Abstract
The aim of this study was to develop and validate an oligonucleotide suspension array for rapid identification of 15 bacterial species responsible for bacteremia, particularly prevalent in Chinese hospitals. The multiplexed array, based on the QIAGEN LiquiChip Workstation, included 15 oligonucleotide probes which were covalently bound to different bead sets. PCR amplicons of a variable region of the bacterial 23S rRNA genes were hybridized to the bead-bound probes. Thirty-eight strains belonging to 15 species were correctly identified on the basis of their corresponding species-specific hybridization profiles. The results show that the suspension array, in a single assay, can differentiate isolates over a wide range of strains and species, and suggest the potential utility of suspension array system to clinical laboratory diagnosis.
Keywords: Oligonucleotide array, Bacteremia, 23S rRNA, Multiplexed detection
INTRODUCTION
Bacteremia is the presence of bacteria circulating in the blood, and is the principal means by which local infections spread to distant organs. The situation demands timely and intensive management, especially with antimicrobial therapy, lest it progresses to generalized sepsis with shock (Khayr et al., 2003). The causative agents of bacteremia typically involve about 50 common species (Anthony et al., 2000), a number which could theoretically be encompassed by a single array-based assay, whose speed and comprehensiveness could play an important role in clinical diagnosis and therapy. Traditional blood culture techniques are slow and often insufficiently sensitive, especially in the case of fastidious organisms or where preliminary antibiotics treatment has already been attempted (Peters et al., 2004).
For the purpose of rapid detection of multiple pathogens, oligonucleotide array analysis following universal PCR amplification has been commonly recognized as having many advantages, and numerous detection and identification systems have been developed (Fukushima et al., 2003; Peters et al., 2004; Troesch et al., 1999).
A highly significant difference among methods is the array substrate. Planar glass microarrays are currently the most widely used platform, and offer an almost unlimited multiplex capacity. However, the requisite hardware for array printing and signal reading is expensive. Moreover, once designed such “fixed” arrays are not easily modified. In short, for most clinical laboratories, fixed planar arrays are neither affordable nor practicable (Bryant et al., 2004). Where dimensionality is not an issue, that is, where the number of separate oligonucleotide probes synchronously required for a particular detection task is below one hundred per analysis sample, suspension arrays seem an attractive alternative, and offer high-throughput automated analysis (Bovers et al., 2007; Nolan and Sklar, 2002; Schmitt et al., 2006).
The principle of suspension array is that target molecules interact with bead-bound probes in aqueous suspension. The particular bead set, and thus probe-type, to which a given target molecule binds is identified by its characteristic color code (classification code) which is determined from the different rations of red fluorophores incorporated into the beads. The quantity of target DNA hybridizing to a particular bead-type is measured by green fluorescence, and imparted by a specific reporter molecule attached to the target; conjunction of probe fluorescence with the classification code fluorescence is recorded by the instrumentation. A current limitation is that only 100 different classifications codes are commercially available.
Bead-based technology allows miniaturization of assay reaction volumes and measurement of multiplexed biological reactions simultaneously in a single reaction vessel (Kettman et al., 1998). Because the hybridization is carried out in aqueous suspension, reaction kinetics is faster than that with planar, solid-phase microarrays (Cai et al., 2000; Henry et al., 1999). Assay design is also more flexible; for instance, addition of a new probe to an assay is a simple matter of adding an additional bead-type to the existing bead set.
The ultimate resolution of any microarray (whether suspension or planar) depends on the combination of PCR primers and gene probes selected (Fukushima et al., 2003; Troesch et al., 1999). By choosing more conserved sequences in the 16S rRNA gene, one can construct more “universal” primers and probes (Vandamme et al., 1996; Woese, 1987) at the expense of specificity. For resolving closely related species or subspecies, e.g., among the Enterobacteriaceae (Dauga, 2002), the more variable (and twice as long) 23S rRNA gene offers advantages (Christensen et al., 1998; Leffers et al., 1987).
