Abstract
Excessive dexamethasone (Dex) administrated into pregnant mice during critical periods of palatal development can produce a high incidence of cleft palate. Its mechanisms remain unknown. Vitamin B12 has been shown to antagonize the teratogenic effects of Dex, which, however, remains controversial. In this study, we investigated the effects of Dex and vitamin B12 on murine embryonic palatal shelf fusion using organ culture of murine embryonic shelves. The explanted palatal shelves on embryonic day 14 (E14) were cultured for 24, 48, 72 or 96 h in different concentrations of Dex and/or vitamin B12. The palatal shelves were examined histologically for the morphological alterations on the medial edge epithelium (MEE) and fusion rates among different groups. It was found that the palatal shelves were not fused at 72 h or less of culture in Dex group, while they were completely fused in the control and vitamin B12-treated groups at 72 and 96 h, respectively. The MEE still existed and proliferated. In Dex+vitamin B12 group the palatal shelves were fused at each time point in a similar rate to controls. These results may suggest that Dex causes teratogenesis of murine embryonic palatal shelves and vitamin B12 prevents the teratogenic effect of Dex on palatogenesis on murine embryos in vitro.
Keywords: Dexamethasone, Vitamin B12, Organ culture, Cleft palate, Medial edge epithelial cells
INTRODUCTION
Cleft lip and palate is one of the most frequent congenital malformations in humans. Its etiology is complex and multifactorial. Both genetic and environmental factors are involved and regulated spatially and temporally by complicated molecular mechanisms (Gritli-Linde, 2007; Rice, 2005). The formation of the mammalian secondary palate requires several critical steps, including growth, elevation, contact, medial edge epithelium (MEE) disappearance, and, finally, bilateral palatal shelves fusion. Interruption of any of these steps may cause cleft palate.
B-vitamins are a group of water-soluble vitamins, including B6, B12, folic acid, etc. Deficiency of vitamins is associated with many illness and birth defects (Botto et al., 2004; Herrmann et al., 2007; Krapels et al., 2004). Epidemiological studies of cleft lip and palate showed that a pregnant female taking certain doses of vitamin B12 may decrease the incidence of cleft and palate in her offspring (van Rooij et al., 2003). However, others also reported that children with congenital malformations did not show any serum vitamin deficiencies compared with a normal population (Stoll et al., 1999).
It was reported that palatal development in vitro was similar to in vivo (Koch and Smiley, 1981; Shimizu et al., 2001). In this study we have investigated the teratogenic effect of dexamethasone (Dex) on the palate and employed vitamin B12 to antagonize the teratogenic effects of Dex on palate fusion in mouse embryos in vitro, using the organ culture method (Hassell, 1975).
MATERIALS AND METHODS
Animals
Adult C57BL/6J mice, weighting 15~20 g, one male and two females per cage, were mated overnight in a temperature-controlled (22 °C) SPF (specified-pathogens free) room, water and food ad libitum. Vaginal plugs appeared in the following morning, which was designated as the embryonic day 0 (E0).
Dissection and organ culture
On E14, mouse embryos were quickly immersed in GMF-PBS (Ca2+ and Mg2+ free-phosphate buffed solution, pH 7.2) and flushed lightly several times. Then the palatal shelves were removed using microsurgical shears and forceps under a dissecting microscope. Isolated palatal shelves were cultured according to the previous methods (Hassell, 1975). Briefly, the palatal shelves were placed in pairs on 0.4 μm porosity millipore filters (Millipore Corporation, USA), nasal epithelium down, media edges in contact, on 35 mm-tissue culture dishes (FALCON, France). The culture medium was composed of DMEM/F12 (Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 Ham’s) (Hyclone Corporation, USA), 10% fetal bovine serum (FBS, Hyclone Corporation), and 100 U/ml Penicillin-Streptomycin, with or without 20 ng/ml Dex (Sigma, USA) and/or 10 ng/ml vitamin B12 (Sigma, USA).
Transplants were divided into the control, the Dex-treated, the vitamin B12-treated, and the Dex+vitamin B12-treated groups, with 8 samples in each group. Samples were cultured for 24, 48, 72 or 96 h at 37 °C in an incubator with 5% CO2 (Leica, Germany). All the medium and treatments were replaced every 24 h.
Histology
At the end of incubation, transplants were immediately immersed in 4% neutral paraformaldehyde and embedded in paraffin. Then, 5 μm-thick serial sections were cut and stained with eosin and haematoxylin. These sections were then photographed using a Leica photographic system (Germany).
Scanning electron microscopy (SEM) preparation
Transplants were immersed in 2.5% glutaraldehyde solution for 2 h at room temperature, and then followed were ethanol gradient dehydration, isoamylacetate replacement, critical point drying, and gold plating. At last, the samples were scanned to observe surface changes of the palatal shelves.
