Abstract
The histopathological features and the associated clinical findings of ulcerative colitis (UC) are due to persistent inflammatory response in the colon mucosa. Interventions that suppress this response benefit UC patients. We tested whether sodium arsenite (SA) benefits rats with dextran sulfate sodium (DSS)-colitis. The DSS-colitis was induced by 5% DSS in drinking water. SA (10 mg/kg; intraperitoneally) was given 8 h before DSS treatment and then every 48 h for 3 cycles of 7, 14 or 21 d. At the end of each cycle rats were sacrificed and colon sections processed for histological examination. DSS induced diarrhea, loose stools, hemoccult positive stools, gross bleeding, loss of body weight, loss of epithelium, crypt damage, depletion of goblet cells and infiltration of inflammatory cells. The severity of these changes increased in the order of Cycles 1, 2 and 3. Treatment of rats with SA significantly reduced this severity and improved the weight gain.
Keywords: Ulcerative colitis, Dextran sulfate sodium, Sodium arsenite, Rats
INTRODUCTION
Inflammatory bowel diseases (IBD) that include Crohn’s disease (CD), ulcerative colitis (UC), diversion colitis and pouchitis are multifactorial diseases of unknown etiology. Advances in research have identified the interaction between genetic, infectious, immunological and environmental factors to play role in their pathogenesis (Gaudio et al., 1999; Vetuschi et al., 2002). Although their exact cause remains unclear, immunological dysfunction seems to initiate a self-sustaining inflammatory process (Podolsky, 1991) manifested by elevated levels of the chemoattractant interleukin (IL)-8 that is responsible for the attraction and infiltration of neutrophils to the colon mucosa and the subsequent pathology.
Being one of the IBD, UC is a disorder involving the large intestine and is characterized by contiguous inflammation of the colonic lamina propria with subsequent injury and disruption of the mucosal barrier (Herias et al., 2005). The initial change of this inflammation is marked by neutrophil infiltration in the colon mucosa followed by other lesions such as loss of the epithelium, loss of goblet cells, and damage of the crypt. These lesions typically characterize UC. Together with the entire disease process, the lesions can be studied in animal models of colitis. The disease can be induced experimentally in rats by oral administration of dextran sulfate sodium (DSS). This is advantageous because of the following: firstly, it exhibits many symptoms which are similar to those seen in human UC including diarrhea, bloody feces, loss of body weight, mucosal ulceration and shortening of the large intestine (Okayasu et al., 1990); secondly, it is possible to induce both acute and chronic phases of the disease (Okayasu et al., 1990; Cooper et al., 1993); and lastly, the model is reproducible in terms of the time course, the development and severity of colitis in individual animals, and the relative uniformity of the lesions (Murthy et al., 1993; Arai et al., 1998; Chen et al., 2007).
Sodium arsenite (SA) contains a heavy metal, which has been proven to be a potential protective agent against several conditions including colitis (Nover, 1991; Welch, 1992; Wijeweera et al., 1995; Pritts et al., 2002; Malago, 2003). When administered at non-lethal concentrations SA induces synthesis of a specific class of proteins, known as heat shock proteins (Hsps) and development of tolerance (Lee and Hahn, 1988; Nover, 1991; Welch, 1992). The phenomenon of SA-induced tolerance is described as a stress-induced cellular resistance to a lethal stress treatment (Wiegant et al., 1993). This phenomenon forms the basis of SA-induced cellular protection against many stressful agents in both animals and cultured mammalian cells.
Studies on different models of colitis have demonstrated that SA pre-treatment has a significant protection against the decrease in cell viability, increase in mucosal permeability, and polymorphonuclear neutrophil infiltration in colitis (Venkatraman et al., 2000; Malago, 2003; Malago et al., 2003). Polymorphonuclear neutrophil infiltration is regarded as the primary and central lesion of colitis that is usually followed by loss of the epithelium, loss of goblet cells and crypt damage (Iba et al., 2003). Stemming on the evidence that SA decreases the neutrophil infiltration in the colon mucosa of colitis models and the fact that infiltration of neutrophils to this mucosa is central to colitis, it can be argued that SA may inhibit development of other lesions of colitis and subsequently prevent colitis. In this study, we aimed at exploring the features of DSS-induced colitis in rats, both acute and chronic states, and then assessing whether SA can inhibit the development of these features.
MATERIALS AND METHODS
Animals
A total of 48 male Wistar rats (Sokoine University of Agriculture, Morogoro, Tanzania) aging 26 weeks and weighing 130~160 g were used in this experiment. They were kept in cages in a restricted access room with controlled temperature and 12 h light/dark cycle and allowed to acclimatize for 2 weeks before commencement of the experiment. The animals were randomly allocated in 4 groups with a maximum of 12 animals per cage. Standard laboratory diet and DSS-treated or -untreated drinking water were available ad libitum. The study was approved by the Animal Research Committee of the Sokoine University of Agriculture, Tanzania.
Induction of colitis
UC was induced by oral administration of 5% (w/v) DSS (average M w>500 000, Sigma, St. Louis, USA) ad libitum in drinking water for 1, 2 or 3 cycles. A cycle constituted of 7 d of treatment with DSS. When rats proceeded to Cycles 2 and 3, the 7 d of treatment with DSS were followed by 7 d of return to normal drinking water between consecutive cycles. Rats (4 in each group; 4 groups) were sacrificed at the end of each cycle by chloroform. One set of the entire experiment took 35 d. The DSS-containing drinking water was changed daily.
Effect of SA on DSS colitis
UC was induced as described above. SA-treated rats received SA (Sigma, St. Louis, USA) at 10 mg/kg intraperitoneally. Treatments with SA started 8 h before exposure to DSS and were continued every 48 h during DSS treatment but withheld on return to normal drinking water. Matched control groups received either SA or physiological saline at equal dose, volume and time.
Evaluation of clinical colitis
In order to determine the clinical activity of DSS colitis, we examined the rats for stool consistency (loose stool, diarrhea), occult and/or bleeding (hemoccult positivity, gross bleeding) and body weight on daily basis during DSS administration. The body weight was expressed as percentage weight change for each individual rat and was calculated compared with that on Day 0. These data were used to calculate a disease activity index as described earlier by Cooper et al.(1993) (Table 1).
Table 1.
