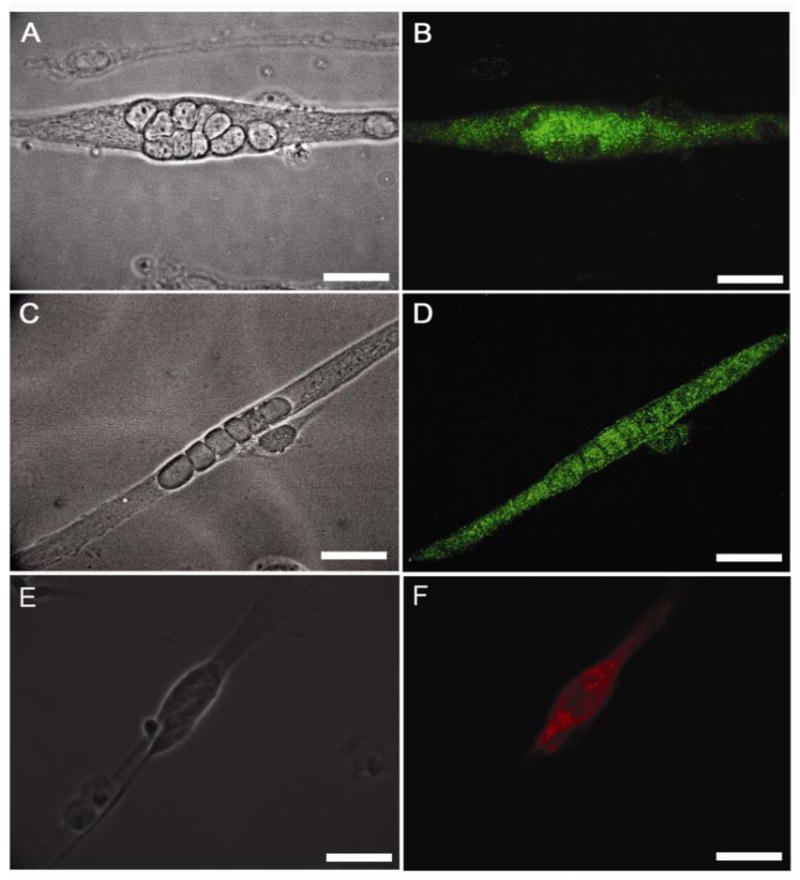
Phase contrast and immunocytochemical characterization of nuclear bag and chain fibers using the intrafusal fiber MHC antibody BA-G5. (A) phase contrast image of characteristic nuclear bag fibers after Nrg 1-β-1 treatment, (B) alpha cardiac-like MHC expression using BA-G5 antibody immunocytochemistry, (C) phase contrast image illustrating characteristic nuclear chain fibers in culture after Nrg 1-β-1 treatment, (D) representative alpha cardiac-like MHC immunocytochemical expression in nuclear chain fibers using the BA-G5 antibody. (E) phase contrast image of nuclear bag fibers after Nrg 1-β-1 treatment, (F) slow-developmental MHC immunocytochemical expression in nuclear bag fiber. Scale bars = 60μm.
Table 3.
Immunocytochemical determination of nuclear bag fiber morphogenesis after Neuregulin 1-β-1 EGF treatment
| ErbB2
positive |
P-ErbB2
positive |
Egr3
positive |
|
|---|---|---|---|
| BA-G5 (+) nuclear bag fibers | 30.67±1.14 | 30.00±0.65 | 30.89±0.68 |
| BA-G5 (-) myotubes | 279.78±4.05 | 0.00±0.00 | 0.00±0.00 |
| Total myotubes | 310.44±4.85 | 30.00±0.65 | 30.89±0.68 |
Values are reported as the mean ± SEM. Experiments were performed three different times in triplicate. Differentiation index = 0.60 ± 0.02.