Statistical analysis
Statistical analysis was performed with the chi-square test and variance analysis (SPSS 13.0). Different groups in the same designated time and in the same group across different incubation times were compared. Values of P<0.05 were considered significant.
RESULTS
The numbers of palatal shelf fusion in various experimental groups are presented in Table 1. The palatal shelves were not fused at the 72 h or less of culture in Dex group, compared with controls (P<0.0025), indicating the teratogenesis of Dex. In Dex+vitamin B12 group the palatal shelves were fused at each time point in a similar rate to the control and vitamin B12 groups, significantly different from the Dex group (P<0.05). These results indicate the antagonistic effect of vitamin B12 against Dex.
Table 1.
The numbers of palatal shelf fusion in various experimental groups
| Group | Number of palatal shelf fusion |
|||
| 24 h | 48 h | 72 h | 96 h | |
| Control | 3 | 7 | 8 | 8 |
| Dex | 0 | 1 | 2 | 2 |
| Vitamin B12 | 4 | 6 | 6 | 8 |
| Dex+vitamin B12 | 5 | 6 | 7 | 7 |
Light microscope
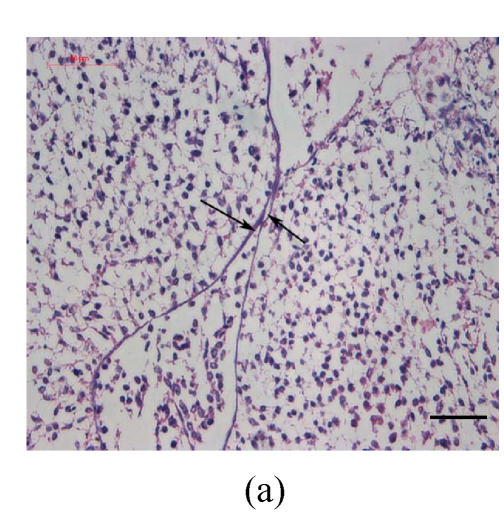
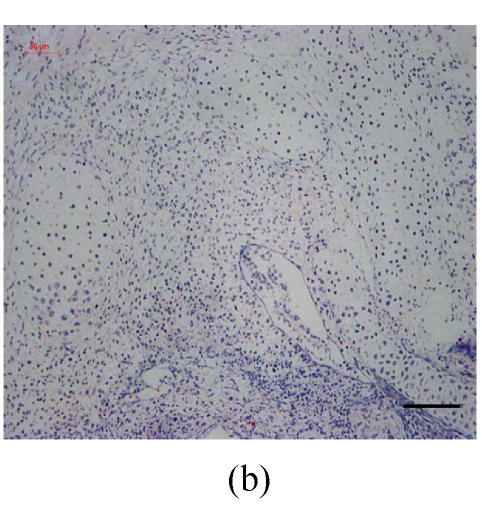
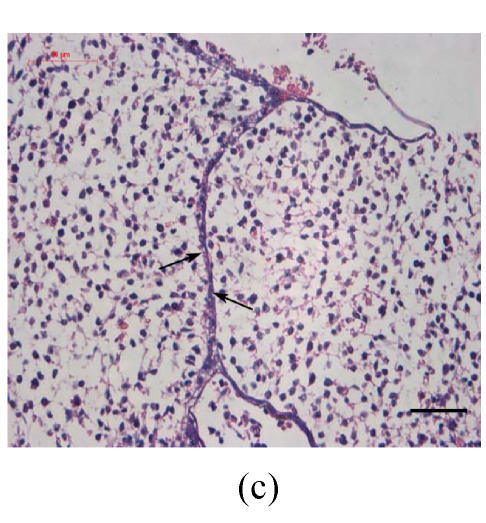