Scoring of DSS-induced colitis activity index as described by Cooper et al.(1993)
| Score | Weight loss (%) | Stool consistency1 | Occult/gross bleeding |
| 0 | None | Normal | Normal |
| 1 | 1~5 | − | − |
| 2 | 5~10 | Loose stools | Hemoccult positive |
| 3 | 10~20 | − | − |
| 4 | >20 | Diarrhea | Gross bleeding |
DSS colitis activity index=(combined scores of weight loss (compared to Day 1 of the experiment), stool consistency and bleeding)/3;
Normal stools: Well formed pellets; Loose stools: Pasty and semi-formed stools which do not stick to the anus; Diarrhea: Liquid stools that stick to the anus
Evaluation of histopathological findings
Sacrificed rats were opened by midline laparotomy. The colon was longitudinally opened, fixed with 10% neutral buffered formalin and embedded in paraffin. After deparaffinizing the thin tissue sections on glass slides, they were stained with hematoxylin and eosin (H & E). For histological evaluation of the tissue damage, mucosal integrity was assessed in a blinded manner and quantitated by two independent pathologists using a slightly modified method by Iba et al.(2003). The scoring was on a 0~3 scale as shown in Table 2. The histological evaluation was done on the distal colon.
Table 2.
Histological score of colitis according to Iba et al.(2003)
| Score | Loss of epithelium (%) | Crypt damage1(%) | Depletion of goblet cells | Infiltration of inflammatory cells |
| 0 | None | None | None | None |
| 1 | 0~5 (mild) | 0~10 (mild) | Mild | Mild |
| 2 | 5~10 (moderate) | 10~20 (moderate) | Moderate | Moderate |
| 3 | >10 (severe) | >20 (severe) | Severe | Severe |
Crypt damage was evaluated as percentage loss of crypt
Statistical analysis
All numerical values are presented as mean±SEM. Statistical significance between the mean values of control, DSS-, SA+DSS-, and SA-treated rats was assessed by one-way analysis of variance (ANOVA) with comparison of means. Unless indicated, the differences were considered significant at 95% confidence interval using the Student’s t-test.
RESULTS
Clinical findings of DSS colitis and the effect of SA
1. Acute and chronic colitis
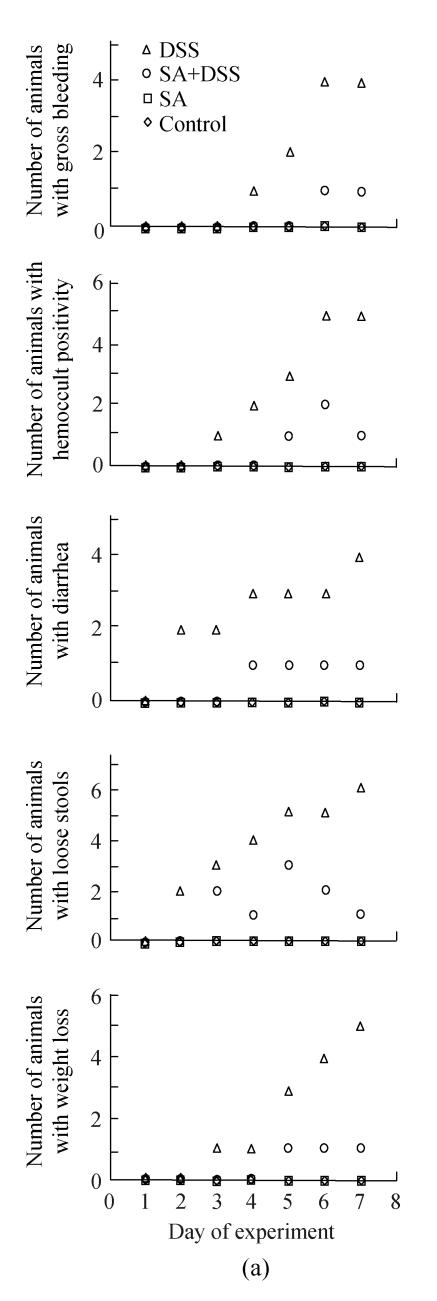
Fig.1 shows the clinical signs of both acute (Cycle 1) and chronic (Cycles 2 and 3) DSS colitis. As shown in Fig.1, the earliest noted clinical signs for animals in Cycle 1 were diarrhea and loose stools, which occurred on Day 2, followed by hemoccult positive stools and weight loss on Day 3, and gross bleeding on Day 4. The number of animals developing the signs was increasing steadily to reach half (gross bleeding and diarrhea) and more than half (hemoccult positive stools, loose stools, and weight loss) of the animals at the end of the cycle.
Fig. 1.
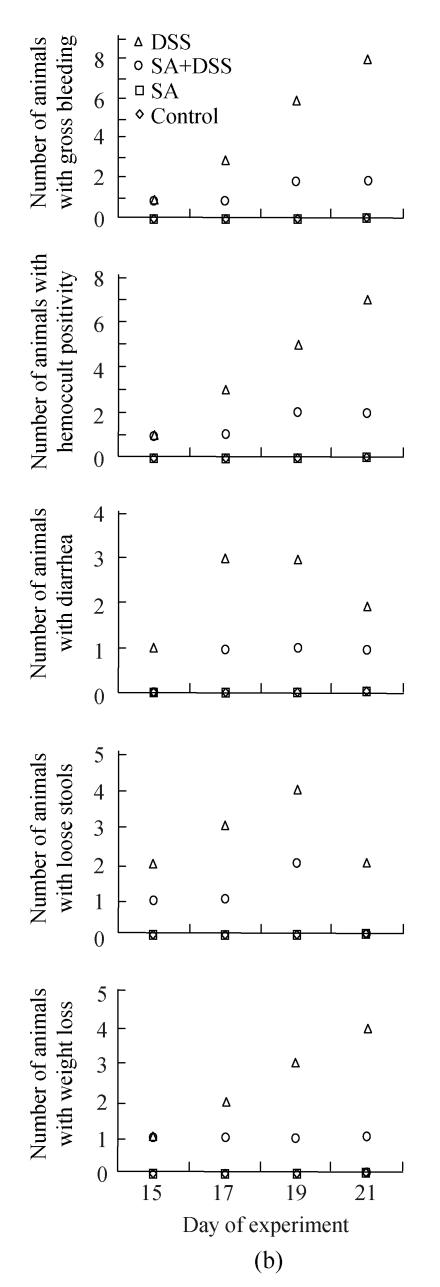
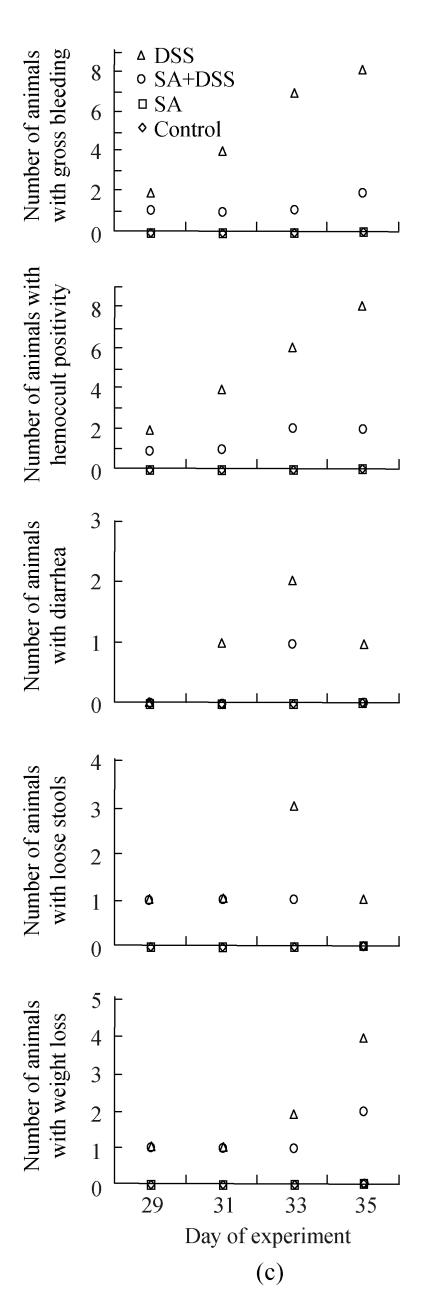
Effect of sodium arsenite (SA) on clinical indices of colitis. Rats were treated with 5% dextran sulfate sodium (DSS) orally, SA (10 mg/kg, intraperitoneally) followed by DSS or SA alone. Values for control rats are also indicated but overlap with those of SA. (a) Cycle 1; (b) Cycle 2; (c) Cycle 3
Weight loss is in comparison to Day 1 of DSS administration
In Cycle 2, all the clinical findings were first observed in 1 or 2 animals on Day 15 of the experiment. Animals showing gross bleeding, hemoccult positivity and weight loss increased steadily so that at the end of the cycle all animals had gross bleeding; all but one had hemoccult positive stools; and half of them had lost their weight. The numbers of animals with diarrhea or loose stools were the highest on Day 17 or 19.