The 23S rRNA gene has been assessed for several bacteria pertinent to bacteremia diagnosis by Anthony et al.(2000), who implemented these probes on planar membrane-based arrays. Their strategy employs non-specific quasi-universal PCR primers, with specific probes capable of detecting species-dependent sequence variations within the PCR product. This system of primers and probes identifies almost all bacteria commonly causing bacteremia in China (Ding et al., 2004). In this study, we have taken the universal 23S PCR primers and specific detection probes developed by Anthony et al.(2000), and have adopted them to implementation in a suspension array environment.
MATERIALS AND METHODS
Bacterial strains
The strains used in this study were 34 clinical isolates collected from the First Affiliated Hospital of Zhejiang University, Hangzhou, China, from April 2004 to August 2005. These isolates were identified by standard culture techniques (using either biochemical methods or the Vitek-AMS test system of BioMerieux, France). Four reference strains were also included, namely Enterococcus faecalis (ATCC 29212), Pseudomonas aeruginosa (ATCC27853), Staphylococcus aureus (ATCC25923), and Listeria monocytogenes (NICPBP54001); these were obtained from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (NICPBP).
DNA extraction from pure cultures of organisms
Each isolate was streaked on either blood agar or Mueller-Hinton agar medium (Oxoid Ltd., UK). A single colony of overnight culture was suspended in 50 µl DNA extraction buffer (pH 7.6, 10 mmol/L Tris-HCl, 5 mmol/L EDTA, 0.5% SDS; filtered through a filter of 0.22-μm pore size), boiled for 10 min, and then centrifuged at 15 000×g for 5 min. The supernatant samples were stored at −20 °C for use.
Universal PCR
Standard laboratory practice to minimize contamination was adhered to at all times: dedicated rooms, pipettors, centrifuges and the use of ultra-pure sterilized water (Kwok and Higuchi, 1989). Primers were synthesized by Invitrogen Biotechnology Company (Shanghai, China), and designed to amplify conserved regions of 23S rRNA gene for bacterial species. The sequences of the primers (Anthony et al., 2000) were as follows: forward, 5′-GCG ATT TCY GAA YGG GGR AAC CC; and reverse, 5′-biotin-TTC GCC TTT CCC TCA CGG TAC (where Y is C or T, and R is A or G). PCR amplification was carried out in a total volume of 50 µl. The reaction mixture contained 10× PyrobestTaq PCR buffer (Takara Biotechnology Ltd., Dalian, China), 200 µmol/L (each) deoxynucleotide triphosphate (Takara Biotechnology Ltd.), 1.25 U of PyrobestTaq DNA polymerase (Takara Biotechnology Ltd.), 20 pmol of forward primer, 40 pmol of reverse primer, and 2 µl of template DNA solution. Ultra-pure sterilized water was used in place of DNA as negative control. Amplification was performed using a PTC-200 Peltier thermal cycler (MJ Research Inc., USA). Thermal cycles were 5 cycles of 95 °C for 15 s, 55 °C for 15 s and 72 °C for 15 s, followed by 21 cycles of 95 °C for 15 s and 65 °C for 30 s. PCR products were verified by 2.0% agarose electrophoresis and ethidium bromide staining, and no bands were detected in negative controls.
Coupling of oligonucleotide probes to bead sets
The 15 oligonucleotide probes used for covalent coupling to beads were as described by Anthony et al.(2000) (Table 1). Each probe was tailed at the 5′ end with 18 dTTP spacer element, and terminated 5′ with a chemically active primary amino group. Spacers were applied to minimize the interference of steric hindrance during hybridization.
Table 1.