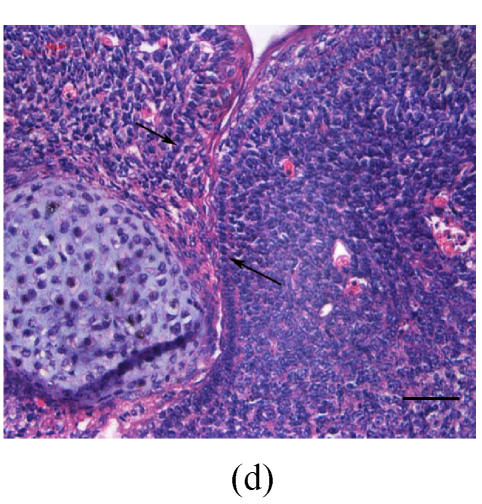
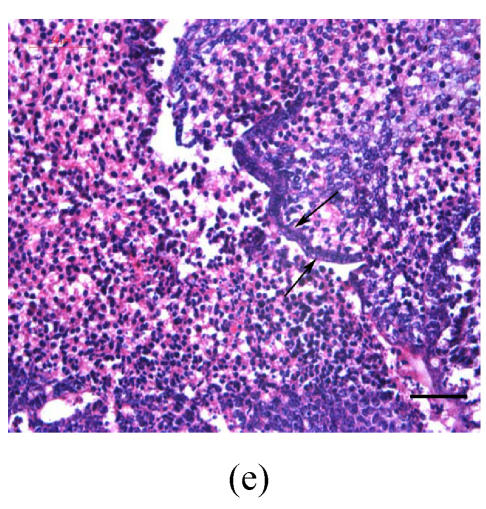
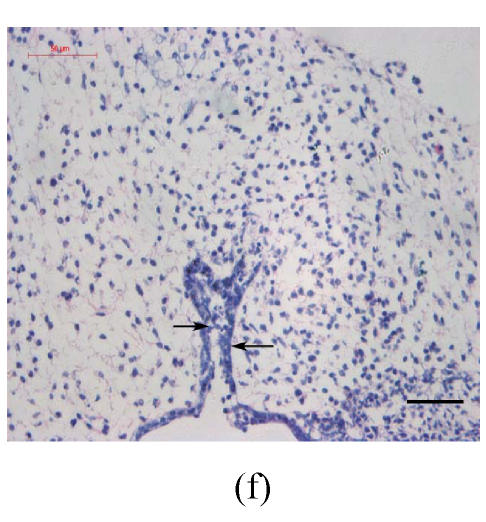
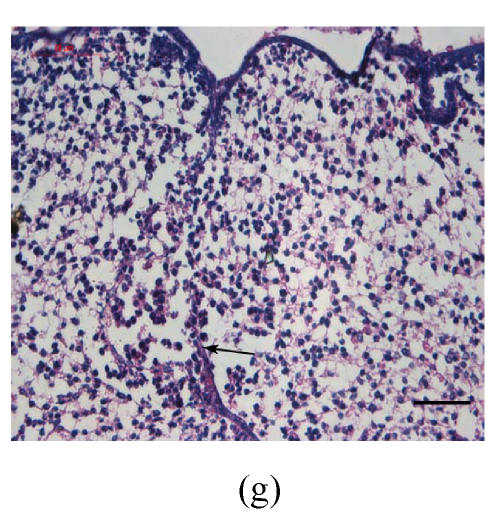
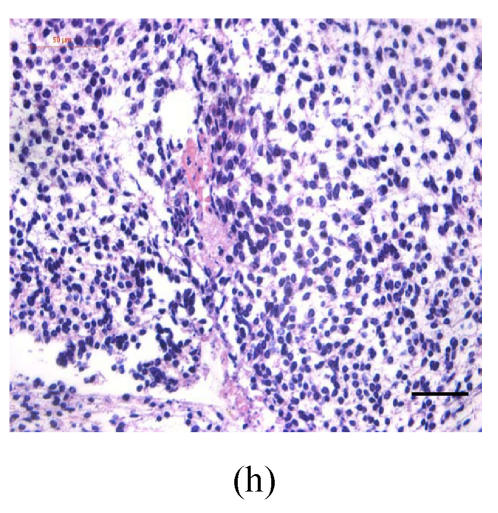
Transplants were classified in three groups according to Vargas (1967) method: no fusion, partial fusion, and complete fusion. Complete fusion or mesenchymal coalescence means no MEE is observed, and the epithelial laminae have been broken down and resorbed. All of the palates in the control group completely fused after 48 h (Fig.1b), and no MEE was observed. All of the palates in the vitamin B12-treated group also completely fused at 96 h. There was no significant difference between control and vitamin B12-treated groups (P>0.5) after 48, 72 and 96 h, which suggests that vitamin B12 had no effect on normal palatal fusion (Figs.1e and 1f). In the Dex-treated group at 24 h no fusion was observed (Fig.1c) and the MEE existed; at 48 h only 1 out of 8 (Fig.1d) and at 72 h 2 out of 8 fused (Fig.1g), and MEE did not disappear but thickened. In Dex+vitamin B12 group at 24 (Fig.1g), 48 (Fig.1h), 72 and 96 h, 5, 6, 7 and 7 out of 8 samples fused, respectively, indicating the antagonism of vitamin B12 against Dex.
Fig. 1.