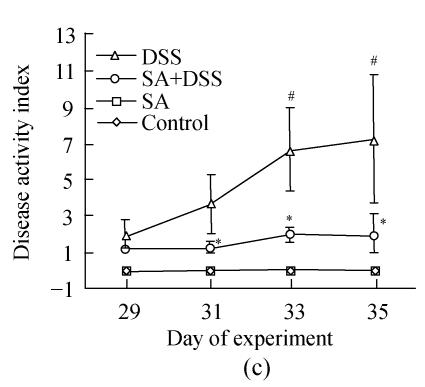
In Cycle 3, the clinical signs were observed on the first day of the cycle except diarrhea, which was first seen on Day 31. In this cycle, the number of animals showing gross bleeding and hemoccult positive stools increased markedly to involve half and all animals on Days 31 and 35, respectively; half of the animals had lost their weight at the end of the cycle; and the highest numbers of animals with diarrhea or loose stools were observed on Day 33. Diarrhea was the least finding observed in both Cycles 2 and 3.
Fig.1 also shows that administration of SA delayed and suppressed the clinical findings of DSS colitis. In Cycle 1, the first appearance of gross bleeding, hemoccult positive stools, diarrhea and weight loss was delayed by two days while that of loose stools was delayed by one day. Only diarrhea was delayed in Cycles 2 and 3 for two days. SA also lowered the number of rats developing the DSS colitis signs right from the day of their first appearance (Cycle 1) and continued throughout the cycle reaching highest on Day 7. The fall was also the highest for most signs at the end of Cycles 2 and 3 whereby the number of animals showing gross bleeding and hemoccult positive stools were only two; one animal or none showed diarrhea or loose stools; and weight loss was observed in one and two animals in Cycles 2 and 3, respectively.
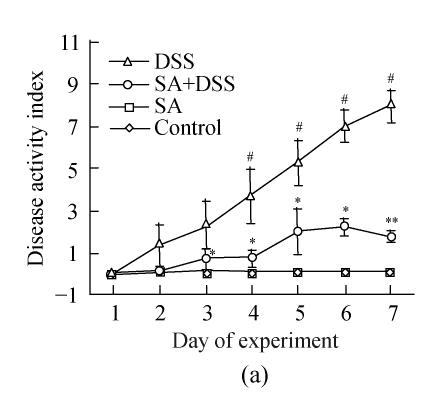
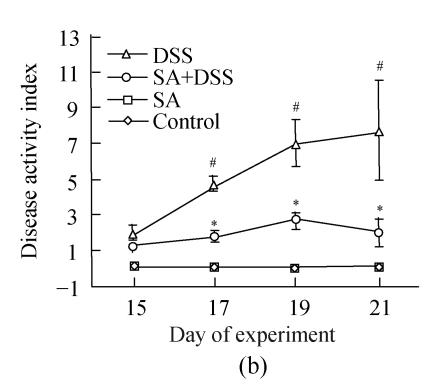
2. DSS colitis activity index
Up to Day 3 of DSS administration the disease activity index of animals in Cycle 1 was insignificant. It progressively and significantly increased from Day 4 to the end of the cycle where it was the highest (Fig.2). In both Cycles 2 and 3 the disease activity index was significantly high on the first day and increased significantly every second day in Cycle 2, and on Days 33 and 35 in Cycle 3. Treatment with SA lowered the index in all cycles. In Cycle 1, already on Day 3 SA had significantly suppressed it by about 3-fold while on Day 7 the suppression was maximal (about 5-fold). In Cycles 2 and 3 SA significantly suppressed the index from the second day of each cycle and reached maximum at the end of the cycles (about 3.5-fold).
Fig. 2.
Effect of sodium arsenite (SA) administration on dextran sulfate sodium (DSS) colitis disease activity index. Rats were treated with 5% DSS orally, SA (10 mg/kg, intraperitoneally) followed by DSS or SA alone. Values for control rats are also indicated but overlap with those of SA. (a) Cycle 1; (b) Cycle 2; (c) Cycle 3
Disease activity index=(combined scores of weight loss (compared to Day 1 of the experiment), stool consistency and bleeding)/3; Results are expressed as the mean±SEM (n=8); * P<0.05, ** P<0.01, significantly different from DSS colitis group; # P<0.05, compared to those of DSS at the beginning of respective cycle
Gross pathology
The colon mucosa of DSS-treated animals sacrificed at the end of the first cycle appeared edematous with hemorrhagic erosions scattered along the entire colon. In the second and third cycles, marked congestion of mesenteric blood vessels, edema of the colon, and multiple erosions of mucosa were observed. These lesions were more pronounced in the distal colon than in the proximal and middle colon. Animals treated with a combination of DSS and SA showed less marked congestion of the colon without edema. The SA-treated and control animals had no gross pathological changes (data not shown).
