Abstract
A novel pH-sensitive polymeric micellar system composed of poly(l-Histidine)-b-poly(ethylene glycol) and poly(l-Lactide)-b-poly(ethylene glycol) block copolymers was studied by dynamic/static light scattering, spectrofluorimetry and differential scanning calorimetry. The mixed micelles displayed ultra pH sensitivity which could be tuned by varying the mixing ratio of the two polymers. In particular, mixed micelles composed of 25 wt. % poly(l-Lactide)-b-poly(ethylene glycol) exhibited desirable pH dependency which could be used as a drug delivery system that selectively targeted the extracellular pH of acidic solid tumors. Micelles were quite stable from pH 7.4 to 7.0 but underwent a two-stage destabilization as pH decreased further. A significant increase in size and aggregation number was observed when pH dropped to 6.8. Further disruption of the micelle core eventually caused phase separation in the micelle core and dissociation of ionized poly(l-Histidine)-b-poly(ethylene glycol) molecules from the micelles as pH decreased to 6.0. Increased electrostatic repulsions which arise from the progressive protonation of imidazole rings overwhelming the hydrophobic interactions among uncharged neutral blocks is considered to be the mechanism for destabilization of the micelle core.
Keywords: polymeric mixed micelles, pH sensitive, drug delivery, poly(l-Histidine), tumor pH
1. Introduction
Amphiphilic block copolymers consisting of a hydrophilic segment and a hydrophobic segment self-assemble into polymeric micelles having a hydrophobic core structure stabilized by a hydrophilic shell in aqueous solution. In recent years, polymeric micelles have been extensively investigated for pharmaceutical applications because of their attractive features as drug delivery vehicles [1–6]. Polymeric micelles mimic aspects of the biological transport system in terms of structure and function. A hydrophilic surface prolongs their blood circulation while smaller size (typically 20–200 nm in diameter) prevents recognition and uptake by the reticuloendothelial system. As a result, these nano-vehicles can have relatively long circulation times after intravenous administration and can passively accumulate in solid tumors for example due to the enhanced permeability and retention effect [7]. Furthermore, the critical micelle concentration of polymeric micelles is usually much lower than low molecular weight surfactant micelles, which ensures improved physical stability against dilution after injection into the blood stream.
An important issue in determining the effectiveness of a micellar drug carrier is efficient drug release after reaching target sites (i.e., cancers). This challenge has motivated the development of micelle systems with a triggered release mechanism which enables the carriers to release drug in response to specific external or internal stimuli such as temperature [8, 9], pH [10, 11], ultrasound [12, 13] or enzymes[14]. Among these stimuli, changes in acidity are particularly useful in the development of miceller drug carriers for treating solid-tumor cancers. First, the relatively acidic tumor extracellular pH (pHe) is a distinguishing phenotype of solid tumors from surrounding normal tissues. The measured pHe values of most solid tumors in patients range from pH 5.7 to pH 7.2 [15] while normal blood remains well-buffered and constant at pH 7.4. Moreover, changes in pH are also encountered once the micelle enters cells via endocytosis pathways where pH can drop as low as 5.5–6.0 in endosomes and approaches 4.5–5.0 in lysosomes. In order to take advantage of the acidic nature of tumor tissue and endocytic vesicles, two strategies have been used thus far to introduce pH sensitivity into a micellar system. One approach is to incorporate an acid labile linkage between the drug and the polymer forming the micelles. The cleavage of such chemical bonds by acidic pH can accelerate antitumor drug release from nanovehicles [10]. Another approach is to incorporate pH-sensitive groups such as amines or carboxylic acids into the block copolymers so that the carriers undergo structural destabilization at acidic pH by protonation of these groups [11, 16].
Copolymers with hydrophilic blocks such as PEG and hydrophobic blocks composed of biodegradable poly(amino acids) have the strong potential to be used as drug carriers due to their non-toxicity and biocompatibility. Recently, our group developed such a novel pH-sensitive poly(amino acid) based diblock copolymer —poly(l-Histidine) (Mn~5000)-b-poly(ethylene glycol) (Mn 2000) (referred as PH-PEG) [17]. Poly(l-Histidine) (Mn~5000) was synthesized by ring opening polymerization of l-Histidine N-carboxyanhydride and coupled to poly(ethylene glycol) (Mn 2000) via an amide linkage. The polymer exhibited pKb around 7.0 and a buffering pH region of pH 5.5–8.0 due to the amphoteric nature of imidazole rings on the PH blocks. Polymer micelles constructed from PH-PEG copolymer were about 110 nm in size at pH 8.0 but began to dissociate below pH 7.4. In order to tailor the triggering pH of the polymeric micelles to the more acidic extracellular pH of tumors while improving their stability at pH 7.4, another biocompatible polymer, poly(l-Lactic acid) (Mn 3000)-b-poly(ethylene glycol) (Mn 2000) (referred as PLLA-PEG) was blended with PH-PEG to form mixed micelles [18]. The anticancer drug doxorubicin (DOX) was successfully incorporated into the mixed micelles with a relatively high loading content (15–17 wt.%) and the mixed micelles containing 25 wt.% PLLA-PEG was found to be selectively responsive to extracellular tumor pH. However the physicochemical nature of the mixed micelles and the mechanism of pH dependent structural transitions still remained unexplored. In this work, the pH sensitivity and interior structural features of the mixed micelles were systematically examined, including morphology and anisotropy, thermodynamic and kinetic stability, miscibility of PH and PLLA blocks in the micellar core and pH dependent structural transitions. Based on experimental results, the destabilization mechanism of the mixed micelles is discussed in detail below.