Nrg 1-β-1 EGF treatment of myotube cultures
The ability of Nrg 1-β-1 to influence skeletal myocyte differentiation was evaluated in a dose and time dependent manner (Table 2). Nrg1-β-1 EGF treatment of the myocytes using 10 ng/ml and 20 ng/ml at the time of plating, resulted in significant cell death by day 3 (images not shown). Next, treatments utilizing 10ng/ml and 20ng/ml concentrations were administered at day 3 while the myocytes were further along in the fusion process. This resulted in a culture of myotubes comparable to controls; there was no significant increase in intrafusal fiber formation or a substantially different number of total myotubes present (Table 2). Having established that day 3 application of Nrg 1-β-1 permitted cell growth and myocyte fusion, the treatment concentration was increased to 100ng/ml. Morphologically, this treatment led to the formation of significantly greater numbers of nuclear bag fibers in culture (Figure 1, Table 2). These cultures were then stained using the anti-alpha cardiac-like antibody to confirm the development of intrafusal fibers due to Nrg 1-β-1 treatment. At this point, we quantified the myotube fusion index of treated cultures and determined it to be 0.60 ± 0.02. This value was found not to be significantly different from untreated culture fusion indexes. We then quantified the average number of bag fiber myotubes in treated cultures. The mean number of nuclear bag fibers was 30.90 ± 0.69 compared to 6.28 ± 0.32 in untreated cultures (Table 2). This difference was shown to be significant using a Student's T-test (P < 0.001). Sporadically, tandem double bag fibers were observed in treated cultures and are noteworthy due to their observed presence in vivo (Figure 1D) [45].
Table 2.
Nuclear bag fiber differentiation by Neuregulin 1-β-1 EGF treatment.
| Surface | Nrg1-β-1
(ng/ml) |
# of Bag Fibers | # of Myotubes | Intrafusal fiber
Differentiation Index |
|---|---|---|---|---|
| DETA | 0.00 | 6.00±0.32 | 306.17 | 0.0196 |
| DETA | 10.00 | 6.00±44 | 333.33 | 0.0209 |
| DETA | 20.00 | 7.66±0.38 | 330.33 | 0.0233 |
| DETA | 100.00 | 30.83±0.69 | 310.17 | 0.1002 |
| Collagen | 0.00 | 6.13±0.53 | 325.00 | 0.0189 |
| Collagen | 100.00 | 32.67±1.53 | 325.00 | 0.1005 |
The number of bag fibers and the number of myotubes are averages of ten experiments all performed in triplicate. Data is reported as the mean ± the standard error of the mean. The differentiation index is the ratio of observed bag fibers to total myotubes with greater than four nuclei.
After establishing that Nrg 1-β-1 EGF induced the morphological changes in nuclear bag fiber formation described above, other neuregulin isoforms were evaluated for their ability to induce similar changes. We tested Nrg 1-SMDF, Nrg 1-α-EGF and Nrg 1-β-ECD with the same sequence of applications as we used for the Nrg 1-β-1 EGF and they showed no phenotypic changes (data not shown).
Additionally, as collagen fibers have been identified inside muscle spindles and in contact with intrafusal fibers, the potential role of collagen adsorbed to culture surfaces was also evaluated to determine if it further influenced intrafusal fiber differentiation [12, 23]. On the collagen surface the average number of bag fibers on treated coverslips was 32.67 ± 1.53 compared to 6.13 ± 0.53 on untreated coverslips (Table 2). These data sets evaluated together show no significant difference between the collagen and DETA surfaces for bag fiber differentiation using a Student's T-test (P < 0.001).
Expression and activation of the neuregulin receptor ErbB2
In order to further evaluate the role of Nrg 1-β-1 signaling on the development of nuclear bag fibers, we tested the expression of the ErbB2 signaling receptor on the surface of treated and untreated myotubes in vitro. Furthermore, after Nrg 1-β-1 treatment, we evaluated the activation of the ErbB2 receptors using the phosphorylated [activated] ErbB2 receptor using the phospho-ErbB2 antibody (Figure 3). In both treated and untreated cultures, the presence of the inactivated form of the ErbB2 receptor was seen in all myotubes [(Table 3) (Figure 2E, K)] making this receptor a poor determinant of intrafusal fiber formation. Therefore, we determined the activation of the ErbB2 receptor in both treated and untreated cultures. In the untreated cultures, no activation of the ErbB2 receptor was seen (Figure 3 G, H, I). Conversely, in Nrg 1-β-1 treated cultures, the activated ErbB2 receptor was present in all treated myotubes exhibiting the nuclear bag phenotype (Figure 3 A, B, C). Concurrently, we evaluated the expression of BA-G5 MHC in these myotubes. We showed that the BA-G5 MHC was present in treated myotubes expressing the active phosphorylated ErbB2 receptor, but not present on any untreated myotubes. This makes the phosphorylated ErbB2 receptor a better determinant of intrafusal fiber formation and supports the evidence from Figure 2 (Figure 3 F, I, L). The summary of these expression experiments was quantified in Table 3.
Figure 3.