Histopathology
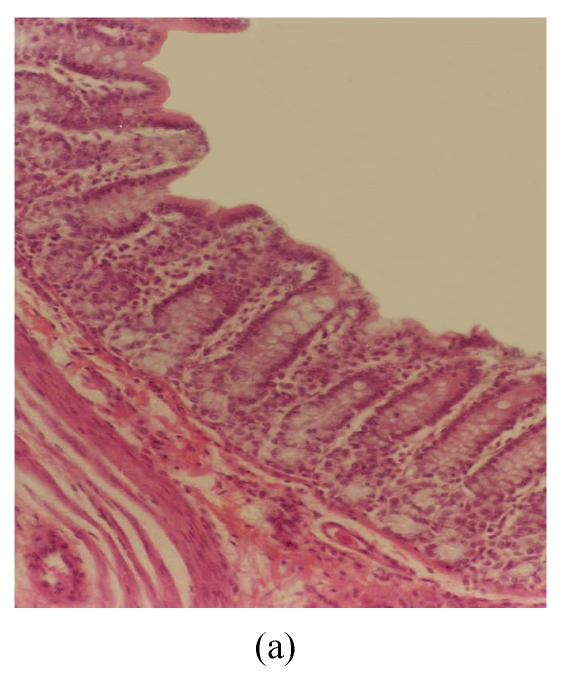
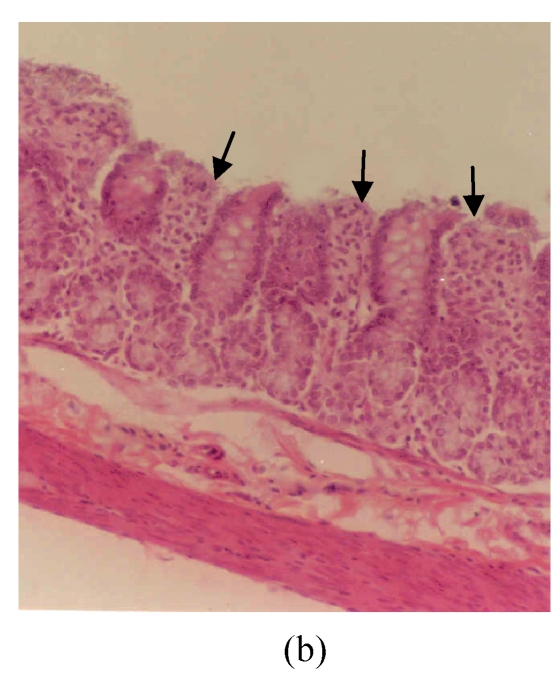
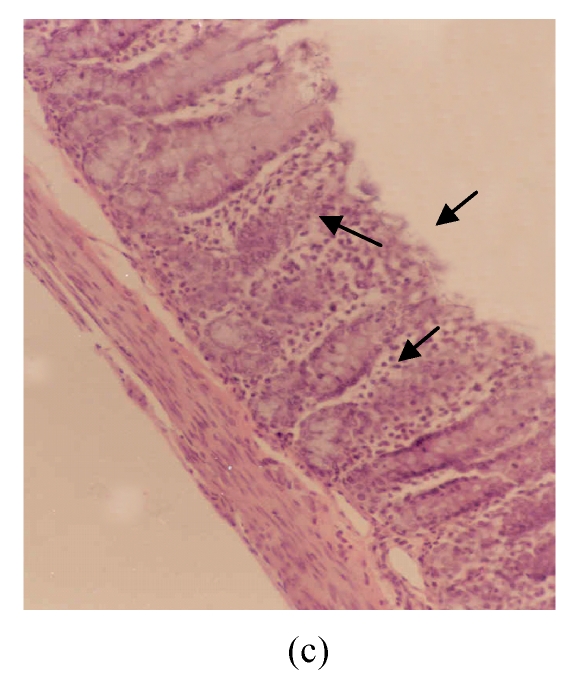
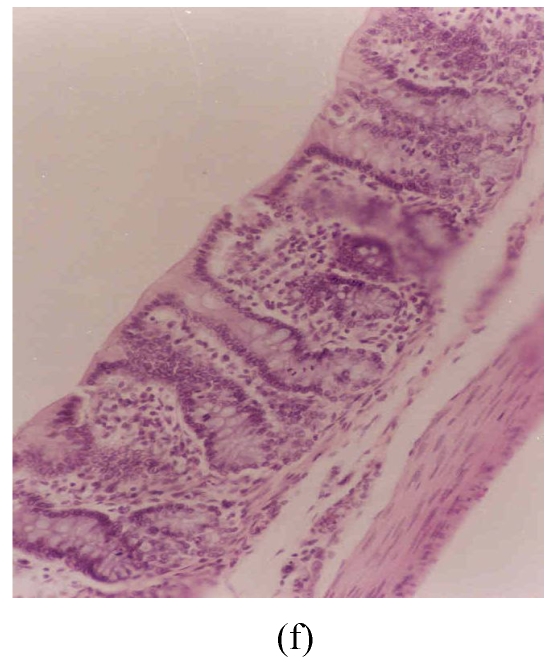
The observed histological lesions are shown in Fig.3. Figs.3a~3c show the colon mucosa of rats exposed to DSS and sacrificed at the end of Cycles 1, 2 and 3 of the experiment, respectively. Rats sacrificed at the end of Cycle 1 (Fig.3a) were least severely affected when compared to those of Cycles 2 and 3. They had quite a good epithelial lining and crypts that were spared, and little infiltration of inflammatory cells in the mucosa, and the goblet cells were depleted slightly. Rats in Cycle 2 (Fig.3b) were more severely affected. Their epithelium was highly damaged with little epithelial lining on the mucosal surface; many crypts were damaged with subsequent loss of goblet cells; and the infiltration of inflammatory cells was more vivid. At the end of Cycle 3, rats had complete absence of epithelial lining on the mucosal surface, loss of epithelium lining the crypts, severe crypt damage, loss of goblet cells (the remaining goblet cells were hyperplastic), and severe infiltration of inflammatory cells (Fig.3c). Compared to rats sacrificed at the end of Cycle 1 (Fig.3a) and Cycle 2 (Fig.3b), these rats (Fig.3c) were the most severely affected.