2. Experimental Section
Materials
Z-His(Bzl)-OH, isopropylamine, triethylamine, PEG (Mn: 2000 Da), diethyl -aminoethyl, (DEAE) Sephadex A-25, potassium tetraborate, ammonium bicarbonate, N-hydroxysuccinimide (NHS), N,N′-dicyclohexylcarbodiimide (DCC), anhydrous dimethylformamide (DMF), anhydrous 1,4-dioxane and dimethylsulfoxide (DMSO) were purchased from Sigma Co. Thionyl chloride was purchased from Fluka Co. Potassium tert-butoxide and ethyl bromoacetate were purchased from Acros Organics. Pyrene and diphenyl hexatriene (DPH) were purchased from Sigma Co. and used as received.
Polymer Synthesis
(1) poly(l-Histidine)-b-poly(ethylene glycol)
Synthesis and purification of poly(l-Histidine) (Mn: ~5000 Da)-b-poly(ethylene glycol) (Mn: 2000 Da) followed the methodology established by our group, which can be found in details elsewhere [17, 19]. The molecule weight of the poly(l-Histidine) block determined from lH NMR was 5200 Da (see Figure S1 in the Supporting Information).
(2) poly(l-Lactic acid)-b-poly(ethylene glycol)
PLLA-PEG diblock copolymer was synthesized by ring opening polymerization of l-Lactide initiated by hydroxy group of PEG monoacid (Mn: 2000 Da) in the presence of stannous octoate as a catalyst [20]. The molecule weight of the poly(l-Lactide) block as determined from lH NMR was 2860 Da (Figure S2).
Preparation of polymeric micelles
Since the polymer mixtures are not readily dissolved in water, a dialysis method was employed to fabricate polymeric micelles [2]. 20 mg of PH-PEG and PLLA-PEG mixtures were weighed respectively at predetermined mixing ratios and dissolved in 3 mL DMSO. Subsequently, 2 mL phosphate buffer (pH 8.0, 10mM) was added dropwise into the solution. The resulting solution was vigorously stirred for half an hour and then transferred into a pre-swollen dialysis membrane (SPECTRA/POR; MWCO 3500) and dialyzed against 10 mM phosphate buffer (pH 9.0). The outer phase was replaced with fresh buffer solution at 1, 2, 4, 6, and 12 h. After 24 h, the micelle solution inside membrane was recovered. The yield of mixed micelles from dialysis was c.a. 90 w/w %. Afterwards, the micelle solution was diluted and adjusted to a predetermined pH with a CORNING 443i pH meter by adding 1 N HCl stock solution.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were carried out with a Brookhaven Instruments Corp. system consisting of a BI-200SM goniometer and a BI-9000AT autocorrelator. The solutions were filtered prior to measurements using a 0.80-μm disposable membrane filter. The results were analyzed by the constrained regularized CONTIN method to yield information on the distribution of the characteristic line width (Γ). The normalized distribution function of the characteristic line width <Γ> so obtained can be used to determine an average apparent diffusion coefficient.
| (1) |
where q=4πnsin(θ/2)/λ is the magnitude of the scattering wave vector.
The apparent hydrodynamic radius Rh,app is related to Dapp via the Stocks-Einstein equation:
| (2) |
where κB is the Boltzmann constant and η is the viscosity of water at temperature T. From DLS measurements, we can obtain the particle-size distribution in solution from a plot of normalized ΓG(Γ) versus Rh,app, with ΓiG(Γi) being proportional to the scattering intensity of particle i having an apparent hydrodynamic radius Rh,i. The value of the relative variance (u2/<Γ>2) obtained from the size distribution plot is considered as the size polydispersity (PI).
Static light scattering
Static light scattering (SLS) measures the angular dependence of the excess absolute time-averaged scattered intensity, known as the excess Rayleigh ratio ΔR(q). In the limit of a dilute solution, the reciprocal δR(q) follows the relationship
| (3) |
where K =4π2n2(dn/dC)2/(NAλ4), n is the solvent refractive index, dn/dC is the specific refractive index increment, NA is Avogadro’s number, λ is the wavelength of theincident beam in vacuo (632.8 nm), q is the scattering wave vector, <Rg> is the z-average gyration of radius, Mw is the weight-average molar mass, and A2 is the second virial coefficient. The dn/dC for PH-PEG/PLLA-PEG (75/25, wt.%) mixtures in the buffer solution is 0.139 mL.g−1, which was obtained using a OPTILAB DSP Interferometeric Refractometer (Wyatt Tech. Co.).