Oligonucleotide probes used for multiplex assay
| Oligo IDa | Species from which the sequence was derived | Accession No.b | Probe sequence (5′-3′) | Length |
| 1b | Proteus mirabilis | AF146762 | ATA GCC CCG TAT CTG AAG ATG CT | 23 |
| 2a | Klebsiella oxytoca | AF146763 | TCC CGT ACA CTA AAA CGC ACA GG | 23 |
| 3c | Salmonella enterica | U77923 | AGA GCC TGA ATC AGC ATG TGT | 21 |
| 4b | Pseudomonas aeruginosa | Y00432 | GCT TCA TTG ATT TTA GCG GAA C | 22 |
| 4c | Haemophilus influenzae | U32742 | GTG AGG AGA ATG TGT TGG GAA G | 22 |
| 5a | Streptococcus pneumoniae | M60763 | GGT TGT AGG ACT GCA ATG TGG ACT C | 25 |
| 5b | Enterococcus faecalis | AF146765 | GGT AGT CTG TTA GTA TAG TTG AAG | 24 |
| 5c | Aeromonas hydrophila | X87281 | TGG AAC GGT CCT GGA AAG GC | 20 |
| 6b | Enterococcus faecium | AF146766 | GGT AGT TCT TTC AGA TAG TCG G | 22 |
| 7a | Staphylococcus aureus | X68425 | ACG GAG TTA CAA AGG ACG ACA TTA | 24 |
| 8a | Staphylococcus epidermidis | AF146770 | ACG GAG TTA CAA AAG AAC ATG TTA GTT TTT | 30 |
| 8c | Staphylococcus haemolyticus | AF146772 | ACG GAG TTA CAA AGG AAT ATA TTA GTT TTT | 30 |
| 9a | Burkholderia cepacia | X16368 | CGT ATT GTT AGC CGA ACG CTC T | 22 |
| 9b | Stenotrophomonas maltophilia | AF146773 | AGC CCT GTA TCT GAA AGG GCC A | 22 |
| 9c | Listeria spp. | X64533 | ACG GAG TTA CAA AAG AAA GTT ATA A | 25 |
As published by Anthony et al.(2000);
Sequence used as a reference for the corresponding probe
The QIAGEN LiquiChip Workstation (Hilden, Germany) was employed for suspension array work. For each probe/bead set combination, 5×106 LiquiChip carboxylated beads were resuspended in 50 µl 100 mmol/L 2-(N-morpholino)ethanesulfonic acid (MES) buffer at pH 4.5. To perform coupling, 1 nmol of amino-substituted oligonucleotide probe was added, followed by the addition of 25 μg N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) (Pierce Chemical, Rockford, IL, USA) and incubated in the dark for 30 min. The EDC addition and incubation were repeated and the beads were washed in 1 ml of PBS with 0.02% Tween-20. Coupled beads were calculated in a cell-counting chamber and stored in TE buffer (pH 8.0, 10 mmol/L Tris-HCI, 1 mmol/L EDTA) at 4 °C in the dark. Coupling efficiency was assessed by hybridizing coupled beads with a molar excess of biotinylated oligonucleotide that was complementary to the 18 dTTP spacer linker, and the extent of hybridization was similarly evaluated, following the standard assay procedure detailed below. Beads with a median fluorescent intensity (MFI) less than 1000 were replaced.
Hybridization of array
The biotin-labeled PCR products were purified using the PCR purification mini kit (Watson Biotechnologies, China) in preparation for hybridization. Each hybridization reaction was performed in a thermal cycler in a total volume of 50 μl containing 10 μl purified template, 20× SSC (final concentration, 4×), and a mixture of 15 probe-coupled bead sets, containing 2 500 beads each. Hybridization protocol was as follows: an initial denaturing step of 5 min at 95 °C, followed by incubation for 20 min at 65 °C. Then the reaction system was transferred to a 96-well filter plate (Millipore Corporation, USA) and 100 μl of 2× SSC-0.02% Tween-20 was added to each well for washing the beads. Each well corresponded to a test sample, and the product of PCR negative control was used as hybridization negative control. After washing two times, each test reaction system was resuspended in 75 μl of 2× SSC-0.02% Tween-20. Subsequently, 25 μl of 10 μg/ml streptavidin-R-PE diluted in 2× SSC-0.02% Tween-20 was added to each well and incubated for 15 min at room temperature with gentle shaking on a plate shaker. The 96-well plate was placed in the Microplate Handler of the LiquiChip reader. For each probe (bead set) in a certain sample well, an MFI value was calculated from the signals of at least 100 beads. The experiment was repeated two to three times for each test sample to confirm the results.
Interpretation of the hybridization results
Signals generated from probes reacting with their non-targets, together with MFI values of 10~40, were considered as background signals. A threshold value was defined for each probe as 2.5 times of the average background signal for that probe. Anything over this was considered as a positive hybridization signal.