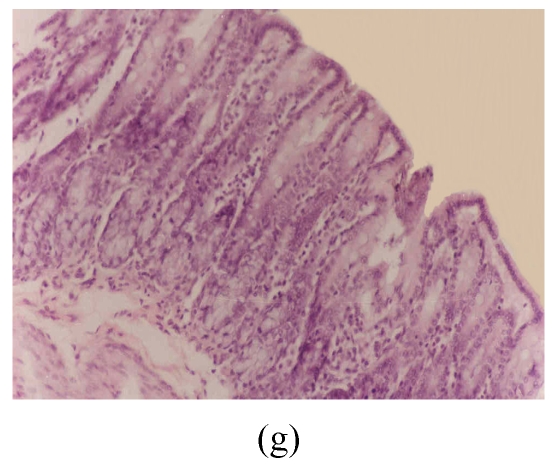
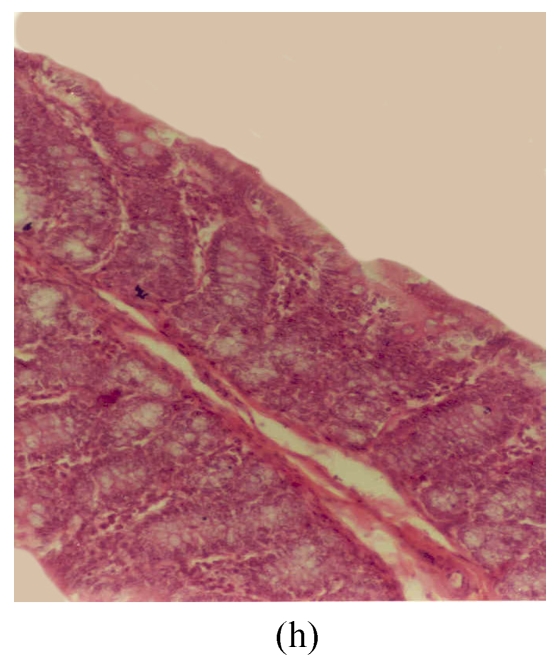
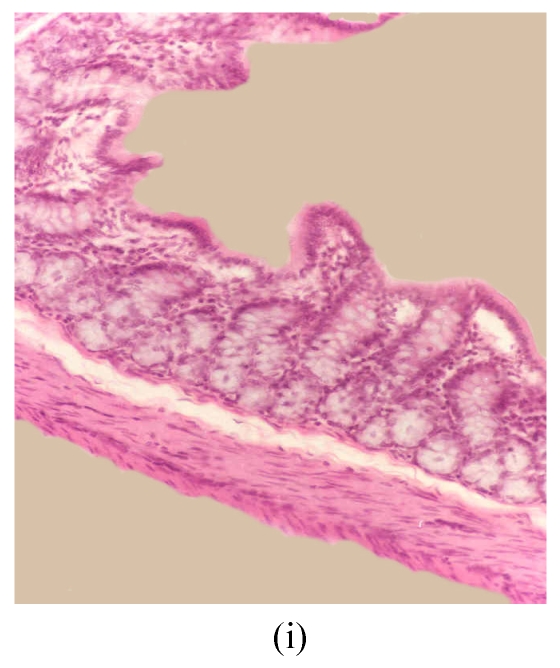
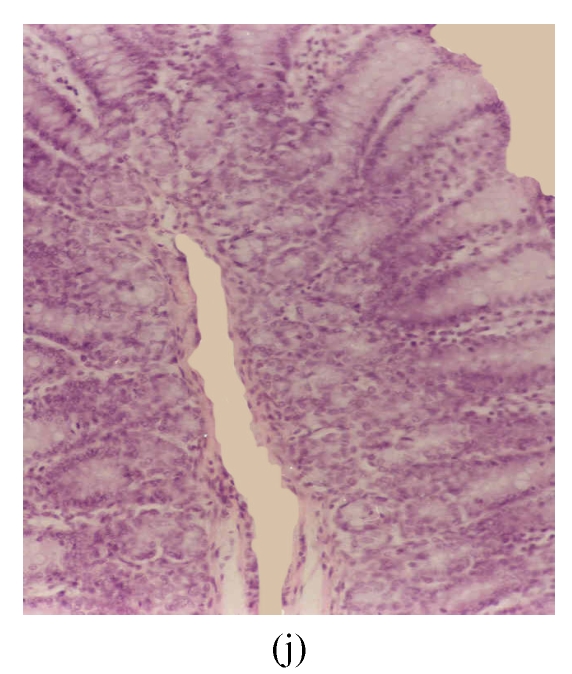
Fig. 3.
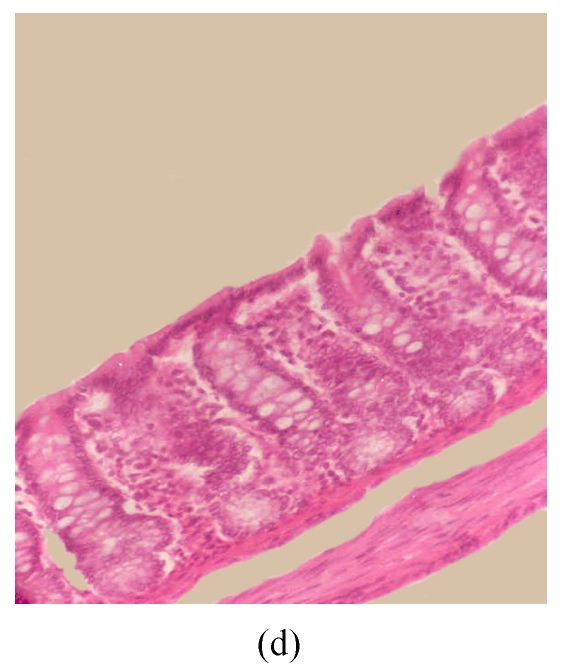
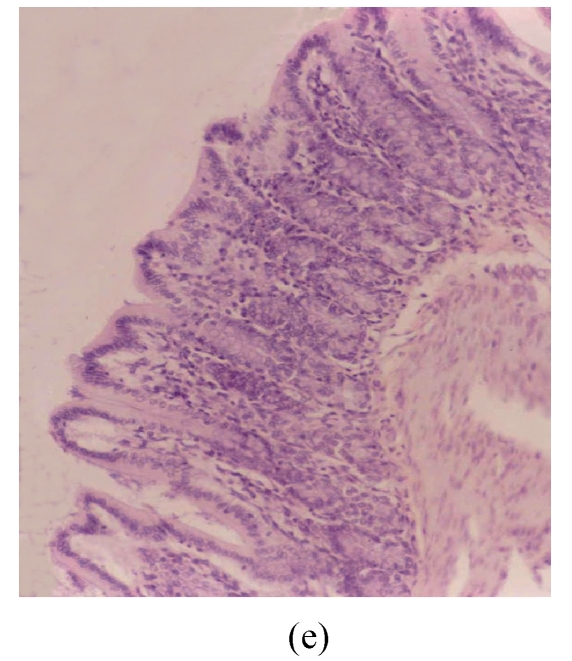
Histological sections of rat colon. (a), (b), (c) Rats were treated with 5% dextran sulfate sodium (DSS) orally; (d), (e), (f) Rats were treated with SA (10 mg/kg, intraperitoneally) followed by DSS; (g), (h), (i) Rats were treated with SA alone; (j) The control animal. (a), (d), (g) Cycle 1; (b), (e), (h) Cycle 2; (c), (f), (i) Cycle 3
Hematoxylin and eosin (H & E); 100× original magnification; Arrows indicating loss of epithelium; Each photo representing 8 animals (4 animals per treatment in duplicate experiments)
Figs.3d~3f depict the effect of pre-treatment of rats with SA on DSS-induced colitis. At the end of Cycle 1 (Fig.3d) rats had a considerably intact epithelial lining, little crypt damage, hyperplastic goblet cells that were not significantly lost, and mild infiltration of inflammatory cells. There was little or no difference between these rats and those exposed to DSS alone sacrificed at the end of Cycle 1 (Fig.3a). At the end of Cycle 2, rats had mild crypt damage, mild loss of goblet cells, and moderate infiltration of inflammatory cells (Fig.3e). When compared to rats exposed to DSS alone (Fig.3b), it is obvious that the epithelial lining in these rats (Fig.3e) was significantly improved. Rats in Cycle 3 exhibited a good epithelial lining on the mucosal surface, almost no crypt damage, mild loss of goblet cells, and little infiltration of inflammatory cells (Fig.3f). These rats had highly improved from the disease when compared to those not receiving SA (Fig.3c).
The results of rats exposed to SA alone and sacrificed at the end of Cycles 1, 2 and 3 of the experiment are shown in Figs.3g, 3h and 3i, respectively. In all these figures the epithelial lining is intact, there is no crypt damage, there is no or very little goblet cell depletion, and there is very mild infiltration of inflammatory cells. These results do not differ much from the normal control rats (Fig.3j).
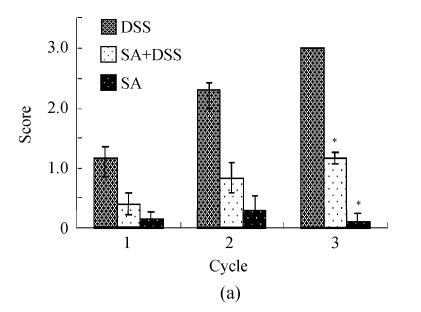
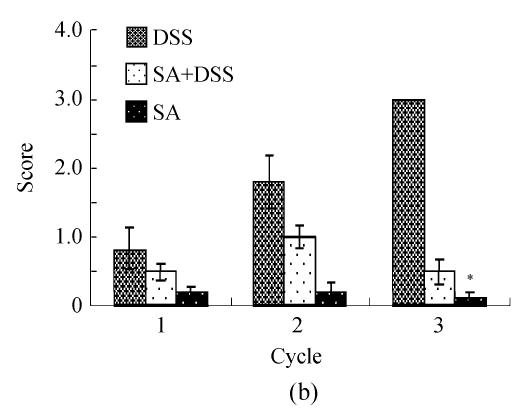
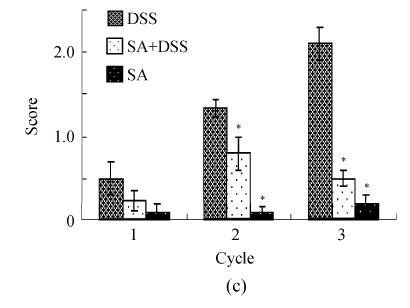
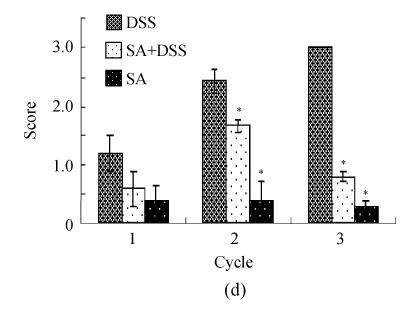
Scores of histopathological lesions