Hematoxylin and eosin (H & E)-stained sections of the palatal shelves. In the control group at 24 h of culture (a), the MEE was observed, and at 48 h (b), the MEE disappeared and the bilateral palatal shelves fused. In Dex group at 24 h (c) and 48 h (d), the MEE was still seen and after 48 h the MEE thickened. In vitamin B12-treated group at 24 h (e) and 48 h (f), the MEE also disappeared. In Dex+vitamin B12 group at 24 h (g) and 48 h (h) the bilateral palatal shelves fused
Black arrows show the MEE; Scale bars=50 µm
Scanning electron microscopy
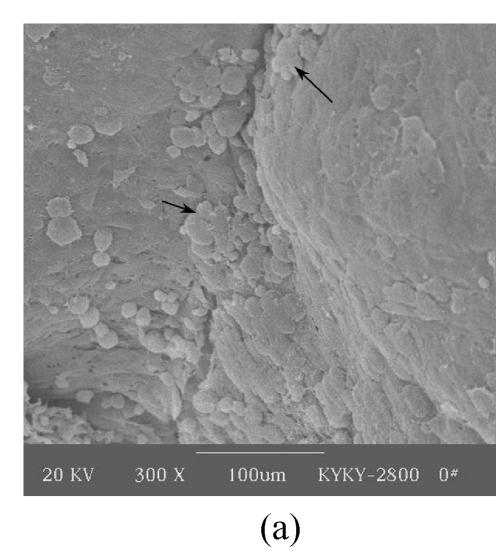
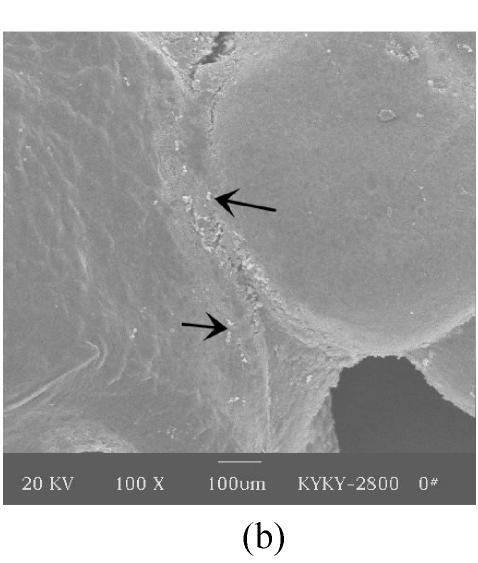
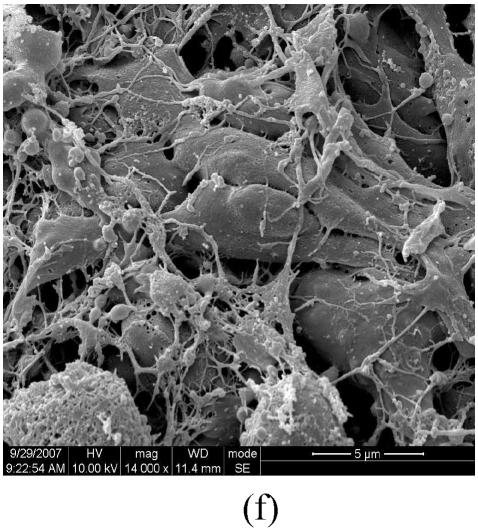
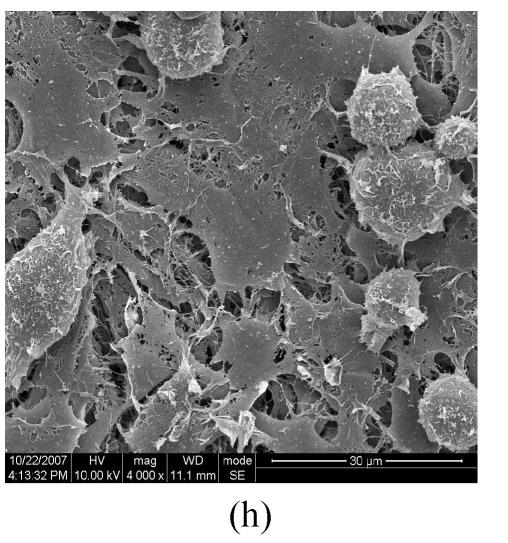
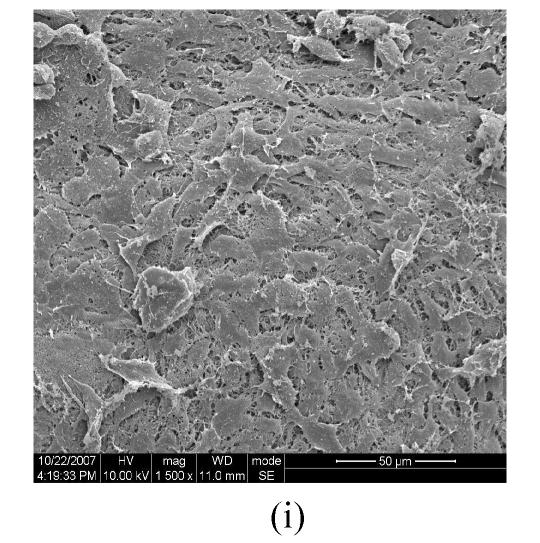
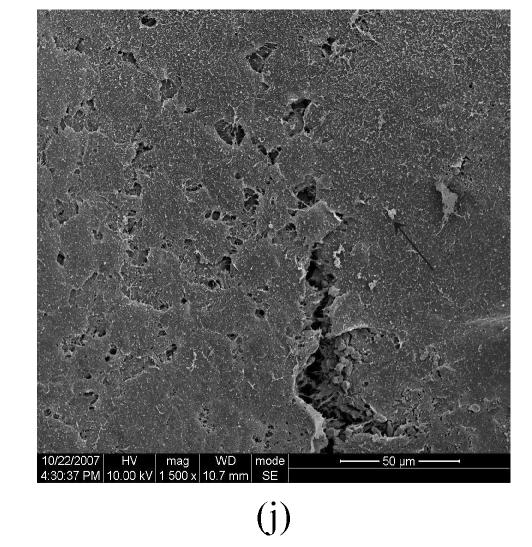
Figs.2a and 2b showed situation of the palatal shelves in the control group, and incubated after 24 h, bilateral palatal shelves have achieved completely fusion. The spheroidal structures in MES (medial epithelial seam) seemed likely deciduous perithelial cells from MEE and migrated to surface. The observations of palatal shelves of Dex-treated group showed in Figs.2c and 2d. Opposing palatal shelves seemed fused incompletely and retained seam between bilateral palates. The spheroidal structures in MES and palatal surface were less than the control group, and the spheroidal structures trapped, which suggested perithelial cells that underwent slough and migration were less than the control group. The perithelial cells did not slough and migrate, which resulted in basal cell of MEE and could not contact and induced cleft palate. Some fibroblast-like cells were observed on the surface of palatal shelves after vitamin B12 treatment (Figs.2e and 2f), but fusion of shelves was normal (Fig.2g). Possibly, function of vitamin B12 involved in cell morphous was needed further study. In vitamin B12+Dex-treated group, surface morphous of palatal shelves was similar to that of the control group, and bilateral shelves fused but retained some feature like vitamin B12-treated group (Figs.2h, 2i and 2j).
Fig. 2.
SEM observation of palates fusion. In the control group (a), the black arrows show some spheroidal structures in the medial edge area at 24 h and fusion at 48 h (b). In the Dex-treated group at 24 h the bilateral palatal shelves contacted and few filopodia were observed at 24 h (c). In Dex-treated group at 48 h, the spheroidal structures did not disappear and seemed trapped in the medial edge seam (d). In the vitamin B12-treated group at 24 h (e), compared with Dex-treated group (c), more spheroidal structures and some fibroblast-like cells were seen as the black arrows indicate. The square (f) in (e) is shown at a higher magnification. Few spheroidal structures were observed in the medial area at 48 h in vitamin B12 group (g). Vitamin B12+Dex group had some spheroidal structures and some fibroblast-like cells were seen at 24 h (h), and at 48 h, many fibroblast-like cells were observed, with a few spheroidal structures (i); and at 72 h no spheroidal structures were observed and in some areas a few apoptosis cells were seen, with few filopodia (j)