Sensitivity test of the suspension array
In order to evaluate the sensitivity of the system, a 10-fold dilution series of a fresh overnight culture of Listeria monocytogenes strain NICPBP54001 in Brain-Heart Infusion (BHI, Oxoid Ltd., UK) was prepared and 1 ml of each dilution was used for DNA extraction. This dilution series was carried through the standard PCR amplification (26 cycles), as well as extended amplification to 35 cycles. Samples with 35 amplification cycles were taken to ascertain the lowest limit of our detection system. Actual numbers of viable bacteria in this dilution series were counted on blood agar plates.
RESULTS
PCR amplification
The 23S rRNA primers were tested on DNA extracts from all 38 strains representing 15 bacterial species, together with a Candida albicans isolate. All bacterial isolates produced PCR products, evident as electrophoresis bands of approximately 400 bp for Gram-positive bacteria and 350 bp for Gram-negative bacteria. No bands were produced from a negative eukaryotic control, C. albicans.
PCR cycle number can be expected to affect the concentration of target present for hybridization, and thus, the sensitivity and specificity of array analysis (Dunbar et al., 2003a). Below saturating concentration of target, hybridization is driven kinetically in a concentration-dependent manner (Wetmur, 1991); above that level, as equilibrium is approached, the hybridization efficiency decreases (Armstrong et al., 2000; Nolan and Mandy, 2001) and cross-hybridization may become apparent (Dunbar et al., 2003b). Under the conditions and protocols used here, we determined that 26 amplification cycles were optimum, yielding efficient hybridization without sacrificing discrimination.
Bacterial identification on the multiplex suspension array
The results for all the amplicons hybridized to a mixture of 15 distinct bead sets are shown in Table 2. No positive hybridization signal was produced from C. albicans, nor was any appreciable cross-hybridization observed between species that did give positive signals.
Table 2.
Hybridization detection of 23S rRNA gene PCR products from 39 pure cultures. PCR products were hybridized to a mixture of 15 distinct bead sets, each set containing a specific oligonucleotide probe
| Species | Test samples | Oligo IDa |
| Aeromonas hydrophila | 2 | 5c |
| Burkholderia cepacia | 2 | 9a |
| Enterococcus faecalis | 3 | 5b |
| Enterococcus faecium | 2 | 6b |
| Haemophilus influenzae | 4 | 4c |
| Klebsiella oxytoca | 1 | 2a |
| Listeria monocytogenes | 1 | 9c |
| Pseudomonas aeruginosa | 2 | 4b |
| Proteus mirabilis | 2 | 1b |
| Salmonella typhimurium | 4 | 3c |
| Stenotrophomonas maltophilia | 6 | 9b |
| Staphylococcus aureus | 4 | 7a |
| Staphylococcus epidermidis | 1 | 8a |
| Staphylococcus haemolyticus | 2 | 8c |
| Streptococcus pneumoniae | 2 | 5a |
| Candida albicans | 1 | NAb |
Oligos with positive signal;
NA (non-available) indicated that no positive amplicons or hybridization signals were obtained from the pure yeast culture, which was included as a negative control
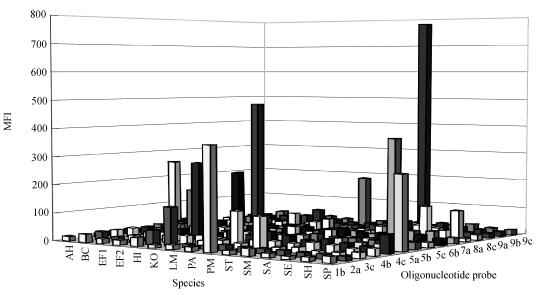
Fig.1 shows representative hybridization signals generated from PCR products of the 15 bacterial samples hybridized against all 15 probes, whereby each species hybridized only to the probe intended for it (Table 1). The MFI of positive signals (Fig.1) ranged from 110 to 779, and the MFI of background signals from 10~40, giving signal-to-noise ratios from 2.5~50.
Fig. 1.