Scores of the histopathological lesions are demonstrated in Fig.4. It is clear from Fig.4a that exposure to DSS led to a progressive increase in the loss of epithelium throughout the experiment. The loss was about 5% (average score=1) at the end of Cycle 1 to more than 10% (average score=3) at the end of Cycle 3. Pre-treatment with SA significantly reduced the epithelial loss to about half in Cycle 1 and about 5% (average score=1) at the end of both Cycles 2 and 3. Control rats and those treated with SA alone had insignificant losses of epithelium.
Fig. 4.
Histological scores of loss of epithelium (a), crypt damage (b), goblet cell depletion (c), and infiltration of inflammatory cells (d). Rats were treated with 5% dextran sulfate sodium (DSS) orally, SA (10 mg/kg, intraperitoneally) followed by DSS or SA alone
Results are mean±SEM of at least 8 individual rats. * P<0.05, significantly different from DSS exposed rats
Fig.4b reveals that DSS caused about 10% loss of crypt (average score=1) at the end of Cycle 1; about 20% (average score=2) and over 20% (average score=3) at the end of Cycles 2 and 3, respectively. SA reduced the severity of crypt damage insignificantly in Cycle 1, but significantly in Cycles 2 and 3. In Cycle 2 the reduction was about one third, while in Cycle 3 it was about 6 times. SA itself had little or no effect on crypt damage.
Results presented in Fig.4c show that DSS induced a very mild (average score=0.5), mild (average score=1.2), and moderate (average score=2) depletion of goblet cells at the end of Cycles, 1, 2 and 3, respectively. SA had insignificant effect in Cycle 1, but significantly decreased the goblet cell depletion to about one third and to about 4-fold at the end of Cycles 2 and 3, respectively. In all cycles, SA alone did not cause depletion of goblet cells.
The infiltration of inflammatory cells in the colon mucosa of rats treated with DSS was mild (average score=1) at the end of Cycle 1, moderate (average score=2.5) at the end of Cycle 2; and severe (average score=3) at the end of Cycle 3 (Fig.4d). Pre-treatment with SA had insignificant effect at the end of Cycle 1, but significantly decreased this infiltration at the end of Cycle 2 (about one third fold) and Cycle 3 (more than 3-fold). In each of the three cycles SA alone did not cause any significant infiltration of inflammatory cells into the colon mucosa.