(a), (b) Scale bars=100 µm; (c), (d), (e), (i), (j) Scale bars=50 µm; (g), (h) Scale bars=30 µm; (f) Scale bars=5 µm
DISCUSSION
Bilateral palatal fusion includes two phases: the first phase in which palates move from a vertical position to a horizontal position, and the second phase in which the two palates contact and fuse to form the secondary palate. The potential of the palatal shelves to fuse in vitro depends on the age of embryo, which varies among species. Pourtois (1966) found that in the rat the potential to fuse was acquired 24 h before the actual time of the secondary palatal fusion, whereas Vargas (1967) found that in the mouse it was at least 40 h before the actual time of fusion in vivo. Teratogens that disturb any of these developmental events may induce a failure of the palates to fuse.
Studies both in vivo and in vitro demonstrated that environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a cleft palate teratogen, not only disrupts the differentiation and proliferation of the bilateral MEE in mice, but also has effects on the expression of some growth factors (Abbott and Birnbaum, 1990a; 1990b; Abbott et al., 1989a; 1992; 2003; 2005b; Bock and Köhle, 2006; Hassoun and Dencker, 1982; Miettinen et al., 2004; Ryan et al., 1989). Other teratogens also showed effects on the fusion of the palatal shelves, such as retinoic acid (RA), which induces cleft palate when administered in excess to the maternal mouse, depending on the stage of embryonic development exposed (Abbott et al., 1989b; Newall and Edwards, 1981). Both in vivo and in vitro studies showed that RA changed the regulation of cell proliferation, which was often associated with the altered expression of epidermal growth factor receptor (EGFR) (Abbott and Birnbaum, 1990c; Abbott et al., 2005a) and transforming growth factor beta (TGF-β) (Degitz et al., 1998; Nugent et al., 1998). Dex, one of the glucocorticoids (GC) that are well-known teratogens, is a fat-soluble hormone that penetrates the cell membrane and binds to GC receptor (GR) in the cytoplasm, inhibiting palatal mesenchymal proliferation, and resulting in smaller palates that fail to contact and fuse (Greene and Kochhar, 1975; Hackney, 1980; Pratt et al., 1984; Shah et al., 1989). Sensitivity to Dex varies by species and stages of pregnancy, and the species differences in susceptibility are related to H-2 histocompatility (Kusanagi, 1984; Montenegro and Palomino, 1989). A population-based case-control study of cleft lip and palate showed that maternal corticosteroid use during pregnancy is associated with moderately increased risk of delivering an infant with an orofacial cleft (Carmichael et al., 2007).