A histogram of representative hybridization signals generated by hybridization of bead-immobilized probes to PCR products from all bacteria studied
AH: Aeromonas hydrophila; BC: Burkholderia cepacia; EF1: Enterococcus faecalis; EF2: Enterococcus faecium; HI: Haemophilus influenzae: KO: Klebsiella oxytoca; LM: Listeria monocytogenes; PA: Pseudomonas aeruginosa; PM: Proteus mirabilis; ST: Salmonella typhimurium; SM: Stenotrophomonas maltophilia; SA: Staphylococcus aureus; SE: Staphylococcus epidermidis; SH: Staphylococcus haemolyticus; SP: Streptococcus pneumoniae
For the clinical samples identified previously by culture methods, all 34 identities could be confirmed using the current assay. These included discrimination of Gram-negative species: Aeromonas hydrophila, Burkholderia cepacia, Haemophilus influenzae, Klebsiella oxytoca, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhimurium, Stenotrophomonas maltophilia, and Gram-positive species: Enterococcus faecalis, Enterococcus faecium, Listeria monocytogenes, Staphlococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus and Streptococcus pneumoniae.
Sensitivity tests with Listeria monocytogenes
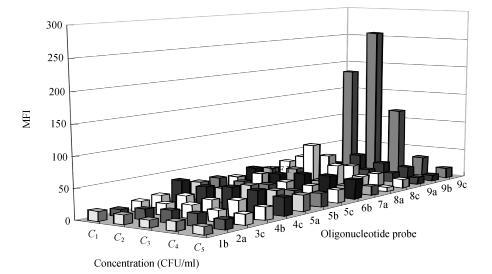
Using L. monocytogenes DNA and 26 amplification cycles, a specific amplicon was visible in gels only when the suspension used for DNA extraction contained ≥9.3×107 cells/ml. By increasing PCR cycles to 35, amplicons were also generated in samples with as low as 9.3×106 cells/ml. Only the PCR products generated at sample concentration ≥9.3×106 CFU/ml at 35 cycles could react with the combined bead sets (Fig.2). The hybridization signals produced by 9.3×107 CFU/ml at 26 cycles were similar with those of 9.3×106 CFU/ml at 35 cycles, and better than those of 9.3×107 CFU/ml at 35 cycles. For probe 8a, presented a cross-hybridization value of a little higher 50 under the circumstances. At higher amplification levels, however, our preliminary experiments indicate that cross-reactivity quickly becomes a problem. Since the concentration of 1×108 cells/ml is readily achieved with samples of pure cultures, it is prudent to use larger DNA input and lesser amplification.
Fig. 2.
The detection limit of the suspension array with 15 bead sets for Listeria monocytogenes NICPBP54001. PCR product of 9.3×107 CFU/ml at 26 cycles and amplicons at 35 thermal cycles from different concentrations (9.3×107, 9.3×106 and 9.3×105 CFU/ml) and template-negative control (PCR NC), were hybridized to the multiplex array and analyzed by LiquiChip reader. On the basis of 9c positive signals, Listeria monocytogenes was identified. The detection limit at 35 cycles for Listeria monocytogenes was 9.3×106 CFU/ml
C 1: 9.3×107 (26 cycles); C 2: 9.3×107 (35 cycles); C 3: 9.3×106 (35 cycles); C 4: 9.3×105 (35 cycles); C 5: PCR NC (35 cycles)
DISCUSSION
Based on sequence-specific hybridization of 23S rRNA gene amplicons produced using a common primer set, 38 strains representing 15 species were discriminated unambiguously at species level in this study using suspension arrays. The results are in good agreement with those using the membrane-array platform (Anthony et al., 2000). The suspension array based on 23S rRNA gene can provide quantitative data with computer analysis as compared to visual analysis used in membrane-assay. Moreover, the system is easier to manipulate and less labor-intensive than membrane-assay. A new protocol for rapid bacterial diagnostics at the molecular level with oligonucleotide arrays was established.
In this study, just one probe derived from the 23S rRNA gene, was used to identify one bacterial species and all members of that species. With this, possible multiplex infection could be relatively easily discriminated by the combination mode of positive hybridization signals of different probes.