Fluorescence measurements
All fluorescence measurements were performed using an SHIMADZU RF-5301PC spectrofluorometer with a cylindrical quartz cuvette. Pyrene and 1,6-diphenyl-1,3,5-hexatriene (DPH) were used as fluorescence probes to analyze the block copolymers in the buffer solution. The final concentration of pyrene and DPH in the test sample was 0.6 μM and 4 μM respectively. The pyrene excitation at λem=393 nm was recorded for the critical micelle concentration (CMC) determinations.
The steady-state fluorescence anisotropy value of DPH was determined in the L-format geometry of detection. The excitation wavelength was 357 nm, and the emission was measured at 430 nm. The anisotropy value (r) was calculated from the following relationship:
| (4) |
where IS is the contribution of scattered light from a sample solution without DPH; G= IHV/IHH is the instrumental correction factor; and IVV, IVH, IHV, and IHH refer to the resultant emission intensities polarized in the vertical or the horizontal detection planes (second subindex) when excited with vertically or horizontally polarized light (first subindex) [21].
DSC measurements
Thermal properties of polymers were observed by differential scanning calorimetry (DSC). Samples weighing 3–5 mg were analyzed with a METTLER TOLEDO 821e DSC in sealed aluminium pans. The flowing rate of N2 was controlled at 80 mL.min−1. The samples were cooled to −50 °C and heated to 250 °C with a heating rate of 20 °C.min−1. The sample was prepared by lyophilizing the mixed PH-PEG/PLLA-PEG micellar solution. The heating–cooling cycle was repeated twice and the glass transition temperature (Tg) was obtained at the second cycle.
3. Results and discussion
3.1 pH dependency of mixed micelles as a function of the PH-PEG/PLLA-PEG mixing ratios
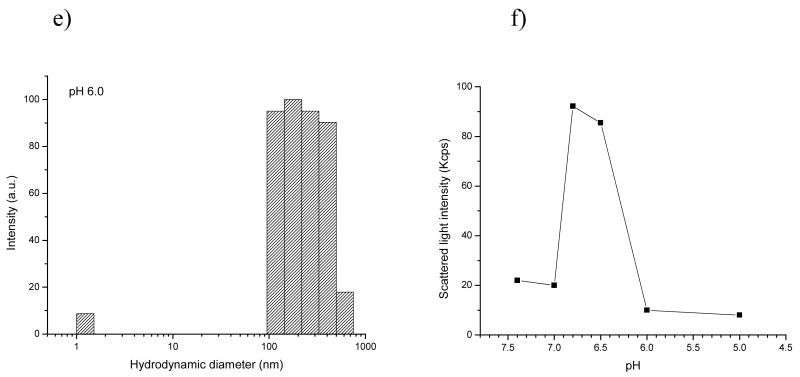
The pH dependent transitions of single PH-PEG and PLLA-PEG micelles were respectively studied by DLS at first as the control experiments (Figure S3). The PH-PEG micelles were stable at pH 8.0 while the micelles began to dissociate into unimers below pH 7.4 till complete disintegration at pH 6.0. For the PLLA-PEG micelles, no obvious size change was detected within the studied pH range due to its pH insensitivity. Subsequently, pH dependency of the PH-PEG/PLLA-PEG mixed micelles was investigated at different mixing ratios (95/5, 90/10, 75/25, and 60/40 wt.%). Examples of intensity fraction distributions of micelle size are shown in Figure 1 for the PH-PEG/PLLA-PEG (75/25 wt.%) system using a total polymer concentration of 0.1 mg.mL−1. At pH 7.4, the system exhibited a single modal distribution with a hydrodynamic diameter of 129±10 nm (Figure 1.a). From pH 7.4 to 7.0, size of the micelle size remained unchanged (132±11 nm at pH 7.0 in diameter, Figure 1.b). However, an obvious size increase (195±18 nm ) took place as pH decreased from 7.0 to 6.8 (Figure 1.c). At the same time, a significant increase in scattered intensity at 90° was detected (Figure 1.f). The above results indicate that the micelles underwent a remarkable structural transition upon changing pH from 7.0 to 6.8 and correspondingly pH 6.8 is referred as the pHt (triggering pH) for the system. The size of the micelles further increased to 232±25 nm as pH decreased to 6.5 (Figure 1.d). Upon dropping the pH to 6.0, a new peak appeared in the DLS plot with the size 2.0±0.5 nm in diameter (Figure 1.e), accompanied by a dramatic decrease in scattered intensity (Figure 1.f). Similar transitions were observed for the other PH-PEG/PLLA-PEG mixing ratios (95/5, 90/10, 60/40, wt.%) (Figure S4). Interestingly, the triggering pH (pHt) was found to decrease as the PLLA-PEG fraction in the system increased (Figure 2). However, when the PLLA-PEG fraction reached 50% (wt.), the size of the mixed micelle did not change from pH 8.0 to 6.0 (139±5 nm in diameter, DLS data were not shown here), suggesting the micelles probably lost pH sensitivity within the studied pH range. It can be seen that among all the mixing ratios investigated, the 75/25 wt.% mixed system showed a desirable pHt (6.8), which may be specifically responsive to the relatively acidic extracellular pH of tumors [15]. In the following sections, systematic studies were performed using this system.
Figure 1.