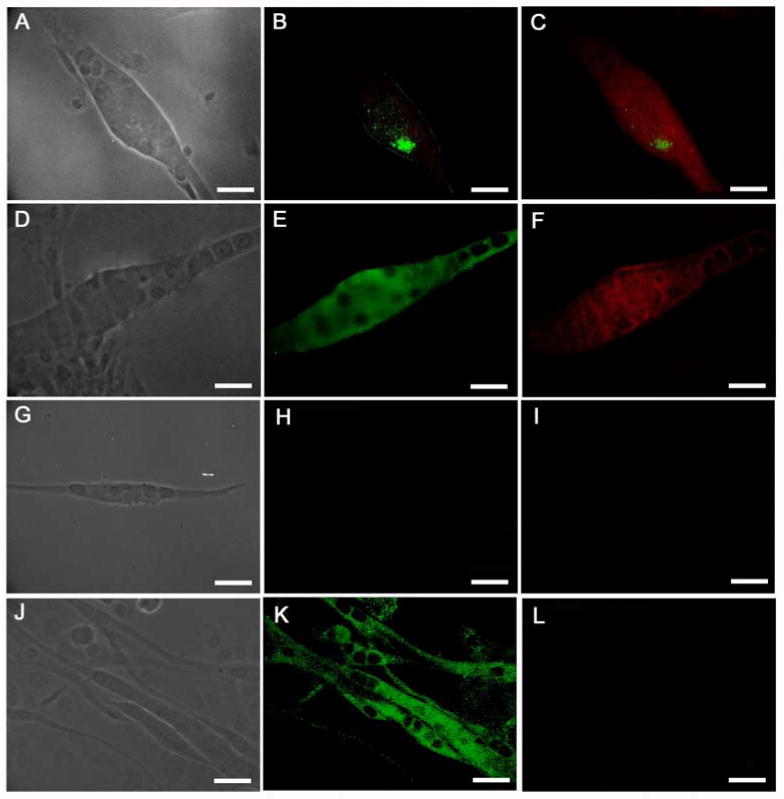
Expression of the phosphorylated and unphosphorylated forms of the ErbB2 receptor in Nrg1-B-1 treated and untreated cultures. (A-C) Nrg 1-β-1 treated myotube, (A) phase contrast image of a bag fiber, (B) phospho-ErbB2 immunostaining shows an activated receptor cluster, (C) BA-G5 + phosphor-ErbB2 merge image, (D-F) Nrg 1-β-1 treated myotube, (D) phase contrast image, (E) unphosphorylated ErbB2 staining, (F) BA-G5 + unphosphorylated ErbB2 merge image, (G-I) myotube from untreated culture, (G) phase contrast image, (H) phosphor-ErbB2 staining shows no receptor activation, (I) BA-G5 staining shows no immunoreactivity, (J-L) untreated myotubes, (J) phase contrast, (K) unphosphorylated ErbB2 immunostaining shows reactivity in untreated myotube cultures, (L) BA-G5 staining is absent in non-intrafusal myotubes. Scale bars = 40μm.
Activation of intrafusal fiber specific transcription factor Egr3
In order to further verify our finding that Nrg 1-β-1 induced nuclear bag fiber formation, we also evaluated the expression of the intrafusal fiber specific transcription factor Egr3 in treated and untreated myotube cultures. We concurrently analyzed the myotubes co-expression of either intrafusal specific BA-G5 MHC or the nonspecific slow MHC (Figure 4). In Nrg 1-β-1 treated intrafusal fibers, the co-expression of BA-G5 MHC and the Egr3 transcription factor was present in all myotubes evaluated over three experiments [(Table 3) (Figure 4A, B, C)]. In untreated cultures, myotubes did not express either the Egr3 transcription factor or the BA-G5 MHC (Table 3). Consequently, these myotubes were visualized using the slow MHC (Figure 4 D, E, F). In summary, while expression of the ErbB2 receptor was not sufficient to distinguish intrafusal from extrafusal fibers, the phosphorylation of ErbB2 after Nrg 1-β-1 treatment and the expression of the transcription factor Egr3 conclusively distinguishes the formation of nuclear bag fibers from other non-bag fibers on the nonbiological substrate DETA.
Figure 4.