Initially we obtained positive PCR signals with negative controls, a phenomenon already reported elsewhere during amplification with universal primer sets for bacterial 16S rRNA (Corless et al., 2000) and 5S rRNA (Maiwald et al., 1994). However, these PCR products produced negative signals in the following hybridization assay, indicating that the false positive PCR products were caused by contaminating DNA derived from an unknown source. PCR reaction mixtures were likely to have been contaminated with bacterial DNA, possibly derived from PCR amplification reagents, including Taq DNA polymerase, buffers, dNTPs and primers supplied by manufacturers (Zehr et al., 2003). Therefore, above all, we changed the manufacturer providing primers to preclude this phenomenon, and just as we expected, no bands in PCR negative controls were generated again.
Of course, the issue of ultimate sensitivity of the suspension array approach is also linked to the method and scale of DNA extraction employed. In the sensitivity test on L. monocytogenes, the detection cutoff value was approximately 1×107 CFU/ml for the starting PCR template. This is 1000 times above that usual PCR reaction should work, mainly because here a very “low-tech” extraction method was employed: boiling in 0.5% SDS, a method accessible to any clinical laboratory. An effort to dramatically increase sensitivity of the current method would most likely have to include more sophisticated, miniaturized extraction.
The LiquiChip system is more highly automated, thus less labor-intensive. Here quantitative data can be yielded within 2 h after achieving single colony isolates from the blood culture. Because current bead sets provide 100 classification codes, increasing multiplexity to accommodate uncommon or unanticipated species or strains is technically straightforward through the addition of appropriate probes. As 23S rRNA gene sequence data constantly increases to include ever more exotic species, this continually increased discriminatory power will prove to be a major advantage. The suspension array technique holds great promise for microbial diagnostics in the routine laboratory.
Acknowledgments
We thank Yi-dong Wu and Edward Zumbika for critical discussion, and Bob Wohlhueter and Thomas Riley for revising this manuscript.
Footnotes
Project (Nos. 2003C13015 and 021103128) supported by Science and Technology Department of Zhejiang Province, China
References
- 1.Anthony RM, Brown TJ, French GL. Rapid diagnosis of bacteremia by universal amplification of 23S ribosomal DNA followed by hybridization to an oligonucleotide array. J Clin Microbiol. 2000;38(2):781–788. doi: 10.1128/jcm.38.2.781-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong B, Stewart M, Mazumder A. Suspension arrays for high throughput, multiplexed single nucleotide polymorphism genotyping. Cytometry. 2000;40(2):102–108. doi: 10.1002/(SICI)1097-0320(20000601)40:2<102::AID-CYTO3>3.3.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Bovers M, Diaz MR, Hagen F, Spanjaard L, Duim B, Visser CE, Hoogveld HL, Scharringa J, Hoepelman IM, Fell JW, et al. Identification of genotypically diverse Cryptococcus neoformans and Cryptococcus gattii isolates by Luminex xMAP technology. J Clin Microbiol. 2007;45(6):1874–1883. doi: 10.1128/JCM.00223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant PA, Venter D, Robins-Browne R, Curtis N. Chips with everything: DNA microarrays in infectious diseases. Lancet Infect Dis. 2004;4(2):100–111. doi: 10.1016/S1473-3099(04)00930-2. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, White PS, Torney D, Deshpande A, Wang Z, Keller RA, Marrone B, Nolan JP. Flow cytometry-based minisequencing: a new platform for high-throughput single-nucleotide polymorphism scoring. Genomics. 2000;66(2):135–143. doi: 10.1006/geno.2000.6218. [DOI] [PubMed] [Google Scholar]
- 6.Christensen H, Nordentoft S, Olsen J. Phylogenetic relationships of Salmonella based on rRNA sequences. Int J Syst Bacteriol. 1998;48(Pt 2):605–610. doi: 10.1099/00207713-48-2-605. [DOI] [PubMed] [Google Scholar]