DLS Plots for PH-PEG/PLLA-PEG (75/25, wt.%) mixed micelles (C=0.1 mg.mL−1) as a function of pH (a–e) and scattered light intensity at 90° vs. pH (f).
Figure 2.

The change of triggering pH (pHt) for the structural transition as a function of PLLA-PEG fraction in the mixed micelles.
3.2 Physicochemical properties of the mixed micelles at pH 7.4
3.2.1 CMC, pyrene partition equilibrium constant and microviscosity
The physicochemical properties of PH-PEG/PLLA-PEG (75/25 wt.%) mixed micelles were studied in pH 7.4 phosphate buffer with an ion strength of 0.15 mol.kg−1 to mimic physiological conditions. The CMC was determined by the characteristic feature of pyrene excitation spectra where a shift of the (0,0) band from 333 to 336 nm was observed upon micellization [22, 23]. The CMC was 6.5 μg.mL−1 for the mixed system as derived from Figure 3.a, which is much lower than low molecular weight surfactants, e.g., 2.0 mg.mL−1 for sodium dodecyl sulfate (SDS) in water and comparable to other polymeric amphiphiles [22, 23].
Figure 3.
Plots of a) I336/I333 (from pyrene excitation spectra) vs. polymer concentration (the solid arrow indicated the CMC) and b) (F − Fmin)/(Fmax − F) vs. C − CMC for the PH-PEG/PLLA-PEG (75/25, wt.%) mixed micelles (C=0.1 mg.mL−1).
The hydrophobicity of the micellar core was estimated by measuring the partition equilibrium constant Kv of pyrene according to the method reported by Wilhelm et al.[23]:
| (5) |
where Fmax and Fmin correspond to the average magnitude of I336/I333 in the flat region of high and low concentration ranges in Figure 3.a, and F is the intensity ratio (I336/I333) in the intermediate concentration range; Vm and Vw are the volume of the hydrophobic core and water phase in the micelles respectively. For the mixed system, eq. 5 can be expressed as:
| (6) |
where C is the total mixed polymer concentration; xi is the weight fraction of hydrophobic blocks in each polymer and ρi (i=PLLA, PH) is the density of the hydrophobic blocks in each polymer, which is assumed to be the value of bulk poly(l-Lactide) (1.25 g.cm−3) [24] and poly(l-Histidine) (1.44 g.cm−3) [25] respectively. From the slope of the graph in Figure 3.b, the Kv value of the system was determined to be 2.4×104. The microviscosity of the micellar hydrophobic microdomain was estimated by the measurement of the steady-state fluorescence anisotropy originating from the depolarization of DPH fluorescence. Generally the anisotropy value increases with increasing microviscosity of the micellar core because the rotational diffusion of DPH is increasingly hindered [26]. The anisotropy value, r, measured for the mixed micelles is 0.315 at pH 7.4. It is worth comparing that the r value is much higher than that for micelles from low molecular surfactants (0.070 for SDS) [27]. Both the pyrene partition constant and anisotropy results indicate the mixed micelles have a relatively hydrophobic core with a high degree of rigidity.
3.2.2 Microstructure and Morphology
The average hydrodynamic diameter measured by DLS at 90° is 129±10 nm at a total polymer concentration of 0.1 mg.mL−1 with a relatively low polydispersity index (PDI=0.02) (Figure 1.a). In addition, the apparent diffusion rate (Dapp) was found to be identical as the scattering vector (q) changed (Figure S5), indicating that the micelles are spherical and isotropic because of their undetectable rotational motion [28]. In fact, the regular spherical morphology was also demonstrated by AFM observation for PH-PEG micelles in our previous report [17]. In general, the size of individual core-shell type micelles from an amphiphilic diblock polymer is in the range of several tens of nanometers [23]. However, micelles with a size range of several hundred nanometers are often observed due to intermicellar aggregation in amphiphilic block copolymer systems [28–30]. Therefore, we assume that the micelles formed from PH-PEG/PLLA-PEG mixtures would also be secondary aggregates further associated with individual micelles [31]. Nevertheless, it is interesting to notice that the mixed micelles still possess a spherical morphology in spite of having a multi-core structure.