Various in vitro methods have been employed in the investigation on the effects of Dex on the palate, including mesenchymal culture, or palatal epithelial and mesenchymal co-incubation, and organ culture. The methods vary in their ability to stimulate palate development and fusion, and the organ culture has significant advantages in this regard (Chou et al., 2004). In the current study, we observed that Dex appeared to stimulate the thickening of the MEE. When exposed to 20 ng/ml Dex for 24 h, the palatal shelves from E14 pregnant mice retained the MEE, and even after 96 h the MEE still existed. SEM images show that the spheroidal structures did not disappear and seemed trapped in the medial edge seam. Compared with the control group, few filopodia were observed.
Vitamin B12 is one of the most important coenzymes and participates in many biochemical reactions in vivo (Selhub, 2002). Population-based epidemiological investigations showed that pregnant women taking certain doses of vitamin B12 could reduce the risk of non-syndromic craniofacial clefts (van Rooij et al., 2003; Vujkovic et al., 2007). It was demonstrated that the vitamin B12 concentration of 185 pmol/L or less and the pyridoxal-5′-phosphate (PLP) concentration of 44 nmol/L or less in mothers increased the risk of having a child with cleft lip with or without cleft palate (van Rooij et al., 2003). Animal experiments showed that when vitamin B12 level decreased, the level of amniotic homocysteine, a risk factor for the malformation of the palate, increased (Weingärtner et al., 2007). Experiments on mice from E10~E13 demonstrated that vitamin B12 could antagonize the Dex-induced cleft palate (Natsume et al., 1986). However, the main mechanism of how vitamin B12 antagonized the Dex-induced craniofacial clefts is still unknown.
In the current studies, in the vitamin B12-treated group 10 ng/ml vitamin B12 was added in the medium, and the palate fuse rate and SEM images were similar to the control, suggesting that vitamin B12 had no effect on palatal MEE. With exposure to 20 ng/ml Dex+10 ng/ml vitamin B12, at 48 h incubation, the fuse rate significantly increased to near the one in controls and SEM images became similar to the control as well. These results demonstrate that vitamin B12 could antagonize the Dex-induced failure of the palatal fusion in mouse embryos.
CONCLUSION
In summary, our results may suggest that Dex causes teratogenesis of murine embryonic palatal shelves and vitamin B12 prevents the teratogenic effect of Dex on palatogenesis on murine embryos in vitro. We, therefore, propose that a proper concentration of vitamin B12 may effectively antagonize the effects of Dex that leads to MEE thickening, which is possibly a consequence of excessive MEE proliferation or reduced cell death. Investigations into the molecular mechanisms of how Dex interacted with vitamin B12 were not examined and further studies are needed to clarify the preventive effects of vitamin B12 on cleft lip and palate.
Footnotes
Project (No. 30530730) supported by the National Natural Science Foundation of China
References
- 1.Abbott BD, Birnbaum LS. TCDD-induced altered expression of growth factors may have a role in producing cleft palate and enhancing the incidence of clefts after coadministration of retinoic acid and TCDD. Toxicol Appl Pharmacol. 1990;106(3):418–432. doi: 10.1016/0041-008x(90)90337-t. [DOI] [PubMed] [Google Scholar]
- 2.Abbott BD, Birnbaum LS. Rat embryonic palatal shelves respond to TCDD in organ culture. Toxicol Appl Pharmacol. 1990;103(3):441–451. doi: 10.1016/0041-008X(90)90317-N. [DOI] [PubMed] [Google Scholar]
- 3.Abbott BD, Birnbaum LS. Retinoic acid-induced alterations in the expression of growth factors in embryonic mouse palatal shelves. Teratology. 1990;42(6):597–610. doi: 10.1002/tera.1420420604. [DOI] [PubMed] [Google Scholar]
- 4.Abbott BD, Diliberto JJ, Birnbaum LS. 2,3,7,8-tetrachlorodibenzo-p-dioxin alters embryonic palatal medial epithelial cell differentiation in vitro. Toxicol Appl Pharmacol. 1989;100(1):119–131. doi: 10.1016/0041-008X(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 5.Abbott BD, Harris MW, Birnbaum LS. Etiology of retinoic acid-induced cleft palate varies with the embryonic stage. Teratology. 1989;40(6):533–553. doi: 10.1002/tera.1420400602. [DOI] [PubMed] [Google Scholar]