- 7.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Kaczmarski EB, Fox AJ. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J Clin Microbiol. 2000;38(5):1747–1752. doi: 10.1128/jcm.38.5.1747-1752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauga C. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. Int J Syst Evol Microbiol. 2002;52(Pt 2):531–547. doi: 10.1099/00207713-52-2-531. [DOI] [PubMed] [Google Scholar]
- 9.Ding LP, Sun YH, Wang Q, Nian H. Drug resistance of common bacteria isolated from blood and bone marrow. J Chin Med Univ. 2004;33(1):83–85. (in Chinese) [Google Scholar]
- 10.Dunbar S, Godbout R, Newkirk H, Hetzel J. Microsphere suspension array technology for SNP detection in cattle. IEEE Eng Med Biol Mag. 2003;22(4):158–162. doi: 10.1109/MEMB.2003.1237526. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar SA, Vander Zee CA, Oliver KG, Karem KL, Jacobson JW. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAP system. J Microbiol Methods. 2003;53(2):245–252. doi: 10.1016/S0167-7012(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima M, Kakinuma K, Hayashi H, Nagai H, Ito K, Kawaguchi R. Detection and identification of Mycobacterium species isolates by DNA microarray. J Clin Microbiol. 2003;41(6):2605–2615. doi: 10.1128/JCM.41.6.2605-2615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry MR, Wilkins Stevens P, Sun J, Kelso DM. Real-time measurements of DNA hybridization on microparticles with fluorescence resonance energy transfer. Anal Biochem. 1999;276(2):204–214. doi: 10.1006/abio.1999.4344. [DOI] [PubMed] [Google Scholar]
- 14.Kettman JR, Davies T, Chandler D, Oliver KG, Fulton RJ. Classification and properties of 64 multiplexed microsphere sets. Cytometry. 1998;33(2):234–243. doi: 10.1002/(SICI)1097-0320(19981001)33:2<234::AID-CYTO19>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Khayr WF, CarMichael MJ, Dubanowich CS, Latif RH. Epidemiology of bacteremia in the geriatric population. Am J Ther. 2003;10(2):127–131. doi: 10.1097/00045391-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 17.Leffers H, Kjems J, Ostergaard L, Larsen N, Garrett RA. Evolutionary relationships amongst archaebacteria. A comparative study of 23S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J Mol Biol. 1987;195(1):43–61. doi: 10.1016/0022-2836(87)90326-3. [DOI] [PubMed] [Google Scholar]
- 18.Maiwald M, Ditton HJ, Sonntag HG, von Knebel Doeberitz M. Characterization of contaminating DNA in Taq polymerase which occurs during amplification with a primer set for Legionella 5S ribosomal RNA. Mol Cell Probes. 1994;8(1):11–14. doi: 10.1006/mcpr.1994.1002. [DOI] [PubMed] [Google Scholar]
- 19.Nolan JP, Mandy FF. Suspension array technology: new tools for gene and protein analysis. Cell Mol Biol (Noisy-le-grand) 2001;47(7):1241–1256. [PubMed] [Google Scholar]
- 20.Nolan JP, Sklar LA. Suspension array technology: evolution of the flat-array paradigm. Trends Biotechnol. 2002;20(1):9–12. doi: 10.1016/S0167-7799(01)01844-3. [DOI] [PubMed] [Google Scholar]
- 21.Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. New developments in the diagnosis of bloodstream infections. Lancet Infect Dis. 2004;4(12):751–760. doi: 10.1016/S1473-3099(04)01205-8. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;44(2):504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troesch A, Nguyen H, Miyada CG, Desvarenne S, Gingeras TR, Kaplan PM, Cros P, Mabilat C. Mycobacterium species identification and rifampin resistance testing with high-density DNA probe arrays. J Clin Microbiol. 1999;37(1):49–55. doi: 10.1128/jcm.37.1.49-55.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandamme P, Pot B, Gillis M, de Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60(2):407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetmur JG. DNA probes: applications of the principles of nucleic acid hybridization. Crit Rev Biochem Mol Biol. 1991;26(3):227–259. doi: 10.3109/10409239109114069. [DOI] [PubMed] [Google Scholar]
- 26.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehr JP, Crumbliss LL, Church MJ, Omoregie EO, Jenkins BD. Nitrogenase genes in PCR and RT-PCR reagents: implications for studies of diversity of functional genes. Biotechniques. 2003;35(5):996–1002. 1004–1005. doi: 10.2144/03355st08. [DOI] [PubMed] [Google Scholar]