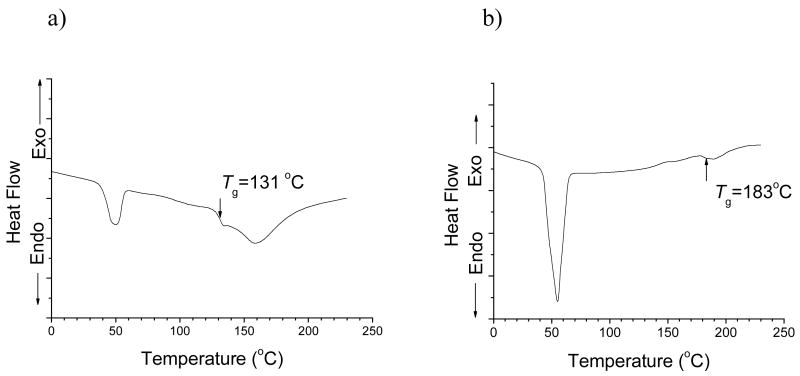
When the two polymers are blended to form secondary micelles as mentioned above, three possibilities should be considered with respect to composition. The first situation is to form two different kinds of secondary structures composed of merely PH-PEG or PLLA-PEG. The second situation is to form a uniform secondary structure through the association of primary micelles with heterogeneous cores, i.e. PLLA core or PH core. The third situation is to form a uniform secondary structure through the association of primary micelles with homogenous cores where PH and PLLA coexist. The narrow size distribution polydispersity (PDI=0.02) of the micelles (Figure 1.a) implies the co-micellization of the two copolymers, which excludes the first situation. Indeed, considering the positive transfer of entropy of mixing, formation of a homogenous core for the primary micelles should be favored. Differential scanning calorimetry (DSC) was performed to further study the thermal properties of the PH-PEG/PLLA-PEG mixtures. The glass transition temperatures (Tg) of a polymer blend is known as an important criteria for the miscibility of components. The thermograms obtained for PH-PEG/PLLA-PEG mixtures (75/25, wt.%) is shown in Figure 4.a. The two endothermic peaks appearing at 50°C and 158°C should be assigned to the melting behaviors of PEG block and PLLA block respectively [32]. In addition, a characteristic secondary transition was observed at 131°C, which would probably be attributed to the glass transition of PH/PLLA blends. It is also worth to note that the Tg is intermediate between those of PLLA (40~50°C) [32] and PH (183°C, Figure 4.b). The presence of a single Tg in a broad composition range situated between the Tg’s of the individual components indicates miscibility of the poly(l-Histidine)/poly(l-Lactide) blends [33]. Therefore, it could be deduced that the secondary structure is probably caused by the association of single primary micelles with a homogenously-mixed PH/PLLA core.
Figure 4.
DSC heating curves of a) PH-PEG/PLLA-PEG mixtures (75/25,wt.%) and b) PH-PEG. The glass transition temperature for each sample was indicated by a solid arrow.
The radius of gyration (Rg), the weight-average molecular weight (Mw(mic)) and the second viral coefficient (A2) were determined by SLS. Figure S6 shows a typical Zimm plot of PH-PEG/PLLA-PEG mixed micelles in pH 7.4 buffer solution. On the basis of eq. 6, Mw(mic), Rg and A2 were calculated to be (5.6±0.1)×106 g.mol−1, 104±1 nm and –(2.35±0.36)×10−5 respectively. The negative value of A2 indicates a relatively high hydrophobic micelle core [34, 35]. The aggregation number (Nagg) for the mixed micelles was determined to be 848 using the value of Mw(mic). It is worth mentioning that the obtained Mw(mic) should be attributed to the entire secondary structure rather than single core-shell primary micelles, which is also consistent with the quite large Nagg value. The density of the micelles was calculated on the basis of its spherical feature: where Na is Avogadro’s number and Rh is the hydrodynamic radius from DLS. ρ mic is 8.0×10−3 g.mL−1 for this system which is much lower than the density of water, probably due to the stretching of hydrated PEG blocks. In addition, it was found the micelle size remained unchanged for 2 weeks with only a slight increase in size observed afterwards, indicating that the micelles had considerably high thermodynamic stability although the secondary structure was adopted.
3.3 pH-induced structural transitions from pH 7.4 to 6.0
As previously demonstrated by DLS, the size of the micelles remained unchanged from pH 7.4 to 7.0 whereas an obvious increase in size was detected when pH further decreased to 6.8. SLS measurements were also performed for mixed micelles at pH 7.0, 6.8 and 6.5 respectively. A series of physicochemical properties including micelle weight-average molecular weight (Mw(mic)), radius of gyration (Rg), micelle aggregation number (<Nagg>), micelle density (ρ(mic)) and the second virial coefficient (A2) etc. were obtained from SLS and are compared in Table 1. At pH 7.0, all the physicochemical parameters are very similar to those at pH 7.4. Therefore it can be considered that the micelles still maintained stability from pH 7.4 to 7.0. However, as pH decreased to 6.8, an obvious increase in Mw(mic), <Nagg> and Rg was observed, implying a significant structural change in the micelles. The increase in A2 (from −2.20×10−5 to 1.26×10−5 cm3.mol.g−2) indicates increasing solubility of the polymer in the solution, which is probably attributed to the decreasing hydrophobicity of the micelle core. At the same time, it is worth mentioning that the spherical morphology of the micelles was retained at pH 6.8 due to its angle independence of Dapp (Figure S5). The transition continued and micelles became even larger as pH decreased to 6.5, as can be seen from Table 1. As pH reached 6.0, small particles with ~2 nm in diameter were detected by DLS, which indicates another transition step took place. We assume that these small particles were probably ionized hydrophilic PH-PEG unimers dissociated from the micelles.
Table 1.