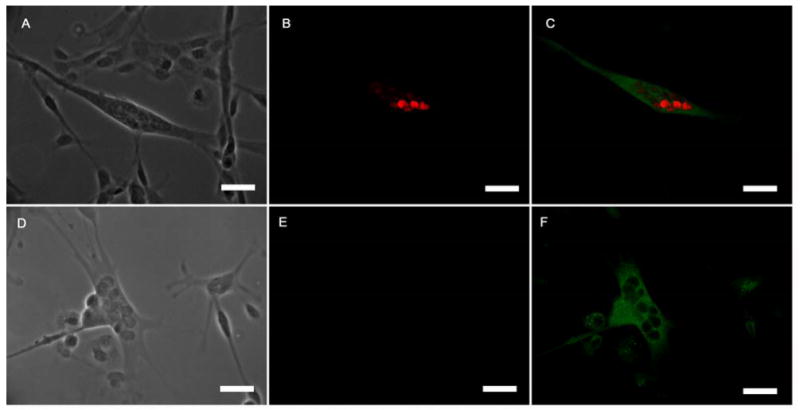
Activation of the transcription factor Egr3 in Nrg 1-β-1 intrafusal fibers. (A-C) Nrg 1-β-1 treated myotube, (A) phase contrast image of the treated bag fiber, (B) positive Egr3 transcription factor immunoreactivity localized in the nuclei, (C) BA-G5 + Egr3 immunoreactivity merge image (D-F) untreated myotube, (D) phase contrast image of an untreated two untreated myotubes, (E) absent Egr3 transcription factor immunoreactivity, (F) Slow MHC immunoreactivity of the untreated myotube. Scale bars = 60μm.
Electrophysiological evaluation of Nrg 1-β-1 treated myotubes
Electrophysiology was used to characterize the Nrg 1-β-1 treated myotubes capacity for inward Na+ currents and outward K+ currents. These experiments represent the first in vitro evaluation of the electrophysiological properties associated with the individual intrafusal fibers rather than examination of the entire muscle spindle. The resting membrane potential of the nuclear bag fibers was -50.6 ± 1.6mV (n = 5). The current traces of the treated cultures that showed characteristics consistent with nuclear bag fibers exhibited maximal inward Na+ currents of -5900pA and outward K+ currents of 1800pA (Figure 5A). Additionally, the Nrg 1-β-1 treated bag fiber myotubes' ability to fire action potentials was also investigated. All myotubes consistent with the nuclear bag fiber morphology demonstrated single action potential firing (Figure 5C).
Figure 5.
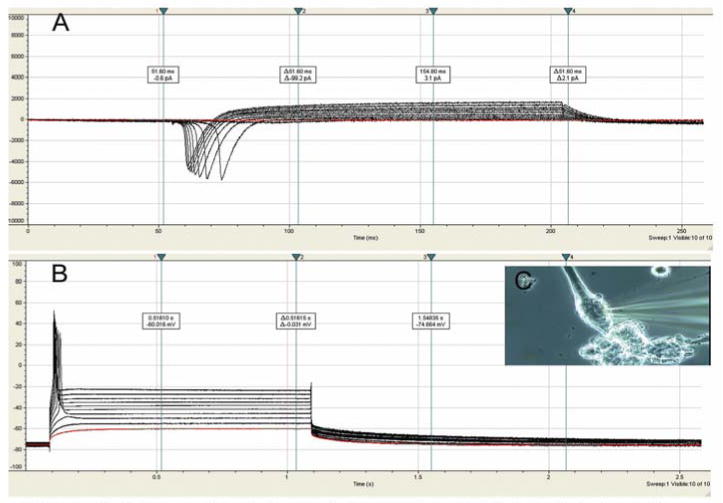
Electrophysiological properties of Nrg 1-β-1 treated nuclear bag fiber myotubes on DETA. (A) voltage clamped, current traces of Nrg 1-β-1 treated myotube showing maximal inward Na+ currents of -5900pA and outward K+ currents of 1800pA, (B) current clamped, single action potentials of a Nrg 1-β-1 treated myotube, (C) phase contrast image of a patched nuclear bag fiber.
Discussion
Neuregulin 1-β-1 treatment in a minimalistic in vitro system of developing myotubes resulted in a significant increase in nuclear bag fiber formation. This demonstrates that Nrg 1-β-1 EGF plays a positive role in intrafusal fiber development. This finding supports in vivo and in vitro experiments indicating that Nrg 1-β-1 activates transcription factors highly expressed in muscle spindle fibers and is required for their development. Although the incomplete differentiation of all myotubes into nuclear bag or chain fibers in culture would suggest a role for other factors in this process as well [25-27]. Additionally, the incomplete differentiation could represent that certain myotubes are refractory to Nrg 1-β-1 treatment. This suggests the possibility of two distinct populations of cells, one that can become intrafusal fibers, and one that will become extrafusal fibers. This idea is supported by the finding that while all myotubes were positive for ErbB2 receptor expression, only a subset of the myotubes was positive for the activated form of the receptor after Nrg 1-β-1 treatment.