- 6.Abbott BD, Diliberto JJ, Birnbaum LS. Mechanisms of TCDD-induction of cleft palate: insights from in vivo and in vitro approaches. Chemosphere. 1992;25(1-2):75–78. doi: 10.1016/0045-6535(92)90483-8. [DOI] [Google Scholar]
- 7.Abbott BD, Buckalew AR, DeVito MJ, David RP, Bryant L, Schmid JE. EGF and TGF-α expression influence the developmental toxicity of TCDD: dose response and AhR phenotype in EGF, TGF-α, and EGF+TGF-α knockout mice. Toxicol Sci. 2003;71(1):84–95. doi: 10.1093/toxsci/71.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Abbott BD, Best DS, Narotsky MG. Teratogenic effects of retinoic acid are modulated in mice lacking expression of epidermal growth factor and transforming growth factor-alpha. Birth Defects Research Part A Clinical and Molecular Teratology. 2005;73(4):204–217. doi: 10.1002/bdra.20117. [DOI] [PubMed] [Google Scholar]
- 9.Abbott BD, Buckalew AR, Leffler KE. Effects of epidermal growth factor (EGF), transforming growth factor (TGF), and 2,3,7,8-tetrachlorodibenzo-p-dioxin on fusion of embryonic palates in serum-free organ culture using wild-type, EGF knockout, and TGF-α knockout mouse strains. Birth Defects Research Part A Clinical and Molecular Teratology. 2005;73(6):447–454. doi: 10.1002/bdra.20133. [DOI] [PubMed] [Google Scholar]
- 10.Bock KW, Köhle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72(4):393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Botto LD, Richard SJ, Erickson D. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. Am J Med Genet Part C: Semin Med Genet. 2004;125C(1):12–21. doi: 10.1002/ajmg.c.30004. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael SL, Shaw GM, Ma C, Werler MM, Rasmussen SA, Lammer EJ. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol. 2007;197(6):585.e1–585.e7. doi: 10.1016/j.ajog.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Chou MJ, Kosazuma T, Takigawa T, Yamada S, Takahara S, Shiota K. Palatal shelf movement during palatogenesis: a fate map of the fetal mouse palate cultured in vitro. Anat Embryol. 2004;208(1):19–25. doi: 10.1007/s00429-004-0379-0. [DOI] [PubMed] [Google Scholar]
- 14.Degitz SJ, Morris D, George L, Foley B, Francis M. Role of TGF-β in RA-induced cleft palate in CD-1 mice. Teratology. 1998;58(5):197–204. doi: 10.1002/(SICI)1096-9926(199811)58:5<197::AID-TERA6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Greene RM, Kochhar DM. Some aspects of corticosteroid-induced cleft palate: a review. Teratology. 1975;11(1):47–55. doi: 10.1002/tera.1420110106. [DOI] [PubMed] [Google Scholar]
- 16.Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301(2):309–326. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 17.Hackney JF. A glucocorticoid receptor in fetal mouse: its relationship to cleft palate formation. Teratology. 1980;21(1):39–51. doi: 10.1002/tera.1420210106. [DOI] [PubMed] [Google Scholar]
- 18.Hassell JR. The development of rat palatal shelves in vitro: An ultrastructural analysis of the inhibition of epithelial cell death and palate fusion by the epidermal growth factor. Dev Biol. 1975;45(1):90–102. doi: 10.1016/0012-1606(75)90244-4. [DOI] [PubMed] [Google Scholar]
- 19.Hassoun EM, Dencker L. TCDD embryo toxicity in the mouse may be enhanced by β-naphthoflavone, another ligand of the Ah-receptor. Toxicol Lett. 1982;12(2-3):191–198. doi: 10.1016/0378-4274(82)90185-0. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann M, Schmidt J, Umanskaya N, Colaianni G, Al Marrawi F, Widmann T, Zallone A, Wildemann B, Herrmann W. Stimulation of osteoclast activity by low B-vitamin concentrations. Bone. 2007;41(4):584–591. doi: 10.1016/j.bone.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Koch WE, Smiley GR. In-vivo and in-vitro studies of the development of the avian secondary palate. Arch Oral Biol. 1981;26(3):181–187. doi: 10.1016/0003-9969(81)90128-X. [DOI] [PubMed] [Google Scholar]
- 22.Krapels IPC, van Rooij IALM, Ocké MC, van Cleef BAGL, Kuijpers-Jagtman AM, Steegers-Theunissen RPM. Maternal dietary B vitamin intake, other than folate, and the association with orofacial cleft in the offspring. Eur J Nutr. 2004;43(1):7–14. doi: 10.1007/s00394-004-0433-y. [DOI] [PubMed] [Google Scholar]