Physicochemical parameters obtained from SLS for the PH-PEG/PLLA-PEG (75/25, wt.%) mixed micelles
| PH | <Mw(mic)> / g.mol−1 | <Nagg> | Rg / nm | ρ(mic) /g.cm−3 | A2 / cm3.mol.g−2 |
|---|---|---|---|---|---|
| 7.4 | (5.6±0.1)×106 | 0.85×103 | 104±1 | 8.1×10−3 | −(2.35±0.36)×10−5 |
| 7.0 | (5.5±0.1)×106 | 0.83×103 | 102±2 | 7.9×10−3 | −(2.2±0.6)×10−5 |
| 6.8 | (8.1±1.2)×106 | 1.22×103 | 198±14 | 3.5×10−3 | (1.26±0.94)×10−5 |
| 6.5 | (1.1±0.3)×107 | 1.67×103 | 267±40 | 0.95×10−3 | (1.9±1.2)×10−5 |
3.4 Effect of PLLA-PEG on the stability of mixed micelles and the mechanism of pH triggered micelle destabilization
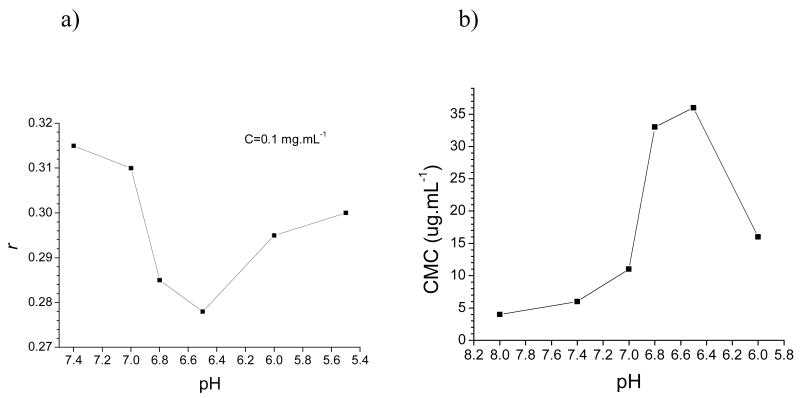
The above results reveal that the micelles were quite stable from pH 7.4 to 7.0 while a significant destabilization in the micelle core took place as pH decreased to 6.8. We consider that ionization of the PH block plays an important role in the pH responsive transition of the micelles. As was reported, PH-PEG showed pKb around 7.0 and had a buffering pH region of pH 5.5–8.0 owing to the amphoteric nature of the imidazole ring on the PH block [17]. As pH decreased, the progressive protonation of the imidazole groups increased the electrostatic repulsions between PH blocks and made them less hydrophobic, leading to the dissociation of the PH-PEG micelles below pH 7.4. On the other hand, PLLA-PEG itself can form micelles with a relatively lower CMC (4.0 μg.mL−1) and higher partition constant of pyrene (Kv=1.5×105) (Figure S7) than those of PH-PEG. Considering that both polymers have the same PEG block length, it is likely that the former one has a more hydrophobic core than the latter. It can be expected that compared to PH-PEG micelles, the intra-micellar hydrophobic interactions in the mixed micelles will be strengthened by the presence of PLLA-PEG. This trend combined with the pH insensitivity of PLLA-PEG will give the micelle core enhanced stability against electrostatic repulsions. Therefore micelle stability was hardly compromised from pH 7.4 to 7.0 and its structure remained almost unaltered. When pH decreased to 6.8, however, electrostatic repulsions between PH blocks began to overcome the hydrophobic interactions, leading to destabilization of the micelle core. As a result, the micelles began to swell to balance the increasing electrostatic repulsions between PH blocks and a significant increase in micelle size and aggregation number took place. Nevertheless, the intra-micellar hydrophobic interactions were still effective to maintain a homogenously mixed core. This transition continued as the pH decreased to 6.5. When pH reached 6.0, the electrostatic repulsions became so strong that the PH blocks could no longer hold together inside the core. As a consequence, phase separation occurred in the core and PH-PEG unimers dissociated from the mixed micelles, leaving aggregates mainly constituted by PLLA-PEG. The microstructure transitions were further revealed by changes of the DPH anisotropy (r) and CMC with pH (Figure 5.a, Figure 5.b). A sharp decrease in r value and an obvious increase of CMC were detected when pH changed from 7.0 to 6.5, which indicates the decreasing microviscosity and hydrophobicity of the destabilized micelle core. However as pH dropped from 6.5 to 6.0, there was an increase in r value instead, probably reflecting a fair degree of rigidity in the microdomains of the remaining aggregates. At the same time, the CMC also decreased (Figure 5.b) and the equivalent value for PLLA-PEG (4.5 ug.mL−1) at pH 6.0 is close to CMC of the single PLLA-PEG micelles (4 ug.mL−1). This implies the micelles formed by the polymer mixtures at CMC were probably mostly composed of PLLA-PEG while PH-PEG existed as unimers, consistent with the phase separation found in the mixed micelle core in this instance. Based on the above discussion, schematic illustrations of the micelle destabilization mechanism are suggested in Figure 6. It should be mentioned that these illustrations may not be thoroughly verified and further studies are still going on in our laboratory. In addition our results also revealed the pH dependency of the mixed system can be tailored within certain range by variations of the mixing ratio of the two polymers. Increasing the fraction of PLLA-PEG will enhance the stability of the micelles against pH drops and lower the triggering pH for the structural destabilization and vice versa.