The additional experiments using the ErbB2 signaling and the activation of the transcription factor Egr3 by the Nrg 1-β-1 growth factor further suggest that these fibers are in fact intrafusal fibers of the nuclear bag phenotype. These experiments show that the combination of BA-G5 MHC expression, activated ErbB2 signaling and expression of the transcription factor Egr3 are more precise indicators of nuclear bag fiber formation than BA-G5 MHC expression alone. Furthermore, the expression of the inactivated ErbB2 receptor in seemingly non-intrafusal fibers could indicate that these myotubes were unable to complete the morphogenesis to the intrafusal fiber phenotype, an area of development needing further research. In contrast, it is possible that by embryonic day 18 certain fate specification processes have occurred that limit the ability of every myotube to become an intrafusal fiber.
The similarity in bag fiber formation on the DETA surface compared to the collagen surface supports previous work illustrating the utility of DETA as a growth promoting surface for neurons and muscle [18, 28-30, 38, 40]. While a collagen adsorbed surface did not significantly affect bag fiber formation, other extracellular matrix components are good candidates for further influencing muscle spindle morphogenesis through integrin signaling [46]. Specifically, the close proximity of tenascin-c, s-laminin and chondroitin sulfate proteoglycan to the intrafusal fibers invites speculation about the role these ECM components play in muscle spindle mechanoreceptor development and physiology [47, 48]. This defined in vitro system provides an ideal environment to further evaluate the role of additional factors in intrafusal fiber development.
The necessity and utility of each growth factor present in the media formulation was derived from previous experiments from our groups and others [28, 31, 38]. The addition of nerve growth factor (NGF) to the formulation came as a result of experiments done by Murphy et al. showing an active form of NGF was secreted by rat skeletal muscle cells and a myogenic cell line [42]. They postulated the molecule acted in a support role for associated nerves. Here we hypothesized the molecule could also play an autogenic role in the support of muscle cells.
Using electrophysiology, we have begun to describe the electrical properties of nuclear bag fibers in this defined system. These findings are significant because previous experiments on the electrical properties of muscle spindle fibers had to be conducted in vivo on the whole muscle spindle [49, 50]. Here we were able to being evaluating the electrical properties of isolated nuclear bag fibers in vitro. Compared to recording properties of non-bag fiber myotubes, we found no significant difference in the properties of nuclear bag fibers [28]. This data set raises questions regarding the cell-cell signaling events that might modulate ion channel expression in the intrafusal fibers. In future studies, it will be interesting to compare motor neuron innervated myotubes to sensory neuron innervated myotubes. Furthermore, defining ion channel expression in intrafusal fibers is essential in understanding the functional behavior of the mammalian mechanoreceptor system, a still poorly understood structure, and is something that can be readily studied using this in vitro system.
The important role of sensory innervation by Ia afferent proprioceptive neurons for the development and maintenance of muscle spindles, as well as the retrograde support provided by NT-3 from innervated intrafusal fibers, is well documented [22, 51, 52]. Additionally, the role that various neurotrophic factors play in the interaction between the Ia afferents and the motor neurons for the maintenance of the monosynaptic stretch reflex arc have been elucidated [1, 53, 54]. Although ion channel expression has been related to these findings, a clear picture of the trophic factors required for expression of appropriate ion channels necessary for functioning of the reflex arc has also not been clearly defined either and could also benefit from studies with this system.
Conclusion
In conclusion, we used an in vitro serum-free system to further explore the role of neuregulin 1-β-1 on intrafusal fiber development. We characterized the dose and time dependent nature of the neuregulin 1-β-1 in the formation of nuclear bag fibers on the nonbiological substrate DETA. We also showed the permissive nature of DETA in supporting the formation of nuclear bag fibers. Finally, we began to characterize the electrical properties of isolated intrafusal fibers in vitro, something that has not been done until now. The high reproducibility of this system will facilitate further investigation into intrafusal fiber and muscle spindle morphogenesis. The complete development of such a system will permit the systematic evaluation of mechanoreceptor formation, maintenance and function, as well as its integration into an in vitro model of the stretch reflex arc.