DISCUSSION
The primary aim of this study was to evaluate the effect of administration of SA to DSS-induced colitis in rats. Our data extend the fact that DSS is capable of inducing colitis in rats characterized clinically by diarrhea, loose stools, hemoccult positive stools, bleeding, and loss of body weight; and histologically by loss of epithelium, crypt damage, depletion of goblet cells, and infiltration of inflammatory cells in the colon mucosa. The severity of the disease increases with chronicity. Treatment with SA delays the appearance of clinical signs in acute colitis and greatly reduces the severity in both acute and chronic rat models of DSS colitis.
The DSS colitis clinical signs and morphological alterations observed in our study are consistent with findings of various studies. In several rat models of DSS colitis, diarrhea, loose stools, hemoccult positive stools, gross bleeding, and loss of body weight are common signs observed (Gaudio et al., 1999; Kullmann et al., 2001; Vetuschi et al., 2002; Iba et al., 2003). They can occur as early as Day 2 in acute colitis rat models and as Day 4 in chronic models (Kullmann et al., 2001). Consistently, our first observation of DSS colitis clinical signs was on day 2 in acute colitis (Cycle 1) and on Day 1 of Cycles 2 and 3 (chronic model) (Fig.1). Our observation of clinical signs on Day 1 of Cycles 2 and 3 suggests that they are a result of previous treatments in preceding cycles. This harmonizes with other experimental setups in which chronic colitis signs were observed several days after replacement of DSS with normal drinking water. According to other researchers, these signs can be observed as long as 10 d after replacement of DSS with drinking water (Kullmann et al., 2001; Iba et al., 2003). Since there were only 7 d of treatment with drinking water between cycles in our setup, the observed clinical signs on the beginning of Cycles 2 and 3 are tied to the preceding cycle of DSS treatment.
Our data further show that in each cycle the disease activity index was increasing and reached the highest at the end of each cycle. In the entire experiment, it was maximal at the end of Cycle 1 (Day 7). In addition, this study indicates that the disease activity index increases as long as animals are under DSS treatment. These observations are in consistent with findings by Kullmann et al.(2001) who noted a maximal disease activity on last days of DSS treatment in various cycles of both acute and chronic colitis models. The disease activity decreased when DSS administration was replaced by drinking water. This is in harmony with our finding that rats, proceeding to Cycle 2 or 3 and being given drinking water between consecutive cycles, had lower disease activity index at the beginning of a cycle than that at the end of the preceding cycle.
The clinical manifestations observed in our experiment were a reflection of histological changes. Most of these histological changes have been observed by others in several DSS colitis rat models (Gaudio et al., 1999; Iba et al., 2003; Herias et al., 2005). Peculiar to our study, we noted that the severity of these lesions increased with time so that the most severe lesions were observed in Cycle 3, less severe in Cycle 2, and the least severe in Cycle 1. The variations in severity of these histological findings were also reflected in the clinical signs. We observed more animals with severe clinical signs (gross bleeding and hemoccult positive stools) in Cycles 2 and 3 than in Cycle 1. In fact, all animals had gross bleeding and hemoccult positive stools (except one in Cycle 2) at the end of Cycles 2 and 3. On the contrary, the less severe signs of diarrhea and loose stools were observed in fewer animals in Cycles 2 and 3 than in Cycle 1. This progressive increase in the severity of the clinical signs of the disease from Cycle 1 to Cycle 3 could be accounted for by the increasing damage to the colon by DSS. According to Iba et al.(2003), damage to the intestinal crypt is not yet repaired even at Day 20 of replacement of DSS solution with drinking water. In a way, this is in consistency with our findings depicted by the progressive loss of epithelium and crypt damage through the 3 cycles. The severest crypt damage in Cycle 3 could, therefore, be a cumulative effect of all the DSS treatment cycles since the 7 d of replacement with water cannot offer enough time for the crypt to regenerate (Iba et al., 2003). The histological lesions, together with the clinical signs of loose stools, diarrhea, hemoccult positive stools, and gross bleeding were severe enough to compromise the normal colon function (such as absorption) and could thus account for the losses in the body weight.
It is now becoming clear that the development and pathology of UC are due to persistently elevated levels of the neutrophil chemoattractant IL-8 in the colon mucosa (Hecht and Savkovic, 1997; Hata et al., 2001). This chemoattractant is produced following activation of the transcription factor, nuclear factor kappa B (NF-κB) (Malago et al., 2002). DSS can activate this factor and could in turn lead to synthesis and secretion of IL-8 (Marrero et al., 2000). The IL-8 then attracts inflammatory cells that infiltrate the colon mucosa, and accounts for the morphological alterations and the subsequent clinical signs. In inflammatory bowel diseases, continual activation of NF-κB and persistent elevation of IL-8 seem to be the common course of the intestinal inflammations for many causes. DSS colitis could also follow this pathogenesis.
The findings that colitis lesions in rats pre-treated with SA (Figs.3d~3f) were less severe than those in rats without pre-treatment (Figs.3a~3c) suggest that SA conferred a protective effect against the DSS induced colitis. The phenomenon of SA-mediated protection has been reported in several other conditions such as hepatic, heart muscle and neuronal necroses, sepsis and endotoxaemia in rats and human (Lowenstein et al., 1991; Ribeiro et al., 1994; Chen et al., 2005). In the gut, Wang and Hasselgren (2002) explored the administration of SA in septic mice that had increased intestinal permeability. They found out that SA pre-treatment reduced the sepsis-induced increase in intestinal permeability. Other studies have demonstrated that SA pre-treatment has a significant protection against the decrease in cell viability, increase in mucosal permeability, and polymorphonuclear neutrophil infiltration in colitis (Venkatraman et al., 2000; Malago, 2003; Malago et al., 2003). Since polymorphonuclear neutrophil infiltration is the primary and central lesion of colitis and is usually followed by loss of the epithelium, loss of goblet cells and crypt damage (Iba et al., 2003), it follows that the observed SA protection against colitis in this study could be mediated via its ability to inhibit the polymorphonuclear neutrophil infiltration and the subsequent pathology. SA is well known to mediate most of its effects via activation of the heat shock response that, in turn, suppresses the inflammatory response (Malago et al., 2002). The dose of SA used in this study (10 mg/kg, intraperitoneally, every 48 h) had a broad safety margin, as none of the rats died or showed abnormal behavior due to SA.