Figure 5.
Plots of a) anisotropy vs. pH and b) CMC vs. pH for the PH-PEG/PLLA-PEG (75/25 wt.%) mixed micelles.
Figure 6.
Schematic illustrations of micelle destabilization. From pH 7.4 to 7.0, the polymer mixtures formed a spherical secondary structure which was formed by the association of individual core-shell micelles with relatively hydrophobic cores; from pH 6.8 to 6.5, an obvious increase of size and aggregation number was induced by the significant destabilization of the micelle core; when pH reached 6.0, phase separation took place in the micelle core, leading to dissociation of PH-PEG from the micelles.
3.5 Dissociation kinetics of the mixed micelles upon dilution to a concentration below CMC
It is known that a drug delivery system is subject to a “sink condition” or severe dilution upon intravenous injection into an animal or human subject. The kinetic stability, i.e. the rate of micelle dissociation into unimers is also an important parameter in evaluating the micellar delivery system. Here, DPH was incorporated into the micelles to monitor the dissociation kinetics of the mixed micelles upon dilution to a concentration below CMC (see the experimental section). As the micelles dissociated, the fluorescence intensity of DPH decreased gradually from the initial intensity (I0) until equilibration, and the infinite intensity (Ii) was recorded. At time t, the fluorescent intensity was It. The fraction of polymers that remained as micelles at time t after dilution can be expressed as [36]:
| (7) |
The changes of fi as a function of time at 7.4, 7.0 and 6.8 were calculated using eq. 7 and shown in Figure 7. The initial rate of dissociation followed first-order kinetics; the rate constants for the three pHs were 0.008, 0.009, and 0.087 s−1, respectively. It can be noticed the system had a relatively slow dissociation rate at pH 7.4 and 7.0 with the corresponding half life (t1/2) c.a. 85 min and 75 min probably due to the high rigidity of the micelle core. However, a much faster dissociation rate was observed with the t1/2 only 8 min when pH decreased to 6.8. The decrease in kinetic stability can be mainly attributed to the overwhelming electrostatic repulsions that disrupted the micelle core in this instance.
Figure 7.
Dissociation kinetics of the PH-PEG/PLLA-PEG (75/25, wt.%) mixed micelles as indicated by the fraction of polymers that remained as micelles upon dilution below CMC as a function of time. At the time of the study, 20 μl mixed micelle solution (100 μg.mL−1) was injected into 2.0 ml phosphate buffer (pH 7.4, 7.0 and 6.8). The final polymer concentration was c.a. 1 μg.mL−1 which is below its CMC. The fluorescence intensity of DPH λex=357 nm, λem=430 nm) was recorded as a function of time.
4. Conclusion
In conclusion, PH-PEG/PLLA-PEG (75/25 wt.%) mixed micelles exhibited ultra pH sensitivity and could respond to minute pH changes and selectively target extracellular pH of tumors. A two-stage destabilization process was revealed when the pH changed from 7.4 to 6.0. First significant destabilization of the micelle core occurred when pH dropped from 7.0 to 6.8, which induced an increase in micelle size and aggregation number. As pH went down to 6.0, further disruption of the micelle core caused ionized PH-PEG unimers to dissociate from the micelles. In addition, the pH dependency of the mixed micelles was found to be influenced by the mixing ratio of the two polymer components. Thus fabrication of polymeric micelles by mixing a pH sensitive polymer and a pH insensitive polymer may provide us with an innovative way of tuning the pH sensitivity of a mixed system. Future studies will explore the interaction between the polymers and drug (i.e., doxorubicin) and how the interactions influence the physicochemical properties and pH dependency of drug-loaded micelles.