Acknowledgments
We would like to acknowledge that support for this research was provided by the University of Central Florida and NIH grant number 5 R01 NS 050452-03. We confirm that any aspect of the work covered in this manuscript that has involved experimental animals has been conducted with the ethical approval of all relevant bodies. The monoclonal antibody A4.840, developed by Helen M. Blau, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Current Opinion in Neurobiology. 2003;13(1):96–102. doi: 10.1016/s0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 2.Maier A. Development and regeneration of muscle spindles in mammals and birds. Int J Dev Biol. 1997;41(1):1–17. [PubMed] [Google Scholar]
- 3.Kucera J, Dorovini-Zis K. Types of human intrafusal muscle fibers. Muscle Nerve. 1979;2(6):437–51. doi: 10.1002/mus.880020605. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, Tomonaga M, Hirayama K, Narabayashi H. Histochemical study of normal human muscle spindle. Histochemical classification of intrafusal muscle fibers and intrafusal nerve endings. J Neurol. 1977;216(2):79–89. doi: 10.1007/BF00312942. [DOI] [PubMed] [Google Scholar]
- 5.Kucera J, Walro J. Myosin heavy chain expression in developing rat intrafusal muscle fibers. Neuroscience Letters. 1990;109(12):18–22. doi: 10.1016/0304-3940(90)90531-d. [DOI] [PubMed] [Google Scholar]
- 6.Kucera J, Walro JM. Origin of intrafusal muscle fibers in the rat. Histochemistry. 1990;93(6):567–80. doi: 10.1007/BF00272199. [DOI] [PubMed] [Google Scholar]
- 7.Pedrosa F, Soukup T, Thornell LE. Expression of an alpha cardiac-like myosin heavy chain in muscle spindle fibres. Histochemistry. 1990;95(2):105–13. doi: 10.1007/BF00266582. [DOI] [PubMed] [Google Scholar]
- 8.Kucera J, Walro JM, Reichler J. Innervation of developing intrafusal muscle fibers in the rat. Am J Anat. 1988;183(4):344–58. doi: 10.1002/aja.1001830408. [DOI] [PubMed] [Google Scholar]
- 9.Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 2003;130(11):2291–301. doi: 10.1242/dev.00447. [DOI] [PubMed] [Google Scholar]
- 10.Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J. The Transcription Factor Egr3 Modulates Sensory Axon-Myotube Interactions during Muscle Spindle Morphogenesis. Developmental Biology. 2001;232(2):388–99. doi: 10.1006/dbio.2001.0202. [DOI] [PubMed] [Google Scholar]
- 11.Patten RM, Ovalle WK. Muscle spindle ultrastructure revealed by conventional and high-resolution scanning electron microscopy. Anat Rec. 1991;230(2):183–98. doi: 10.1002/ar.1092300206. [DOI] [PubMed] [Google Scholar]
- 12.Schroder JM, Bodden H, Hamacher A, Verres C. Scanning electron microscopy of teased intrafusal muscle fibers from rat muscle spindles. Muscle & Nerve. 1989;12(3):221–32. doi: 10.1002/mus.880120311. [DOI] [PubMed] [Google Scholar]
- 13.Albert Yv, Whitehead J, Eldredge L, Carter J, Gao X, Tourtellotte WG. Transcriptional regulation of myotube fate specification and intrafusal muscle fiber morphogenesis. J Cell Biol. 2005;169(2):257–68. doi: 10.1083/jcb.200501156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucera J, Fan G, Walro J, Copray S, Tessarollo L, Jaenisch R. Neurotrophin-3 and trkC in muscle are non-essential for the development of mouse muscle spindles. Neuroreport. 1998;9(5):905–09. doi: 10.1097/00001756-199803300-00026. [DOI] [PubMed] [Google Scholar]
- 15.Kucera J, Walro JM. Axotomy induces fusimotor-free muscle spindles in neonatal rats. Neuroscience Letters. 1992;136(2):216–18. doi: 10.1016/0304-3940(92)90052-9. [DOI] [PubMed] [Google Scholar]
- 16.Soukup T, Novotova M. Ultrastructure and innervation of regenerated intrafusal muscle fibres in heterochronous isografts of the fast rat muscle. Acta Neuropathol (Berl) 2000;100(4):435–44. doi: 10.1007/s004010000194. [DOI] [PubMed] [Google Scholar]
- 17.Soukup T, Thornell LE. Expression of myosin heavy chain isoforms in regenerated muscle spindle fibres after muscle grafting in young and adult rats--plasticity of intrafusal satellite cells. Differentiation. 1997;62(4):179–86. doi: 10.1046/j.1432-0436.1998.6240179.x. [DOI] [PubMed] [Google Scholar]