CONCLUSION
In conclusion, we have shown in this study that SA has a protective effect on DSS colitis. Although direct link needs further studies, it is argued that the protective effect of SA on DSS colitis revolves around the role of DSS to activate inflammatory response and the effect of SA to suppress this inflammatory response. SA could mediate its protection, at least in part, via its putatively known mechanism of activating the heat shock response that, in turn, suppresses the inflammatory response. Further studies are needed to elucidate this. To our knowledge, this is a first study on the effect of SA on DSS colitis in rats.
References
- 1.Arai Y, Takanashi H, Kitagawa H, Okayasu I. Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine. 1998;10(11):890–896. doi: 10.1006/cyto.1998.0355. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Pan J, Du B, Sun D. Induction of the heat shock response in vivo inhibits NF-kappa B activity and protects murine liver from endotoxemia-induced injury. J Clin Immunol. 2005;25(5):452–461. doi: 10.1007/s10875-005-5636-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Si JM, Liu WL, Cai JT, Du Q, Wang L, Liang J, Gao M. Induction of experimental acute ulcerative colitis in rats by administration of dextran sulfate sodium at low concentration followed by intracolonic administration of 30% ethanol. J Zhejiang Univ Sci B. 2007;8(9):632–637. doi: 10.1631/jzus.2007.B0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 5.Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R. Dextran sulfate sodium colitis in rats: clinical, structural and ultrastructural aspects. Dig Dis Sci. 1999;44(7):1458–1475. doi: 10.1023/A:1026620322859. [DOI] [PubMed] [Google Scholar]
- 6.Hata K, Andoh A, Sato H, Araki Y, Tanaka M, Tsujikawa T, Fujiyama Y, Bamba T. Sequential changes in luminal microflora and mucosal cytokine expression during developing of colitis in HLA-B27/beta2-microglobulin transgenic rats. Scand J Gastroenterol. 2001;36(11):1185–1192. doi: 10.1080/00365520152584824. [DOI] [PubMed] [Google Scholar]
- 7.Hecht G, Savkovic SD. Effector role of epithelia in inflammation: interaction with bacteria. Aliment Pharmacol Ther (Suppl) 1997;3:64–69. doi: 10.1111/j.1365-2036.1997.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 8.Herias MV, Koninkx JFJG, Vos JG, Huis in′t Veld JHJ, van Dijk JE. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. IJFM. 2005;103(2):143–155. doi: 10.1016/j.ijfoodmicro.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Iba Y, Sugimoto Y, Kamei C, Masukawa T. Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. Int Immunopharmacol. 2003;3(4):485–491. doi: 10.1016/S1567-5769(02)00299-0. [DOI] [PubMed] [Google Scholar]
- 10.Kullmann F, Messmann H, Alt M, Gross V, Bocker T, Scholmerich J, Ruschoff J. Clinical and histopathological features of dextran sulfate sodium induced acute and chronic colitis associated with dysplasia in rats. Int J Colorectal Dis. 2001;16(4):238–246. doi: 10.1007/s003840100311. [DOI] [PubMed] [Google Scholar]
- 11.Lee KJ, Hahn GM. Abnormal proteins as the trigger for the induction of stress responses: heat, diamide, and sodium arsenite. J Cell Physiol. 1988;136(3):411–420. doi: 10.1002/jcp.1041360304. [DOI] [PubMed] [Google Scholar]
- 12.Lowenstein DH, Chan PH, Miles MF. The stress protein response in cultured neurons: characterization and evidence for a protective role in excitotoxicity. Neuron. 1991;7(6):1053–1060. doi: 10.1016/0896-6273(91)90349-5. [DOI] [PubMed] [Google Scholar]
- 13.Malago JJ. The Role of the Heat Shock Response in the Cytoprotection of the Intestinal Epithelium. Addix: Wijk bij Duurstede; 2003. (Ph.D Thesis) [Google Scholar]
- 14.Malago JJ, Koninkx JFJG, van Dijk JE. The heat shock response and cytoprotection of intestinal epithelium. Cell Stress Chaperones. 2002;7(2):191–199. doi: 10.1379/1466-1268(2002)007<0191:THSRAC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malago JJ, Koninkx JFJG, Ovelgönne HH, van Asten FJAM, Swennenhuis JF, van Dijk JE. Expression levels of heat shock proteins in enterocyte-like Caco-2 cells after exposure to Salmonella enteritidis . Cell Stress Chaperones. 2003;8(2):194–203. doi: 10.1379/1466-1268(2003)008<0194:ELOHSP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV. Dextran sulfate sodium-induced murine colitis activates NF-κB and increases galanin-1 receptor expression. Am J Physiol Gastrointest Liver Physiol. 2000;278(5):G797–G804. doi: 10.1152/ajpgi.2000.278.5.G797. [DOI] [PubMed] [Google Scholar]
- 17.Murthy SNS, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38(9):1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 18.Nover L. HSFs and HSPs—a stressful program on transcription factors and chaperones. New Biol. 1991;3(9):855–859. [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 21.Pritts TA, Wang Q, Sun X, Fischer DR, Hungness ES, Fischer JE, Wong HR, Hasselgren PO. The stress response decreases NF-kappa B activation in liver of endotoxemic mice. Shock. 2002;18(1):33–37. doi: 10.1097/00024382-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein 72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22(6):922–929. doi: 10.1097/00003246-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Venkatraman A, Ramakrishna BS, Pulimood AB, Patra S, Murthy S. Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand J Gastroenterol. 2000;35(10):1053–1059. doi: 10.1080/003655200451171. [DOI] [PubMed] [Google Scholar]
- 24.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in DSS-induced colitis in rats. Dig Dis Sci. 2002;47(7):1447–1457. doi: 10.1023/A:1015931128583. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Hasselgren PO. Heat shock response reduces intestinal permeability in septic mice: potential role of interleukin-10. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R669–R676. doi: 10.1152/ajpregu.00606.2001. [DOI] [PubMed] [Google Scholar]
- 26.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 27.Wiegant FA, Souren JE, van Rijn H, van Wijk R. Arsenite induced sensitization and self-tolerance of Reuber H35 hepatoma cells. Cell Biol Toxicol. 1993;9(1):49–59. doi: 10.1007/BF00755139. [DOI] [PubMed] [Google Scholar]
- 28.Wijeweera JB, Thomas CM, Gandolfi AJ, Brendel K. Sodium arsenite and heat shock induce stress proteins in precision-cut rat liver slices. Toxicology. 1995;104(1-3):35–45. doi: 10.1016/0300-483X(95)03119-Z. [DOI] [PubMed] [Google Scholar]