- 23.Kusanagi T. Sensitive stages and dose-response analyses of palatal slit and cleft palate in C57BL/6 mice treated with a glucocorticoid. Teratology. 1984;29(2):281–286. doi: 10.1002/tera.1420290214. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen HM, Huuskonen H, Partanen AM, Miettinen P, Tuomisto JT, Pohjanvirta R, Tuomisto J. Effects of epidermal growth factor receptor deficiency and 2,3,7,8-tetrachlorodibenzo-p-dioxin on fetal development in mice. Toxicol Lett. 2004;150(3):285–291. doi: 10.1016/j.toxlet.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Montenegro MA, Palomino H. Inhibition of palatal fusion in vitro by indomethacin in two strains of mice with different H-2 backgrounds. Arch Oral Biol. 1989;34(12):949–955. doi: 10.1016/0003-9969(89)90051-4. [DOI] [PubMed] [Google Scholar]
- 26.Natsume N, Narukawa T, Kawai T. Teratogenesis of dexamethasone and preventive effect of vitamin B12 . Int J Oral Maxillofac Surg. 1986;15(6):752–755. doi: 10.1016/s0300-9785(86)80117-x. [DOI] [PubMed] [Google Scholar]
- 27.Newall DR, Edwards JRG. The effect of vitamin A on fusion of mouse palates. I. Retinyl palmitate and retinoic acid in vivo. Teratology. 1981;23(1):115–124. doi: 10.1002/tera.1420230114. [DOI] [PubMed] [Google Scholar]
- 28.Nugent P, Ma L, Greene RM. Differential expression and biological activity of retinoic acid-induced TGFβ isoforms in embryonic palate mesenchymal cells. J Cell Physiol. 1998;177(1):36–46. doi: 10.1002/(SICI)1097-4652(199810)177:1<36::AID-JCP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Pourtois M. Onset of the acquired potentiality for fusion in the palatal shelves of rats. J Embryol Exp Morphol. 1966;16(1):171–182. [PubMed] [Google Scholar]
- 30.Pratt RM, Perry EL, Chapman LM, Goulding EH. Glucocorticoid teratogenesis in mouse whole embryo culture. Teratology. 1984;30(1):71–81. doi: 10.1002/tera.1420300110. [DOI] [PubMed] [Google Scholar]
- 31.Rice DP. Craniofacial anomalies: from development to molecular pathogenesis. Curr Mol Med. 2005;5(7):699–722. doi: 10.2174/156652405774641043. [DOI] [PubMed] [Google Scholar]
- 32.Ryan RP, Sunahara GI, Lucier GW, Birnbaum LS, Nelson KG. Decreased ligand binding to the hepatic glucocorticoid and epidermal growth factor receptors after 2,3,4,7,8-pentachlorodibenzofuran and 1,2,3,4,7,8-hexachlorodibenzofuran treatment of pregnant mice. Toxicol Appl Pharmacol. 1989;98(3):454–464. doi: 10.1016/0041-008X(89)90174-9. [DOI] [PubMed] [Google Scholar]
- 33.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6(1):39–42. [PubMed] [Google Scholar]
- 34.Shah RM, Chen YP, Burdett DN. Growth of the secondary palate in the hamster following hydrocortisone treatment: shelf area, cell number, and DNA synthesis. Teratology. 1989;40(2):173–180. doi: 10.1002/tera.1420400211. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu N, Aoyama H, Hatakenaka N, Kaneda M, Teramoto S. An in vitro screening system for characterizing the cleft palate-inducing potential of chemicals and underlying mechanisms. Reprod Toxicol. 2001;15(6):665–672. doi: 10.1016/S0890-6238(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 36.Stoll C, Dott B, Alembik Y, Koehl C. Maternal trace elements, vitamin B12, vitamin A, folic acid, and fetal malformations. Reprod Toxicol. 1999;13(1):53–57. doi: 10.1016/S0890-6238(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 37.van Rooij IA, Swinkels DW, Blom HJ, Merkus HM, Steegers-Theunissen RP. Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts. Am J Obstet Gynecol. 2003;189(4):1155–1160. doi: 10.1067/S0002-9378(03)00592-1. [DOI] [PubMed] [Google Scholar]
- 38.Vargas VI. Palatal fusion in vitro in the mouse. Arch Oral Biol. 1967;12(11):1283–1288. doi: 10.1016/0003-9969(67)90130-6. [DOI] [PubMed] [Google Scholar]
- 39.Vujkovic M, Ocke MC, van der Spek PJ, Yazdanpanah N, Steegers EA, Steegers-Theunissen RP. Maternal western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstet Gynecol. 2007;110(2 Pt 1):378–384. doi: 10.1097/01.AOG.0000268799.37044.c3. [DOI] [PubMed] [Google Scholar]
- 40.Weingärtner J, Maile S, Proff P, Reicheneder C, Bienengräber V, Fanghänel J, Gedrange T. Secondary palatal closure in rats in association with relative maternofetal levels of folic acid, vitamin B12, and homocysteine. Ann Anat. 2007;189(3):229–233. doi: 10.1016/j.aanat.2006.10.006. [DOI] [PubMed] [Google Scholar]