- 18.Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, Shaffer K, Schaffner AE, Zcribbs DH, Cotman CW. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. Journal of Neuroscience Methods. 1998;82(2):167–73. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 19.Mulder MM, Hitchcock RW, Tresco PA. Skeletal myogenesis on elastomeric substrates: implications for tissue engineering. J Biomater Sci Polym Ed. 1998;9(7):731–48. doi: 10.1163/156856298x00118. [DOI] [PubMed] [Google Scholar]
- 20.Stett A, Egert U, Guenther E, Hofmann F, Meyer T, Nisch W, Haemmerle H. Biological application of microelectrode arrays in drug discovery and basic research. Analytical and Bioanalytical Chemistry. 2003;377(3):486–95. doi: 10.1007/s00216-003-2149-x. [DOI] [PubMed] [Google Scholar]
- 21.Schnorrer F, Dickson BJ. Muscle Building: Mechanisms of Myotube Guidance and Attachment Site Selection. Developmental Cell. 2004;7(1):9–20. doi: 10.1016/j.devcel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Taylor MD, Holdeman AS, Weltmer SG, Ryals JM, Wright DE. Modulation of muscle spindle innervation by neurotrophin-3 following nerve injury. Experimental Neurology. 2005;191(1):211–22. doi: 10.1016/j.expneurol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Arnesen S, Mosler S, Larsen N, Gadegaard N, Purslow P, Lawson M. The Effects of Collagen Type I Topography on Myoblasts In Vitro. Connective Tissue Research. 2004;45(4 5):238. doi: 10.1080/03008200490888424. [DOI] [PubMed] [Google Scholar]
- 24.Yang LX, Nelson PG. Glia cell line-derived neurotrophic factorregulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience. 2004;128(3):497–509. doi: 10.1016/j.neuroscience.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 25.Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A Role for Neuregulin1 Signaling in Muscle Spindle Differentiation. Neuron. 2002;36(6):1035–49. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. PNAS. 2004;101(33):12218–23. doi: 10.1073/pnas.0404240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet. 1998;20(1):87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- 28.Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27(24):4374. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Schaffner AE, Barkerm JL, Stengerm DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods. 1995;62(12):111–9. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 30.Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell Dev Biol Anim. 2005;41(10):343–8. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 31.Das M, Molnar P, Devaraj H, Poeta M, Hickman JJ. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol Prog. 2003;19(6):1756–61. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 32.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Research. 1993;630(12):136–47. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 33.Kuhl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Developmental Biology. 1982;93(2):344–54. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- 34.Daniels M, Lowe BT, Shah S, Ma J, Samuelsson SJ, Lugo B, Parakh T, Uhm C. Rodent nerve-muscle cell culture system for studies of neuromuscular junction development: Refinements and applications. Microscopy Research and Technique. 2000;49(1):26–37. doi: 10.1002/(SICI)1097-0029(20000401)49:1<26::AID-JEMT4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Woods TL, Smith CW, Zeece MG, Jones SJ. Conditions for the culture of bovine embryonic myogenic cells. Tissue and Cell. 1997;29(2):207–15. doi: 10.1016/s0040-8166(97)80020-1. [DOI] [PubMed] [Google Scholar]
- 36.Rudnicki MA, Jackowski G, Saggin L, McBurney MW. Actin and myosin expression during development of cardiac muscle from cultured embryonal carcinoma cells. Developmental Biology. 1990;138(2):348–58. doi: 10.1016/0012-1606(90)90202-t. [DOI] [PubMed] [Google Scholar]
- 37.Daniels MP. Localization of actin, beta-spectrin, 43 × 10(3) Mr and 58 × 10(3) Mr proteins to receptor-enriched domains of newly formed acetylcholine receptor aggregates in isolated myotube membranes. Journal of Cell Science. 1990;97(Pt 4):615–26. doi: 10.1242/jcs.97.4.615. [DOI] [PubMed] [Google Scholar]