Supplementary Material
Acknowledgments
The first author appreciates Prof. C. A. Wight and Dr. J. Wang’s help with DSC measurements, Dr. J. Y. Yang’s help with dn/dC measurements, and editorial aid from Ph. D candidate Deepa Mishra. This work was supported by NIH CA101850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kakizawa Y, Kataoka K. Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev. 2002;54(2):203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(23):137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 3.Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20(5):357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- 4.Bae YS, Diezi TA, Zhao A, Kwon GS. Mixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agents. Journal of Controlled Release. 2007;122:324–330. doi: 10.1016/j.jconrel.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Yi Y, Yoon HJ, Kim BO, Shim M, Kim SO, Hwang SJ, Seo MH. A mixed polymeric micellar formulation of itraconazole: Characteristics, toxicity and pharmacokinetics. J Control Release. 2007;117(1):59–67. doi: 10.1016/j.jconrel.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Rijcken CJF, Soga O, Hennink WE, van-Nostrum CF. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: An attractive tool for drug delivery. Journal of Controlled Release. 2007;120(3):131–148. doi: 10.1016/j.jconrel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 8.Chung JE, Yokoyama M, Aoyagi T, Sakurai Y, Okano T. Effect of molecular architecture of hydrophobically modified poly(N-isopropylacrylamide) on the formation of thermoresponsive core-shell micellar drug carriers. J Control Release. 1998;53(1–3):119–130. doi: 10.1016/s0168-3659(97)00244-7. [DOI] [PubMed] [Google Scholar]
- 9.Na K, Lee KH, Lee DH, Bae YH. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci. 2006;27(2–3):115–222. doi: 10.1016/j.ejps.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42(38):4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 11.Leroux J, Roux E, Le Garrec D, Hong K, Drummond DC. N-isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J Control Release. 2001;72(1–3):71–84. doi: 10.1016/s0168-3659(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 12.Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102(1):203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery--a general review. Expert opinion on drug delivery. 2004;1(1):37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veronese FM, Schiavon O, Pasut G, Mendichi R, Andersson L, Tsirk A, Ford J, Wu G, Kneller S, Davies R JD. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate Chem. 2005;16(4):775–784. doi: 10.1021/bc040241m. [DOI] [PubMed] [Google Scholar]
- 15.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49(16):4373–4384. [PubMed] [Google Scholar]
- 16.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. Journal of Controlled Release. 2007;118(2):216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90(3):363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91(1–2):103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim GM, Bae YH, Jo WH. pH-induced micelle formation of poly(histidine-co-phenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromolecular Bioscience. 2005;5(11):1118–1124. doi: 10.1002/mabi.200500121. [DOI] [PubMed] [Google Scholar]
- 20.Kil S, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids and Surfaces A: Physicochem Eng Aspects. 2003;214:49–59. [Google Scholar]
- 21.Ringsdorf H, Venzmer J, Winnik FM. Fluorescence studies of hydrophobically modified poly(N-isopropylacrylamides) Macromolecules. 1991;24:1678. [Google Scholar]
- 22.Kabanov AV, Nazarova IR, Astafieva IV, Batrakova EV, Alakhov VY, Yaroslavov AA, Kabanov VA. Micelle Formation and Solubilization of Fluorescent Probes in Poly(oxyethylene-b-oxypropylene-b-oxyethylene) Solutions. Macromolecues. 1995;28(7):2303–2314. [Google Scholar]
- 23.Wilhelm MZ, Wang C, Xu Y, Winnik R, Mura MA, Riess GCJ. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe study. Macromolecues. 1991;24(5):1033–1040. [Google Scholar]
- 24.Chen HL, Liu HH, Lin JS. Microstructure of Semicrystalline Poly(L-lactide)/Poly(4-vinylphenol) Blends Evaluated from SAXS Absolute Intensity Measurement. Macromolecules. 2000;33:4856–4860. [Google Scholar]
- 25.Oshima R, Kumanotani J. Spherulites of Poly(L-histidine hydrochloride) Macromolecules. 1986;19:938–941. [Google Scholar]
- 26.Lakowicz JR. Principles of Fluorescence Spectroscopy. Plenum Press; New York: 1983. [Google Scholar]
- 27.McGlade MJ, Randall FJ, Tcheurekdjian N. Fluorescence probe studies of aqueous solution interaction between sodium dodecyl sulfate and anionic polyelectrolytes. Macromolecules. 1987;20:1782. [Google Scholar]
- 28.Xu RL, Winnik MA, Hallett FR, Riess G, Croucher MD. Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecues. 1991;24(1):87–93. [Google Scholar]
- 29.Chu B. Structure and Dynamics of Block Copolymer Colloids. Langmuir. 1995;11:414–421. [Google Scholar]
- 30.Allen C, Yu Y, Maysinger D, Eisenberg A. Polycaprolactone-b-poly(ethylene Oxide) Block Copolymer Micelles as a Novel Drug Delivery Vehicle for Neurotrophic Agents FK506 and L-685,818. Bioconjugate Chem. 1998;9(5):564–572. doi: 10.1021/bc9702157. [DOI] [PubMed] [Google Scholar]
- 31.The mechanism on the formation of secondary structures was briefly discussed in the Supporting Information.
- 32.Lee SH, Kim SH, Han YK, Kim YH. Synthesis and Characterization of Poly(ethylene oxide)/Polylactide/Poly(ethylene oxide) Triblock Copolymer. Journal of Polymer Science: Part A: Polymer Chemistry. 2002;40:2545–2555. [Google Scholar]
- 33.Meaurio E, Zuza SJR. Miscibility and Specific Interactions in Blends of Poly(L-Lactide) with Poly(Vinylphenol) Macromolecues. 2005;38:1207–1215. [Google Scholar]
- 34.Schatz C, Pichot C, Delair T, Viton C, Domard A. Static Light Scattering Studies on Chitosan Solutions: From Macromolecular Chains to Colloidal. Dispersions Langmuir. 2003;19(23):9896–9903. [Google Scholar]
- 35.Villetti MA, Borsali R, Crespo JS, Soldi V, Fukada K. Static and dynamic light scattering of polyelectrolyte/surfactant solutions: the Na-hyaluronate/(C(10)TAB) system. Macromol Chem Phys. 2004;205:907–917. [Google Scholar]
- 36.Sou K, Endo T, Takeoka S, Tsuchida E. Poly(ethylene glycol)-Modification of the Phospholipid Vesicles by Using the Spontaneous Incorporation of Poly(ethylene glycol)-Lipid into the Vesicles. Bioconjugate Chem. 2000;11:372–379. doi: 10.1021/bc990135y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.