- 38.Das M, Molnar P, Gregory C, Riedel L, Jamshidi A, Hickman JJ. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum-free medium. Biomaterials. 2004;25(25):5643–7. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, McCort SM, Calvert JM. Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: characterization and geometric definition of mammalian cell adhesion and growth. J Am Chem Soc. 1992;114:8435–42. [Google Scholar]
- 40.Ravenscroft MS, Bateman K, Shaffer K. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. J Am Chem Soc. 1998;120(47):12169–77. [Google Scholar]
- 41.Das M, Rumsey JW, Gregory CA, Bhargava N, Kang JF, Molnar P, Riedel L, Guo X, Hickman JJ. Embryonic motoneuron-skeletal muscle co-culture in a defined system. Neuroscience. 2007;146(2):481–8. doi: 10.1016/j.neuroscience.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 42.Murphy RA, Singer RH, Saide JD, Pantazis NJ, Blanchard MH, Byron KS, Arnason BG, Young M. Synthesis and secretion of a high molecular weight form of nerve growth factor by skeletal muscle cells in culture. Proc Natl Acad Sci USA. 1977;74(10):4496–5000. doi: 10.1073/pnas.74.10.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Copray S, Liem R, Mantingh-Otter IJ, Brouwer N. Coculture of rat embryonic proprioceptive sensory neurons and myotubes. Muscle Nerve. 1996 Nov;19(11):1401–12. doi: 10.1002/(SICI)1097-4598(199611)19:11<1401::AID-MUS4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.McWhorter DL, Walro JM, Signs SA, Wang J. Expression of alpha-cardiac myosin heavy chain in normal and denervated rat muscle spindles. Neuroscience Letters. 1995;200(1):2. doi: 10.1016/0304-3940(95)12085-i. [DOI] [PubMed] [Google Scholar]
- 45.Kucera J. One-bag-fiber muscle spindles in tenuissimus muscles of the cat. Histochemistry and Cell Biology. 1982;76(3):315–28. doi: 10.1007/BF00543954. [DOI] [PubMed] [Google Scholar]
- 46.Grinnell AD, Chen BM, Kashani A, Lin J, Suzuki K, Kidokoro Y. The role of integrins in the modulation of neurotransmitter release from motor nerve terminals by stretch and hypertonicity. Journal of Neurocytology. 2003;32(5 8):489–503. doi: 10.1023/B:NEUR.0000020606.58265.b5. [DOI] [PubMed] [Google Scholar]
- 47.Maier A, Mayne R. Basal lamina development in chicken muscle spindles. Dev Dyn. 1995;202(3):284–93. doi: 10.1002/aja.1002020307. [DOI] [PubMed] [Google Scholar]
- 48.Pedrosa-Domellof F, Virtanen I, Thornell LE. Tenascin is present in human muscle spindles and neuromuscular junctions. Neuroscience Letters. 1995;198(3):173. doi: 10.1016/0304-3940(95)11986-7. [DOI] [PubMed] [Google Scholar]
- 49.Ito F, Fujitsuka N, Kim N. The spindle potential in the frog muscle spindle does not require external Na+ Brain Research. 1983;277(2):352–4. doi: 10.1016/0006-8993(83)90944-7. [DOI] [PubMed] [Google Scholar]
- 50.Querfurth H. Action-potential initiation and maintained activity of the isolated frog muscle spindle. European Journal of Neuroscience. 2006;24(4):1147–56. doi: 10.1111/j.1460-9568.2006.04983.x. [DOI] [PubMed] [Google Scholar]
- 51.Peng HB, Yang JF, Dai Z, Lee CW, Hung HW, Feng ZH, Ko CP. Differential Effects of Neurotrophins and Schwann Cell-Derived Signals on Neuronal Survival/Growth and Synaptogenesis. J Neurosci. 2003;23(12):5050–60. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelena J. Inductive effect of primary sensory neurons on the development of encapsulated mechanoreceptors. Cesk Fysiol. 1983;32(5):385–400. [PubMed] [Google Scholar]
- 53.Stephens HE, Belliveau AC, Gupta JS, Mirkovic S, Kablar B. The role of neurotrophins in the maintenance of the spinal cord motor neurons and the dorsal root ganglia proprioceptive sensory neurons. International Journal of Developmental Neuroscience. 2005;23(7):613–20. doi: 10.1016/j.ijdevneu.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Seebach BS, Arvanov V, Mendell LM. Effects of BDNF and NT-3 on Development of Ia/Motoneuron Functional Connectivity in Neonatal Rats. J Neurophysiol. 1999;81(5):2398–405. doi: 10.1152/jn.1999.81.5.2398. [DOI] [PubMed] [Google Scholar]


